Abstract
Manganese (Mn) deficiency is a major factor underlying brittle leaf disease in date palm, yet its root-centered mechanisms under field conditions remain poorly understood. Nine mature palms (three per health category: healthy, asymptomatic Mn-deficient, and BLD-affected) were assessed for soil chemistry (pH, salinity), root Mn concentration and hydraulics, canopy pigments and chlorophyll fluorescence (Fv/Fm), as well as metabolic responses. Elevated soil pH and variable salinity significantly constrained root Mn uptake and water conductance, leading to a ~60% decline in root Mn, a 20% reduction in root water content, an 80% loss of chlorophyll, and a 26% decrease in Fv/Fm. These changes induced strong metabolic reprogramming, including a twofold rise in glucose, increased protein content, and a tenfold enhancement in peroxidase activity. Asymptomatic palms already displayed early declines in pigments and fluorescence, highlighting their diagnostic value. This study demonstrates that soil-driven Mn deficiency impairs root function and cascades to canopy physiology and metabolism, offering realistic avenues for rhizosphere management and early field diagnosis in arid oases.
1. Introduction
The date palm (Phoenix dactylifera L.) is a keystone species in arid and semi-arid agroecosystems of North Africa and the Middle East, where it sustains rural livelihoods and provides vital ecological and cultural services. Beyond its agronomic importance, genomic studies have revealed key adaptations of the date palm genome to drought, salinity, and oxidative stress [1,2,3]. Cultivated on more than 1.3 million ha across North Africa and the Arabian Peninsula, date palm supports both subsistence and commercial farming under extreme aridity. Over recent decades, oasis management has shifted from traditional multi-strata systems integrating palms, fruit trees, and forages toward high-density monocultures emphasizing cultivars such as Deglet Nour. While modernization initially enhanced yields, it increased dependence on groundwater and chemical fertilizers, accelerating salinization and soil alkalinization. Declining organic inputs and limited micronutrient monitoring have gradually reduced the long-term resilience of oasis ecosystems [4]. In Tunisia, Algeria, and Saudi Arabia, increasing groundwater use and mineral fertilization, coupled with recurrent droughts and rinsing temperatures, now threaten the sustainability of oasis-based agroecosystems. Tunisia remains one of the leading producers of high-quality cultivars, particularly Deglet Nour, yet the sustainability of its groves is increasingly jeopardized by brittle leaf disease (BLD), a chronic physiological disorder of major agronomic concern.
First reported in southern Tunisia in the 1980s, BLD has since become endemic in the Djerid region, causing interveinal chlorosis, leaflet brittleness, vascular collapse, and eventual palm decline [5,6]. The disorder has also been reported in adjacent Algerian oases, indicating that BLD occurs under similar environmental and management conditions across the North African date-producing belt [5,7,8]. Unlike infectious diseases, BLD has not been linked to a consistent biotic agent, and multiple independent field and experimental investigations have confirmed its nutritional origin. Converging evidence indicates that manganese (Mn) deficiency is the primary causal factor of this disorder [5,8,9].
Manganese is an essential micronutrient with multiple physiological functions in plant: it stabilizes the oxygen-evolving complex (OEC) of photosystem II, activates antioxidant enzymes, and regulates carbohydrate and protein metabolism [10,11,12]. The Mn4CaO5 cluster of PSII and associated extrinsic proteins are particularly sensitive to Mn storage, leading to impaired oxygen evolution [13,14]. In date palm and other perennial crops, Mn deficiency disrupts PSII photochemistry, decreases Rubisco and ATP synthase activities, and enhances the production of reactive oxygen species through altered SOD isoenzyme expression [15,16,17]. In roots, Mn scarcity intensifies endodermal suberization and disturbs ion homeostasis, ultimately restricting hydraulic conductance [18,19]. Consequently, Mn limitation triggers cascading physiological and metabolic disturbances, leading to reduced chlorophyll content, photosynthetic efficiency, and overall plant vitality.
Although Mn is generally present in oasis soils, BLD-affected palms consistently display reduced Mn in both roots and leaves, suggesting impaired uptake and restricted translocation [10,20]. In alkaline and saline environments, soil pH, redox potential, and carbonate equilibria strongly influence Mn speciation and solubility [16,21,22]. These edaphic constraints, further intensified by moisture fluctuations, markedly reduce Mn bioavailability and may drive the onset of Mn deficiency in palms. Root-level dysfunction, including reduced fine-root density, tissue browning, and decreased hydraulics conductance, is frequently in affected trees reinforcing the hypothesis that root impairment plays a central role in BLD progression [23,24,25]. However, the mechanistic integration of soil chemistry, root physiology and canopy metabolism remains incomplete.
Climate change and increasing aridity exacerbate soil alkalinity, salinity, and water scarcity further altering micronutrient dynamics and predisposing palms to nutritional disorders. These shifts also increase vulnerability to biotic stresses, including root pathogens and secondary oxidative injuries, thereby compounding the decline of oasis systems. The strategic role of date palm in ensuring regional food security underlines the need for climate-smart management approaches. Recent reviews emphasize that enhancing Mn nutrition improves photosynthetic resilience and supports water-saving strategies in perennial crops [26,27].
In southern Tunisia, BLD currently affects an estimated 4–6% of palms, causing substantial yield losses and economic impacts: affected trees can lose more than half their productivity, and severely diseased palms may die within a few seasons [6]. Typically, oasis Soils contain 60–90 mg kg−1 total Mn, whereas BLD-affected sites often exhibit less than 40 mg kg−1 total Mn and <20 µg g−1 DW in leaves. Foliar application of Mn sulfate (0.5–1%) have temporarily alleviated symptoms but have proven inconsistent under highly alkaline irrigation conditions [8].
Within this context, the present study investigates how soil pH, salinity, and moisture, constrain Mn uptake and drive the physiological decline associated with brittle leaf disease in date palm. By integrating analyses of soil chemistry, root traits, and foliar physiological markers, this work aims to clarify the links between Mn deficiency, root dysfunction, and canopy metabolic responses, providing a mechanistic basis for improved nutrient management and early field diagnosis of Mn-related disorders in arid oases.
2. Materials and Methods
2.1. Study Site and Sampling Design
The study was conducted in the Chamsa oasis (33°58′ N, 8°13′ E; Tozeur, southern Tunisia), located 7.5 km northwest of Tozeur city in the Djerid region. This historical oasis, established in 1950 and rehabilitated into a modern aligned plantation, was selected because it represents a well-documented BLD hotspot under homogeneous pedoclimatic and management conditions, minimizing variability in soil type, irrigation, and cultivation. The site is a monoculture exclusively composed of the cultivar Deglet Nour with palms of comparable age (40–50 years) and vigor. Its inclusion in previous BLD surveys [6,9] ensures data comparability and field representativeness.
The oasis experiences a hot desert climate (Köppen–Geiger BWh) with a mean annual temperature of 22.4 °C (range 5–46 °C) and annual rainfall of ≈108 mm. Potential evapotranspiration exceeds 2000 mm year−1, producing a highly arid water balance. Soils are sandy-loam, alkaline (pH 7.8–8.2), and moderately saline (2–3 dS m−1). Fertility indicators were as follows: sand = 62%, silt = 25%, clay = 13%; CEC = 12.6 cmol (+) kg−1; organic matter = 0.4%; CaCO3 = 22%. These characteristics typify weakly fertile, moderately calcareous oasis soils. Irrigation is supplied by groundwater through flood application every 10–12 days.
Nine mature palms (n = 9) were classified into three health categories (n = 3 per category): (i) healthy (no visible symptoms; leaf Mn > 50 µg g−1 DW); (ii) asymptomatic Mn-deficient (no visible symptoms but proximity ≤ 10 m to symptomatic trees; leaf Mn 20–40 µg g−1 DW); and (iii) BLD-affected (stage 2 symptoms with midrib folding, striations, brittleness; Mn < 20 µg g−1 DW). Classification was performed prior to physiological analyses to ensure objective differentiation (see Supplementary Figure S1).
