Effects of Extreme Moisture Events on Greenhouse Gas Emissions and Soil Ecological Functional Stability in Calcaric Cambisols
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Research Area
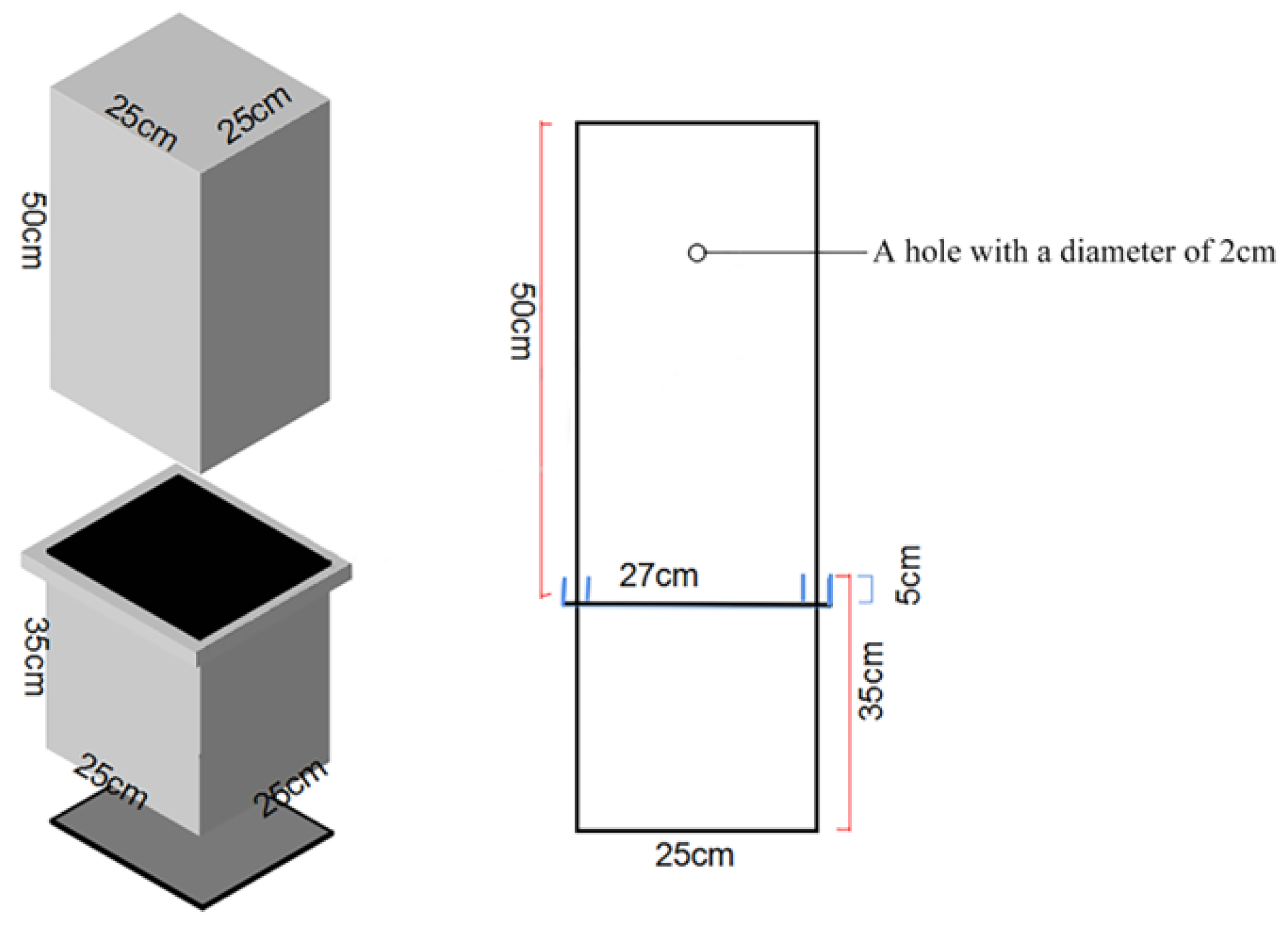
2.2. Experiment Design
2.3. Measuring Index and Method
2.3.1. Soil Basic Index Determination
2.3.2. Determination of Greenhouse Gases in Soils
- F—daily emission flux, measured in mg·m−2·h−1;
- M—atomic weight of C or N in CO2-C and N2O-N, which are 12 g·mol−1 and 28 g·mol−1, respectively;
- H—effective height of the sampling chamber, measured in meters (m);
- dc/dt—gas emission rate, represented as the slope of the regression equation between gas concentration and time (0, 10, 20, and 30 min);
- T—average temperature inside the chamber during sampling, measured in degrees Celsius (°C).
- G—total emission, kg·hm−2;
- Fi—gas emission flux at the i sampling, mg·m−2·h−1;
- di—the number of days between the i sampling and the next sampling;
- D—total number of days.
