DNA-Based Quantification of Fusarium Species in Winter Wheat Grain in Poland from 2014 to 2017 and 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Grain Samples
2.2. Sample Preparation
2.3. DNA Isolation
2.4. Real-Time PCR
2.5. Statistical Analysis
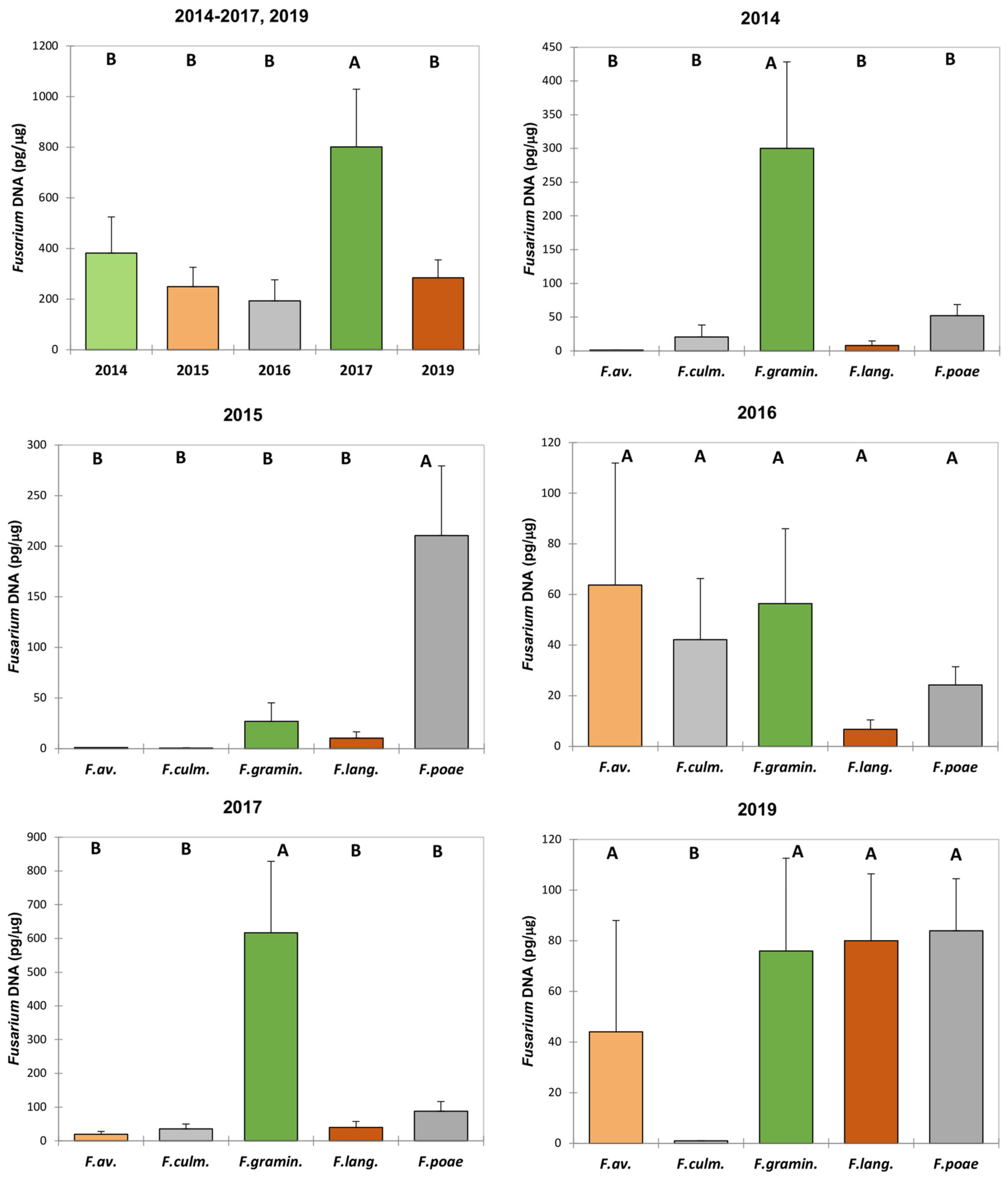
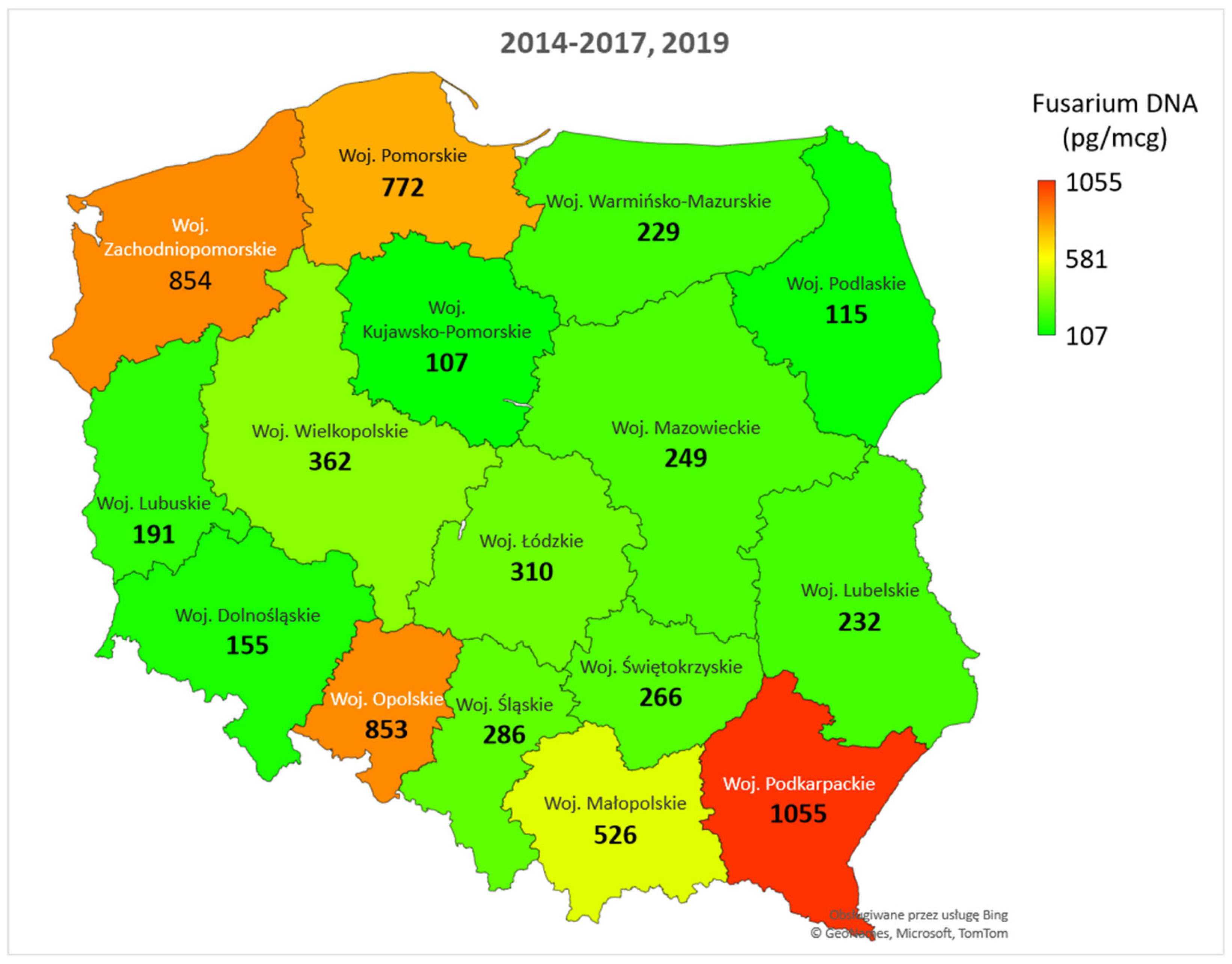
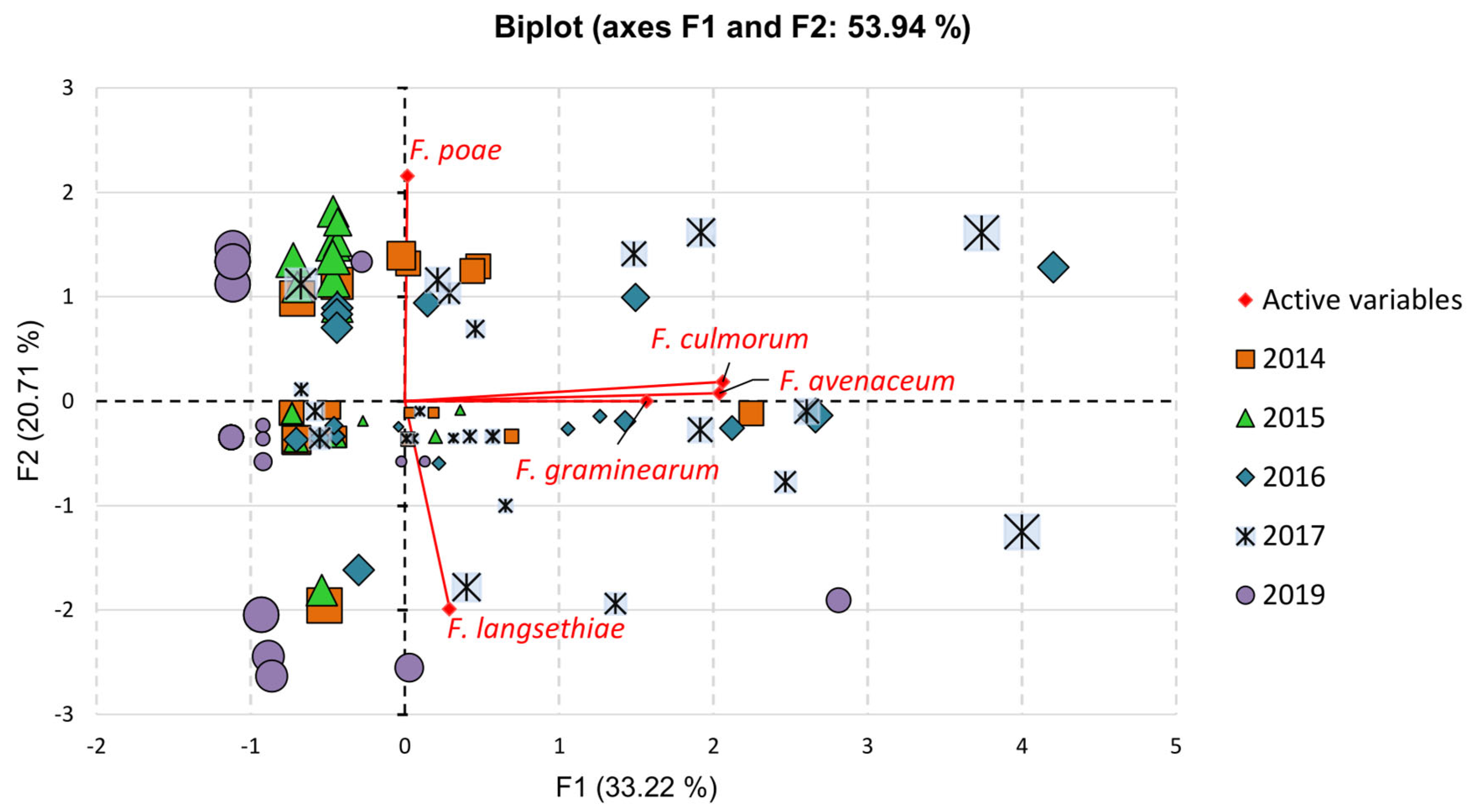
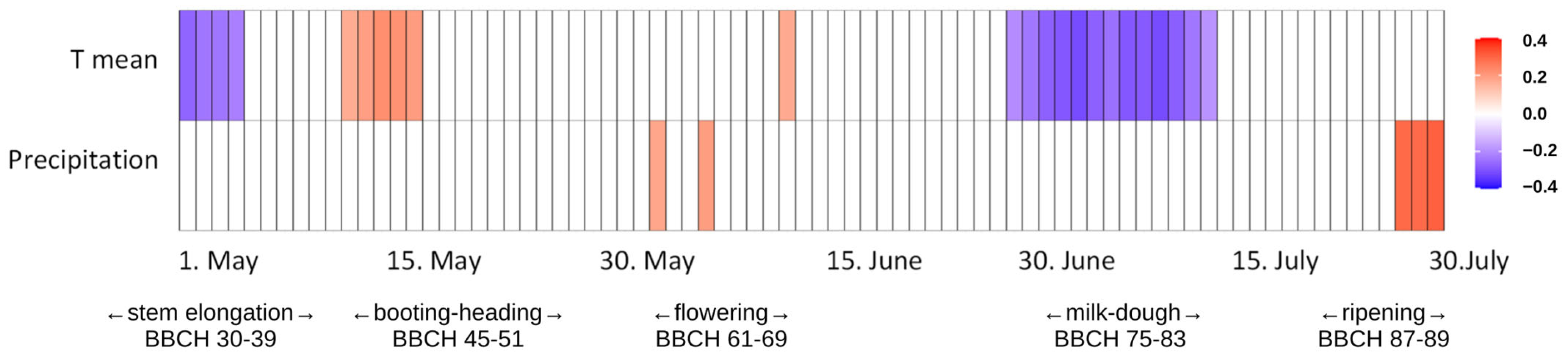
3. Results
3.1. Weather Conditions
3.2. Fusarium Species
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium Ear Blight (Scab) in Small Grain Cereals—A Review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Bottalico, A. Fusarium Diseases of Cereals: Species Complex and Related Mycotoxin Profiles, in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Jestoi, M.N.; Paavanen-Huhtala, S.; Parikka, P.; Yli-Mattila, T. In Vitro and in Vivo Mycotoxin Production of Fusarium Species Isolated from Finnish Grains. Arch. Phytopathol. Plant Prot. 2008, 41, 545–558. [Google Scholar] [CrossRef]
- Somma, S.; Alvarez, C.; Ricci, V.; Ferracane, L.; Ritieni, A.; Logrieco, A.; Moretti, A. Trichothecene and Beauvericin Mycotoxin Production and Genetic Variability in Fusarium poae Isolated from Wheat Kernels from Northern Italy. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2010, 27, 729–737. [Google Scholar] [CrossRef]
- Vanheule, A.; De Boevre, M.; Moretti, A.; Scauflaire, J.; Munaut, F.; De Saeger, S.; Bekaert, B.; Haesaert, G.; Waalwijk, C.; Van Der Lee, T.; et al. Genetic Divergence and Chemotype Diversity in the Fusarium Head Blight Pathogen Fusarium poae. Toxins 2017, 9, 255. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Sulyok, M.; Hecker, A.; Jenny, E.; Krska, R.; Schuhmacher, R.; Forrer, H.-R.R. Toxigenicity and Pathogenicity of Fusarium poae and Fusarium avenaceum on Wheat. Eur. J. Plant Pathol. 2008, 122, 265–276. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jensen, J.D.; Nielsen, G.C.; Jensen, J.E.; Spliid, N.H.; Thomsen, I.K.; Justesen, A.F.; Collinge, D.B.; Jørgensen, L.N. Fusarium Head Blight of Cereals in Denmark: Species Complex and Related Mycotoxins. Phytopathology 2011, 101, 960–969. [Google Scholar] [CrossRef]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum—The North European Situation. Int. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paaavanen-Huhtala, P.; Parikka, P.; Hietaniemi, V.; Jestoi, M.; Rizzo, A. Real-Time PCR Detection and Quantification of Fusarium poae as Compared to Mycotoxin Production in Grains in Finland. In Proceedings of the 2nd International Symposium on Fusarium Head Blight, Incorporating the 8th European Fusarium Seminar, Orlando, FL, USA, 11–15 December 2004; Canty, S.M., Boring, T., Wardwell, J., Ward, R.W., Eds.; Michigan State University: East Lansing, MI, USA, 2004; pp. 422–425. [Google Scholar]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Jestoi, M.; Parikka, P.; Hietaniemi, V.; Gagkaeva, T.; Sarlin, T.; Haikara, A.; Laaksonen, S.; Rizzo, A. Real-Time PCR Detection and Quantification of Fusarium poae, F. graminearum, F. sporotrichioides and F. langsethiae in Cereal Grains in Finland and Russia. Arch. Phytopathol. Plant Prot. 2008, 41, 243–260. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Tacke, B.K.; Casper, H.H. Gene Genealogies Reveal Global Phylogeographic Structure and Reproductive Isolation among Lineages of Fusarium graminearum, the Fungus Causing Wheat Scab. Proc. Natl. Acad. Sci. USA 2000, 97, 7905–7910. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Geiser, D.M.; Kistler, H.C.; Aoki, T. Genealogical Concordance between the Mating Type Locus and Seven Other Nuclear Genes Supports Formal Recognition of Nine Phylogenetically Distinct Species within the Fusarium graminearum Clade. Fungal Genet. Biol. 2004, 41, 600–623. [Google Scholar] [CrossRef] [PubMed]
- Starkey, D.E.; Ward, T.J.; Aoki, T.; Gale, L.R.; Kistler, H.C.; Geiser, D.M.; Suga, H.; Tóth, B.; Varga, J.; O’Donnell, K. Global Molecular Surveillance Reveals Novel Fusarium Head Blight Species and Trichothecene Toxin Diversity. Fungal Genet. Biol. 2007, 44, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, C.; Sharanowski, B.; Dilantha Fernando, W.G. Molecular Phylogenetic Relationships, Trichothecene Chemotype Diversity and Aggressiveness of Strains in a Global Collection of Fusarium graminearum Species. Toxins 2019, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Talas, F.; Parzies, H.K.; Miedaner, T. Diversity in Genetic Structure and Chemotype Composition of Fusarium graminearum Sensu Stricto Populations Causing Wheat Head Blight in Individual Fields in Germany. Eur. J. Plant Pathol. 2011, 131, 39–48. [Google Scholar] [CrossRef]
- van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum Species Complex and Chemotypes: A Review. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2015, 32, 453–460. [Google Scholar] [CrossRef]
- Backhouse, D. Global Distribution of Fusarium graminearum, F. asiaticum and F. boothii from Wheat in Relation to Climate. Eur. J. Plant Pathol. 2014, 139, 161–173. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Moreira, G.M.; Ward, T.J.; O’Donnell, K.; Nicolli, C.P.; Machado, F.J.; Duffeck, M.R.; Alves, K.S.; Tessmann, D.J.; Waalwijk, C.; et al. Fusarium graminearum Species Complex: A Bibliographic Analysis and Web-Accessible Database for Global Mapping of Species and Trichothecene Toxin Chemotypes. Phytopathology 2022, 112, 741–751. [Google Scholar] [CrossRef]
- Karlsson, I.; Mellqvist, E.; Persson, P. Temporal and Spatial Dynamics of Fusarium spp. and Mycotoxins in Swedish Cereals during 16 Years. Mycotoxin Res. 2022, 1, 3–18. [Google Scholar] [CrossRef]
