Infection Dynamics of Zarea fungicola and Its Impact on White Button Mushroom Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Cropping Trials
2.2. Pathogen Inoculation Treatments
2.3. Statistical Analysis
3. Results
3.1. Symptoms Development
3.2. Disease Development (AUDPC)
3.3. Disease Influence on the Mushroom Yield and Biological Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khonsanit, A.; Thanakitpipattana, D.; Mongkolsamrit, S.; Kobmoo, N.; Phosrithong, N.; Samson, R.A.; Crous, P.W.; Luangsa-Ard, J.J. A phylogenetic assessment of Akanthomyces sensu lato in Cordycipitaceae (Hypocreales, Sordariomycetes): Introduction of new genera, and the resurrection of Lecanicillium. Fungal Syst. Evol. 2024, 14, 271–305. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W. A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycol. Res. 2008, 112, 811–824. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Baars, J.J.P.; Kalkhove, S.I.C.; Lugones, L.G.; Wösten, H.A.B.; Bakker, P.A. Lecanicillium fungicola: Causal agent of dry bubble disease in white-button mushroom. Mol. Plant Pathol. 2010, 11, 585–595. [Google Scholar] [CrossRef]
- Gams, W.; Van Zaayen, A. Contribution to the taxonomy and pathogenicity of fungicolous Verticillium species. I. Taxonomy. Neth. J. Plant Pathol. 1982, 88, 57–78. [Google Scholar] [CrossRef]
- Collopy, P.D.; Largeteau-Mamoun, M.L.; Romaine, C.P.; Royse, D.J. Molecular phylogenetic analyses of Verticillium fungicola and related species causing dry bubble disease of the cultivated button mushroom, Agaricus bisporus. Phytopathology 2001, 91, 905–912. [Google Scholar] [CrossRef]
- Calonje, M.; Garcia Mendoza, C.; Perez Cabo, A.; Bernardo, D.; Novaes-Ledieu, M. Interaction between the mycoparasite Verticillium fungicola and the vegetative phase of Agaricus bisporus. Mycol. Res. 2000, 104, 988–992. [Google Scholar] [CrossRef]
