Weed Seedbank Changes Associated with Temporary Tillage After Long Periods of No-Till
Abstract
1. Introduction
2. Materials and Methods
3. Results
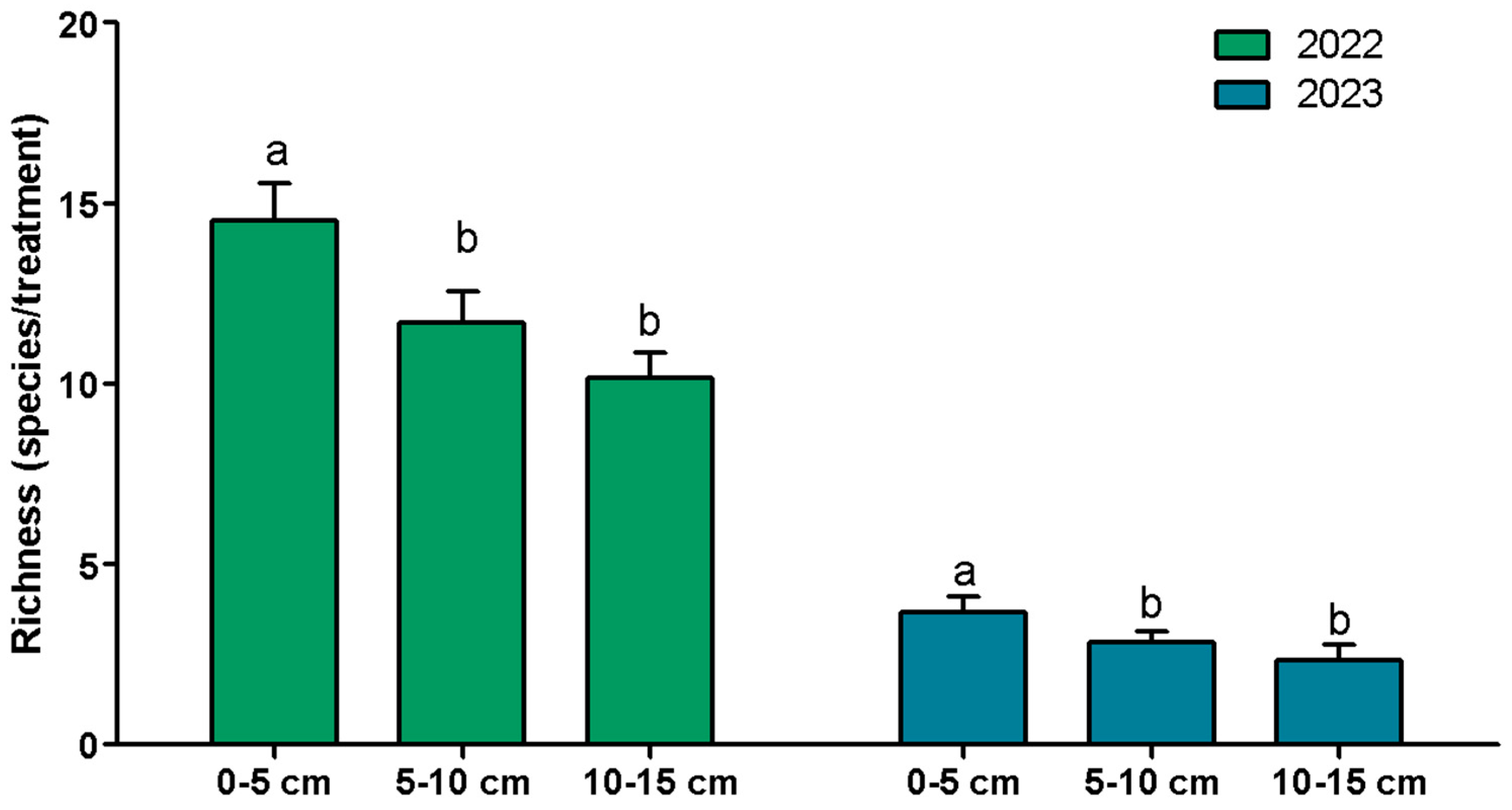
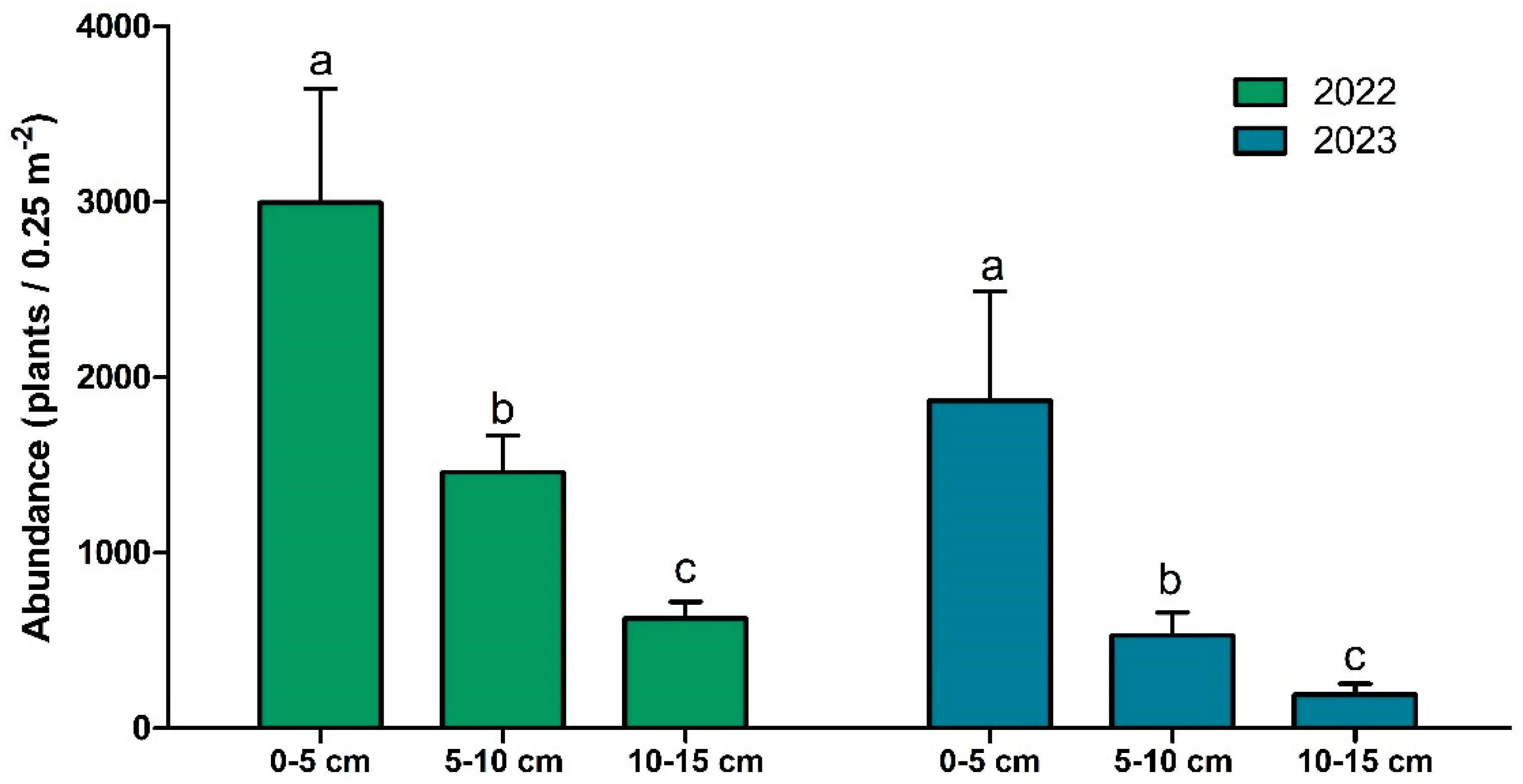
3.1. Weed Species Composition
3.2. Species Richness and Abundance
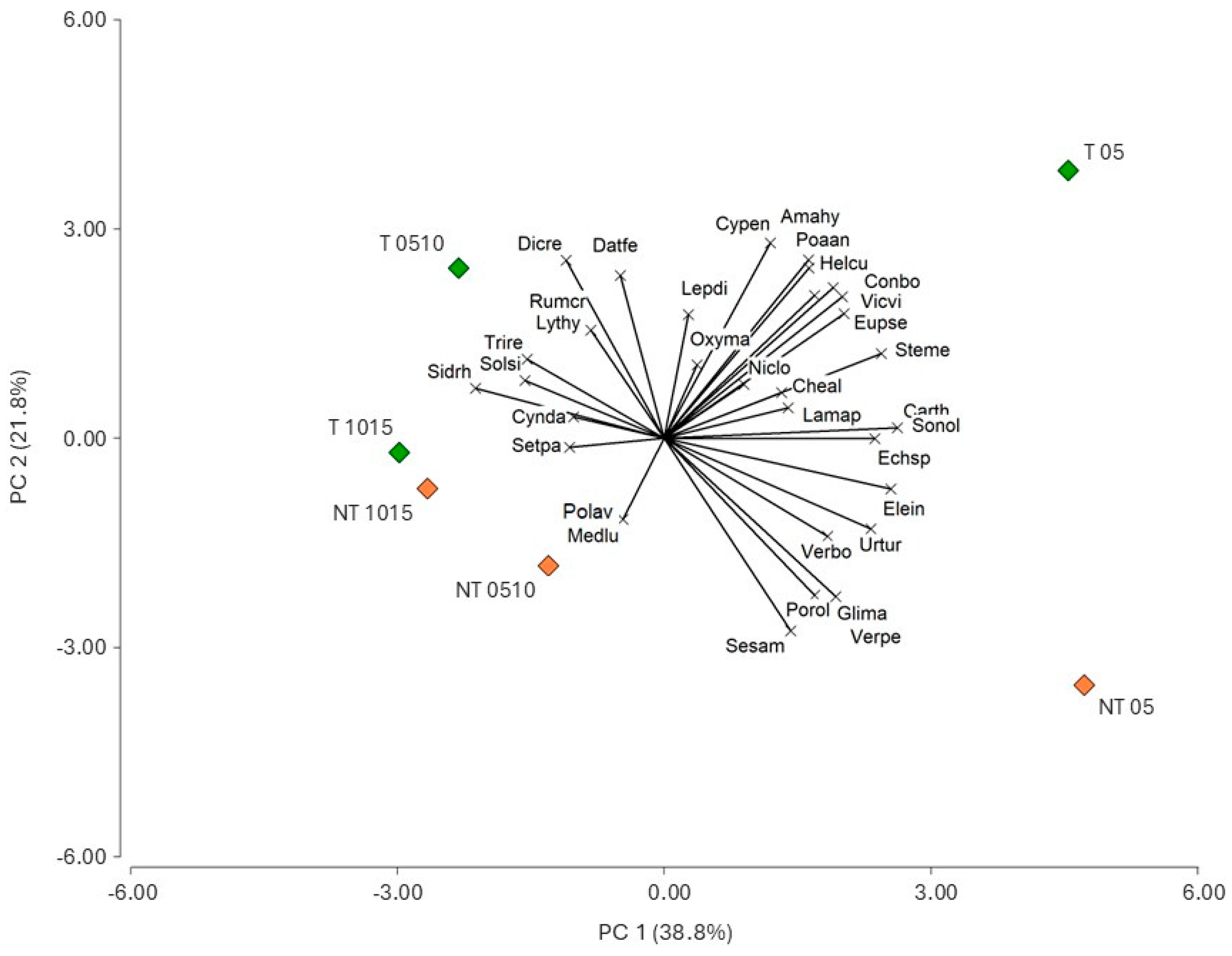
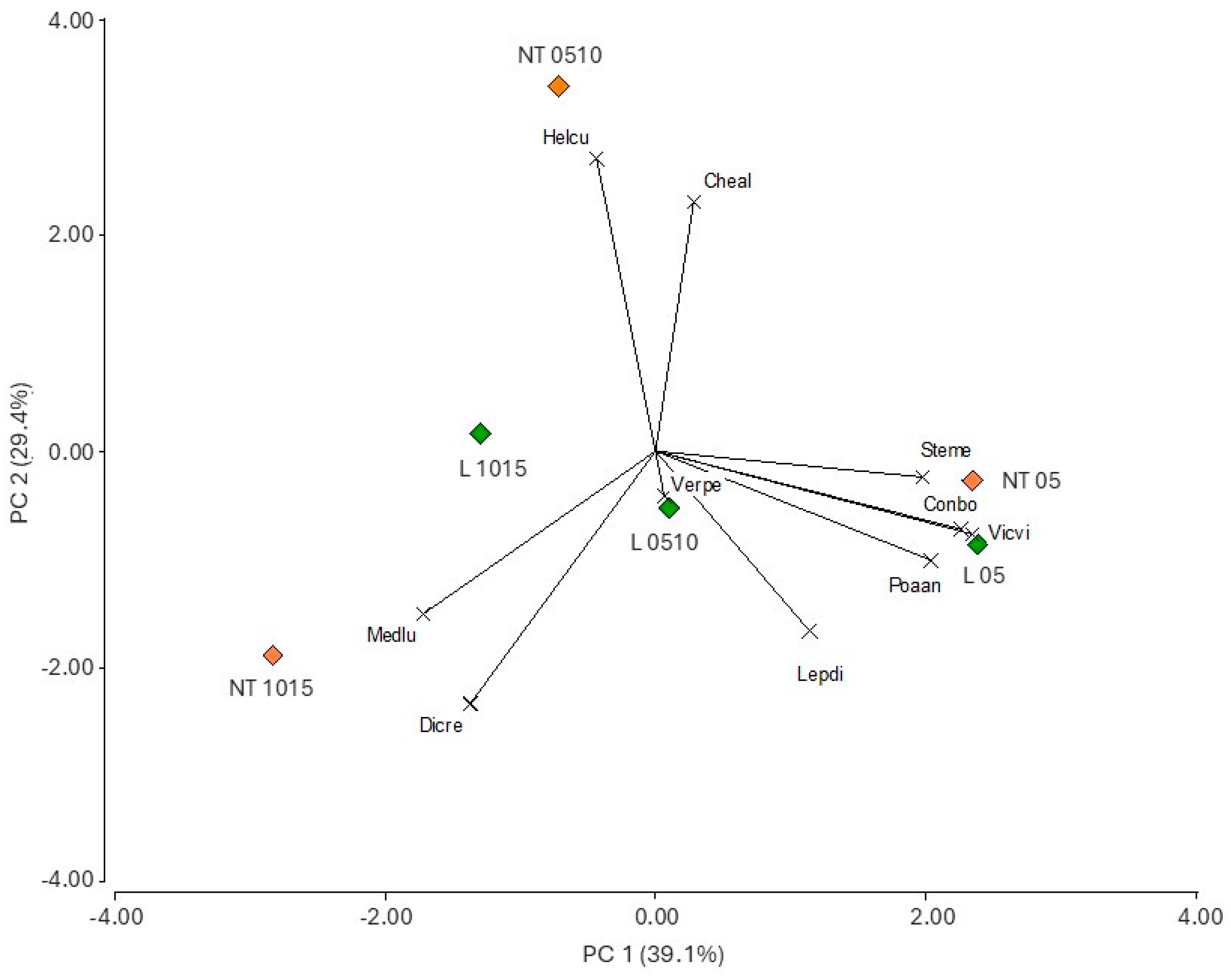
3.3. Treatment Effects on Weed Species Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oreja, F.H.; Moreno, N.; Gundel, P.E.; Vercellino, R.B.; Pandolfo, C.E.; Presotto, A.; Perotti, V.; Permingeat, H.; Tuesca, D.; Scursoni, J.A.; et al. Herbicide-resistant weeds from dryland agriculture in Argentina. Weed Res. 2024, 64, 89–106. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, E.B.; Suárez, S.A.; Ghersa, C.M. Soybean weed community composition and richness between 1995 and 2003 in the Rolling Pampas (Argentina). Agric. Ecosyst. Environ. 2006, 115, 229–236. [Google Scholar] [CrossRef]
- Satorre, E.H.; de la Fuente, E.B.; Mas, M.T.; Suárez, S.A.; Kruk, B.C.; Guglielmini, A.C.; Verdú, A.M. Crop rotation effects on weed communities of soybean (Glycine max L. Merr.) agricultural fields of the Flat Inland Pampa. Crop Prot. 2020, 130, 105068. [Google Scholar] [CrossRef]
- Oreja, F.H.; Vera, A.C.D.; Kruk, B.C.; de la Fuente, E.B.; Scursoni, J.A. Survey of major weed problems, management practices, and herbicide use in extensive row crops from Argentina. Adv. Weed Sci. 2024, 42, e02024. [Google Scholar] [CrossRef]
- de la Fuente, E.B.; Oreja, F.H.; Lenardis, A.E.; Fuentes, M.T.; Agosti, B.; Barrio, A.; Barberis, S.; Robredo, J.; Gil, A.; Marzetti, M.; et al. Intensification of crop rotation affecting weed communities and the use of herbicides in the rolling Pampa. Heliyon 2021, 7, e05874. [Google Scholar] [CrossRef]
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, environmental pollution, and health. In Environmental Health Risk; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2016; pp. 1–27. [Google Scholar]
- Mohler, C.L.; Galford, A.E. Weed seedling emergence and seed survival: Separating the effects of seed position and soil modification by tillage. Weed Res. 1997, 37, 147–155. [Google Scholar] [CrossRef]