- Valverde-Bogantes, E.; Bianchini, A.; Herr, J.R.; Rose, D.J.; Wegulo, S.N.; Hallen-Adams, H.E. Recent Population Changes of Fusarium Head Blight Pathogens: Drivers and Implications. Can. J. Plant Pathol. 2020, 42, 315–329. [Google Scholar] [CrossRef]
- Polišenská, I.; Jirsa, O.; Salava, J.; Sedláčková, I.; Frydrych, J. Fusarium Mycotoxin Content and Fusarium Species Presence in Czech Organic and Conventional Wheat. World Mycotoxin J. 2021, 14, 201–211. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.-A.A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Sundheim, L.; Brodal, G.; Hofgaard, I.S.; Rafoss, T. Temporal Variation of Mycotoxin Producing Fungi in Norwegian Cereals. Microorganisms 2013, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van Der Lee, T.; Brodal, G. Associations between Fusarium Species and Mycotoxins in Oats and Spring Wheat from Farmers Fields in Norway over a Six-Year Period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Maiorano, A.; Blandino, M.; Reyneri, A.; Vanara, F. Effects of Maize Residues on the Fusarium spp. Infection and Deoxynivalenol (DON) Contamination of Wheat Grain. Crop Prot. 2008, 27, 182–188. [Google Scholar] [CrossRef]
- Obst, A.; Lepschy-von Gleissenthall, J.; Beck, R. On the Etiology of Fusarium Head Blight of Wheat in South Germany—Preceding Crops, Weather Conditions for Inoculum Production and Head Infection, Proneness of the Crop to Infection and Mycotoxin Production. Cereal Res. Commun. 1997, 25, 699–703. [Google Scholar] [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected Shifts in Fusarium Species’ Composition on Cereal Grain in Northern Europe Due to Climatic Change. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2012, 29, 1543–1555. [Google Scholar] [CrossRef]
- Miller, J.D. Mycotoxins in Small Grains and Maize: Old Problems, New Challenges. Food Addit. Contam. Part A 2008, 25, 219–230. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal Agent of Foot and Root Rot and Head Blight on Wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Kulik, T.I.; Molcan, T.I.; Bilska, K.; Beyer, M.; Pasquali, M.; van Diepeningen, A.; Myszczynski, K. Two Distinct Fusarium graminearum Populations Colonized European Wheat in the Past Two Decades. PLoS ONE 2023, 18, e0296302. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P. Community Ecology of Fungal Pathogens Causing Wheat Head Blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef]
- FAO Poland Agricultural Census 1990—Main Results. Available online: https://openknowledge.fao.org/handle/20.500.14283/bs464e (accessed on 10 October 2025).
- Eurostat Grain Maize and Corn-Cob-Mix by Area, Production and Humidity. Available online: https://ec.europa.eu/eurostat/databrowser/view/tag00093/default/table?lang=en (accessed on 10 October 2025).
- Eurostat Green Maize by Area, Production and Humidity. Available online: https://ec.europa.eu/eurostat/databrowser/view/tag00101/default/table?lang=en (accessed on 10 October 2025).
