Study of the Effects of Co-Application of Biochar and Biogas Slurry on Nitrogen Cycling Enzyme Activity and Nitrogen Use Efficiency
Abstract
1. Introduction
- Due to the large specific surface area and high porosity of biochar, as well as the abundance of organic matter in biogas slurry, their combined application alters soil physicochemical properties and improves soil quality.
- The improvement of soil quality through the co-application of biochar and biogas slurry helps to reduce gaseous emissions, decrease nitrogen losses, and enhance both the abundance of nitrogen-cycling-related functional genes and the activities of key enzymes.
- These changes induced by the combined application ultimately contribute to higher crop yields and improved nitrogen use efficiency.
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Materials
2.3. Experimental Design
2.4. Soil Sampling and Determination
2.5. Measurement of Soil Ammonia and Nitrous Oxide Emissions
2.6. Determination of Soil Enzyme Activities
2.7. Determination of N Cycle-Related Functional Genes
2.8. Data Analysis
3. Results and Discussion
3.1. Effects of Biochar–Biogas Slurry Co-Application on Basic Soil Physicochemical Properties
3.2. Two-Way ANOVA of Biochar–Biogas Slurry Co-Application on Soil Physicochemical Properties
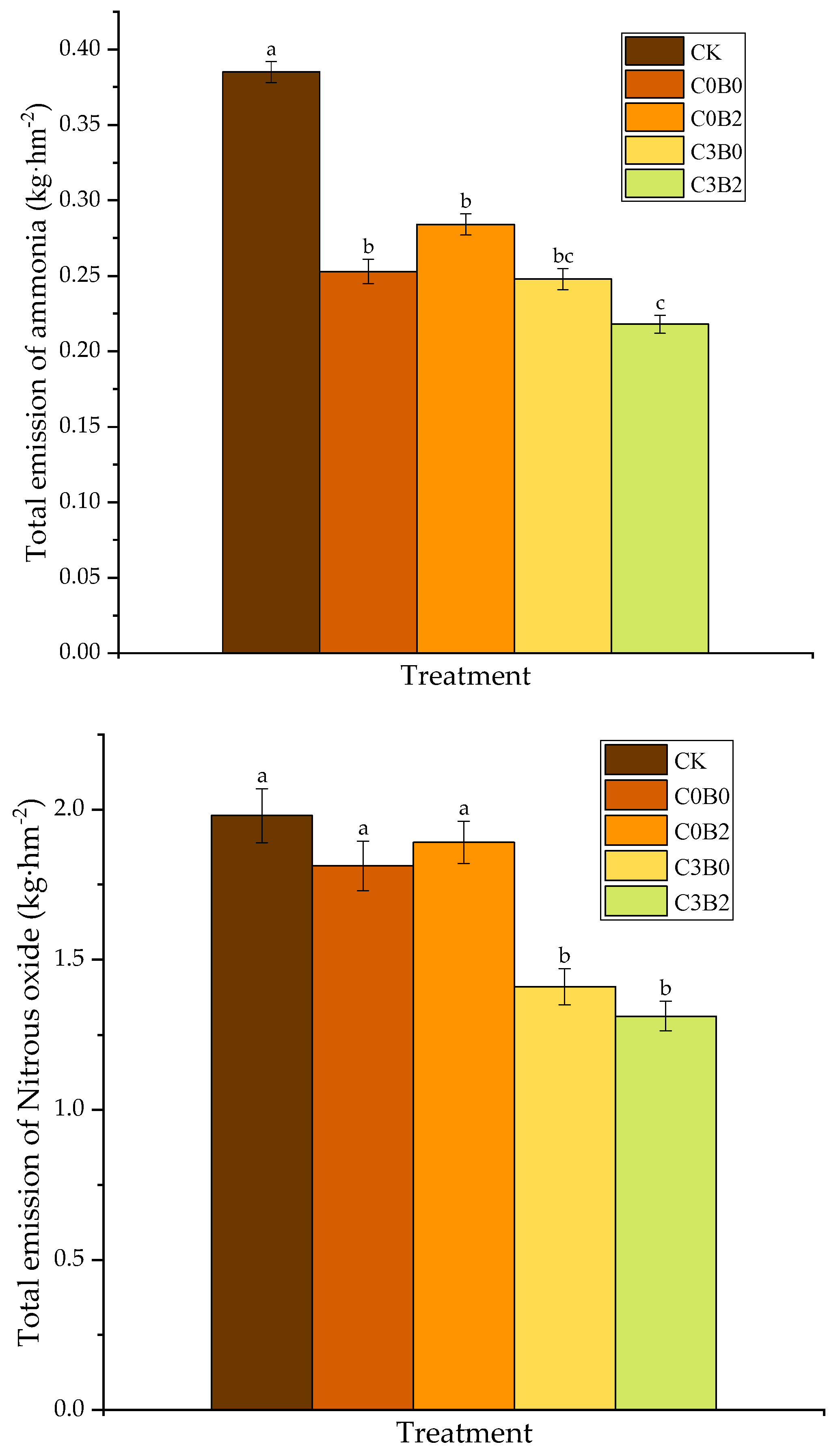