Although replication was limited, each palm yielded multiple stratified subsamples (soil, roots, leaves) forming a nested design. Subsamples were aggregated at the palm level to preserve biological independence. Sampling occurred in early April (spring) during active sap flow and early fruit set, coinciding with maximum BLD visibility. All palms shared identical irrigation and fertilization schedules to minimize short-term variability.
2.2. Soil and Root Sampling
Root access was achieved by destructive trenching (2 m deep × 1 m wide) authorized by the landowner. Roots were collected manually to preserve structural integrity. Samples were stratified by depth (0–50, 50–100, 100–150 cm). At each depth and health category, soil was analyzed for pH (1:2.5 suspension), electrical conductivity (EC), gravimetric moisture, and Mn content.
Roots were classified into four orders based on branching and diameter following [28]: primary (PR, d > 0.7 cm), secondary (SR, 0.5 < d ≤ 0.7 cm), tertiary (TR, 0.3 < d ≤ 0.5 cm), and fine (FR, d ≤ 0.3 cm). Manganese concentrations were quantified for all classes, but tertiary roots were retained for detailed analyses as they offered consistent biomass, homogenous structure, and clear health contrasts. Fine roots were excluded due to insufficient tissue for biochemical assays.
Root water content was determined gravimetrically. Three complementary approaches captured spatial heterogeneity: (i) systematic zoned sampling, (ii) random blind collection, and (iii) targeted fine-root recovery. In total, 162 root subsamples were collected across all depths and categories.
2.3. Leaf Sampling
Leaflets were taken from the mid-crown region to ensure comparable physiological age. Samples were collected from the midsection of fronds ranked 7 ± 1 from the central spear, corresponding to mature, fully expanded leaves with stable metabolic activity. For each palm, a composite sample of 24 leaflets was formed (six per cardinal direction; two per rachis position: proximal, median, distal) from one representative frond. In symptomatic palms, sampled leaflets displayed visible BLD symptoms, whereas in healthy and asymptomatic palms they were green and turgid.
2.4. Analytical Procedures
Soil pH and EC were measured in 1:2.5 soil–water suspensions using calibrated meters. Moisture content was determined gravimetrically after oven-drying at 105 °C for 24 h. Soil Mn was quantified after aqua regia digestion (3:1 HCl:HNO3) by flame atomic absorption spectrophotometry (AAS; PerkinElmer AAnalyst 400) [29].
Root and leaf water content was calculated as ((Pf − Ps)/Pf) × 100, where Pf = fresh weight and Ps = dry weight [30].
Mn concentration in roots and leaves was determined by ashing (500–550 °C) and acid dissolution (20% HCl for roots, 1 N HNO3 for leaves) followed by AAS quantification against certified standards [29].
Chlorophyll pigments were extracted from 0.2 g fresh tissue in 80% acetone with CaCO3; absorbances at 663 and 645 nm were converted using Lichtenthaler & Wellburn [31] and updated coefficients [32]. Chlorophyll fluorescence (Fv/Fm) was measured in situ using a FIM 1500 fluorometer (Hansatech Instruments) after 20 min dark adaptation (12 replicates per palm) [33,34].
Soluble sugars were extracted from 0.5 g tissue with 80% ethanol at 80 °C and quantified enzymatically using a GOD–POD kit (Sigma-Aldrich GAGO20, St. Louis, MO, USA) at 505 nm. Total soluble proteins were extracted in 0.1 M phosphate buffer (pH 7.0) and measured by the Bradford assay (Bio-Rad kit, Hercules, CA, USA; BSA standard).
Total phenolics were determined from 0.2 g tissue extracted in 80% methanol using the Folin–Ciocalteu method; absorbance was read at 760 nm and results expressed as µg gallic acid equivalents g−1 FW [35].
Peroxidase (POD) activity was determined spectrophotometrically at 470 nm using 20 mM guaiacol and 10 mM H2O2 in 50 mM Tris–maleate buffer (pH 6.5). Absorbance change was monitored for 3 min, and activity expressed as ΔA470 min−1 g−1 FW [23].
2.5. Statistical Analyses
Each palm was treated as an independent experimental unit (n = 3 per category). Stratified subsamples were averaged to palm-level means prior to analysis to ensure biological independence and avoid pseudoreplication. Outliers (>3 SD from group mean) were excluded. Data were log-transformed when required to meet assumptions of normality (Shapiro–Wilk test) and homoscedasticity (Levene test).
Soil and root parameters (pH, EC, moisture, Mn concentration, root water content) were analyzed by two-way ANOVA with Health (3 levels: healthy, asymptomatic, BLD-affected) and Depth (3 levels: 0–50, 50–100, 100–150 cm) as fixed factors. Leaf-related traits (Fv/Fm, pigments, sugars, phenolics, POD activity) were analyzed by one-way ANOVA with Health as factor. Significant effects were resolved by Tukey’s HSD (α = 0.05). Effect sizes were expressed as partial η2.
Principal Component Analysis (PCA) was performed on centered and scaled data to visualize multivariate responses. All data are reported as mean ± standard error (SE; n = 3 palms per category). Error bars represent SE in all figures. Analyses were carried out using SAS (Version 9.4) and R v4.3 (p < 0.05).
2.6. Data Availability
All data supporting this study are available within the article and its Supplementary Material. Additional raw data are accessible from the corresponding author upon reasonable request.
3. Results
3.1. Visual and Structural Symptoms
BLD-affected palms exhibited the characteristic foliar alterations of brittle leaf disease, including interveinal chlorosis, leaflet brittleness, midrib folding, and marginal necrosis, whereas healthy palms maintained uniformly green and turgid canopies (Figure 1A–F).
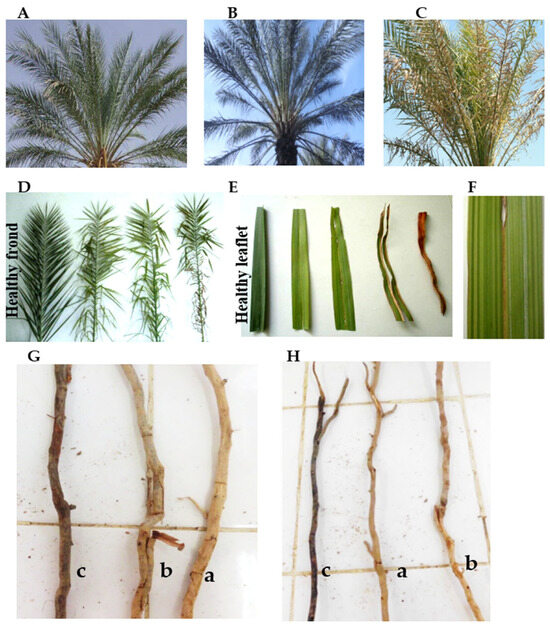
Figure 1.
Visual and structural alterations associated with Brittle Leaf Disease (BLD) in Phoenix dactylifera L. cv. ‘Deglet Nour’. (A–C) Leaf health categories: healthy, apparently healthy (Mn-deficient but asymptomatic), and BLD-affected (Stage 2 with midrib folding, interveinal striations, and leaflet desiccation). (D–F) Progression of symptoms at palm and leaflet scale, including longitudinal chlorotic and necrotic streaks. (G,H) Root system alterations in secondary (G) and tertiary (H) roots across health categories: (a) Healthy; (b) Apparently healthy (Mn-deficient); (c) BLD-affected.
Asymptomatic but Mn-deficient palms appeared symptom-free at first glance yet frequently displayed faint chlorotic streaks, indicative of an early stage of decline.