- Stępień, Ł.; Chełkowski, J. Fusarium Head Blight of Wheat: Pathogenic Species and Their Mycotoxins. World Mycotoxin J. 2010, 3, 107–119. [Google Scholar] [CrossRef]
- Bilska, K.; Jurczak, S.; Kulik, T.; Ropelewska, E.; Olszewski, J.; Żelechowski, M.; Zapotoczny, P. Species Composition and Trichothecene Genotype Profiling of Fusarium Field Isolates Recovered from Wheat in Poland. Toxins 2018, 10, 325. [Google Scholar] [CrossRef]
- Kuzdraliński, A.; Nowak, M.; Szczerba, H.; Dudziak, K.; Muszyńska, M.; Leśniowska-Nowak, J. The Composition of Fusarium Species in Wheat Husks and Grains in South-Eastern Poland. J. Integr. Agric. 2017, 16, 1530–1536. [Google Scholar] [CrossRef]
- Okorski, A.; Milewska, A.; Pszczółkowska, A.; Karpiesiuk, K.; Kozera, W.; Dąbrowska, J.A.; Radwińska, J. Prevalence of Fusarium Fungi and Deoxynivalenol Levels in Winter Wheat Grain in Different Climatic Regions of Poland. Toxins 2022, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Konecki, R.; Snarska, K.; Łozowicka, B. Quantitative Evaluation of Fusarium Species and Crop Quality Traits in Wheat Varieties of Northeastern Poland. J. Plant Prot. Res. 2018, 58, 413–419. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Stępień, Ł.; Waśkiewicz, A.; Beszterda, M.; Góral, T.; Belter, J. Toxigenic Fusarium Species Infecting Wheat Heads in Poland. Cent. Eur. J. Biol. 2014, 9, 163–172. [Google Scholar] [CrossRef]
- Góral, T.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D. Species of the Genus Fusarium and Fusarium Toxins in the Grain of Winter and Spring Wheat in Poland. Biul. Inst. Hod. I Aklim. Roślin 2021, 296, 25–42. [Google Scholar] [CrossRef]
- Postupolski, J.; Starski, A.; Ledzion, E.; Kurpińska-Jaworska, J.; Szczęsna, M. Assessment of Changes in the Occurrence of Fusarium Toxin and Ochratoxin A in Poland Related to Extreme Weather Phenomena. Rocz. Panstw. Zakl. Hig. 2019, 70, 127–135. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Yoshinari, T.; Waśkiewicz, A.; Szymczyk, K. Contamination of Wheat Cultivated in Various Regions of Poland during 2017 and 2018 Agricultural Seasons with Selected Trichothecenes and Their Modified Forms. Toxins 2019, 11, 88. [Google Scholar] [CrossRef]
- Kowalska, G.; Kowalski, R. Occurrence of Mycotoxins in Selected Agricultural and Commercial Products Available in Eastern Poland. Open Chem. 2021, 19, 653–664. [Google Scholar] [CrossRef]
- Marzec-Schmidt, K.; Börjesson, T.; Suproniene, S.; Jędryczka, M.; Janavičienė, S.; Góral, T.; Karlsson, I.; Kochiieru, Y.; Ochodzki, P.; Mankevičienė, A.; et al. Modelling the Effects of Weather Conditions on Cereal Grain Contamination with Deoxynivalenol in the Baltic Sea Region. Toxins 2021, 13, 737. [Google Scholar] [CrossRef] [PubMed]