- Bàrberi, P.; Lo Cascio, B. Long-term tillage and crop rotation effects on weed seedbank size and composition. Weed Res. 2001, 41, 325–340. [Google Scholar] [CrossRef]
- Benech-Arnold, R.L.; Sánchez, R.A.; Forcella, F.; Kruk, B.C.; Ghersa, C.M. Environmental control of dormancy in weed seed banks in soil. Field Crops Res. 2000, 67, 105–122. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.S.; Preston, C. Influence of environmental factors on seed germination and seedling emergence of rigid ryegrass (Lolium rigidum). Weed Sci. 2006, 54, 1004–1012. [Google Scholar] [CrossRef]
- Baraibar, B.; Westerman, P.R.; Carrión, E.; Recasens, J. Effects of tillage and irrigation in cereal fields on weed seed removal by seed predators. J. Appl. Ecol. 2017, 54, 819–828. [Google Scholar] [CrossRef]
- Steckel, L.E.; Sprague, C.L.; Stoller, E.W.; Wax, L.M. Tillage effects on common waterhemp (Amaranthus rudis) emergence and vertical distribution in the soil seedbank. Weed Sci. 2007, 55, 255–260. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P. Ecological Assembly Rules: Perspectives, Advances, Retreats; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Booth, B.D.; Swanton, C.J. Assembly theory applied to weed communities. Weed Sci. 2002, 50, 2–13. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Ryan, M.R.; Smith, R.G.; Mirsky, S.B.; Mortensen, D.A.; Seidel, R. Management filters and species traits: Weed community assembly in long-term organic and conventional systems. Weed Sci. 2010, 58, 265–277. [Google Scholar] [CrossRef]
- Christoffoleti, P.J.; Caetano, R.S.X. Weed seed bank dynamics under no-tillage in southern Brazil. Soil Tillage Res. 1998, 48, 175–183. [Google Scholar]
- Hossain, M.I.; Begum, M. Weed seedbank in different tillage systems in rice cultivation. J. Agric. Crop Res. 2016, 4, 48–56. [Google Scholar]
- Young, S.L. Decision support for integrated weed management. In Integrated Weed Management for Sustainable Agriculture; Naylor, R.E., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 277–292. [Google Scholar]
- Aliaga, V.; Ferrelli, F.; Piccolo, M.C. Regionalization of climate over the Argentine Pampas. Int. J. Climatol. 2017, 37, 1237–1247. [Google Scholar] [CrossRef]