3.3. Effects of Biochar–Biogas Slurry Co-Application on Total Ammonia and Nitrous Oxide Emissions
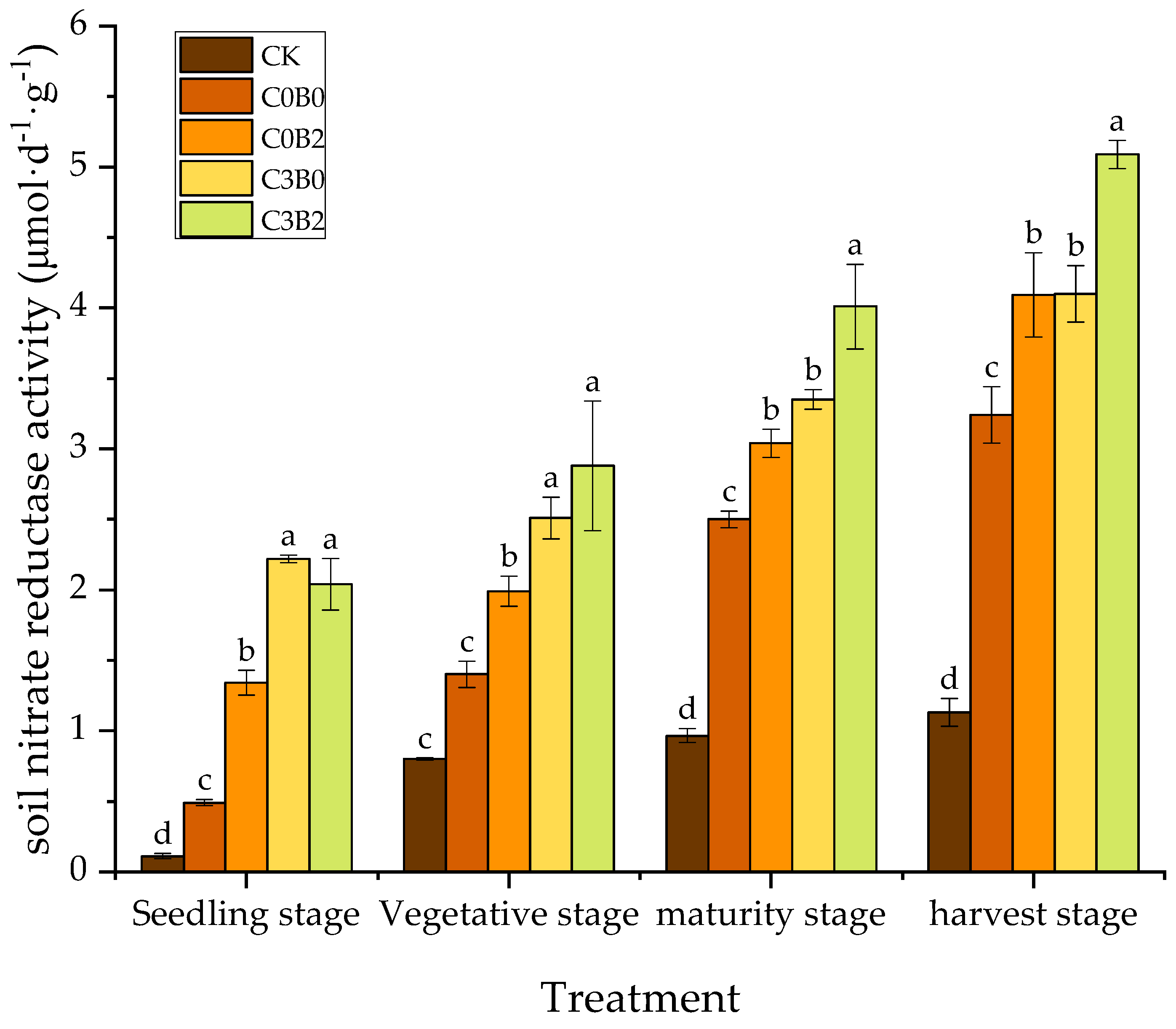
3.4. Effects of Biochar–Biogas Slurry Co-Application on Soil Nitrate Reductase Activity
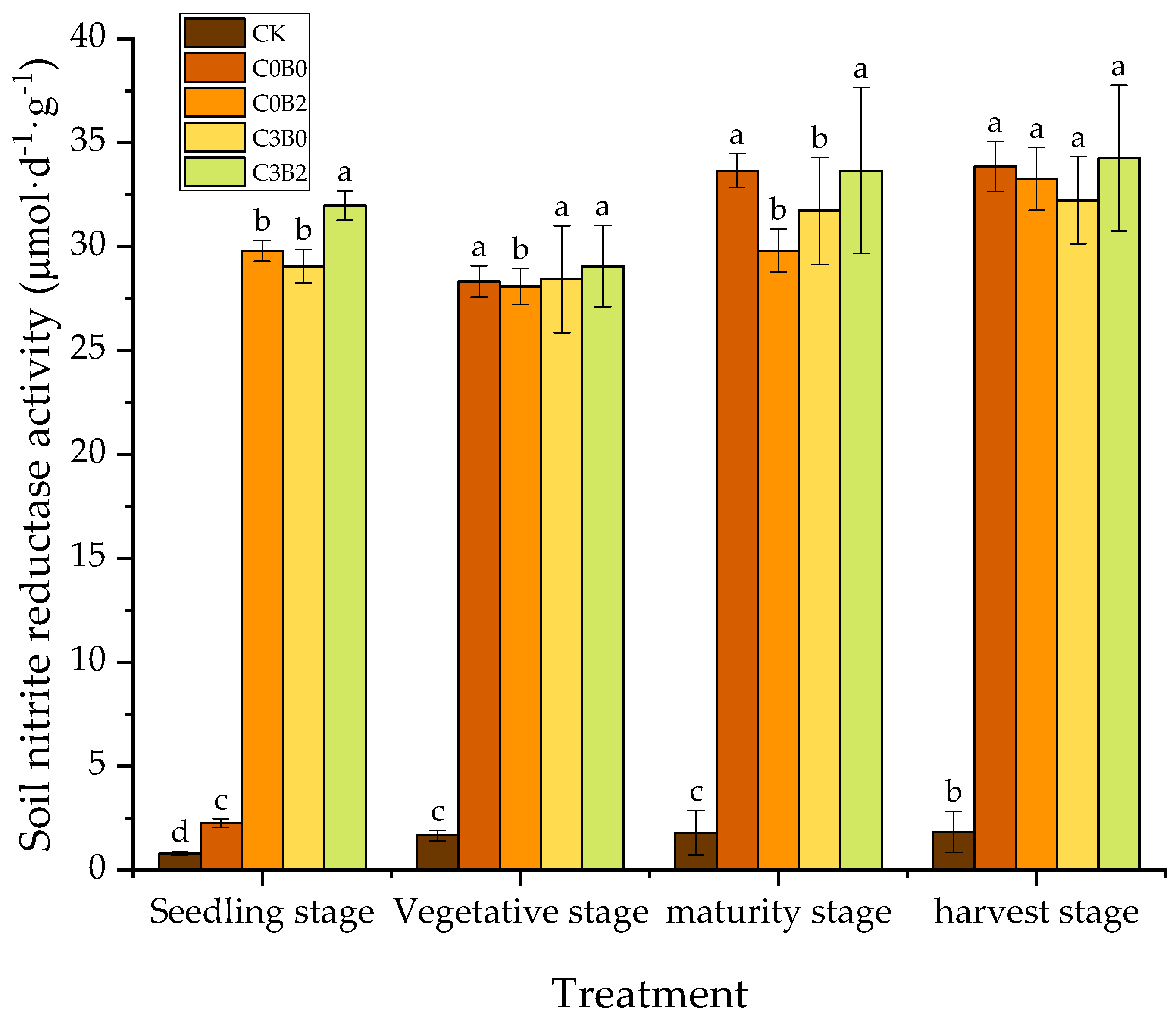
3.5. Effects of Biochar–Biogas Slurry Co-Application on Soil Nitrite Reductase Activity
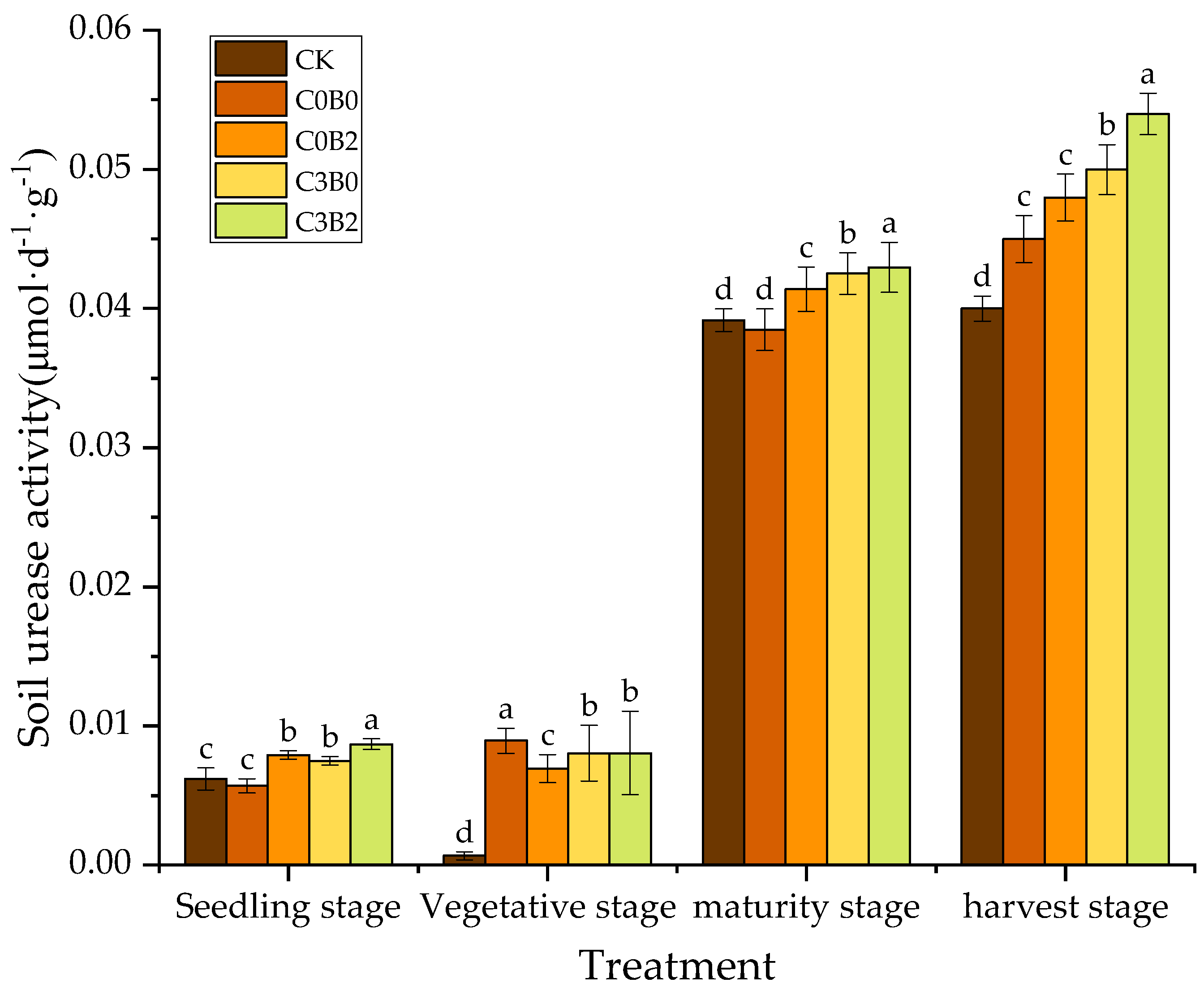
3.6. Effects of Biochar–Biogas Slurry Co-Application on Soil Urease Activity