- Gourdain, E.; Piraux, F.; Barrier-Guillot, B. A Model Combining Agronomic and Weather Factors to Predict Occurrence of Deoxynivalenol in Durum Wheat Kernels. World Mycotoxin J. 2011, 4, 129–139. [Google Scholar] [CrossRef]
- Aleksandrowicz, E. Factors Influencing the Occurrence of Fusarium Mycotoxins in the Grain of Winter Wheat. Pol. J. Agron. 2020, 43, 103–112. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Góral, T. Nowe Dopuszczalne Limity Zawartości Toksyn Fuzaryjnych (Deoksyniwalenol, Toksyny T-2/HT-2) w Ziarnie Zbóż i Produktach Zbożowych. Biul. Inst. Hod. I Aklim. Roślin 2024, 302, 45–47. [Google Scholar] [CrossRef]
- Góral, T.; Walentyn-Góral, D. Zróżnicowanie Podatności Odmian Pszenicy Ozimej i Jarej Na Fuzariozę Kłosów Badanych w Latach 2009–2016. Biul. Inst. Hod. I Aklim. Roślin 2018, 284, 3–11. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-Time PCR for Quantification of Eleven Individual Fusarium Species in Cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef]
- Zhang, X.; Halder, J.; White, R.P.; Hughes, D.J.; Ye, Z.; Wang, C.; Xu, R.; Gan, B.; Fitt, B.D.L. Climate Change Increases Risk of Fusarium Ear Blight on Wheat in Central China. Ann. Appl. Biol. 2014, 164, 384–395. [Google Scholar] [CrossRef]
- Skelsey, P.; Newton, A.C. Future Environmental and Geographic Risks of Fusarium Head Blight of Wheat in Scotland. Eur. J. Plant Pathol. 2015, 142, 133–147. [Google Scholar] [CrossRef][Green Version]
- Madgwick, J.W.; West, J.S.; White, R.P.; Semenov, M.A.; Townsend, J.A.; Turner, J.A.; Fitt, B.D.L. Impacts of Climate Change on Wheat Anthesis and Fusarium Ear Blight in the UK. Eur. J. Plant Pathol. 2011, 130, 117–131. [Google Scholar] [CrossRef]
- Matengu, T.T.; Bullock, P.R.; Mkhabela, M.S.; Zvomuya, F.; Henriquez, M.A.; Ojo, E.R.T.; Fernando, W.G.D. Weather-Based Models for Forecasting Fusarium Head Blight Risks in Wheat and Barley: A Review. Plant Pathol. 2024, 73, 492–505. [Google Scholar] [CrossRef]
- Kharbikar, L.L.; Dickin, E.T.; Edwards, S.G. Impact of Post-Anthesis Rainfall, Fungicide and Harvesting Time on the Concentration of Deoxynivalenol and Zearalenone in Wheat. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2015, 32, 2075–2085. [Google Scholar] [CrossRef]
- Cowger, C.; Patton-Ozkurt, J.; Brown-Guedira, G.; Perugini, L. Post-Anthesis Moisture Increased Fusarium Head Blight and Deoxynivalenol Levels in North Carolina Winter Wheat. Phytopathology 2009, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Duba, A.; Goriewa-Duba, K.; Wachowska, U. Trichothecene Genotypes Analysis of Fusarium Isolates from Di-, Tetra- And Hexaploid Wheat. Agronomy 2019, 9, 698. [Google Scholar] [CrossRef]
- Birzele, B.; Meier, A.; Hindorf, H.; Krämer, J.; Dehne, H.W. Epidemiology of Fusarium Infection and Deoxynivalenol Content in Winter Wheat in the Rhineland, Germany. Eur. J. Plant Pathol. 2002, 108, 667–673. [Google Scholar] [CrossRef]