- Cabrera, A.L. (Ed.) Flora de la Provincia de Buenos Aires. In Colección Científica del Instituto Nacional de Tecnología Agropecuaria, Volume 4; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 1963–1970; Volumes 1–6. [Google Scholar]
- Petetin, C.A.; Molinari, E. Clave Ilustrada para el Reconocimiento de Malezas en el Campo al Estado Vegetativo; Colección Científica del Instituto Nacional de Tecnología Agropecuaria; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 1977; Volume XIV, pp. 1–243. [Google Scholar]
- Molina, A.R. Malezas Argentinas; Molina, A.R.: Buenos Aires, Argentina, 2005–2011; Volumes 1–5. [Google Scholar]
- Zubizarreta, L.; Díaz Panizza, L. Guía de Reconocimiento de Malezas; Syngenta: Vicente López, Argentina, 2014. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2018; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2018. [Google Scholar]
- Kucheruk, L.; Spennemann, P.; Rivera, J.; Naumann, G.; Otkin, J. Olas de Calor Récord del Verano 2023 y su rol en el Desarrollo de Sequías de Rápido Desarrollo en la Pampa Húmeda Argentina. Available online: https://sissa.crc-sas.org/wp-content/uploads/2024/05/IT-Olas-de-calor.pdf (accessed on 29 October 2024).
- Copeland, S.M.; Harrison, S.P.; Latimer, A.M.; Damschen, E.I.; Eskelinen, A.M.; Fernandez-Going, B.; Spasojevic, M.J.; Anacker, B.L.; Thorne, J.H. Ecological effects of extreme drought on Californian herbaceous plant communities. Ecol. Monogr. 2016, 86, 295–311. [Google Scholar] [CrossRef]
- Yamashita, O.M.; Guimarães, S.C. Germination of Conyza canadensis and Conyza bonariensis seeds in function of water availability in the substrate. Planta Daninha 2010, 28, 309–317. [Google Scholar] [CrossRef]
- Storkey, J.; Neve, P. What good is weed diversity? Weed Res. 2018, 58, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Armengot, L.; Jose-Maria, L.; Chamorro, L.; Sans, F.X. Weed harrowing in organically grown cereal crops avoids yield losses without reducing weed diversity. Agron. Sustain. Dev. 2013, 33, 405–411. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.; Preston, C. Seedling recruitment pattern and depth of recruitment of 10 weed species in minimum tillage and no-till seeding systems. Weed Sci. 2006, 54, 658–668. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed seed movement and dispersal strategies in the agricultural environment. Weed Biol. Manag. 2007, 7, 141–157. [Google Scholar] [CrossRef]