As the disorder progressed, fronds became curled and drooping, with longitudinal yellow–brown streaks extending along the midrib, followed by leaflet brittleness and necrosis at advanced stages. The sequential progression of foliar symptoms is illustrated in Supplementary Figure S1.
Structural deterioration was also evident in the root system. BLD-affected palms exhibited markedly shortened, thickened, and extensively browned lateral roots with reduced branching compared to the dense, finely ramified networks of healthy palms (Figure 1G,H). Quantitative assessments confirmed that tertiary root length decreased by approximately 38%, mean diameter increased by 22%, and branching density declined by 41% relative to healthy palms, revealing a pronounced loss of absorptive fine-root architecture under Mn stress. These morphological alterations were closely associated with reduced Mn levels at the same root order (F(2,6) = 24.08, p < 0.001, η2 = 0.13), underscoring the close relationship between root structure and Mn acquisition efficiency.
Apparently healthy palms exhibited intermediate traits, consistent with early root functional decline.
3.2. Soil Chemistry and Root Mn Availability
Soil chemistry differed significantly among health categories (F2,6 = 75.2, p < 0.001, η2 = 0.319). BLD-affected palms exhibited a higher soil pH (7.81 ± 0.16) compared with healthy palms (7.56 ± 0.16), corresponding to an average increase of +0.25 units (Supplementary Table S1).
Soil electrical conductivity (EC) ranged from 1.5 to 2.1 dS m−1 across depths, showing no significant difference among health categories (F2,6 = 2.47, p = 0.163, η2 = 0.052), although the upper 0–50 cm layer tended to show lower EC values in BLD-affected soils (Figure 2). Soil moisture was strongly affected by health status (F2,6 = 61.9, p < 0.001, η2 = 0.278), declining from 10.25 ± 2.67% in healthy to 7.59 ± 2.66% in BLD-affected palms.
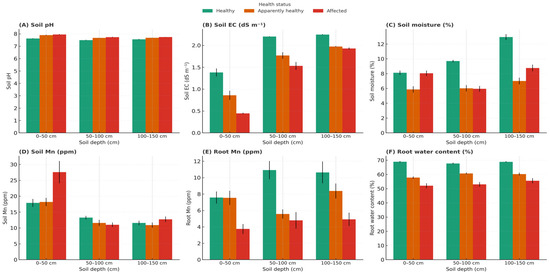
Figure 2.
Depth-resolved variation in soil chemistry and root traits of Phoenix dactylifera L. cv. ‘Deglet Nour’ across three palm health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. (A) Soil pH, (B) Electrical conductivity (EC), (C) Gravimetric soil moisture, (D) Soil Mn concentration, (E) Root Mn concentration, and (F) Root water content were measured at three depth intervals: 0–50, 50–100, and 100–150 cm. Boxplots display medians, interquartile ranges, and outliers; green triangles indicate mean values. Note: Statistical differences were tested using two-way ANOVA (health status x depth) followed by Tukey’s HSD (p < 0.05). Data represent means from three palms per category (n = 3).
Stratified sampling across depths (0–50, 50–100, 100–150 cm) confirmed that Mn deficiency was not due to total soil Mn depletion (F2,6 = 3.86, p = 0.0221, η2 = 0.023) but to lower Mn concentrations in the rhizosphere of affected palms under elevated pH conditions.
Root Mn concentrations declined markedly across all root types (F2,6 = 24.08, p < 0.001, η2 = 0.13; Supplementary Table S2).
Mn levels decreased consistently from primary to feeder roots, indicating a systemic deficiency across the entire root system. Primary and secondary roots showed intermediate Mn concentrations (Supplementary Table S3.4), confirming a consistent gradient of depletion from coarse to fine roots. Mn in feeder roots was also measured (13.80 ± 0.37, 6.58 ± 0.13, and 4.64 ± 1.15 ppm for healthy, asymptomatic, and BLD-affected palms, respectively), but these data were retained in Supplementary Table S3.4 due to the low biomass yield and higher analytical variability of this fine, fragile root class.
Among these, tertiary roots were retained for detailed representation (Figure 3B), as they most directly reflect rhizosphere Mn uptake and provided reproducible measurements across replicates. Their Mn concentration dropped from 14.42 ± 1.21 ppm in healthy palms to 10.11 ± 0.84 ppm in asymptomatic and 5.77 ± 0.84 ppm in diseased palms (see also Supplementary Table S2).
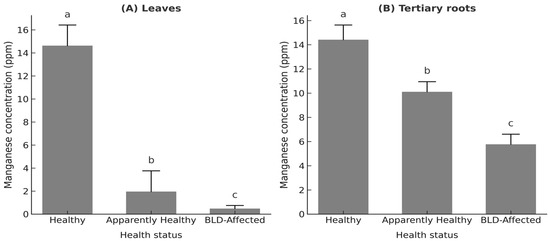
Figure 3.
Manganese (Mn) concentration (ppm) in (A) median crown leaves and (B) tertiary roots of Phoenix dactylifera L. cv. ‘Deglet Nour’ across three palm health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Note: Values represent palm-level means ± standard deviation (n = 3 palms per group). Different lowercase letters indicate statistically significant differences among groups (one-way ANOVA followed by Tukey’s HSD, p < 0.05).
Root water content also decreased significantly (F2,6 = 16.5, p = 0.005, η2 = 0.41), from 69.4 ± 1.8% in healthy to 55.4 ± 2.1% in BLD-affected palms, representing a ≈20% reduction (Table 1). This decline was most pronounced in the 0–50 cm layer where absorptive roots predominate (Supplementary Table S3.2).

Table 1.
Leaf and root water content (%) in Phoenix dactylifera L. cv. ‘Deglet Nour’ across three palm health categories.
Both Health and Depth significantly affected root Mn concentrations (Health: F2,6 = 24.08, p < 0.001; Depth: F2,6 = 8.47, p = 0.018), with a significant Health × Depth interaction (F4,12 = 3.21, p = 0.045). This Health × Depth interaction indicates that depth effects were conditional upon palm health status, with stronger Mn depletion in deeper roots of BLD-affected palms, while healthy palms maintained higher Mn concentrations in upper soil layers. (Figure 2).
3.3. Leaf Mn and Photosynthetic Traits
Leaf Mn concentrations closely followed root Mn patterns, showing a highly significant decline with palm health status (F2,6 = 4435.86, p < 0.001, η2 = 0.965). Leaf and root Mn were strongly correlated (R2 = 0.81, p < 0.01), confirming the tight coupling between below- and aboveground Mn status (Figure 3A).
The maximum quantum yield of PSII (Fv/Fm) decreased sharply from 0.81 ± 0.06 in healthy palms to 0.66 ± 0.10 in asymptomatic and 0.60 ± 0.09 in diseased palms (F2,6 = 25.0, p < 0.001, η2 = 0.81; Figure 4; Supplementary Table S3.3), indicating a severe reduction in photochemical efficiency under Mn stress.
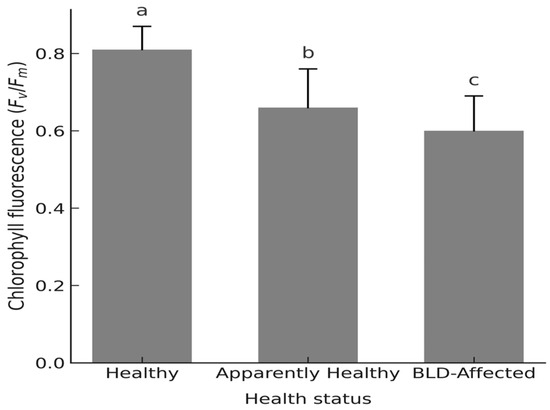
Figure 4.