2.3.3. Calculation of Soil Stability
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Effects of Extreme Moisture on Soil Greenhouse Gas Emissions
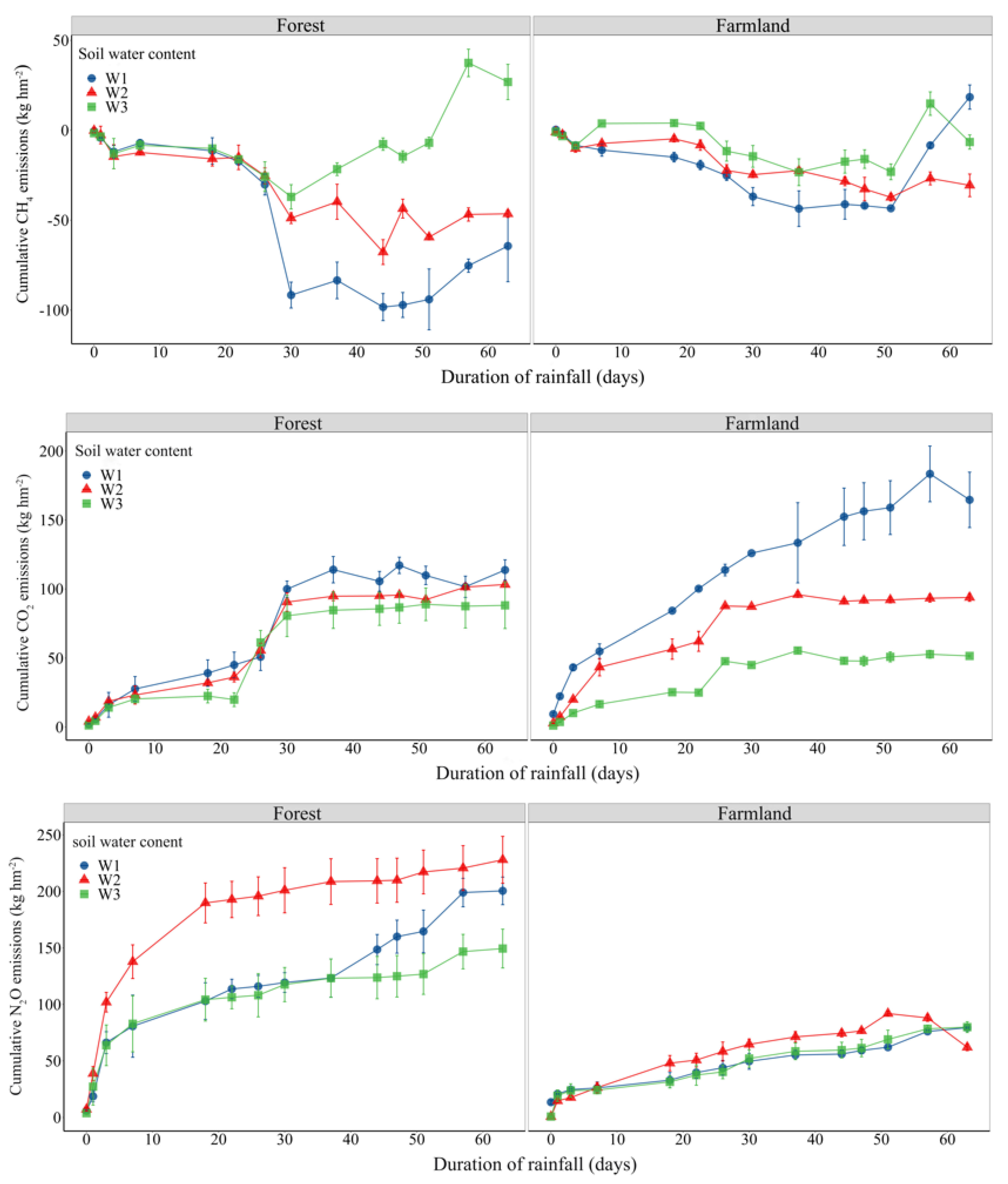
3.1.1. Dynamic Trends of Soil CH4 Under Different Extreme Water Conditions
3.1.2. Variation Trend of Soil CO2 Under Different Extreme Moisture Conditions
3.1.3. Variation Trend of Soil N2O Under Different Extreme Moisture Conditions
3.1.4. Differences in Global Warming Potential
3.2. Soil Carbon and Nitrogen Under Extreme Moisture Conditions
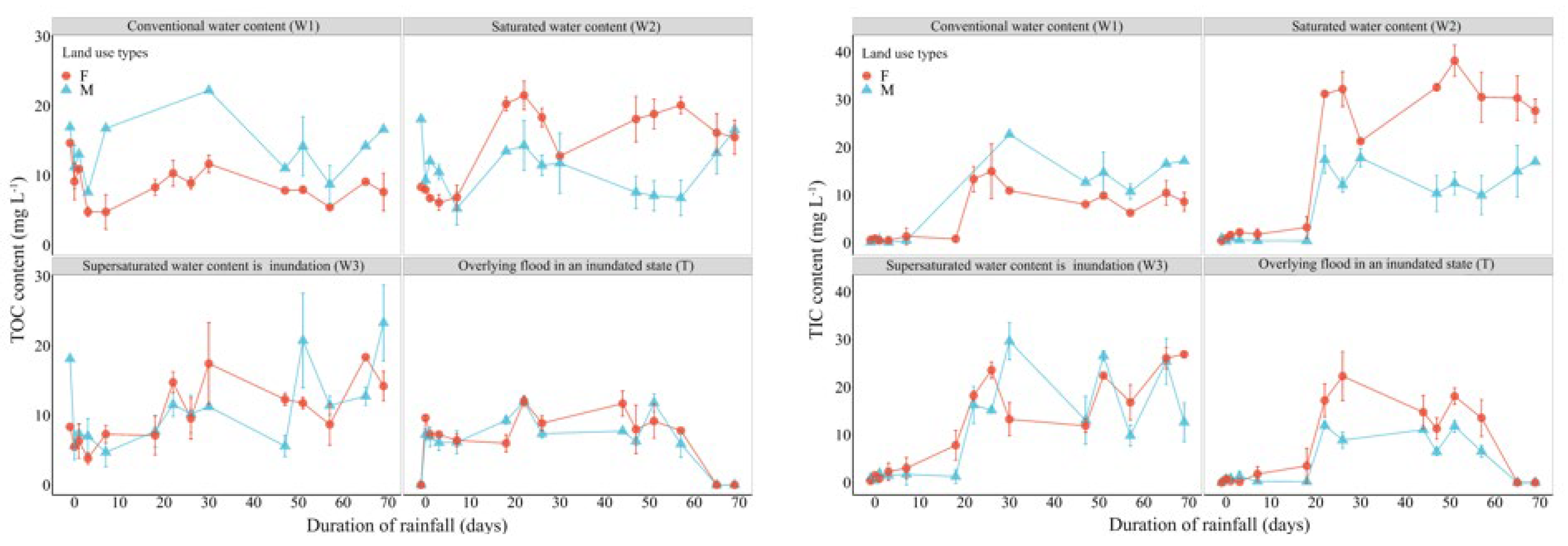
3.2.1. Changes in Soil Carbon Pool Under Different Extreme Moisture Conditions
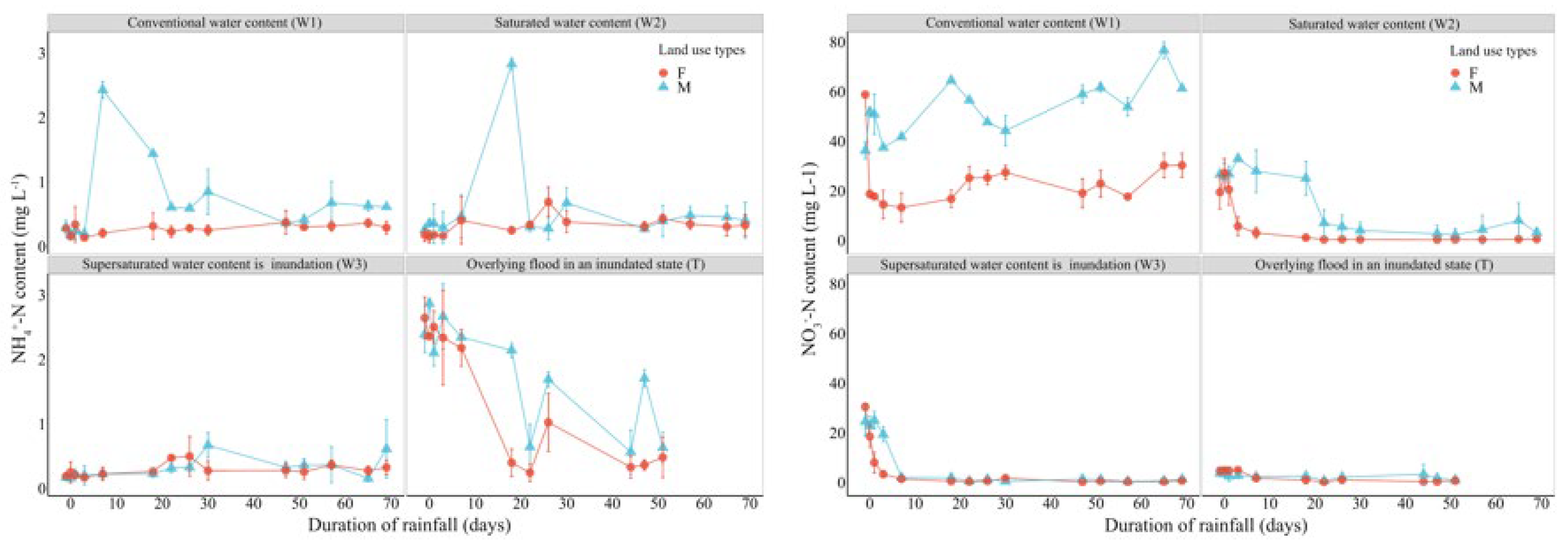
3.2.2. Changes in Soil Nitrogen Pool Under Different Extreme Moisture Conditions
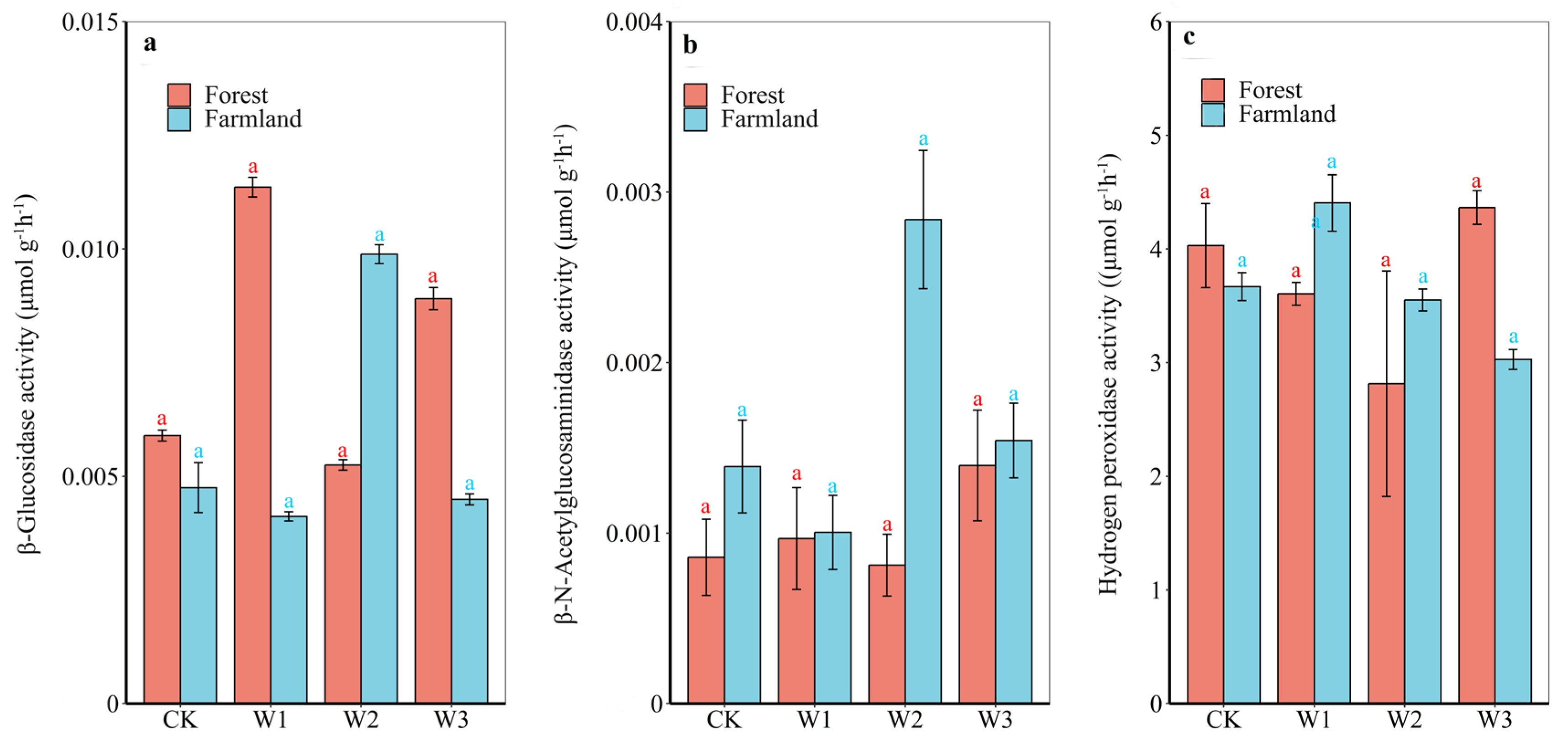
3.3. Soil Enzyme Activity
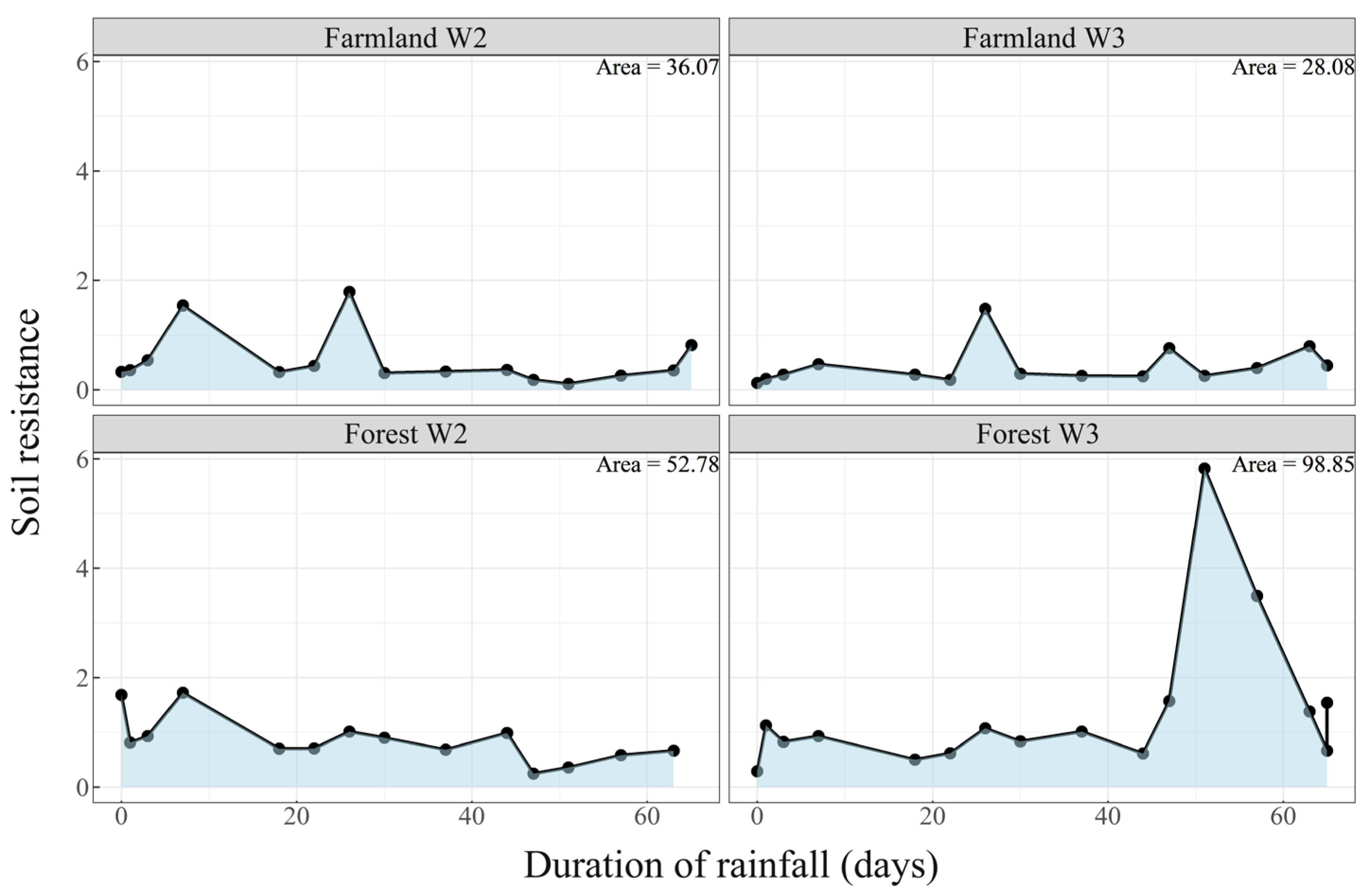
3.4. Soil Resistance
3.5. Evaluation of Soil Functional Stability Under Extreme Moisture Conditions
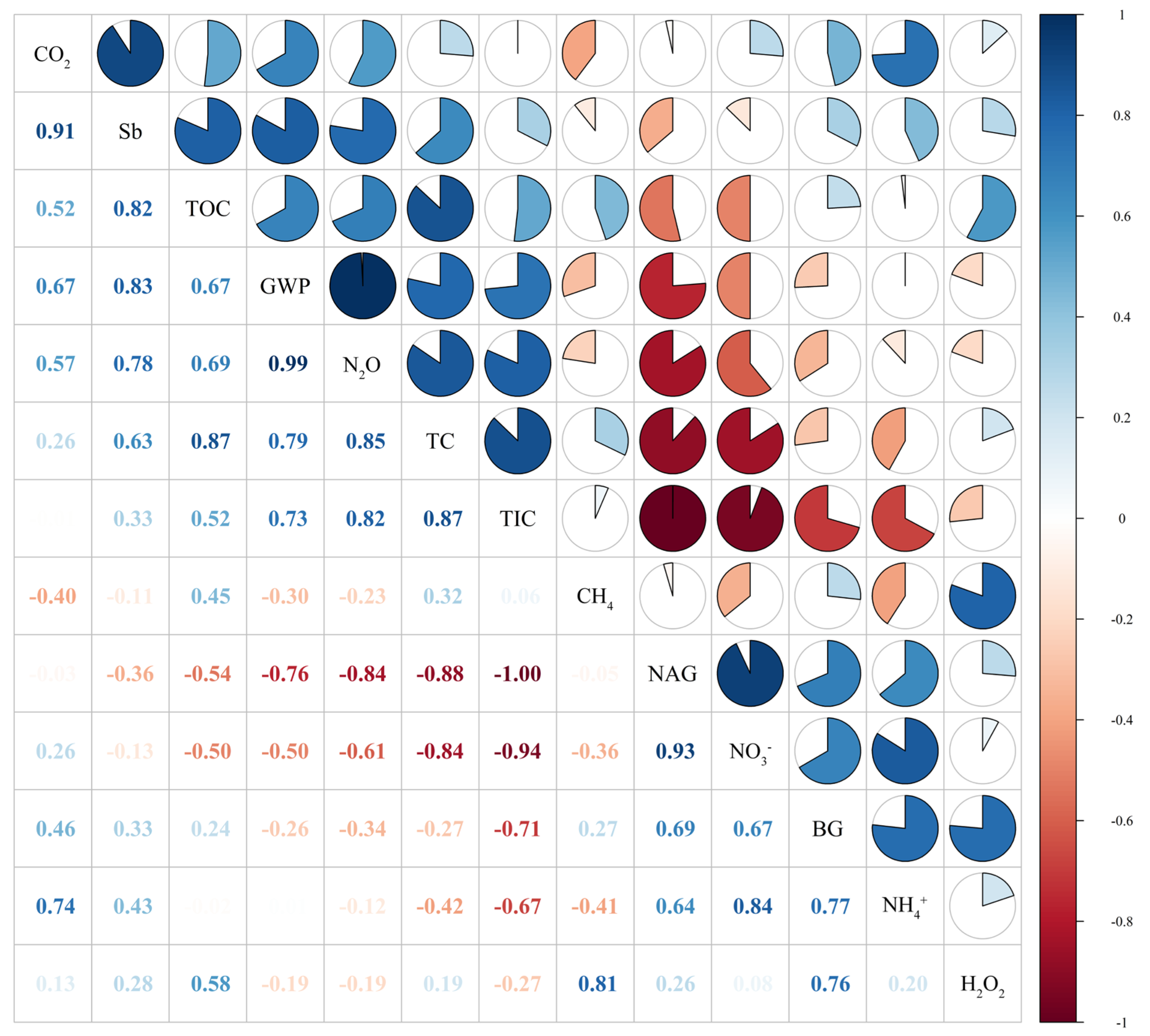
4. Discussion
4.1. The Effects of Extreme Moisture on Soil Greenhouse Gas Emissions
4.2. Effects of Extreme Moisture on Soil Carbon, Nitrogen, and Enzyme Activities
4.3. Effects of Extreme Moisture on Soil Functional Stability
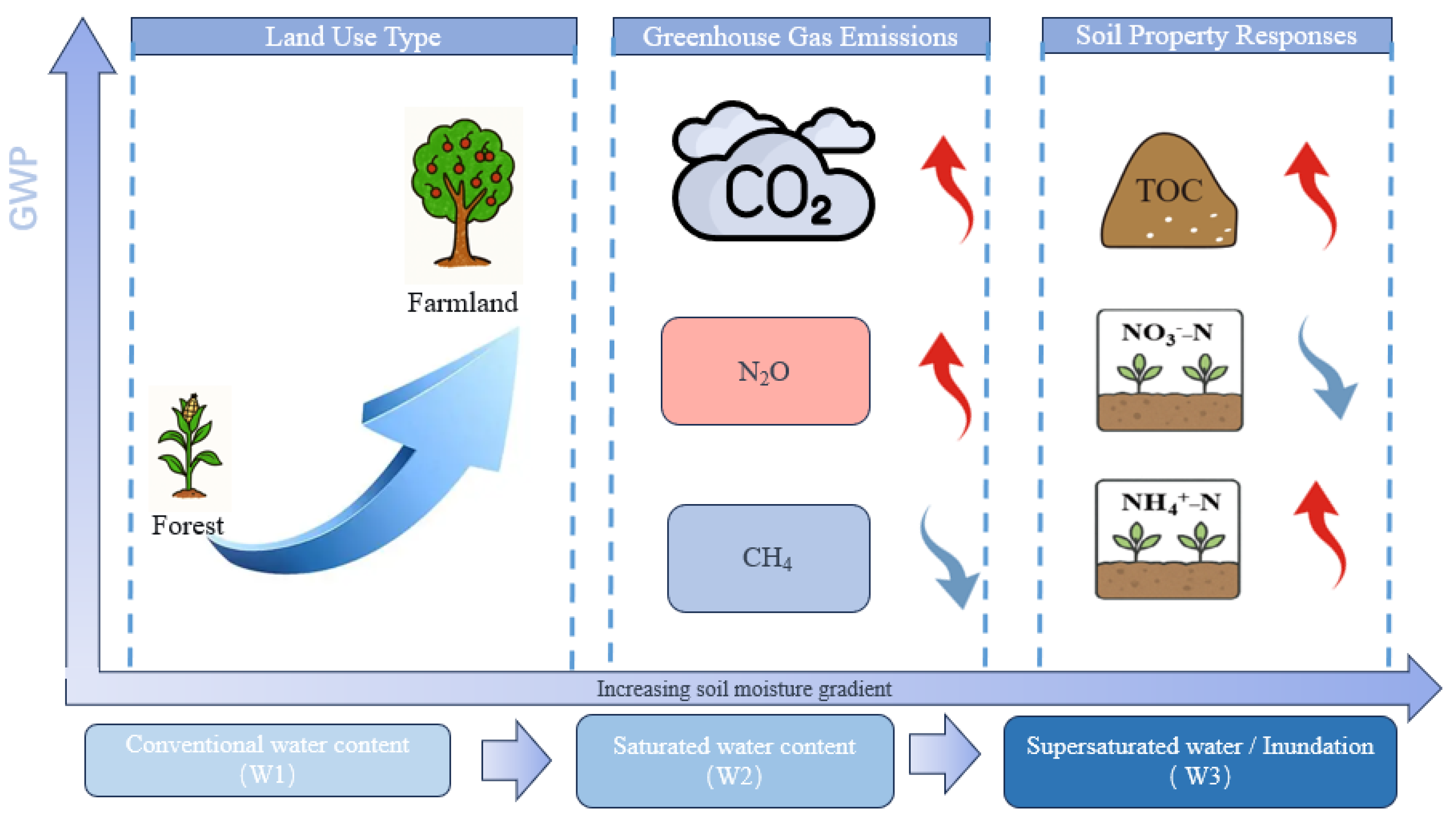
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, R.; Xu, K.; Chen, R.; Chen, W.; Li, F.; Lv, C. Heat waves in summer 2022 and increasing concern regarding heat waves in general. Atmos. Ocean. Sci. Lett. 2022, 16, 100290. [Google Scholar] [CrossRef]
- He, C.; Zhou, T.; Zhang, L.; Chen, X.; Zhang, W. Extremely hot East Asia and flooding western South Asia in the summer of 2022 tied to reversed flow over Tibetan Plateau. Clim. Dyn. 2023, 61, 2103–2119. [Google Scholar] [CrossRef]
- Allan, R.P.; Arias, P.A.; Berger, S.; Canadell, J.G.; Cassou, C.; Chen, D.; Cherchi, A.; Connors, S.L.; Coppola, E.; Cruz, F.A.; et al. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2023: Synthesis Report; Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar]
- Liao, Z.; Chen, Y.; Li, W.; Zhai, P. Growing Threats from Unprecedented Sequential Flood-Hot Extremes Across China. Geophys. Res. Lett. 2021, 48, e2021GL094505. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Chadwick, D.R.; Tatton, G.S.; Hill, P.W.; Jones, D.L. Comparative effects of prolonged freshwater and saline flooding on nitrogen cycling in an agricultural soil. Appl. Soil Ecol. 2018, 125, 56–70. [Google Scholar] [CrossRef]
- Wang, T.; Wei, H.; Ma, W.; Zhou, C.; Chen, H.; Li, R.; Li, S. Response of Taxodium distichum to winter submergence in the water-level-fluctuating zone of the Three Gorges Reservoir region. J. Freshw. Ecol. 2019, 34, 1–17. [Google Scholar] [CrossRef]