- Benvenuti, S. Natural weed seed burial: Effect of soil texture, rain and seed characteristics. Seed Sci. Res. 2007, 17, 211–219. [Google Scholar] [CrossRef]
- Clements, D.R.; Benoit, D.L.; Murphy, S.D.; Swanton, C.J. Tillage effects on weed seed return and seedbank composition. Weed Sci. 1996, 44, 314–322. [Google Scholar] [CrossRef]
- Reuss, S.A.; Buhler, D.D.; Gunsolus, J.L. Effects of soil depth and aggregate size on weed seed distribution and viability in a silt loam soil. Appl. Soil Ecol. 2001, 16, 209–217. [Google Scholar] [CrossRef]
- Hoffman, M.L.; Owen, M.D.; Buhler, D.D. Effects of crop and weed management on density and vertical distribution of weed seeds in soil. Agron. J. 1998, 90, 793–799. [Google Scholar] [CrossRef]
- de la Fuente, E.B.; Perelman, S.; Ghersa, C.M. Weed and arthropod communities in soybean as related to crop productivity and land use in the Rolling Pampas, Argentina. Weed Res. 2010, 50, 561–571. [Google Scholar] [CrossRef]
- Scopel, A.L.; Satorre, E.H.; Benech-Arnold, R.L.; Radosevich, S.R. Modelling the effect of environment on dormancy and germination of weed seed banks in no-tillage systems. Field Crops Res. 1991, 30, 263–277. [Google Scholar]
- Masin, R.; Zuin, M.C.; Otto, S.; Zanin, G. Seed longevity and dormancy of four summer annual grass weeds in turf. Weed Res. 2006, 46, 362–370. [Google Scholar] [CrossRef]
- Taylorson, R.B.; Hendricks, S.B. Seed dormancy in Rumex crispus as related to its endogenous content of abscisic acid and other inhibitors. Plant Physiol. 1972, 50, 635–638. [Google Scholar]
- Mollard, F.P.; Insausti, P. Breaking Setaria parviflora seed dormancy by nitrates and light is part of a mechanism that detects a drawdown period after flooding. Aquat. Bot. 2009, 91, 57–60. [Google Scholar] [CrossRef]
- Ohadi, S.; Rahimian Mashhadi, H.; Tavakkol-Afshari, R.; Beheshtian Mesgaran, M. Modelling the effect of light intensity and duration of exposure on seed germination of Phalaris minor and Poa annua. Weed Res. 2010, 50, 209–217. [Google Scholar] [CrossRef]