Maximum quantum efficiency of Photosystem II (PSII) photochemistry (Fv/Fm) in dark-adapted leaflets of Phoenix dactylifera L. cv. ‘Deglet Nour’ palms across three palm health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Note: Values represent palm-level means ± standard deviation (n = 3 palms per group). Different lowercase letters indicate statistically significant differences among groups (one-way ANOVA followed by Tukey’s HSD, p < 0.05).
Total chlorophyll (a + b) content declined correspondingly from 153.4 ± 52.5 to 32.9 ± 7.1 µg g−1 FW (F2,6 = 565.2, p < 0.001, η2 = 0.779; Figure 5; Supplementary Table S3.4), confirming the strong coupling between pigment loss and impaired PSII performance.
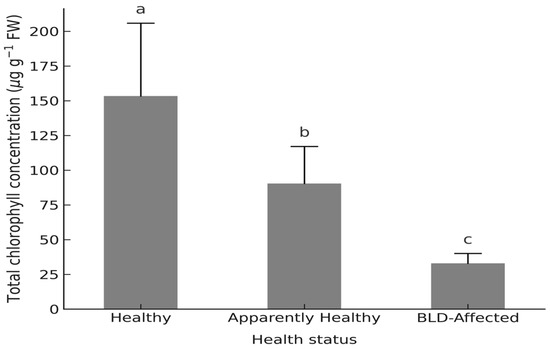
Figure 5.
Total Chlorophyll concentrations (µg g−1 FW) in leaflets of Phoenix dactylifera L. cv. ‘Deglet Nour’ by three palm health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Note: Values represent palm-level means ± standard deviation (n = 3 palms per group). Different lowercase letters indicate statistically significant differences among groups (one-way ANOVA followed by Tukey’s HSD, p < 0.05).
Root Mn was positively correlated with both Fv/Fm (R2 = 0.74, p < 0.01) and total chlorophyll (R2 = 0.78, p < 0.01), emphasizing the mechanistic link between root Mn availability and canopy photosynthetic capacity.
In contrast, leaf water content remained stable or slightly increased in diseased palms (Table 1), suggesting that canopy hydration was preserved despite root-level hydraulic decline.
3.4. Metabolic and Oxidative Responses
Marked biochemical adjustments accompanied disease progression, following a sequential pattern from osmotic regulation to antioxidant activation.
Soluble sugars increased significantly with disease severity (F2,6 = 12.0, p < 0.01, η2 = 0.67), representing an early osmotic adjustment to stress. Total soluble sugars were about 50% higher in diseased palms than in healthy ones, driven mainly by a more than twofold rise in glucose, while sucrose decreased slightly (~20%).
Protein metabolism was subsequently enhanced (F2,6 = 14.0, p < 0.01, η2 = 0.70), with total soluble proteins rising by ~30% (Table 2), suggesting stress-related metabolic reallocation and the activation of protective pathways.

Table 2.
Concentration of glucose, sucrose, total soluble sugars, and soluble protein in leaflets tissues of Phoenix dactylifera L. cv. ‘Deglet Nour’ palms) across three palm health categories.
Antioxidant metabolism intensified sharply, as indicated by elevated peroxidase (POD) activity (F2,6 = 15.32, p < 0.001, η2 = 0.72) and total phenolic content (F2,6 = 934.03, p < 0.001, η2 = 0.853). POD activity increased from 0.26 ± 0.04 to 0.82 ± 0.09 ΔA470 min−1 g−1 FW in leaves and from 0.11 ± 0.02 to 1.02 ± 0.15 in tertiary roots (Figure 6A,B), while phenolics rose from 0.56 ± 0.02 to 0.95 ± 0.15 µg GAE g−1 extract (Table S3.5).
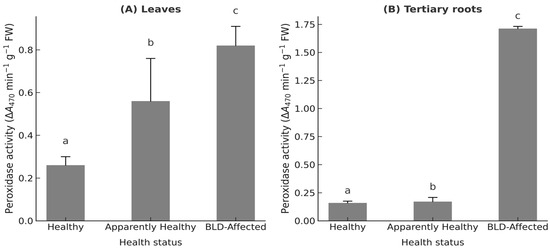
Figure 6.
Peroxidase activity (ΔA470 min−1 g−1 FW) in (A) leaflets and (B) tertiary roots of Phoenix dactylifera L. cv. ‘Deglet Nour’ across three palm health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Note: Values represent palm-level means ± standard deviation (n = 3 palms per group). Different lowercase letters indicate statistically significant differences among groups (one-way ANOVA followed by Tukey’s HSD, p < 0.05).
This ordered sequence, from carbohydrate accumulation to protein synthesis and ultimately antioxidant activation, illustrates a progressive metabolic reprogramming that underlies BLD expression. Asymptomatic palms displayed intermediate values, confirming that biochemical disruption precedes visible symptom expression.
All values are expressed as mean ± SE (n = 3), and error bars representing SE are shown in all figures.
3.5. Multivariate Integration
Correlation analysis revealed strong relationships among root Mn, Fv/Fm (R2 = 0.74, p = 0.005) and total chlorophyll (R2 = 0.78, p = 0.004), whereas soil pH was negatively correlated with root Mn (R2 = 0.65, p = 0.012) (Table S2). These relationships emphasize the close coupling between below- and aboveground Mn dynamics and photosynthetic performance.
Principal Component Analysis (PCA) further separated palms according to their health status. The first component (PC1; ~43%) and the second (PC2; ~21%) together explained about 64% of the total variance. The first component (PC1; ~43% of the total variance) was driven mainly by root Mn, Fv/Fm, chlorophylls, and proteins, while the second component (PC2; ~21%) was associated with soil pH and root water content (Supplementary Figure S2). Healthy, asymptomatic, and diseased palms formed distinct clusters along PC1, supporting a physiological continuum of Mn deficiency.
These multivariate relationships revealed critical quantitative thresholds for field diagnosis: Mn uptake became strongly constrained at soil pH ≥ 7.8, while root Mn concentrations below ~6 µg g−1 DW consistently coincided with reductions of Fv/Fm below 0.72, indicating the onset of photochemical limitation.
A complementary heatmap (Supplementary Figure S3) highlighted strong positive correlations between root Mn, Fv/Fm, and proteins, and negative associations with soil pH, peroxidase activity, and soluble sugars, confirming the inverse relationships between Mn nutrition and oxidative metabolism.
4. Discussion
4.1. Root-Centered Mechanism of BLD
Our analyses confirmed that Mn depletion was systemic across all root classes (Supplementary Table S3.4), reflecting a generalized decline in absorptive and hydraulic efficiency rather than a localized deficiency. Comparable hydraulic impairment under Mn shortage has been documented in other perennials where reduced aquaporin expression limits root conductance [18,19]. Although tertiary roots were selected for detailed representation, this choice was based primarily on their analytical reproducibility and sensitivity to rhizosphere variations, rather than exclusive physiological primacy.
The pronounced Mn depletion in these fine roots, combined with structural collapse and reduced water content, indicates a functional breakdown of the absorptive interface under combined alkaline–saline stress. The magnitude of this effect (η2 = 0.68) is comparable to or higher than those reported for other Mn-deficient woody crops such as citrus and olive trees on calcareous soils (η2 ≈ 0.45–0.60; [6,34]. Such strong effects highlight the robustness of root Mn as a key physiological marker of BLD progression.
Field evidence therefore supports that BLD in date palm is a root-centered physiological disorder, not caused by absolute Mn depletion in bulk soils but by restricted Mn mobility and uptake efficiency under adverse edaphic conditions. Although Mn bioavailability was not directly quantified (e.g., by DTPA extraction), the consistent decline in root Mn concentration and water content across soil depths supports this interpretation. Elevated soil pH around affected palms provides a mechanistic basis for these observations, since Mn2+ solubility decreases sharply with increasing pH [36], and salinity further constrains both Mn uptake and hydraulic conductance [28]. Such pH-driven Mn immobilization follows predictable thermodynamic equilibria of Mn (III/IV) oxides, as described in alkaline calcareous soils [16,22]. These results corroborate broader reports that Mn–pH interactions dominate plant Mn nutrition in diverse cropping systems [16,37].