- Shen, R.; Lan, Z.; Huang, X.; Chen, Y.; Hu, Q.; Fang, C.; Jin, B.; Chen, J. Soil and plant characteristics during two hydrologically contrasting years at the lakeshore wetland of Poyang Lake, China. J. Soils Sediments 2020, 20, 3368–3379. [Google Scholar] [CrossRef]
- Hafeez, F.; Zafar, N.; Nazir, R.; Javeed, H.M.R.; Rizwan, M.; Faridullah; Asad, S.A.; Iqbal, A. Assessment of flood-induced changes in soil heavy metal and nutrient status in Rajanpur, Pakistan. Environ. Monit. Assess. 2019, 191, 234. [Google Scholar] [CrossRef]
- Olivera Viciedo, D.; de Mello Prado, R.; Martínez, C.A.; Habermann, E.; de Cássia Piccolo, M. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Fay, P.A.; Carlisle, J.D.; Knapp, A.K.; Blair, J.M.; Collins, S.L. Productivity responses to altered rainfall patterns in a C 4-dominated grassland. Oecologia 2003, 137, 245–251. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of More Extreme Precipitation Regimes for Terrestrial Ecosystems. BioScience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Durack, P.J.; Wijffels, S.E.; Matear, R.J. Ocean salinities reveal strong global water cycle intensification during 1950 to 2000. Science 2012, 336, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Donat, M.G.; Lowry, A.L.; Alexander, L.V.; O’Gorman, P.A.; Maher, N. More extreme precipitation in the world’s dry and wet regions. Nature Clim. Change 2016, 6, 508–513. [Google Scholar] [CrossRef]
- Pendergrass, A.G.; Knutti, R.; Lehner, F.; Deser, C.; Sanderson, B.M. Precipitation variability increases in a warmer climate. Sci. Rep. 2017, 7, 17966. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Solomon, S.; Dai, A.; Portmann, R.W. How Often Will It Rain? J. Climate 2007, 20, 4801–4818. [Google Scholar] [CrossRef]
- Fishman, R. More uneven distributions overturn benefits of higher precipitation for crop yields. Environ. Res. Lett. 2016, 11, 024004. [Google Scholar] [CrossRef]
- Grimm, N.B.; Chapin, I.I.I.F.S.; Bierwagen, B.; Gonzalez, P.; Groffman, P.M.; Luo, Y.; Melton, F.; Nadelhoffer, K.; Pairis, A.; Raymond, P.A.; et al. The impacts of climate change on ecosystem structure and function. Front. Ecol. Environ. 2013, 11, 474–482. [Google Scholar] [CrossRef]
- Norris, J.; Chen, G.; Neelin, J.D. Thermodynamic versus Dynamic Controls on Extreme Precipitation in a Warming Climate from the Community Earth System Model Large Ensemble. J. Clim. 2019, 32, 1025–1045. [Google Scholar] [CrossRef]
- Wing, O.E.J.; Lehman, W.; Bates, P.D.; Sampson, C.C.; Quinn, N.; Smith, A.M.; Neal, J.C.; Porter, J.R.; Kousky, C. Inequitable patterns of US flood risk in the Anthropocene. Nat. Clim. Chang. 2022, 12, 156–162. [Google Scholar] [CrossRef]