- Batlla, D.; Benech-Arnold, R.L. The role of fluctuations in soil water content on the regulation of dormancy changes in buried seeds of Polygonum aviculare L. Seed Sci. Res. 2006, 16, 47–59. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Seed germination ecology of Portulaca oleracea L.: An important weed of rice and upland crops. Ann. Appl. Biol. 2009, 155, 61–69. [Google Scholar] [CrossRef]
- Sharpe, S.M.; Boyd, N.S. Black medic (Medicago lupulina) emergence and emergence predictors within Florida strawberry fields. Weed Sci. 2019, 67, 253–260. [Google Scholar] [CrossRef]
- Benvenuti, S.; Macchia, M.; Miele, S. Light, temperature and burial depth effects on Rumex obtusifolius seed germination and emergence. Weed Res. 2001, 41, 177–186. [Google Scholar] [CrossRef]
- Wu, H.; Walker, S.; Rollin, M.J.; Tan, D.K.; Robinson, G.; Werth, J. Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist). Weed Biol. Manag. 2007, 7, 192–199. [Google Scholar] [CrossRef]
- Widderick, M.J.; Walker, S.R.; Sindel, B.M.; Bell, K.L. Germination, emergence, and persistence of Sonchus oleraceus, a major crop weed in subtropical Australia. Weed Biol. Manag. 2010, 10, 102–112. [Google Scholar] [CrossRef]
- Saatkamp, A.; Affre, L.; Baumberger, T.; Dumas, P.J.; Gasmi, A.; Gachet, S.; Arène, F. Soil depth detection by seeds and diurnally fluctuating temperatures: Different dynamics in 10 annual plants. Plant Soil 2011, 349, 331–340. [Google Scholar] [CrossRef]
- Hartzler, R.G.; Buhler, D.D.; Stoltenberg, D.E. Emergence characteristics of four annual weed species. Weed Sci. 1999, 47, 578–584. [Google Scholar] [CrossRef]
| Season | Crops | Timing (Approximate Months) | Herbicides | Fertilizers |
|---|---|---|---|---|
| 2018/19 | Soybean | Nov 2018–Apr 2019 | Long fallow: glyphosate (62%) (1.5 L ha−1), 2,4-D (100%) (0.8 L ha−1) | Mix 7–40 (80 kg ha−1) |
| Preemergence: sulfentrazone (50%) (0.38 L ha−1), thiencarbazone-methyl + iodosulfuron-methyl-sodium (46 g ha−1), glyphosate (64%) (2 L ha−1) | ||||
| Postemergence: glyphosate (54%) (1.7 L ha−1), clethodim (0.7 L ha−1) | ||||
| 2019/20 | Wheat—soybean (double crop) | Wheat: Jun–Nov 2019, Soybean: Dec 2019–Apr 2020 | Fallow: Chlorsulfuron + metsulfuron-methyl (10 g ha−1), glyphosate (54%) (1.9 L ha−1), 2,4-D (100%) (0.7 L ha−1) | Wheat: mix 7–40 (80 kg ha−1), urea (244 kg ha−1); Soybean: mix 7–40 (75 kg ha−1) |