- Shamshad, A.; Clift, A.D.; Mansfield, A. Hostparasite interaction between cultivated mushroom, Agaricus bisporus hybrid strain Sylvan A15, and the mycoparasite Verticillium fungicola, a causal agent of dry bubble disease. Aus. Plant Pathol. 2009, 38, 74–78. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Regnault-Roger, C.; Savoie, J.-M. Verticillium disease of Agaricus bisporus: Variations in host contribution to total fungal DNA in relation to symptom heterogeneity. Eur. J. Plant Pathol. 2007, 118, 155–164. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Gaze, R.H. Mushroom Pest and Disease Control: A Color Handbook, 1st ed.; CRC Press: London, UK, 2007; pp. 70–75. [Google Scholar]
- Largeteau, M.L.; Savoie, J.-M. Effect of the fungal pathogen Verticillium fungicola on fruiting initiation of its host, Agaricus bisporus. Mycol. Res. 2008, 112, 825–828. [Google Scholar] [CrossRef]
- Largeteau, M.L.L.; Mata, G.; Savoie, J.-M. Verticillium fungicola var. fungicola affects Agaricus bisporus cultivation in Mexico. FEMS Microbiol. Lett. 2004, 236, 191–196. [Google Scholar] [CrossRef]
- Aminuzzaman, F.M.; Shahi, S.; Thapa, S.; Das, K. Mushroom diseases and their management: A review. In Recent Advances in Mushroom Cultivation Technology and Its Application; Kulshreshtha, S., Ed.; Bright Sky Publications: Delhi, India, 2022; Volume 2, pp. 1–27. [Google Scholar]
- Clarke, J.; Grogan, H.; Fitzpatrick, D. Characterising the proteomic response of mushroom pathogen Lecanicillium fungicola to Bacillus velezensis QST 713 and Kos biocontrol agents. Eur. J. Plant Pathol. 2022, 163, 369–379. [Google Scholar] [CrossRef]
- Wong, W.C.; Preece, T.F. Sources of Verticillium fungicola on a commercial mushroom farm in England. Plant Pathol. 1987, 36, 577–582. [Google Scholar] [CrossRef]
- Bernardo, D.; Cabo, A.P.; Novaes-Ledieu, M.; Mendoza, C.G. Verticillium disease or ‘dry bubble’ of cultivated mushrooms: The Agaricus bisporus lectin recognizes and binds the Verticillium fungicola cell wall glucogalactomannan. Can. J. Microbiol. 2004, 50, 729–735. [Google Scholar] [CrossRef]