- Yan, L.; Chen, S.; Xia, J.; Luo, Y. Precipitation Regime Shift Enhanced the Rain Pulse Effect on Soil Respiration in a Semi-Arid Steppe. PLoS ONE 2014, 9, e104217. [Google Scholar] [CrossRef]
- Cui, F.; Yan, G.; Zhou, Z.; Zheng, X.; Deng, J. Annual emissions of nitrous oxide and nitric oxide from a wheat–maize cropping system on a silt loam calcareous soil in the North China Plain. Soil Biol. Biochem. 2012, 48, 10–19. [Google Scholar] [CrossRef]
- Nearing, M.A.; Jetten, V.; Baffaut, C.; Cerdan, O.; Couturier, A.; Hernandez, M.; Le Bissonnais, Y.; Nichols, M.H.; Nunes, J.P.; Renschler, C.S.; et al. Modeling response of soil erosion and runoff to changes in precipitation and cover. Catena 2005, 61, 131–154. [Google Scholar] [CrossRef]
- Jarvis, P.; Rey, A.; Petsikos, C.; Wingate, L.; Rayment, M.; Pereira, J.; Banza, J.; David, J.; Miglietta, F.; Borghetti, M.; et al. Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: The “Birch effect”. Tree Physiol. 2007, 27, 929–940. [Google Scholar] [CrossRef]
- Lado-Monserrat, L.; Lull, C.; Bautista, I.; Lidón, A.; Herrera, R. Soil moisture increment as a controlling variable of the “Birch effect”. Interactions with the pre-wetting soil moisture and litter addition. Plant Soil 2014, 379, 21–34. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil Enzyme Activities and Biodiversity Measurements as Integrative Microbiological Indicators. In Methods for Assessing Soil Quality; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1997; pp. 247–271. [Google Scholar]
- Burns, A.; Ryder, D.S. Response of bacterial extracellular enzymes to inundation of floodplain sediments. Freshw. Biol. 2001, 46, 1299–1307. [Google Scholar] [CrossRef]
- Gardner, C.M.; Robinson, D.; Blyth, K.; Cooper, J.D. Soil Water Content. In Soil and Environmental Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Weltzin, J.F.; Loik, M.E.; Schwinning, S.; Williams, D.G.; Fay, P.A.; Haddad, B.M.; Harte, J.; Huxman, T.E.; Knapp, A.K.; Lin, G.; et al. Assessing the Response of Terrestrial Ecosystems to Potential Changes in Precipitation. BioScience 2003, 53, 941–952. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Teixeira, K.J.; Delong, J.P.; Fox, A.M.; Brese, D.A.; Litvak, M.E. Differential responses of production and respiration to temperature and moisture drive the carbon balance across a climatic gradient in New Mexico. Glob. Change Biol. 2011, 17, 410–424. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity–stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef]