| 2020/21 | Early corn | Sep 2020–Feb 2021 | Fallow: glyphosate (62%) (1.5 L ha−1), 2,4-D (100%) (0.8 L ha−1), atrazine (90%) (1.5 kg ha−1), picloram (24%) (0.1 L ha−1) | Mix 7–40 (80 kg ha−1) |
| Postemergence: glyphosate (62%) (1.5 L ha−1), 2,4-D (100%) | Urea (196 kg ha−1) | |||
| 2021/22 | Cover crop cereal rye + hairy vetch − corn | Cover: Apr–Oct 2021; Soybean: Nov 2021–Apr 2022 | Fallow CC: glyphosate (62%) (1.5 L ha−1), 2,4-D (100%). Termination: Glyphosate (48%) (1.5 kg ha−1), flumioxazin (48%) | Soybean MAP 11–52-0 (80 kg ha−1) |
| Postemergence: fomesafen (24%) (1.2 L ha−1), haloxyfop-R-methyl (10.4%) (0.19 L ha−1), clopiralyd (30%) (0.4 L ha−1), imazethapyr (10%) (0.23 L ha−1) | ||||
| 2022/23 | Sunflower | Aug 2022–Jan 2023 | Fallow: glyphosate (48%) (2 kg ha−1), pyroxasulfone (8.5%) (0.5 L ha−1), sulfentrazone (50%) (0.45 L ha−1) | Mix 12–11–18 + micronutrients (80 kg ha−1) |
| Postemergence: imazethapyr (10%) (60 g ha−1), clethodim (10%) (0.8 L ha−1) | ||||
| 2023/24 | Cover crop cereal rye − corn | Cover: Apr–Oct 2023; Corn: Nov 2023–Apr 2024 | Fallow CC: glyphosate (48%) (1.5 kg ha−1), pyroxasulfone (8.5%) (1 L ha−1) | CC: Mix 7–40 (60 kg ha−1) |
| Corn preemergence: S-metolachlor (29.7%) + atrazine (27.4%) + mesotrione (3.9%) + bicyclopyrone (0.7%) (0.8 L ha−1), S-metolachlor (96%) (0.85 L ha−1), glyphosate (48%) (1.5 kg ha−1) | Corn: Mix 7–40 (116 kg ha−1), | |||
| Postemergence: glyphosate (48%) (1.5 kg ha−1), atrazine (90%) (0.6 kg ha−1), picloram (24%) (0.15 L ha−1) | urea (116 kg ha−1), Urea solution (18%) (10 L ha−1) |
| Response | Source | df | SS | MS | F | p-Value | Significance |
|---|---|---|---|---|---|---|---|
| Richness | Year | 1 | 28.219 | 28.219 | 368.88 | <0.001 | *** |
| System | 1 | 0.170 | 0.170 | 2.22 | 0.149 | ||
| Depth | 2 | 1.573 | 0.787 | 10.28 | 0.00051 | *** | |
| Year × System | 1 | 0.133 | 0.133 | 1.74 | 0.198 | ||
| Year × Depth | 2 | 0.075 | 0.038 | 0.49 | 0.617 | ||
| System × Depth | 2 | 0.468 | 0.234 | 3.06 | 0.064 | ||
| Residuals | 26 | 1.989 | 0.077 | ||||
| Abundance | Year | 1 | 64.031 | 64.031 | 15.72 | 0.00051 | *** |
| System | 1 | 0.150 | 0.150 | 0.037 | 0.850 | ||