The asymptomatic category, characterized by intermediate Mn levels and partial root dysfunction, likely represents a transitional, pre-symptomatic phase of BLD development, where early absorptive impairment precedes visible canopy decline. This reinforces the concept that root-level dysfunction constitutes the primary trigger of BLD, while foliar symptoms represent its downstream expression.
4.2. Foliar Dysfunctions: PSII Impairment and Pigment Loss
Foliar responses clearly reflected the systemic constraints imposed by root-level Mn deficiency, indicating a systemic loss of absorptive and hydraulic efficiency. Both the maximum quantum yield of PSII (Fv/Fm) and total chlorophyll (a + b) declined progressively from healthy to asymptomatic and BLD-affected palms, demonstrating a strong functional coupling between Mn status and photosynthetic performance. Fv/Fm decreased from 0.81 ± 0.06 to 0.60 ± 0.09 (F2,6 = 25.0, p < 0.001, η2 = 0.81), while chlorophyll content dropped by nearly 80%.
These declines are best explained by the central role of Mn in photosystem II electron transport, where Mn acts as a catalytic cofactor in the oxygen-evolving reactions and supports chlorophyll stability under light stress [10,38,39]. Prior structural evidence in date-palm has also confirmed Mn involvement in the stability of OEC proteins [40]. The Mn4CaO5 cluster and extrinsic proteins PsbO/PsbP are known to destabilize under Mn deprivation, impairing water-splitting efficiency and PSII electron transport [13,14]. Although the OEC was not directly analyzed at the protein level (e.g., PsbO), the observed reduction in Fv/Fm is consistent with functional impairment of Mn-dependent redox reactions within PSII reaction centers.
Importantly, asymptomatic palms already exhibited a 40–50% pigment loss and intermediate Fv/Fm, underscoring the value of chlorophyll fluorescence as an early diagnostic indicator of Mn stress before visible chlorosis develops [33,41]. Comparable patterns of PSII depression under Mn limitation have been reported in other C3 perennials such as citrus and conifers [42,43,44], supporting a common photochemical constraint across woody species, even if the magnitude of response may differ from C4 crops such as rice. Despite photosynthetic pathway differences, Mn-dependent photochemical limitations are structurally conserved across C3 and C4 species [45].
Altogether, these results demonstrate that foliar photoinhibition in BLD palms originates from root-induced Mn limitation, disrupting photosynthetic energy conversion and pigment maintenance rather than being a primary leaf disorder.
4.3. Metabolic and Oxidative Adjustments
The biochemical reprogramming observed in BLD palms reflects a coordinated metabolic response to Mn deficiency and its downstream oxidative consequences. The marked accumulation of soluble sugars, particularly glucose, suggests an early osmotic and redox buffering mechanism that helps maintain cell turgor and counteract oxidative stress under restricted Mn uptake [46,47,48,49]. This accumulation coincided with a moderate rise in total soluble proteins, consistent with stress-related synthesis of protective and antioxidant enzymes, a response often reported in perennial crops exposed to chronic micronutrient limitation [50].
The sharp activation of peroxidase (POD) and phenolic metabolism represents a second-level defense adjustment aimed at detoxifying reactive oxygen species (ROS) generated by the impaired Mn-dependent superoxide dismutase pathway [51]. Such compensatory upregulation of peroxidase and phenolics under Mn limitation is a hallmark of the ROS–Mn-SOD metabolic trade-off [52]. The strong effect sizes recorded (η2 = 0.72 for POD; η2 = 0.85 for phenolics) confirm that oxidative stress regulation is a major component of the BLD syndrome. Rather than repeating the quantitative values, these coordinated increases in enzymatic and non-enzymatic antioxidants highlight a shift from primary metabolism toward redox homeostasis.
Similar hierarchical activation of osmolyte accumulation, protein synthesis, and antioxidant defense has been documented under Mn deficiency in cereals and woody species [53,54,55]. The pattern observed here in date palm indicates that oxidative imbalance is not a secondary symptom, but a core mechanism linking root Mn limitation to canopy decline. This cross-compartmental redox signaling from roots to leaves represents a central aspect of Mn-deficiency syndromes in woody perennials [12].
4.4. Integrative Model and Applied Perspectives
The results converge toward an integrated root-centered pathogenesis model linking soil chemistry, Mn dynamics, and physiological decline.
(1) Soil alkalinity and salinity jointly reduce Mn2+ solubility and root uptake efficiency through decreased cation exchange and impaired hydraulic conductivity [56,57,58].
(2) Absorptive roots progressively lose functionality, exhibiting reduced Mn accumulation and water content, which directly limit the Mn flux to developing fronds [37].
(3) The resulting foliar Mn shortage disrupts PSII photochemistry, lowers Fv/Fm, and accelerates pigment degradation, leading to canopy-level photosynthetic decline [38,39].
(4) Metabolic reprogramming, characterized by the sequential activation of osmolyte accumulation, protein synthesis, and antioxidant defense, marks the terminal phase of BLD progression [59,60].
From a management standpoint, the present findings establish a clear mechanistic basis for nutrient- and pH-targeted interventions. These recommendations are consistent with emerging frameworks advocating nutrient-efficient and climate-smart practices for perennial crops in arid environments [26,27].
From a practical standpoint, four complementary management actions are proposed, starting with soil pH correction as a primary lever for restoring Mn availability. The application of elemental sulfur (200–400 kg ha−1 yr−1), divided into two treatments during spring and autumn, has been shown to effectively lower soil pH by 0.3–0.5 units in oasis soils [8]. Incorporating acidifying organic matter, such as composted date residues or farmyard manure at 10–15 t ha−1, further improves buffering capacity, stimulates microbial Mn reduction, and promotes the gradual re-acidification of calcareous profiles. Together, these practices enhance Mn solubility and root uptake, forming the cornerstone of preventive BLD management under alkaline–saline conditions.
Salinity management: Periodic leaching irrigation using high-quality groundwater or rainfall harvesting can reduce EC by 20–30%. Maintaining leaching fractions above 15% is recommended under arid conditions.
Mn supplementation: In alkaline soils, foliar Mn sprays (MnSO4·H2O at 0.3–0.5% concentration, or chelated forms such as Mn-EDTA or Mn-DTPA at 0.2–0.3%) are more effective than soil applications. Two to three applications during early spring (pre-flowering) and mid-summer (fruit development) maintain foliar Mn above 40 µg g−1 DW.
For field diagnosis and early detection, the combined use of portable chlorophyll fluorometers (Fv/Fm threshold ≤ 0.75), root Mn profiling, and rapid colorimetric assays for POD and phenolics provides a practical toolkit for monitoring Mn stress before visible symptoms appear [42,43,44].
Collectively, these measures align with the root-centered mechanism identified here and offer cost-effective options (≈ 400–600 USD ha−1 yr−1) to mitigate BLD severity under alkaline–saline conditions typical of southern Tunisian oases.
4.5. Limitations and Future Perspectives
While this study provides field-based mechanistic evidence for Mn-linked BLD pathogenesis, several experimental limitations must be acknowledged. Future work should aim to scale these findings under controlled and replicated frameworks to test the specific interactions driving Mn dysfunction. Incorporating multi-scale modeling and isotopic tracing could further resolve Mn fluxes and feedbacks between soil and root compartments [16,22].