- Dragt, J.W.; Geels, F.P.; De Bruijnz, W.C.; Van Griensven, L.J.L.D. Intracellular infection of the cultivated mushroom Agaricus bisporus by the mycoparasite Verticillium fungicola var. fungicola. Mycol. Res. 1996, 100, 1082–1086. [Google Scholar] [CrossRef]
- Calonje, M.; Garcia Mendoza, C.; Galan, B.; Novaes-Ledieu, M. Enzymic activity of the mycoparasite Verticillium fungicola on Agaricus bisporus fruit body cell walls. Microbiology 1997, 143, 2999–3006. [Google Scholar] [CrossRef]
- Santana Nunes, J.; de Brito, M.R.; Zied, D.C.; das Graças Leite, E.A.; Souza Dias, E.; Alves, E. Evaluation of the infection process by Lecanicillium fungicola in Agaricus bisporus by scanning electron microscopy. Rev. Iberoam. Micol. 2017, 34, 36–42. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2021/1450 of 3 September 2021 amending Implementing Regulation (EU) No 540/2011 as regards the approval periods of the active substances acrinathrin and prochloraz. Off. J. 2021, L 313, 25–27. [Google Scholar]
- Marčić, D.; Milijašević-Marčić, S.; Drobnjaković, T.; Luković, J.; Šantrić, L.; Grujić, N.; Potočnik, I. Bioprotection of the button mushroom from pests and diseases. Agronomy 2025, 15, 1323. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J.; Santos, M.; Diánez, F.; Carrasco, J. Control of fungal diseases in mushroom crops while dealing with fungicide resistance: A Review. Microorganisms 2021, 9, 585. [Google Scholar] [CrossRef]
- McGee, C.F. Microbial ecology of the Agaricus bisporus mushroom cropping process. Appl. Microbiol. Biotechnol. 2018, 102, 1075–1083. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Baars, J.P.P.; Regnault-Roger, C.; Savoie, J.-M. Molecular and physiological diversity among Verticillium fungicola var. fungicola. Mycol. Res. 2006, 110, 431–440. [Google Scholar] [CrossRef]
- Jeger, M.; Viljanen-Rollinson, S. The use of the area under the disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theor. Appl. Genet. 2001, 102, 32–40. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Schrier, N.; Kalkhove, S.I.C.; Lugones, L.G.; Baars, J.J.P.; Zijlstra, C.; Weerdt, M.; Wösten, H.A.B.; Bakker, P.A.H.M. Absence of induced resistance in Agaricus bisporus against Lecanicillium fungicola. Antonie Van Leeuwenhoek 2013, 103, 539–550. [Google Scholar] [CrossRef]