- Seybold, C.A.; Herrick, J.E.; Brejda, J.J. Soil resilience: The fundamental component of soil quality. Soil Sci. 1999, 164, 224–234. [Google Scholar] [CrossRef]
- Holt, R.D. Ecology: Asymmetry and stability. Nature 2006, 442, 252–253. [Google Scholar] [CrossRef] [PubMed]
- He, J.-Z.; Hu, H.-W.; Zhang, L.-M. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 2012, 55, 146–154. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.-C.; Zhang, Q.-F.; Cheng, X.-L. Impacts of different land use types on soil nitrogen mineralization in Danjiangkou Reservoir Area, China. Chin. J. Plant Ecol. 2012, 36, 530–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, B.; Xu, Y.; Han, Z. CMIP6 Evaluation and Projection of Precipitation over Northern China: Further Investigation. Adv. Atmos. Sci. 2023, 40, 587–600. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Wu, Y.; Wen, J. Future changes in precipitation characteristics in China. Int. J. Climatol. 2019, 39, 3558–3573. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; IUSS: Vienna, Austria, 2022. [Google Scholar]
- Oram, N.J.; De Deyn, G.B.; Bodelier, P.L.E.; Cornelissen, J.H.C.; van Groenigen, J.W.; Abalos, D. Plant community flood resilience in intensively managed grasslands and the role of the plant economic spectrum. J. Appl. Ecol. 2020, 57, 1524–1534. [Google Scholar] [CrossRef]
- GB/T 7172–1987; Standardization Administration of China. Method for the Determination of Soil Water Content. Standards Press of China: Beijing, China, 1987. (In Chinese)
- NY/T 1121.4–2006; Ministry of Agriculture of the People’s Republic of China. Soil Testing—Part 4: Method for Determination of Soil Bulk Density. China Agriculture Press: Beijing, China, 2006. (In Chinese)
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen—Total. In Methods of Soil Analysis. Part 3—Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series 5; Soil Science Society of America & American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar] [CrossRef]
- Ge, X. Effect of Cover Cropping on Greenhouse Gasemissions in a Winter Wheat Farmland Underdifferent Rainfall Patteren. Master’s thesis, Northwest University, Maryville, MO, USA, 2019. [Google Scholar]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil–atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Morell, F.J.; Álvaro-Fuentes, J.; Lampurlanés, J.; Cantero-Martínez, C. Soil CO2 fluxes following tillage and rainfall events in a semiarid Mediterranean agroecosystem: Effects of tillage systems and nitrogen fertilization. Agric. Ecosyst. Environ. 2010, 139, 167–173. [Google Scholar] [CrossRef]