- Waalwijk, C.; Van Der Heide, R.; De Vries, I.; Van Der Lee, T.; Schoen, C.; Costrel-de Corainville, G.; Häuser-Hahn, I.; Kastelein, P.; Köhl, J.; Lonnet, P.; et al. Quantitative Detection of Fusarium Species in Wheat Using TaqMan. Eur. J. Plant Pathol. 2004, 110, 481–494. [Google Scholar] [CrossRef]
- Isebaert, S.; De Saeger, S.; Devreese, R.; Verhoeven, R.; Maene, P.; Heremans, B.; Haesaert, G. Mycotoxin-Producing Fusarium Species Occurring in Winter Wheat in Belgium (Flanders) during 2002–2005. J. Phytopathol. 2009, 157, 108–116. [Google Scholar] [CrossRef]
- Giraud, F.; Pasquali, M.; El Jarroudi, M.; Vrancken, C.; Brochot, C.; Cocco, E.; Hoffmann, L.; Delfosse, P.; Bohn, T. Fusarium Head Blight and Associated Mycotoxin Occurrence on Winter Wheat in Luxembourg in 2007/2008. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2010, 27, 825–835. [Google Scholar] [CrossRef]
- Chandelier, A.; Nimal, C.; André, F.; Planchon, V.; Oger, R. Fusarium Species and DON Contamination Associated with Head Blight in Winter Wheat over a 7-Year Period (2003–2009) in Belgium. Eur. J. Plant Pathol. 2011, 130, 403–414. [Google Scholar] [CrossRef]
- Laszlo, E.; Varga, B.; Veisz, O. Composition of Fusarium Species Causing Natural Spike Infection in Wheat. Acta Agron. Hung. 2011, 59, 255–260. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; de Rijk, T.C.; Booij, C.J.H.; Goedhart, P.W.; Boers, E.A.M.; Zhao, C.; Waalwijk, C.; Mol, H.G.J.; van der Lee, T.A.J. Occurrence of Fusarium Head Blight Species and Fusarium Mycotoxins in Winter Wheat in the Netherlands in 2009. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2012, 29, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Goliński, P.; Perkowski, J.; Kostecki, M.; Grabarkiewicz-Szczȩsna, J.; Chełkowski, J. Fusarium Species and Fusarium Toxins in Wheat in Poland—A Comparison with Neighbour Countries. Sydowia 1996, 48, 12–22. [Google Scholar]
- Kulik, T.; Jestoi, M. Quantification of Fusarium poae DNA and Associated Mycotoxins in Asymptomatically Contaminated Wheat. Int. J. Food Microbiol. 2009, 130, 233–237. [Google Scholar] [CrossRef]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and Association of Pathogenic Fungi Causing Fusarium Ear Blightin Wheat in Four European Countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Sakalauskas, S.; Stumbriene, K.; Suproniene, S.; Svegzda, P. Changes in Fusarium Link Species Composition From Lithuanian Wheat Grain in Years 2005–2007 to 2011–2013. Proc. Latv. Univ. Agric. 2014, 32, 45–50. [Google Scholar] [CrossRef]
- Audenaert, K.; van Broeck, R.; van Bekaert, B.; de Witte, F.; Heremans, B.; Messens, K.; Höfte, M.; Haesaert, G.; Broeck, R.; Bekaert, B.; et al. Fusarium Head Blight (FHB) in Flanders: Population Diversity, Inter-Species Associations and DON Contamination in Commercial Winter Wheat Varieties. Eur. J. Plant Pathol. 2009, 125, 445–458. [Google Scholar] [CrossRef]