- Hayes, W.; Keenan, C.; Wilson, J.; Onarinde, B.A. Early detection of dry bubble disease in Agaricus bisporus using volatile compounds. Food Chem. 2024, 435, 137518. [Google Scholar] [CrossRef]
- Noble, R.; Dobrovin-Pennington, A.; Hobbs, P.J.; Pederby, J.; Rodger, A. Volatile C8 compounds and pseudomonads influence primordium formation of Agaricus bisporus. Mycologia 2009, 101, 583–591. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Kalkhove, S.I.C.; Lugones, L.G.; Baars, J.J.P.; Wösten, H.A.B.; Bakker, P.A.H.M. Effects of the mushroom-volatile 1-octen-3-ol on dry bubble disease. Appl. Microbiol. Biotechnol. 2013, 97, 5535–5543. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Kalkhove, S.I.C.; Lugones, L.G.; Wösten, H.A.B.; Bakker, P.A.H.M. Germination of Lecanicillium fungicola in the mycosphere of Agaricus bisporus. Environm. Microbiol. Rep. 2012, 4, 227–233. [Google Scholar] [CrossRef]
- Gea, F.J.; Carrasco, J.; Santos, M.; Diánez, F.; Navarro, M.J. Incidence of Lecanicillium fungicola in white-button mushroom (Agaricus bisporus) cultivated with two types of casing soil. J. Plant Pathol. 2013, 95, 161–164. [Google Scholar]
- Quiroz, L.F.; Ciosek, T.; Grogan, H.; McKeown, P.C.; Spillane, C.; Brychkov, G. Unravelling the Transcriptional response of Agaricus bisporus under Lecanicillium fungicola Infection. Int. J. Mol. Sci. 2024, 25, 1283. [Google Scholar] [CrossRef]
- North, L.H.; Wuest, P.J. The infection process and symptom expression of verticillium disease of Agaricus bisporus. Can. J. Plant Pathol. 1993, 15, 74–80. [Google Scholar] [CrossRef]
- Nair, N.G.; Macauley, B.J. Dry bubble disease of Agaricus bisporus, and its control by prochloraz-manganese complex. N. Z. J. Agric. Res. 1987, 30, 107–116. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Rodier, A.; Rousseau, T.; Juarez del Carmen, S.; Védie, R.; Savoie, J.M. Agaricus susceptibility to Verticillium fungicola. Mush. Sci. 2004, 16, 504–522. [Google Scholar]