- Chen, J.; Nie, Y.; Liu, W.; Wang, Z.; Shen, W. Ammonia-Oxidizing Archaea Are More Resistant Than Denitrifiers to Seasonal Precipitation Changes in an Acidic Subtropical Forest Soil. Front. Microbiol. 2017, 8, 1384. [Google Scholar] [CrossRef]
- Molodovskaya, M.; Singurindy, O.; Richards, B.K.; Warland, J.; Johnson, M.S.; Steenhuis, T.S. Temporal Variability of Nitrous Oxide from Fertilized Croplands: Hot Moment Analysis. Soil Sci. Soc. Am. J. 2012, 76, 1728–1740. [Google Scholar] [CrossRef]
- López-Ballesteros, A.; Serrano-Ortiz, P.; Sánchez-Cañete, E.P.; Oyonarte, C.; Kowalski, A.S.; Pérez-Priego, Ó.; Domingo, F. Enhancement of the net CO2 release of a semiarid grassland in SE Spain by rain pulses. J. Geophys. Res. Biogeosciences 2016, 121, 52–66. [Google Scholar] [CrossRef]
- Zhu, M.; De Boeck, H.J.; Xu, H.; Chen, Z.; Lv, J.; Zhang, Z. Seasonal variations in the response of soil respiration to rainfall events in a riparian poplar plantation. Sci. Total Environ. 2020, 747, 141222. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.-L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Change Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Krichels, A.; DeLucia, E.H.; Sanford, R.; Chee-Sanford, J.; Yang, W.H. Historical soil drainage mediates the response of soil greenhouse gas emissions to intense precipitation events. Biogeochemistry 2019, 142, 425–442. [Google Scholar] [CrossRef]
- Van Straaten, O.; Veldkamp, E.; Köhler, M.; Anas, I. Spatial and temporal effects of drought on soil CO2 efflux in a cacao agroforestry system in Sulawesi, Indonesia. Biogeosciences Discuss. 2010, 7, 1223–1235. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Li, G.; Liu, Z.; Wang, H.; Zhang, D. Contributions of nitrification and denitrification to N2O emissions from aged refuse bioreactor at different feeding loads of ammonia substrates. Waste Manag. 2017, 68, 319–328. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Munson, S.M.; Benton, T.J.; Lauenroth, W.K. Soil carbon flux following pulse precipitation events in the shortgrass steppe. Ecol. Res. 2009, 25, 205–211. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Fine Root Biomass, Production, Turnover Rates, and Nutrient Contents in Boreal Forest Ecosystems in Relation to Species, Climate, Fertility, and Stand Age: Literature Review and Meta-Analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Zhang, W. Studies on Main Factors that Influence Organic Carbon in Purple Paddy Soils. Master’s Thesis, Southwest University, El Paso, TX, USA, 2007. [Google Scholar]
- Sánchez-García, C.; Oliveira, B.R.F.; Keizer, J.J.; Doerr, S.H.; Urbanek, E. Water repellency reduces soil CO2 efflux upon rewetting. Sci. Total Environ. 2020, 708, 135014. [Google Scholar] [CrossRef]