Three research priorities emerge:
- Mn × pH × salinity interaction surfaces: Controlled hydroponic or lysimetric trials with micropropagated Phoenix dactylifera plantlets should quantify how incremental changes in soil pH (7.0–8.5) and salinity (1–6 dS m−1) affect Mn speciation, uptake kinetics, and physiological thresholds (e.g., root Mn < 6 ppm predicting Fv/Fm < 0.7).
- Molecular responses and transporter regulation: Expression analyses of Mn transporters (Nramp, MTP, ZIP) and antioxidant genes (POD, CAT, SOD) could reveal whether BLD involves transcriptional downregulation or post-translational inhibition of Mn transport under alkaline stress.
- Seasonal and phenological dynamics: Longitudinal monitoring across flowering, fruit set, and dormancy phases would clarify whether Mn demand and translocation vary with carbon allocation, providing early diagnostic windows for field intervention.
Integrating these approaches will enable the definition of quantitative thresholds for Mn deficiency and the development of decision-support tools for oasis management. Such predictive frameworks are critical for sustaining productivity of date palm agroecosystems under increasing aridity and soil degradation pressures in North Africa and the Middle East.
4.6. Broader Context, Alternative Hypotheses, and Future Outlook
The present findings integrate well within the broader body of research on Brittle Leaf Disease (BLD) and Mn deficiency in date palm. Previous surveys in southern Tunisia and Algeria consistently reported normal total soil Mn levels but reduced foliar Mn [7,9], supporting a functional, rather than absolute, deficiency. Our results refine this understanding by identifying root Mn depletion, reduced hydraulic conductance, and PSII impairment as sequential events linking soil chemistry to canopy decline.
While Mn scarcity appears to be the proximal trigger of BLD symptoms, alternative hypotheses cannot be excluded. Root dysfunction could, in theory, arise from genetic factors, oxidative aging, or latent microbial colonization (e.g., Thielaviopsis punctulata, Fusarium proliferatum), with Mn deficiency acting as a secondary amplifier of physiological stress. However, the absence of consistent pathogen isolation, coupled with the reproducibility of Mn-related traits in healthy vs. affected palms, suggests that Mn bioavailability remains the dominant driver under oasis conditions. Controlled inoculation and transcriptomic studies could help disentangle these potential interactions.
High-throughput genomic and metabolomic profiling of contrasting cultivars may also uncover tolerance genes and Mn transport efficiency traits [1,2,3,60]. The implications of these results extend beyond date palm. Similar Mn-related chlorosis and hydraulic impairment have been described in Phoenix canariensis, Washingtonia robusta, and Cocos nucifera under alkaline soils [16,22]. Thus, the physiological thresholds identified here, soil pH ≥ 7.8, root Mn < 6 µg g−1 DW, and Fv/Fm ≤ 0.72, could serve as early warning indicators across other Palmoideae and Mn-sensitive crops such as citrus, grapevine, and maize.
A remaining limitation concerns the seasonal dimension. Sampling occurred during spring, coinciding with sap flow resurgence, flowering, and initial fruit set, when Mn demand for photosynthetic and enzymatic processes is maximal. In later phenological stages (summer–autumn), nutrient partitioning toward fruit and senescing tissues may exacerbate or partially mask Mn stress signals. Continuous seasonal monitoring of Mn translocation, Fv/Fm, and antioxidant dynamics will be essential to establish phenology-specific thresholds for diagnosis and intervention.
These findings reposition brittle leaf disease (BLD) as a model system for micronutrient–root interaction disorders in arid perennial crops, emphasizing the need for preventive, rhizosphere-centered management rather than symptom-based correction at the canopy level.
5. Conclusions
This field-based study provides integrative evidence that brittle leaf disease (BLD) in Phoenix dactylifera L. cv. ‘Deglet Nour’ reflects a strong interaction between soil alkalinity and root manganese (Mn) nutrition, rather than a pathogen-driven disorder. While bioavailable Mn (e.g., DTPA-extractable Mn) was not directly quantified, the consistent associations among elevated soil pH (7.8–8.2), reduced root Mn (<6 µg g−1 DW), and photochemical decline (Fv/Fm < 0.72) provide compelling field-level evidence that Mn limitation is a dominant physiological constraint under alkaline–saline conditions.
Physiological degradation followed a hierarchical pattern: reduced root water content and Mn accumulation preceded canopy-level dysfunction. Across palms, Fv/Fm decreased by approximately 26%, and total chlorophyll declined by nearly 80%, marking a critical loss of PSII efficiency and pigment stability. These quantitative thresholds delineate an early warning range for Mn deficiency in oasis environments.
Asymptomatic but Mn-deficient palms exhibited intermediate Mn concentrations and photosynthetic values, reinforcing their diagnostic relevance for early detection. In practical terms, field intervention should be initiated when Fv/Fm falls below 0.70 or root Mn drops below 6 µg g−1 DW, signaling the onset of oxidative and metabolic stress.
Beyond Deglet Nour, similar Mn-related declines have been reported in Phoenix canariensis, Cocos nucifera, and other species cultivated on calcareous soils. The integrative strategy combining soil pH correction, foliar Mn supplementation (e.g., Mn-EDTA or Mn-citrate at 0.2–0.5%), and fluorescence-based monitoring is therefore transferable to other Mn-sensitive palm and fruit tree systems.
Future research should refine these thresholds under controlled Mn × pH × salinity conditions and characterize the regulation of Mn transporters (Nramp, MTP, ZIP) and Mn-dependent antioxidant enzymes. Such advances will strengthen predictive models of BLD susceptibility and support nutrient-based, climate-resilient management of oasis agroecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112490/s1. Figure S1. Three-stage progression of brittle leaf disease (BLD) symptoms in Phoenix dactylifera L. fronds. Figure S2. Principal Component Analysis (PCA) of soil, root, and leaf variables in Phoenix dactylifera L. cv. ‘Deglet Nour’ across three palm health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Figure S3. Heatmap of standardized (Z-score) soil, root, and leaf variables across three palm health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Table S1. Results of one-way ANOVA for soil, root, and leaf variables in Phoenix dactylifera L. cv. ‘Deglet Nour’ palms across three health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Table S2. Mean values (± SD) of soil, root, and leaf parameters in Phoenix dactylifera L. cv. ‘Deglet Nour’ palms across three health categories: Healthy, Apparently healthy (Mn-deficient but asymptomatic), and BLD-affected. Table S3.1. Manganese concentrations (ppm) in primary, secondary, and feeder roots of Phoenix dactylifera L. cv. ‘Deglet Nour’ palms according to health status. Table S3.2. Root water content (%) of Phoenix dactylifera L. cv. ‘Deglet Nour’ palms at three soil depths (0–50, 50–100, 100–150 cm) according to health status. Table S3.3. Chlorophyll fluorescence parameters (excluding Fv/Fm) in dark-adapted leaflets of Phoenix dactylifera L. cv. ‘Deglet Nour’ palms according to health status. Table S3.4. Chlorophyll a and b concentrations (µg g−1 FW) in leaflets of Phoenix dactylifera L. cv. ‘Deglet Nour’ palms according to health status. Table S3.5. Total phenolic content (µg GAE g−1 extract) in leaflets and tertiary roots of Phoenix dactylifera L. cv. ‘Deglet Nour’ palms according to health status.