- Holmes, J. Effect of time of inoculation on incidence and control of Verticillium disease of the commercial mushroom. Plant Dis. Rprt. 1971, 55, 643–645. [Google Scholar]
- Largeteau, M.L.; Savoie, J.M. Microbially induced diseases of Agaricus bisporus: Biochemical mechanisms and impact on commercial mushroom production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef]
- Carrasco, J.; Tello, M.L.; De Toro, M.; Tkacz, A.; Poole, P.; Pérez-Clavijo, M.; Preston, G. Casing microbiome dynamics during button mushroom cultivation: Implications for dry and wet bubble diseases. Microbiology 2019, 165, 611–624. [Google Scholar] [CrossRef]
- Foulongne-Oriol, M.; Rodier, A.; Rousseaau, T.; Largetau, M.; Savoie, J.M. Quantitative genetics to dissect the fungal-fungal interaction between Lecanicillium verticillium and the white button mushroom Agaricus bisporus. Fungal Biol. 2011, 115, 421–431. [Google Scholar] [CrossRef]
- Caitano, C.E.C.; Iossi, M.R.; Pardo-Giménez, A.; Vieira Junior, W.G.; Dias, E.S.; Zied, D.C. Design of a useful diagrammatic scale for the quantification of Lecanicillium fungicola disease in Agaricus bisporus cultivation. Curr. Microbiol. 2020, 77, 4037–4044. [Google Scholar] [CrossRef]
- Mills, P.R.; Fermor, T.; Muthumeenakshi, S.; Lincoln, S. Cell wall degrading enzymes produced by Verticillium spp. and their relationship to infection in Agaricus bisporus. In Proceedings of the 15th International Congress on the Science and Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; Volume 15, pp. 601–605. [Google Scholar]
- Mamoun, M.; Olivier, J.M.; Vedie, R. Discussions on assessment of artificial infections with Verticillium fungicola for breeding programmes. In Proceedings of the 14th International Congress on the Science and Cultivation of Edible Fungi, Oxford, UK, 17–22 September 1995; Volume 14, pp. 669–677. [Google Scholar]