- Song, K.; Lee, S.-H.; Mitsch, W.J.; Kang, H. Different responses of denitrification rates and denitrifying bacterial communities to hydrologic pulsing in created wetlands. Soil Biol. Biochem. 2010, 42, 1721–1727. [Google Scholar] [CrossRef]
- Mchergui, C.; Besaury, L.; Langlois, E.; Aubert, M.; Akpa-Vinceslas, M.; Buatois, B.; Quillet, L.; Bureau, F. A comparison of permanent and fluctuating flooding on microbial properties in an ex-situ estuarine riparian system. Appl. Soil Ecol. 2014, 78, 1–10. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Hentz, C.S.; Pinto, O.B.; Carneiro, E.; de Souza Nogueira, J. Soil N, P, and C dynamics of upland and seasonally flooded forests of the Brazilian Pantanal. Glob. Ecol. Conserv. 2017, 12, 227–240. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patrick, W.H. Effect of alternate aerobic and anaerobic conditions on redox potential, organic matter decomposition and nitrogen loss in a flooded soil. Soil Biol. Biochem. 1975, 7, 87–94. [Google Scholar] [CrossRef]
- Tanner, C.C.; D’Eugenio, J.; McBride, G.B.; Sukias, J.P.S.; Thompson, K. Effect of water level fluctuation on nitrogen removal from constructed wetland mesocosms. Ecol. Eng. 1999, 12, 67–92. [Google Scholar] [CrossRef]
- Ye, C.; Cheng, X.; Zhang, Q. Recovery approach affects soil quality in the water level fluctuation zone of the Three Gorges Reservoir, China: Implications for revegetation. Environ. Sci. Pollut. Res. 2014, 21, 2018–2031. [Google Scholar] [CrossRef]
- Zhang, Y. Ecological Effects of Farmland Landscape Pattern on Aphid and Ladybug Populations. Ph.D. Dissertation, Hunan Agricultural University, Changsha, China, 2019. (In Chinese). [Google Scholar]
- Zhang, Y. Ecological Effects of Agricultural Landscape Patterns Onaphids and Ladybeetles. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2019. [Google Scholar]
- Zhang, J.; Wang, L.; Liao, Y.; Tang, L.; Liu, Q. Effects of irrigation amount on soil enzyme activity, yield and nutrient utilization offacilities pepper. Southwest. J. Agric. Sci. 2023, 36, 1216–1221. [Google Scholar]
- Yao, Y.; Liu, X.; Zhu, H.; Tang, D. Effects of Vegetation Restoration Patterns on the Physical and Chemical Properties of Soil. Shaanxi For. Sci. Technol. 2020, 48, 74–79. [Google Scholar]
- Du, X.; Li, Y.B.; Liu, F.; Su, X.L.; Li, Q. Structure and ecological functions of soil micro-food web. Chin. J. Appl. Ecol. 2018, 29, 403–411. [Google Scholar]
- Hueso, S.; García, C.; Hernández, T. Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol. Biochem. 2012, 50, 167–173. [Google Scholar] [CrossRef]