| Depth | 2 | 193.294 | 96.647 | 23.73 | 1.37 × 10−6 | *** | |
| Year × System | 1 | 7.648 | 7.648 | 1.88 | 0.182 | ||
| Year × Depth | 2 | 0.830 | 0.415 | 1.02 | 0.903 | ||
| System × Depth | 2 | 5.153 | 2.577 | 0.63 | 0.539 | ||
| Residuals | 26 | 105.889 | 4.073 |
| Species | Codes | Abundance (Plants Treatment−1) | Frequency (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Occasional Tillage | No-Till | |||||||
| 0–5 cm | 5–10 cm | 10–15 cm | 0–5 cm | 5–10 cm | 10–15 cm | |||
| Chenopodium album L. | Cheal | 61 | 42 | 6 | 40 | 66 | 22 | 100 |
| Oxybasis macrosperma (Hook.f.) S.Fuentes, Uotila & Borsch | Oxyba | 20 | 34 | 8 | 20 | 23 | 11 | 100 |
| Echinochloa sp. | Echsp | 4 | 1 | 2 | 4 | 1 | 2 | 100 |
| Eleusine indica (L.) Gaertn. | Elein | 18 | 4 | 1 | 27 | 5 | 4 | 100 |
| Heliotropium curassavicum L. | Helcu | 46 | 40 | 13 | 32 | 25 | 19 | 100 |
| Lepidium didymum L. | Lepdi | 8 | 12 | 4 | 5 | 8 | 1 | 100 |
| Nicotiana longiflora Cav. | Niclo | 11 | 18 | 5 | 14 | 9 | 7 | 100 |
| Stellaria media (L.) Vill. | Steme | 117 | 4 | 2 | 64 | 2 | 3 | 100 |
| Amaranthus hybridus L. | Amahy | 138 | 13 | 4 | 5 | 3 | 83 | |
| Cyperus entrerianus Boeckeler | Cypen | 8 | 2 | 1 | 2 | 1 | 83 | |
| Dichondra repens J.R.Forst. & G.Forst. | Dicre | 4 | 4 | 3 | 1 | 5 | 83 | |
| Verbena bonariensis L. | Verbo | 2 | 1 | 3 | 2 | 2 | 83 | |
| Datura ferox L. | Datfe | 2 | 2 | 3 | 50 | |||
| Poa annua L. | Poaan | 4 | 17 | |||||
| Lythrum hyssopifolia L. | Lythy | 1 | 17 | |||||
| Setaria parviflora (Poir.) Kerguélen | Setpa | 1 | 17 | |||||
| Rumex crispus L. | Rumcr | 2 | 17 | |||||
| Glycine max | Glyma | 1 | 17 | |||||
| Portulaca oleracea L. | Porol | 3 | 17 | |||||
| Sesuvium americanum (Gillies ex Arn.) A.I.Jocou & C.R.Minué | Sesam | 2 | 1 | 33 | ||||
| Medicago lupulina L. | Medlu | 1 | 17 | |||||
| Polygonum aviculare L. | Polav | 1 | 17 | |||||
| Sonchus oleraceus L. | Sonol | 1 | 1 | 33 | ||||
| Vicia villosa Roth | Vicvi | 10 | 2 | 33 | ||||
| Conyza bonariensis (L.) Cronquist | Conbo | 7 | 1 | 33 | ||||
| Carduus thoermeri Weinm. | Carth | 1 | 1 | 33 | ||||
| Urtica urens L. | Urtur | 4 | 1 | 10 | 1 | 67 | ||
| Lamium amplexicaule L. | Lamam | 1 | 2 | 2 | 50 | |||
| Solanum sisymbriifolium Lam. | Solsi | 3 | 1 | 2 | 50 | |||