- Van Zaayen, A.; Rutjens, A.A. Thermal death points for two Agaricus species and for the spores of some major pathogens. Mush. Sci. 1981, 11, 393–402. [Google Scholar]
- Zied, D.C.; Nunes, J.S.; Nicolini, V.F.; Pardo Gimenez, A.; Rinker, D.L.; Dias, E.S. Tolerance to Lecanicillium fungicola and yield of Agaricus bisporus strains used in Brazil. Sci. Horti. 2015, 190, 117–122. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.C.; Navarro, M.J. Occurrence of Verticillium fungicola var. fungicola on Agaricus bitorquis mushroom crops in Spain. J. Phytopathol. 2003, 151, 98–100. [Google Scholar] [CrossRef]
- Grogan, H. Detecting Dry Bubble Disease on Mushroom Farms. Teagasc, 2012, Project Number 5695. Available online: https://teagasc.ie/wp-content/uploads/2025/05/5695-Vert-CELUP-Technology-Update.pdf (accessed on 16 October 2025).
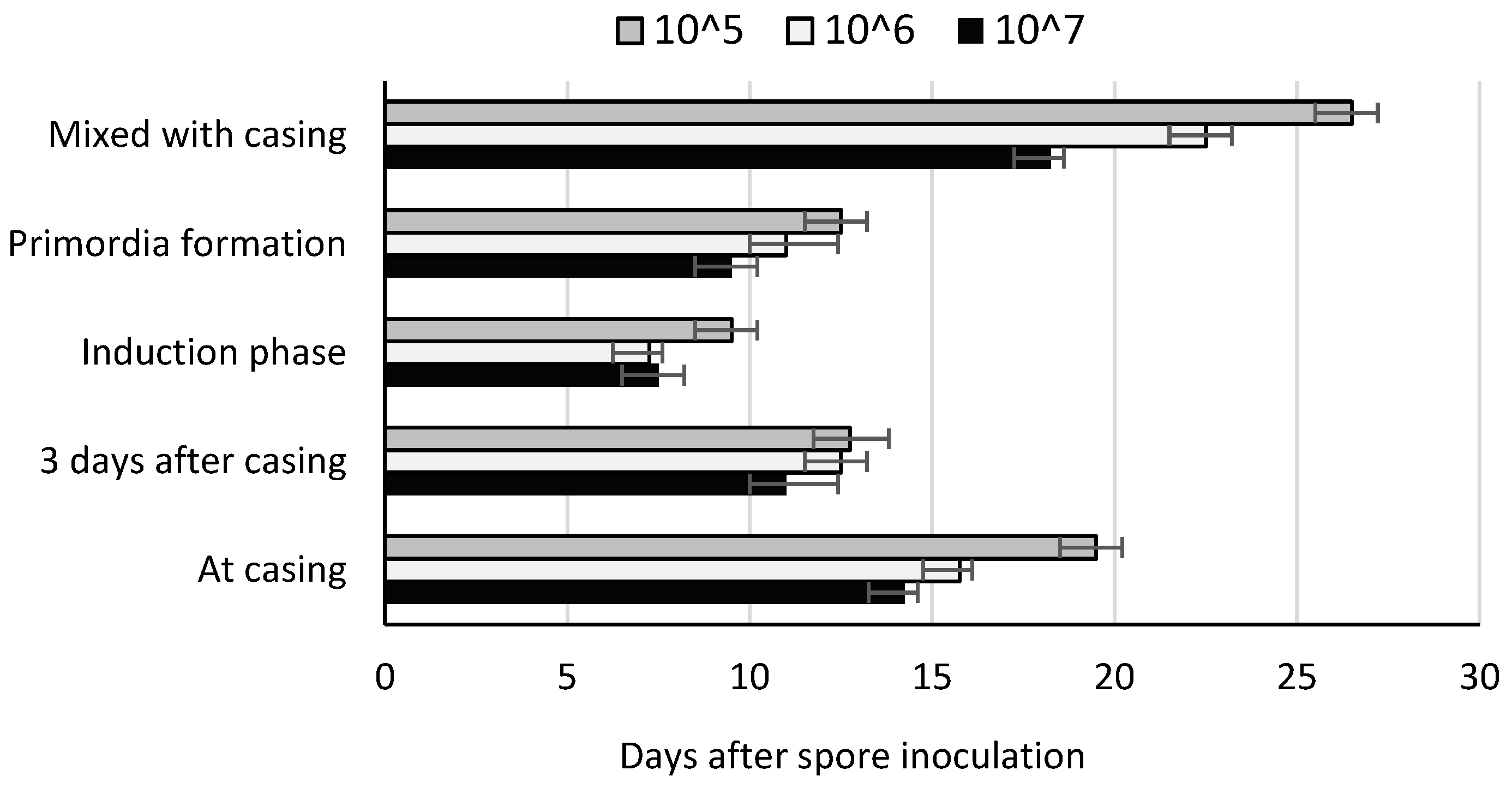
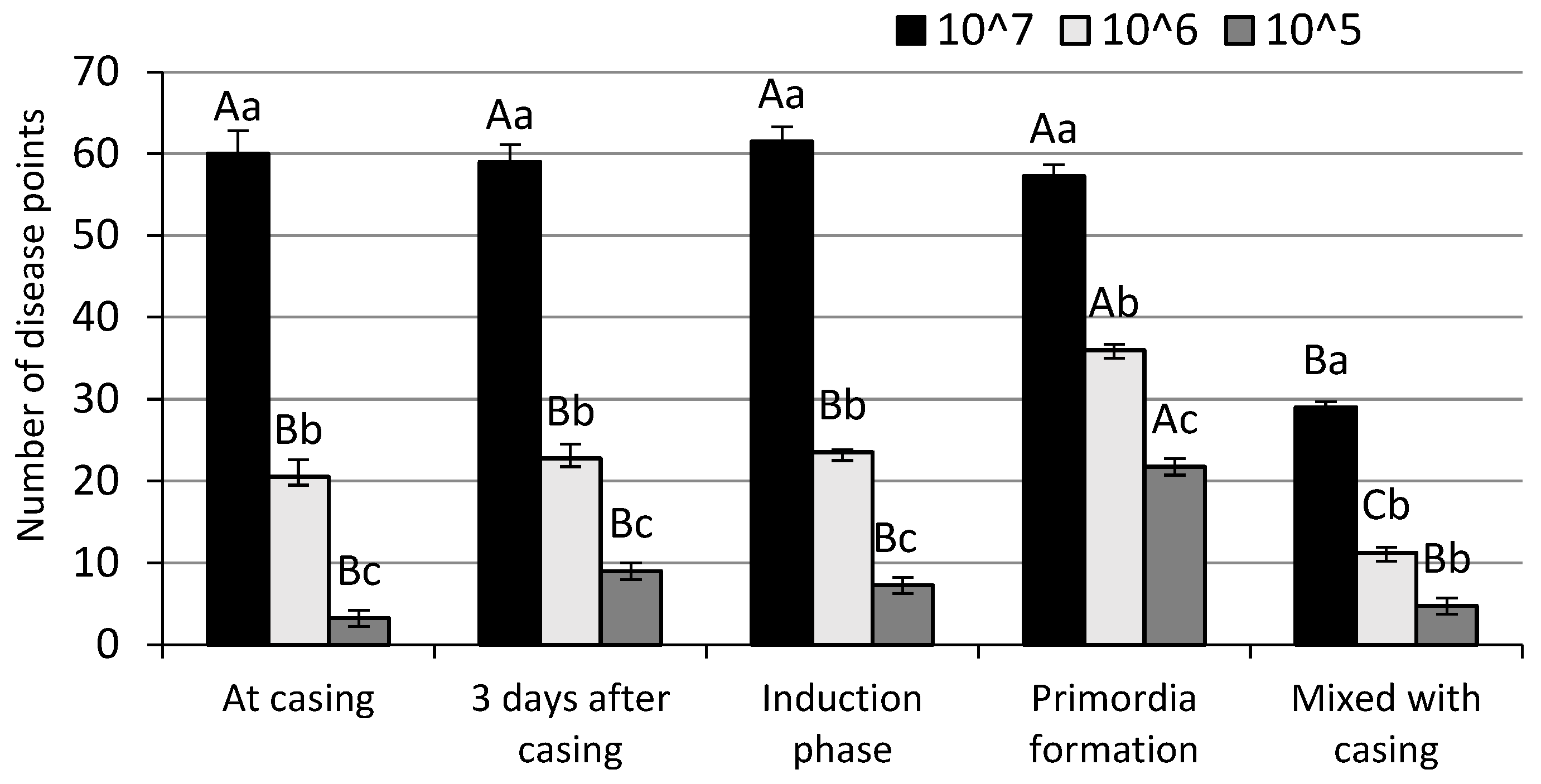



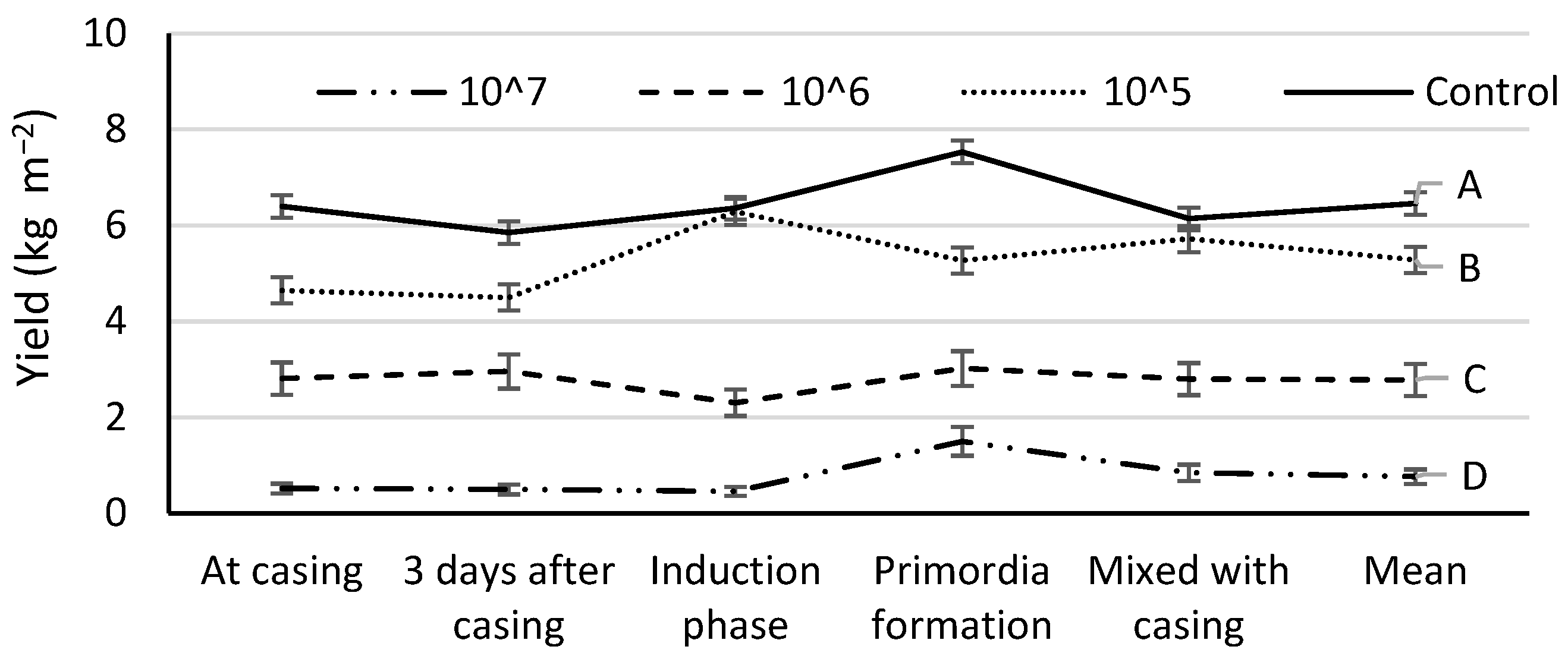
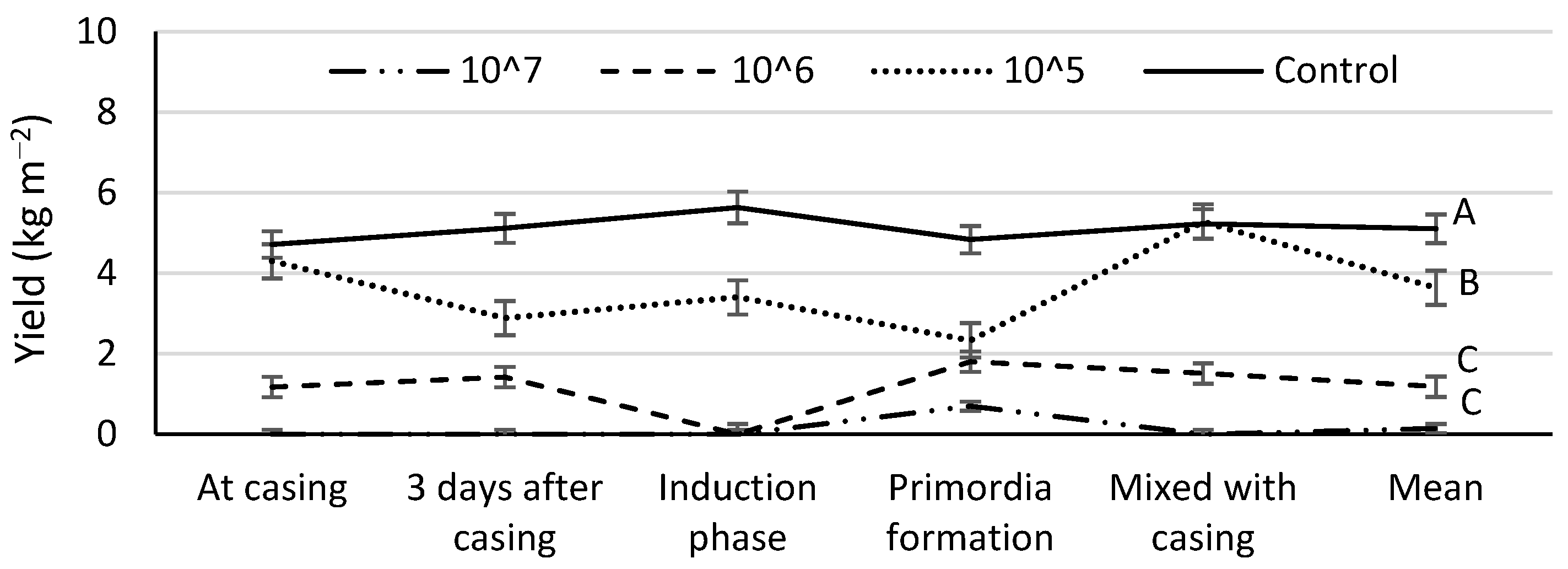
| Inoculation Time | Mushroom Yield (kg m−2) by Number of Spores per m2 Casing | ||||
|---|---|---|---|---|---|
| 107 Spores m−2 | 106 Spores m−2 | 105 Spores m−2 | Control | Mean | |
| At casing | 6.3 ± 0.5 Db | 11.2 ± 1.7 Cbc | 14.4 ± 1.5 Bb | 16.9 ± 0.2 Aa | 12.2 ± 4.8 b |
| 3 days after casing | 3.8 ± 1.4 Dc | 10.1 ± 1.0 Cc | 13.4 ± 1.0 Bb | 15.9 ± 0.4 Aa | 10.8 ± 5.6 c |
| Induction phase | 2.1 ± 1.8 Cc | 8.1 ± 1.7 Bd | 16.4 ± 1.7 Aa | 17.1 ± 0.7 Aa | 11.0 ± 7.2 c |
| Primordia formation | 11.2 ± 1.6 Ca | 12.3 ± 1.0 Ca | 13.9 ± 0.5 Bb | 17.2 ± 0.4 Aa | 13.7 ± 2.8 a |
| Mixed with casing | 7.0 ± 1.5 Db | 11.6 ± 1.6 Cab | 14.6 ± 2.2 Bb | 16.7 ± 0.3 Aa | 12.2 ± 3.9 b |
| Mean | 6.1 ± 3.4 D | 10.6 ± 1.6 C | 14.6 ± 1.4 B | 16.7 ± 0.6 A | – |
| Yield loss (%) by number of spores per m2 casing | |||||
| 107 spores m−2 | 106 spores m−2 | 105 spores m−2 | Control | Mean | |
| At casing | 63.8 ± 3.6 Aab | 34.1 ± 8.6 Bb | 14.9 ± 4.9 Cab | - | 37.6 ab |
| 3 days after casing | 77.3 ± 8.3 Aa | 36.8 ± 6.9 Bb | 15.2 ± 6.6 Cab | - | 43.1 ab |
| Induction phase | 87.6 ± 12.6 Aa | 52.4 ± 10.2 Ba | 3.8 ± 2.7 Cb | - | 47.9 a |