- Liu, C.; Holst, J.; Brüggemann, N.; Butterbach-Bahl, K.; Yao, Z.; Han, S.; Han, X.; Zheng, X. Effects of irrigation on nitrous oxide, methane and carbon dioxide fluxes in an Inner Mongolian steppe. Adv. Atmos. Sci. 2008, 25, 748–756. [Google Scholar] [CrossRef]
| Property | Farmland Soil | Forest Soil |
|---|---|---|
| Soil texture | Silt loam | Silt loam |
| pH (1:2.5 H2O) | 7.8 ± 0.1 | 8.1 ± 0.1 |
| EC (μS·cm−1) | 95.3 ± 8.6 | 68.4 ± 7.2 |
| SOM (g kg−1) | 18.5 ± 1.5 | 12.2 ± 1.1 |
| TN (g kg−1) | 1.2 ± 0.1 | 0.8 ± 0.1 |
| NH4+–N (mg kg−1) | 5.8 ± 0.5 | 4.1 ± 0.4 |
| NO3−–N (mg kg−1) | 12.5 ± 1.3 | 7.3 ± 0.9 |
| Available P (mg kg−1) | 25.6 ± 2.8 | 8.5 ± 1.2 |
| Available K (mg kg−1) | 145.6 ± 12.4 | 98.7 ± 10.5 |
| Bulk density (g cm−3) | 1.32 ± 0.05 | 1.45 ± 0.06 |
| Field capacity (%, mass) | 24.5 ± 1.0 | 22.8 ± 1.2 |
| Treatment | Cumulative CH4 Emissions (kg·hm−2) | Cumulative CO2 Emissions (kg·hm−2) | Cumulative N2O Emissions (kg·hm−2) | GWP (kg·hm−2) |
|---|---|---|---|---|
| farmland W1 | 0.019 ± 0.012 abA | 164.62 ± 20.09 aA | 79.43 ± 0.59 cA | 23,834.26 ± 197.12 cA |
| farmland W2 | −0.031 ± 0.016 abA | 93.82 ± 2.83 abA | 62.08 ± 2.99 bcA | 18,595.01 ± 895.04 bcA |
| farmland W3 | −0.007 ± 0.004 abA | 51.59 ± 0.75 cA | 79.92 ± 4.79 bcA | 23,866.57 ± 1428.78 bcA |
| forest W1 | −0.064 ± 0.020 bA | 113.72 ± 7.42 bA | 200.45 ± 22.21 abB | 59,848.45 ± 6626.46 abB |
| forest W2 | −0.046 ± 0.020 abA | 103.20 ± 8.34 bA | 227.94 ± 72.70 abB | 68,030.37 ± 21,672.80 aB |
| forest l W3 | 0.027 ± 0.050 aA | 88.1 ± 16.81 bA | 149.41 ± 47.17 abcB | 44,613.41 ± 14,072.66 abcB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Qiang, M.; Zuo, J.; Wang, K.; Han, J.; Tong, X.; Zhang, M. Effects of Extreme Moisture Events on Greenhouse Gas Emissions and Soil Ecological Functional Stability in Calcaric Cambisols. Agronomy 2025, 15, 2461. https://doi.org/10.3390/agronomy15112461
Wang W, Qiang M, Zuo J, Wang K, Han J, Tong X, Zhang M. Effects of Extreme Moisture Events on Greenhouse Gas Emissions and Soil Ecological Functional Stability in Calcaric Cambisols. Agronomy. 2025; 15(11):2461. https://doi.org/10.3390/agronomy15112461
Chicago/Turabian StyleWang, Weixin, Minmin Qiang, Jichao Zuo, Kaixuan Wang, Jianqiao Han, Xin Tong, and Man Zhang. 2025. "Effects of Extreme Moisture Events on Greenhouse Gas Emissions and Soil Ecological Functional Stability in Calcaric Cambisols" Agronomy 15, no. 11: 2461. https://doi.org/10.3390/agronomy15112461
APA StyleWang, W., Qiang, M., Zuo, J., Wang, K., Han, J., Tong, X., & Zhang, M. (2025). Effects of Extreme Moisture Events on Greenhouse Gas Emissions and Soil Ecological Functional Stability in Calcaric Cambisols. Agronomy, 15(11), 2461. https://doi.org/10.3390/agronomy15112461