- Lenc, L.; Czecholiński, G.; Wyczling, D.; Turów, T.; Kaźmierczak, A. Fusarium Head Blight (FHB) and Fusarium spp. on Grain of Spring Wheat Cultivars Grown in Poland. J. Plant Prot. Res. 2015, 55, 266–277. [Google Scholar] [CrossRef]
- Hörberg, H.M. Patterns of Splash Dispersed Conidia of Fusarium poae and Fusarium culmorum. Eur. J. Plant Pathol. 2002, 108, 73–80. [Google Scholar] [CrossRef]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship Between the Fungal Complex Causing Fusarium Head Blight of Wheat and Environmental Conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef]
- Thrane, U.; Adler, A.; Clasen, P.-E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in Metabolite Production by Fusarium Langsethiae, Fusarium Poae, and Fusarium Sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural Occurrence of 16 Fusarium Toxins in Grains and Feedstuffs of Plant Origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Perkowski, J.; Stachowiak, J.; Kiecana, I.; Golinski, P.; Chelkowski, J. Natural Occurrence of Fusarium Mycotoxins in Polish Cereals. Cereal Res. Commun. 1997, 25, 379–380. [Google Scholar] [CrossRef]
- Tomczak, M.; Wiśniewska, H.; Stȩpień, Ł.; Kostecki, M.; Chełkowski, J.; Goliński, P. Deoxynivalenol, Nivalenol and Moniliformin in Wheat Samples with Head Blight (Scab) Symptoms in Poland (1998–2000). Eur. J. Plant Pathol. 2002, 108, 625–630. [Google Scholar] [CrossRef]
- Miller, J.D.; Greenhalgh, R.; Wang, Y.; Lu, M. Trichothecene Chemotypes of Three Fusarium Species. Mycologia 1991, 82, 121–130. [Google Scholar] [CrossRef]
- Stȩpień, Ł.; Popiel, D.; Koczyk, G.; Chełkowski, J. Wheat-Infecting Fusarium Species in Poland—Their Chemotypes and Frequencies Revealed by PCR Assay. J. Appl. Genet. 2008, 49, 433–441. [Google Scholar] [CrossRef]
- Uhlig, S.; Torp, M.; Heier, B.T. Beauvericin and Enniatins A, A1, B and B1 in Norwegian Grain: A Survey. Food Chem. 2006, 94, 193–201. [Google Scholar] [CrossRef]
- Stenglein, S.A. Fusarium poae: A Pathogen That Needs More Attention. J. Plant Pathol. 2009, 91, 25–36. [Google Scholar]
- Hellin, P.; Dedeurwaerder, G.; Duvivier, M.; Scauflaire, J.; Huybrechts, B.; Callebaut, A.; Munaut, F.; Legrève, A. Relationship between Fusarium spp. Diversity and Mycotoxin Contents of Mature Grains in Southern Belgium. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2016, 33, 1228–1240. [Google Scholar] [CrossRef]
- Kelly, A.C.; Clear, R.M.; O’Donnell, K.; McCormick, S.; Turkington, T.K.; Tekauz, A.; Gilbert, J.; Kistler, H.C.; Busman, M.; Ward, T.J. Diversity of Fusarium Head Blight Populations and Trichothecene Toxin Types Reveals Regional Differences in Pathogen Composition and Temporal Dynamics. Fungal Genet. Biol. 2015, 82, 22–31. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and Other Selected Fusarium Toxins in Swedish Oats—Occurrence and Correlation to Specific Fusarium Species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef]