| Primordia formation | 33.8 ± 9.8 Ac | 29.1 ± 3.2 ABb | 19.1 ± 8.6 Bab | - | 34.0 bc |
| Mixed with casing | 56.8 ± 5.6 Ab | 28.6 ± 9.4 Bb | 14.3 ± 8.1 Bab | - | 32.8 c |
| Mean | 63.9 ± 19.4 A | 36.2 ± 9.6 B | 13.5 ± 6.1 C | - | - |
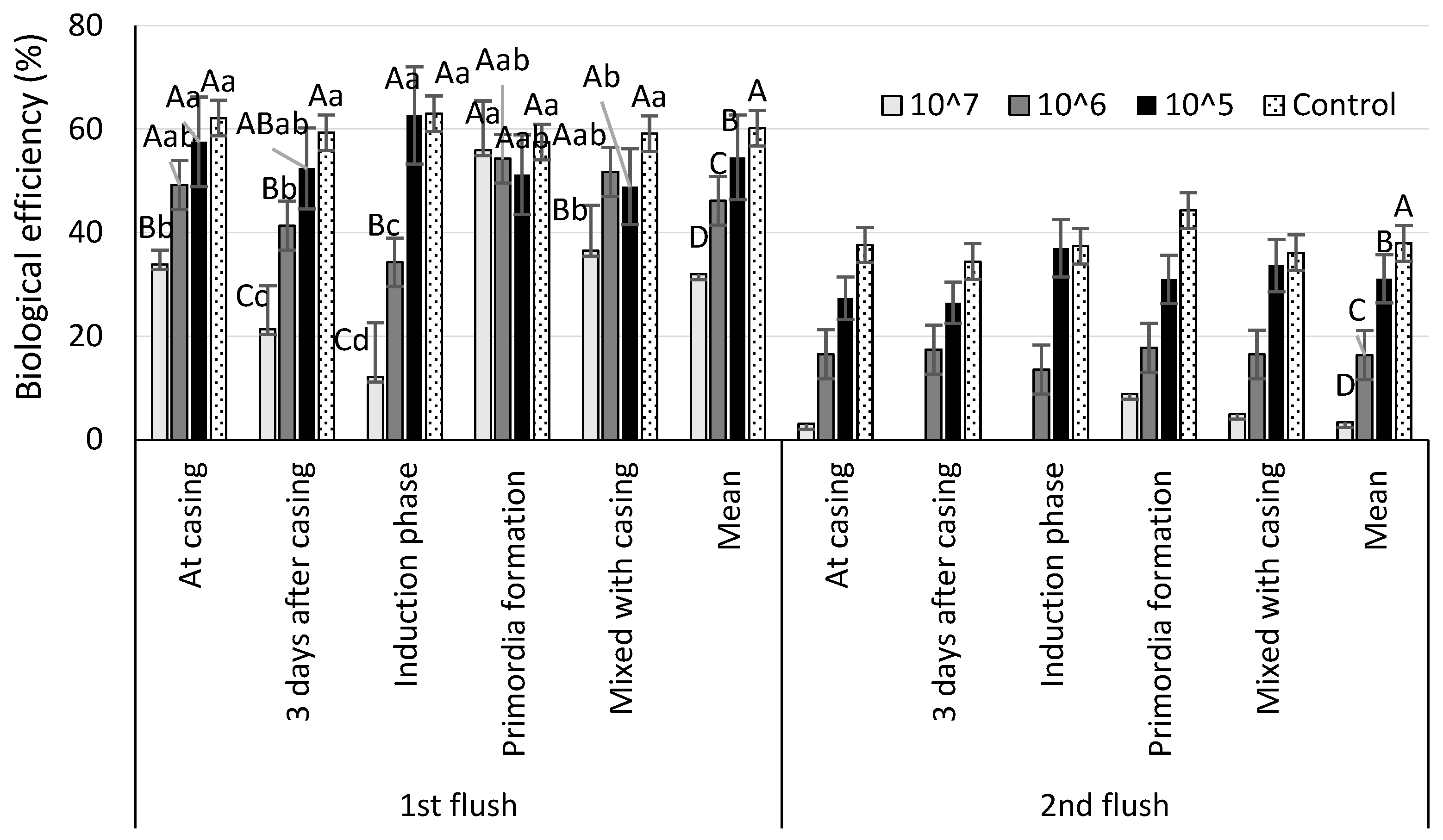
| Biological efficiency (%) by number of spores per m2 casing | |||||
| 107 spores m−2 | 106 spores m−2 | 105 spores m−2 | Control | Mean | |
| At casing | 36.0 ± 4.4 Db | 65.7 ± 9.4 Cbv | 84.8 ± 8.7 Bb | 99.7 ± 4.4 Aa | 71.6 ± 5.8 b |
| 3 days after casing | 21.3 ± 8.4 Dc | 59.0 ± 7.6 Cc | 78.9 ± 10.4 Bb | 94.8 ± 5.2 Aa | 63.3 ± 8.0 c |
| Induction phase | 12.1 ± 6.5 Cc | 47.8 ± 13.4 Bd | 91.7 ± 13.3 Aa | 96.4 ± 6.4 Aa | 62.0 ± 11.4 c |
| Primordia formation | 64.8 ± 15.4 Ca | 72.1 ± 4.8 Ca | 82.2 ± 9.4 Bb | 98.2 ± 5.9 Aa | 79.3 ± 8.6 a |
| Mixed with casing | 41.6 ± 6.5 Dd | 68.2 ± 13.1 Cab | 82.5 ± 22.4 Bb | 95.2 ± 2.3 Aa | 71.9 ± 11.2 b |
| Mean | 35.2 ± 8.0 D | 62.6 ± 9.4 C | 84.0 ± 13.4 B | 96.9 ± 5.2 A | - |
| Inoculation Time | Mushroom Yield (kg m−2) by Number of Spores per m2 Casing | ||||
|---|---|---|---|---|---|
| 107 Spores m−2 | 106 Spores m−2 | 105 Spores m−2 | Control | Mean | |
| At casing | 6.3 ± 0.8 Cb | 11.8 ± 1.7 Bb | 15.6 ± 1.8 Aa | 16.2 ± 0.8 Aa | 12.5 ± 4.6 a |
| 3 days after casing | 2.2 ± 0.9 Cc | 9.6 ± 1.6 Bc | 13.0 ± 1.4 Aa | 15.4 ± 0.4 Aa | 10.1 ± 5.3 b |
| Induction phase | 2.0 ± 1.8 Cc | 7.2 ± 0.7 Bc | 13.3 ± 0.7 Aa | 16.1 ± 0.7 Aa | 9.7 ± 6.3 b |
| Primordia formation | 11.3 ± 1.6 Ba | 13.1 ± 1.6 ABa | 13.4 ± 1.5 Aba | 15.2 ± 0.6 Aa | 13.3 ± 1.5 a |
| Mixed with casing | 9.4 ± 1.5 Ba | 12.0 ± 1.6 Bb | 15.1 ± 1.1 Aa | 15.8 ± 0.9 Aa | 13.1 ± 3.0 a |
| Mean | 6.2 ± 3.4 D | 10.7 ± 2.4 C | 14.1 ± 1.2 B | 15.7 ± 0.5 A | – |
| Yield loss (%) by number of spores per m2 casing | |||||
| 107 spores m−2 | 106 spores m−2 | 105 spores m−2 | Control | Mean | |
| At casing | 60.5 ± 13.5 Ab | 26.2 ± 8.4 Bab | 3.0 ± 2.4 Ca | - | 29.9 bc |
| 3 days after casing | 85.8 ± 7.5 Aa | 37.0 ± 11.7 Ba | 18.3 ± 8.6 Ba | - | 38.0 b |