Author Contributions
S.B.M. and A.N. conceived and designed the study and performed the field experiments and sampling. A.N. conducted the data analysis and interpretation. Both authors contributed to the writing and critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, for funding this research work (Grant number: KFU253755). The APC was funded by the same grant.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BLD | Brittle Leaf Disease |
| Mn | Manganese |
| PSII | Photosystem II |
| OEC | Oxygen-Evolving Complex |
| Fv/Fm | Maximum quantum efficiency of PSII photochemistry |
| EC | Electrical Conductivity |
| POD | Peroxidase |
| ROS | Reactive Oxygen Species |
| FW | Fresh Weight |
| DW | Dry Weight |
| ANOVA | Analysis of Variance |
| PCA | Principal Component Analysis |
| SOD | Superoxide Dismutase |
| MS medium | Murashige and Skoog medium |
| BSA | Bovine Serum Albumin (standard in Bradford assay) |
| HSD | Honestly Significant Difference (Tukey’s test) |
References
- Gros-Balthazard, M.; Hazzouri, K.M.; Flowers, J.M. Genomic insights into date palm origins. Genes 2018, 9, 502. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Flowers, J.M.; Visser, H.J.; Khierallah, H.S.M.; Rosas, U.; Pham, G.M.; Meyer, R.S.; Johansen, C.K.; Fresquez, Z.A.; Masmoudi, K.; et al. Whole genome re-sequencing of date palms yields insights into diversification of fruit color and flowering time. Nat. Commun. 2015, 6, 8824. [Google Scholar] [CrossRef]
- Al-Dous, E.K.; George, B.; Al-Mahmoud, M.E.; Al-Jaber, M.Y.; Wang, H.; Salameh, Y.M.; Al-Azwani, E.K.; Chaluvadi, S.; Pontaroli, A.C.; DeBarry, J.; et al. De novo genome sequencing and comparative genomics of date palm. Nat. Biotechnol. 2011, 29, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Salem, H.M. Salinity-induced desertification in oasis ecosystems: Challenges and future directions. Environ. Monit. Assess. 2024, 196, 696. [Google Scholar] [CrossRef] [PubMed]
- Abassi, R.; Namsi, A.; Bennasri, M.; Ben Abdalah, H.; Ben Maachia, S.; Ouerghi, Z.; Duran-Vila, N. Manganese deficiency is associated with anatomical changes in date palm leaflets showing brittle leaf disease symptoms. J. Plant Pathol. 2014, 96, 29–34. [Google Scholar] [CrossRef]
- Saidi, M.N.; Romdhane, S.B.; Bourogaâ, A.; Namsi, A.; Drira, N.; Gargouri-Bouzid, R. Modulated expression of ion transporters may be responsible for manganese deficiency in brittle leaf disease affected date palm (Phoenix dactylifera L.) trees. Physiol. Mol. Plant Pathol. 2013, 84, 61–69. [Google Scholar] [CrossRef]
- Saadi, I.; Namsi, A.; Mahamoud, O.B.; Takrouni, M.L.; Zouba, A.; Bové, J.M.; Duran-Vila, N. First report of brittle leaf disease of date palm in Algeria. Plant Pathol. 2006, 55, 572. [Google Scholar] [CrossRef]
- Saidi, M.N.; Jbir, R.; Ghorbel, I.; Namsi, A.; Drira, N.; Gargouri-Bouzid, R. Brittle leaf disease induces an oxidative stress and decreases the expression of manganese-related genes in date palm (Phoenix dactylifera L.). Plant Physiol. Biochem. 2012, 50, 1–7. [Google Scholar] [CrossRef]
- Namsi, A.; Montarone, M.; Serra, P.; Takrouni, M.L.; Ben Mahamoud, O.; Zouba, A.; Khoualdia, O.; Bové, J.M.; Duran-Vila, N. Manganese and brittle leaf disease of date palm trees. J. Plant Pathol. 2007, 89, 125–136. [Google Scholar]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, P. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Blasco, B.; Navarro-León, E.; Ruiz, J.M. Oxidative stress in relation with micronutrient deficiency or toxicity. In Plant Micronutrient Use Efficiency; Academic Press: London, UK, 2018; pp. 181–194. [Google Scholar] [CrossRef]
- Cakmak, I.; Brown, P.; Colmenero-Flores, J.M.; Husted, S.; Kutman, B.; Nikolic, M.; Rengel, Z.; Schmidt, S.B.; Zhao, F. Micronutrients. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Ed.; Academic Press: London, UK, 2023; pp. 283–385. [Google Scholar] [CrossRef]
- Pagliano, C.; Saracco, G.; Barber, J. Structural, functional and auxiliary proteins of photosystem II. Photosynth. Res. 2013, 116, 167–188. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese deficiency in plants: The impact on photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef]
- Chen, A.T.; Husted, S.; Salt, D.E.; Schjoerring, J.K.; Persson, D.P. The intensity of manganese deficiency strongly affects root endodermal suberization and ion homeostasis. Plant Physiol. 2019, 181, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Bravo, S.; Amorós, J.A.; Pérez-De-Los-Reyes, C.; García, F.J.; Moreno, M.M.; Sánchez-Ormeño, M.; Higueras, P. Influence of the soil pH in the uptake and bioaccumulation of heavy metals in vine leaves, Castilla-La Mancha (Spain). J. Geochem. Explor. 2017, 174, 79–83. [Google Scholar] [CrossRef]
- Campbell, L.C.; Nable, R.O. Physiological functions of manganese in plants. In Manganese in Soils and Plants; Springer: Dordrecht, The Netherlands, 1988; pp. 139–154. [Google Scholar]
- Bramley, H.; Turner, N.C.; Turner, D.W.; Tyerman, S.D. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol. 2009, 150, 348–364. [Google Scholar] [CrossRef]
- Mimoun, A.; Rey, H.; Jourdan, C.; Banamar, H.; Yakoubi, F.; Babou, F.; Bennaceur, M. Moderate salinity stimulates root plasticity and growth parameters of date palm seedlings (Phoenix dactylifera L.). Rhizosphere 2024, 30, 100876. [Google Scholar] [CrossRef]
- Allen, M.D.; Kropat, J.; Tottey, S.; Del Campo, J.A.; Merchant, S.S. Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function. Plant Physiol. 2007, 143, 263–277. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2011; p. 505. [Google Scholar]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- El Hadrami, I.; Baaziz, M. Somatic embryogenesis and analysis of peroxidases in Phoenix dactylifera L. Biol. Plant. 1995, 37, 197–203. [Google Scholar] [CrossRef]
- Franzisky, B.L.; Mueller, H.M.; Du, B.; Lux, T.; White, P.J.; Carpentier, S.C.; Geilfus, C.M. Date palm acclimates to aridity by diverting organic osmolytes for root osmotic adjustment in parallel with leaf membrane remodeling and ROS scavenging. BioRxiv 2024. [Google Scholar] [CrossRef]
- Gantayat, R.R.; Elumalai, V. Salinity-induced changes in heavy metal behavior and mobility in semi-arid coastal aquifers: A comprehensive review. Water 2024, 16, 1052. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Padilla, Y.G.; Álvarez, S.; Calatayud, Á.; Colmenero-Flores, J.M.; Gómez-Bellot, M.J.; Hernández, J.A.; Martínez-Alcalá, I.; Penella, C.; Pérez-Pérez, J.G.; et al. Advancements in water-saving strategies and crop adaptation to drought: A comprehensive review. Physiol. Plant. 2025, 177, e70332. [Google Scholar] [CrossRef]
- Raza, A.; Khare, T.; Zhang, X.; Rahman, M.M.; Hussain, M.; Gill, S.S.; Varshney, R.K. Novel strategies for designing climate-smart crops to ensure sustainable agriculture and future food security. J. Sustain. Agric. Environ. 2025, 4, e70048. [Google Scholar] [CrossRef]
- Guo, D.; Mitchell, R.J.; Withington, J.M.; Fan, P.P.; Hendricks, J.J. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: Root branch order predominates. J. Ecol. 2008, 96, 737–745. [Google Scholar] [CrossRef]