| Trifolium repens L. | Trire | 2 | 5 | 3 | 8 | 67 | ||
| Cynodon dactylon (L.) Pers. | Cynda | 1 | 1 | 33 | ||||
| Sida rhombifolia L. | Sidrh | 2 | 2 | 2 | 50 | |||
| Euphorbia serpens Kunth | Eupse | 5 | 2 | 2 | 3 | 67 | ||
| Veronica peregrina L. | Verpe | 1 | 4 | 1 | 1 | 67 | ||
| Species | Codes | Abundance (Plants Treatment−1) | Frequency (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Occasional Tillage | No-Till | |||||||
| 0–5 cm | 5–10 cm | 10–15 cm | 0–5 cm | 5–10 cm | 10–15 cm | |||
| Lepidium didymum L. | Lepdi | 21 | 12 | 1 | 5 | 1 | 9 | 100 |
| Stellaria media (L.) Vill. | Steme | 99 | 55 | 14 | 283 | 41 | 13 | 100 |
| Dichondra repens J.R.Forst. & G.Forst. | Dicre | 2 | 2 | 3 | 1 | 4 | 83 | |
| Poa annua L. | Poaan | 18 | 10 | 9 | 50 | |||
| Vicia villosa Roth | Vicvi | 4 | 1 | 5 | 50 | |||
| Conyza bonariensis (L.) Cronquist | Conbo | 1 | 1 | 33 | ||||
| Chenopodium album L. | Cheal | 1 | 4 | 33 | ||||
| Veronica peregrina L. | Verpe | 1 | 17 | |||||
| Heliotropium curassavicum L. | Helcu | 2 | 17 | |||||
| Medicago lupulina L. | Medlu | 2 | 17 | |||||
| Trifolium repens L. | Trire | 2 | 17 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oreja, F.; Torcat Fuentes, M.; Barrio, A.; Schiavinato, D.J.; Rosso, V.; de la Fuente, E. Weed Seedbank Changes Associated with Temporary Tillage After Long Periods of No-Till. Agronomy 2025, 15, 1410. https://doi.org/10.3390/agronomy15061410
Oreja F, Torcat Fuentes M, Barrio A, Schiavinato DJ, Rosso V, de la Fuente E. Weed Seedbank Changes Associated with Temporary Tillage After Long Periods of No-Till. Agronomy. 2025; 15(6):1410. https://doi.org/10.3390/agronomy15061410
Chicago/Turabian StyleOreja, Fernando, Marianne Torcat Fuentes, Antonio Barrio, Dario Javier Schiavinato, Virginia Rosso, and Elba de la Fuente. 2025. "Weed Seedbank Changes Associated with Temporary Tillage After Long Periods of No-Till" Agronomy 15, no. 6: 1410. https://doi.org/10.3390/agronomy15061410
APA StyleOreja, F., Torcat Fuentes, M., Barrio, A., Schiavinato, D. J., Rosso, V., & de la Fuente, E. (2025). Weed Seedbank Changes Associated with Temporary Tillage After Long Periods of No-Till. Agronomy, 15(6), 1410. https://doi.org/10.3390/agronomy15061410