| Induction phase | 86.1 ± 9.7 Aa | 54.4 ± 10.8 Ba | 16.1 ± 9.5 Ca | - | 52.2 a |
| Primordia formation | 24.4 ± 5.1 Ac | 12.1 ± 7.5 Ab | 10.7 ± 9.4 Aa | - | 15.7 d |
| Mixed with casing | 40.4 ± 8.3 Ac | 24.1 ± 7.1 Aab | 3.7 ± 3.6 Ca | - | 22.7 cd |
| Mean | 59.4 ± 17.7 A | 30.8 ± 15.4 B | 10.4 ± 7.1 C | - | - |
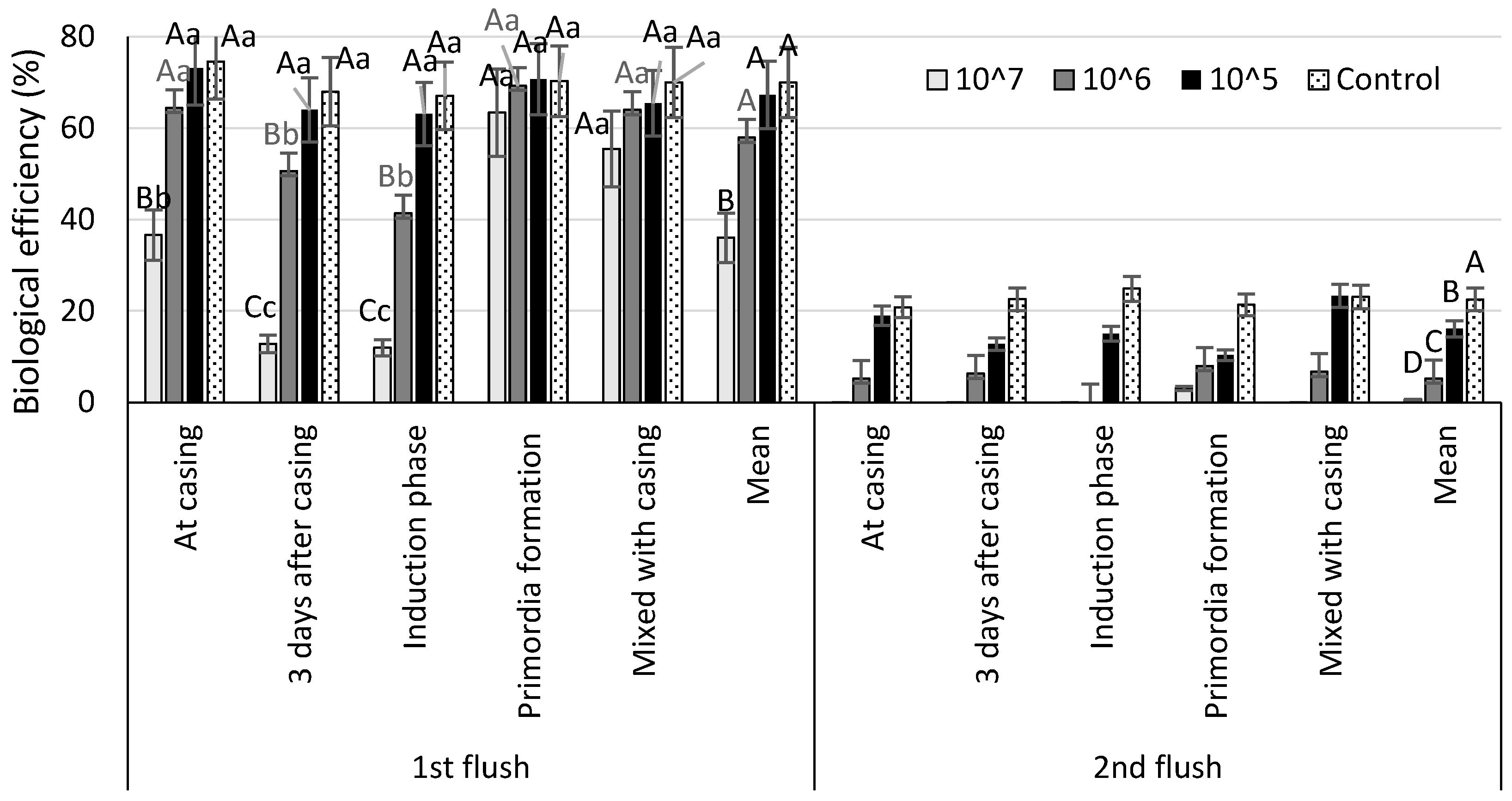
| Biological efficiency (%) by number of spores per m2 casing | |||||
| 107 spores m−2 | 106 spores m−2 | 105 spores m−2 | Control | Mean | |
| At casing | 36.7 ± 8.2 Cb | 69.7 ± 7.8 Bb | 92.0 ± 5.9 Aa | 95.7 ± 9.2 Aa | 73.4 ± 8.7 a |
| 3 days after casing | 12.8 ± 6.4 Cc | 56.8 ± 13.6 Bc | 76.7 ± 18.2 Aa | 90.2 ± 3.3 Aa | 59.2 ± 10.9 b |
| Induction phase | 11.8 ± 5.1 Cc | 41.4 ± 14.3 Bc | 78.0 ± 20.6 Aa | 92.0 ± 12.9 Aa | 55. 8 ± 12.1 b |
| Primordia formation | 66.8 ± 4.9 Ba | 77.3 ± 9.7 ABa | 78.5 ± 10.9 Aba | 87.9 ± 4.5 Aa | 77.5 ± 7.1 a |
| Mixed with casing | 55.5 ± 4.3 Ba | 70.7 ± 9.6 Bb | 88.8 ± 11.6 Aa | 95.2 ± 8.9 Aa | 77.0 ± 9.2 a |
| Mean | 34.4 ± 6.3 D | 63.2 ± 10.2 C | 82.6 ± 12.9 B | 92.2 ± 8.7 A | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumigaj-Tarnowska, J.; Ślusarski, C.; Uliński, Z. Infection Dynamics of Zarea fungicola and Its Impact on White Button Mushroom Yield. Agronomy 2025, 15, 2464. https://doi.org/10.3390/agronomy15112464
Szumigaj-Tarnowska J, Ślusarski C, Uliński Z. Infection Dynamics of Zarea fungicola and Its Impact on White Button Mushroom Yield. Agronomy. 2025; 15(11):2464. https://doi.org/10.3390/agronomy15112464
Chicago/Turabian StyleSzumigaj-Tarnowska, Joanna, Czesław Ślusarski, and Zbigniew Uliński. 2025. "Infection Dynamics of Zarea fungicola and Its Impact on White Button Mushroom Yield" Agronomy 15, no. 11: 2464. https://doi.org/10.3390/agronomy15112464
APA StyleSzumigaj-Tarnowska, J., Ślusarski, C., & Uliński, Z. (2025). Infection Dynamics of Zarea fungicola and Its Impact on White Button Mushroom Yield. Agronomy, 15(11), 2464. https://doi.org/10.3390/agronomy15112464