| Year | Number of Locations | Number of Samples |
|---|---|---|
| 2014 | 20 | 40 |
| 2015 | 20 | 40 |
| 2016 | 22 | 44 |
| 2017 | 24 | 48 |
| 2019 | 25 | 50 |
| Total | 111 | 222 |
| Target | Primer Name | Sequence (5′-3′) |
|---|---|---|
| F. graminearum | Fgram F | CCATTCCCTGGGCGCT |
| species complex | Fgram R | CCTATTGACAGGTGGTTAGTGACTGG |
| F. culmorum | Fcul F | CACCGTCATTGGTATGTTGTCACT |
| Fcul R | CGGGAGCGTCTGATAGTCG | |
| F. langsethiae | Flang F | CAAGTCGACCACTGTGAGTACCTCT |
| Flang R | TGTCAAAGCATGTCAGTAAAGATGAC | |
| F. avenaceum | Fave F | TATGTTGTCACTGTCTCACACCACC |
| Fave R | AGAGGGATGTTAGCATGATGAAG | |
| F. poae | Fpoae F | ACCGAATCTCAACTCCGCTTT |
| Fpoae R | GTCTGTCAAGCATGTTAGCACAAGT | |
| EF1α | Hor1F | TCTCTGGGTTTGAGGGTGAC |
| Hor2R | GGCCCTTGTACCAGTCAAGGT |
| Year (Samples) | F. avenaceum | F. culmorum | F. graminearum | F. langsethiae | F. poae | Fusarium |
|---|---|---|---|---|---|---|
| 2014 (20) | 95/100 * | 45/89 | 100/55 | 50/90 | 100/70 | 100/40 |
| 2015 (20) | 100/100 | 55/100 | 90/89 | 50/80 | 100/50 | 100/35 |
| 2016 (22) | 100/82 | 91/60 | 100/68 | 91/70 | 100/68 | 100/36 |
| 2017 (24) | 79/74 | 67/56 | 100/25 | 75/67 | 100/58 | 100/8 |
| 2019 (25) | 92/96 | 88/100 | 96/79 | 84/48 | 96/50 | 100/28 |
| all years (111) | 93/90 | 70/79 | 97/62 | 71/67 | 99/59 | 100/29 |
| Variables (n = 111) | F. avenaceum | F. culmorum | F. graminearum s.s. | F. langsethiae |
|---|---|---|---|---|
| F. culmorum p-value | 0.441 0.000 | |||
| F. graminearum s.s. p-value | 0.190 0.046 | 0.196 0.040 | ||
| F. langsethiae p-value | 0.029 0.766 | 0.088 0.360 | 0.055 0.570 | |
| F. poae p-value | −0.040 0.675 | 0.042 0.659 | 0.024 0.800 | −0.025 0.794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góral, T.; Grelewska-Nowotko, K.; Ochodzki, P.; Wiewióra, B. DNA-Based Quantification of Fusarium Species in Winter Wheat Grain in Poland from 2014 to 2017 and 2019. Agronomy 2025, 15, 2430. https://doi.org/10.3390/agronomy15102430
Góral T, Grelewska-Nowotko K, Ochodzki P, Wiewióra B. DNA-Based Quantification of Fusarium Species in Winter Wheat Grain in Poland from 2014 to 2017 and 2019. Agronomy. 2025; 15(10):2430. https://doi.org/10.3390/agronomy15102430
Chicago/Turabian StyleGóral, Tomasz, Katarzyna Grelewska-Nowotko, Piotr Ochodzki, and Barbara Wiewióra. 2025. "DNA-Based Quantification of Fusarium Species in Winter Wheat Grain in Poland from 2014 to 2017 and 2019" Agronomy 15, no. 10: 2430. https://doi.org/10.3390/agronomy15102430
APA StyleGóral, T., Grelewska-Nowotko, K., Ochodzki, P., & Wiewióra, B. (2025). DNA-Based Quantification of Fusarium Species in Winter Wheat Grain in Poland from 2014 to 2017 and 2019. Agronomy, 15(10), 2430. https://doi.org/10.3390/agronomy15102430