- Pauwels, J.M.; Verloo, M. Manuel de Laboratoire de Pédologie: Méthodes D’analyses de Sols et de Plantes, Equipement, Gestion de Stocks de Verrerie et de Produits Chimiques; Administration Générale de la Coopération au Développement: Brussels, Belgium, 1992. [Google Scholar]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Doddavarapu, B.; Crasta, G.L.; Shankar, M. Comparative studies on chlorophyll concentration in some important plant families. J. Pharmacogn. Phytochem. 2021, 10, 214–220. [Google Scholar] [CrossRef]
- Lima Neto, A.J.D.; Krug, A.V.; Moura-Bueno, J.M.; Rozane, D.E.; Natale, W.; Hindersmann, J.; Brunetto, G. Proposal of critical nutrient levels in soil and citrus leaves using the boundary line method. Plants 2025, 14, 1764. [Google Scholar] [CrossRef]
- Long, L.; Kristensen, R.K.; Guo, J.; Chen, F.; Pedas, P.R.; Zhang, G.; Yuan, L. Assessing the variation in traits for manganese deficiency tolerance among maize genotypes. Environ. Exp. Bot. 2021, 183, 104344. [Google Scholar] [CrossRef]
- Mohammed, E.A.; Abdalla, I.G.; Alfawaz, M.A.; Mohammed, M.A.; Al Maiman, S.A.; Osman, M.A.; Yagoub, A.E.A.; Hassan, A.B. Effects of extraction solvents on the total phenolic content and antioxidant activity in root vegetables. Agriculture 2022, 12, 1820. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Zhang, Y.; Liu, Y.; Zhang, H.; Tang, M. Arbuscular mycorrhizal fungi alter carbohydrate distribution and amino acid accumulation in Medicago truncatula under lead stress. Environ. Exp. Bot. 2020, 171, 103950. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Pan, Y.; Chen, J.; Liu, Y. Metabolic responses to manganese toxicity in soybean roots and leaves. Plants 2023, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Xie, Y.Z.; Chen, X.F.; Zhang, J.; Chen, H.H.; Ye, X.; Chen, L.S. Growth, mineral nutrients, photo-synthesis and related physiological parameters of Citrus in response to nitrogen deficiency. Agronomy 2021, 11, 1859. [Google Scholar] [CrossRef]
- Keeley, M.; Rowland, D.; Vincent, C. Citrus photosynthesis and morphology acclimate to phloem-affecting huanglongbing disease at the leaf and shoot levels. Physiol. Plant. 2022, 174, e13662. [Google Scholar] [CrossRef] [PubMed]
- Marqués, J.; Duran-Vila, N.; Daròs, J.A. The Mn-binding proteins of the photosystem II oxygen-evolving complex are decreased in date palms affected by brittle leaf disease. Plant Physiol. Biochem. 2011, 49, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P. Manganese adsorption, availability, and uptake in citrus under microsprinkler irrigation. Agrosyst. Geosci. Environ. 2020, 3, e20061. [Google Scholar] [CrossRef]
- Moitazedi, S.; Sayfzadeh, S.; Haghparast, R.; Zakerin, H.R.; Jabari, H. Mitigation of drought stress effects on wheat yield via the foliar application of boron, zinc, and manganese nano-chelates and supplementary irrigation. J. Plant Nutr. 2023, 46, 1988–2002. [Google Scholar] [CrossRef]
- Mueller, H.M.; Franzisky, B.L.; Messerer, M.; Du, B.; Lux, T.; White, P.J.; Geilfus, C.M. Integrative multi-omics analyses of date palm (Phoenix dactylifera) roots and leaves reveal how the halophyte land plant copes with sea water. Plant Genome 2024, 17, e20372. [Google Scholar] [CrossRef]
- Li, J.; Ackah, M.; Amoako, F.K.; Cui, Z.; Sun, L.; Li, H.; Tsigbey, V.E.; Zhao, M.; Zhao, W. Metabolomics and physio-chemical analyses of mulberry plant leaves response to manganese deficiency and toxicity reveal key metabolites and their pathways in manganese tolerance. Front. Plant Sci. 2024, 15, 1349456. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hakata, M. Atlas of rice grain filling-related metabolism under high temperature: Joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010, 51, 795–809. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Pashkovskiy, P.P.; Ivanova, A.I.; Kartashov, A.V.; Kuznetsov, V.V. Manganese deficiency suppress-es growth and photosynthetic processes but increases expression of photosynthetic genes in Scots pine seedlings. Cells 2022, 11, 3814. [Google Scholar] [CrossRef]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarraj, S.; Albasher, G.; Schnitzler, J.P.; Rennenberg, H. Metabolic responses of date palm leaves to drought differ in summer and winter climate. Tree Physiol. 2021, 41, 1685–1700. [Google Scholar] [CrossRef]
- Hani, U.; Krieger-Liszkay, A. Manganese deficiency alters photosynthetic electron transport in Marchantia polymorpha. Plant Physiol. Biochem. 2024, 215, 109042. [Google Scholar] [CrossRef]
- Rajpoot, R.; Srivastava, R.K.; Rani, A.; Pandey, P.; Dubey, R.S. Manganese-induced oxidative stress, ultrastructural changes, and proteomics studies in rice plants. Protoplasma 2021, 258, 319–335. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Millaleo, R.; Alvear, M.; Aguilera, P.; González-Villagra, J.; de la Luz Mora, M.; Alberdi, M.; Reyes-Díaz, M. Mn toxicity differentially affects physiological and biochemical features in highbush blueberry (Vaccinium corymbosum L.) cultivars. J. Soil Sci. Plant Nutr. 2020, 20, 795–805. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.; Mora, M.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Sghaier-Hammami, B.; Saidi, M.N.; Castillejo, M.A.; Jorrin-Novo, J.V.; Namsi, A.; Drira, N.; Gargouri-Bouzid, R. Proteomic analysis of date palm leaves across three stages of brittle leaf disease. Planta 2012, 236, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Shareef, H.J.; Al-Khayri, J.M. Salt and drought stress exhibits oxidative stress and modulated protein patterns in roots and leaves of date palm (Phoenix dactylifera L.). Acta Agric. Slov. 2021, 117, 1–10. [Google Scholar] [CrossRef]
- Skórka, M.; Sieprawska, A.; Telk, A. The implication of manganese surplus on plant cell homeostasis: A review. J. Plant Growth Regul. 2023, 42, 1327–1341. [Google Scholar] [CrossRef]
- Suhim, A.A.; Awad, K.M.; Jaffer, O.N.; Abass, M.H. The impact of salicylic and jasmonic acid in mitigating salinity stress on date palm Phoenix dactylifera L. Barhi cv. Basrah J. Agric. Sci. 2023, 36, 120–130. [Google Scholar] [CrossRef]
- Khan, I.; Lubna Bilal, S.; Abdelbacki, A.M.; Kang, S.M.; AL-Harrasi, A.; Lee, I.J. Genome-wide and transcriptome analysis of PdWRKY transcription factors in date palm (Phoenix dactylifera) revealing insights into heat and drought stress tolerance. BMC Genom. 2025, 26, 589. [Google Scholar] [CrossRef]
- Yaish, M.W.; Patankar, H.V.; Assaha, D.V.; Zheng, Y.; Al-Yahyai, R.; Sunkar, R. Genome-wide expression profiling in leaves and roots of date palm (Phoenix dactylifera L.) exposed to salinity. BMC Genom. 2017, 18, 3633. [Google Scholar] [CrossRef]
- Parveen, A.; Perveen, S.; Tariq, S.; Atif, M.; Saeed, F.; Zafar, S. Protective role of manganese, proline and rice straw extract in wheat against drought-driven oxidative stress. Acta Physiol. Plant. 2024, 46, 28. [Google Scholar] [CrossRef]
- Ali-Dinar, H.; Munir, M.; Mohammed, M. Drought-tolerance screening of date palm cultivars under water stress conditions in arid regions. Agronomy 2023, 13, 2811. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).