3.7. Effects of Combined Biochar and Biogas Slurry Application on Soil N-Cycling Functional Genes
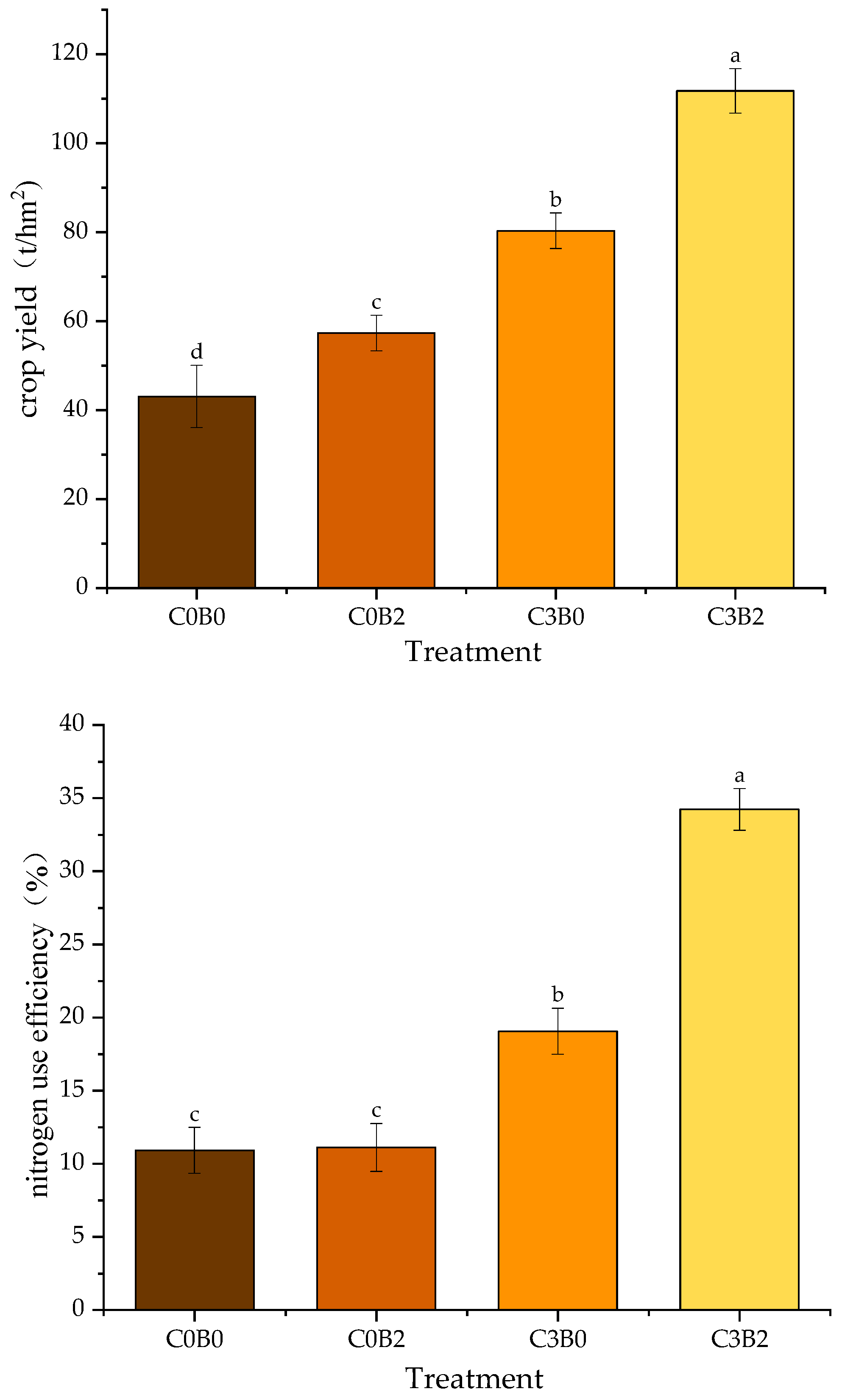
3.8. Effects of Biochar–Biogas Slurry Co-Application on Cabbage Yield and Agronomic N-Use Efficiency
3.9. Two-Factor Analysis of Different Biochar and Biogas Slurry Application Rates on Gas Volatilization, Nitrogen-Cycle-Related Functional Genes and Enzymes, Crop Yield, and Nitrogen Utilization Efficiency
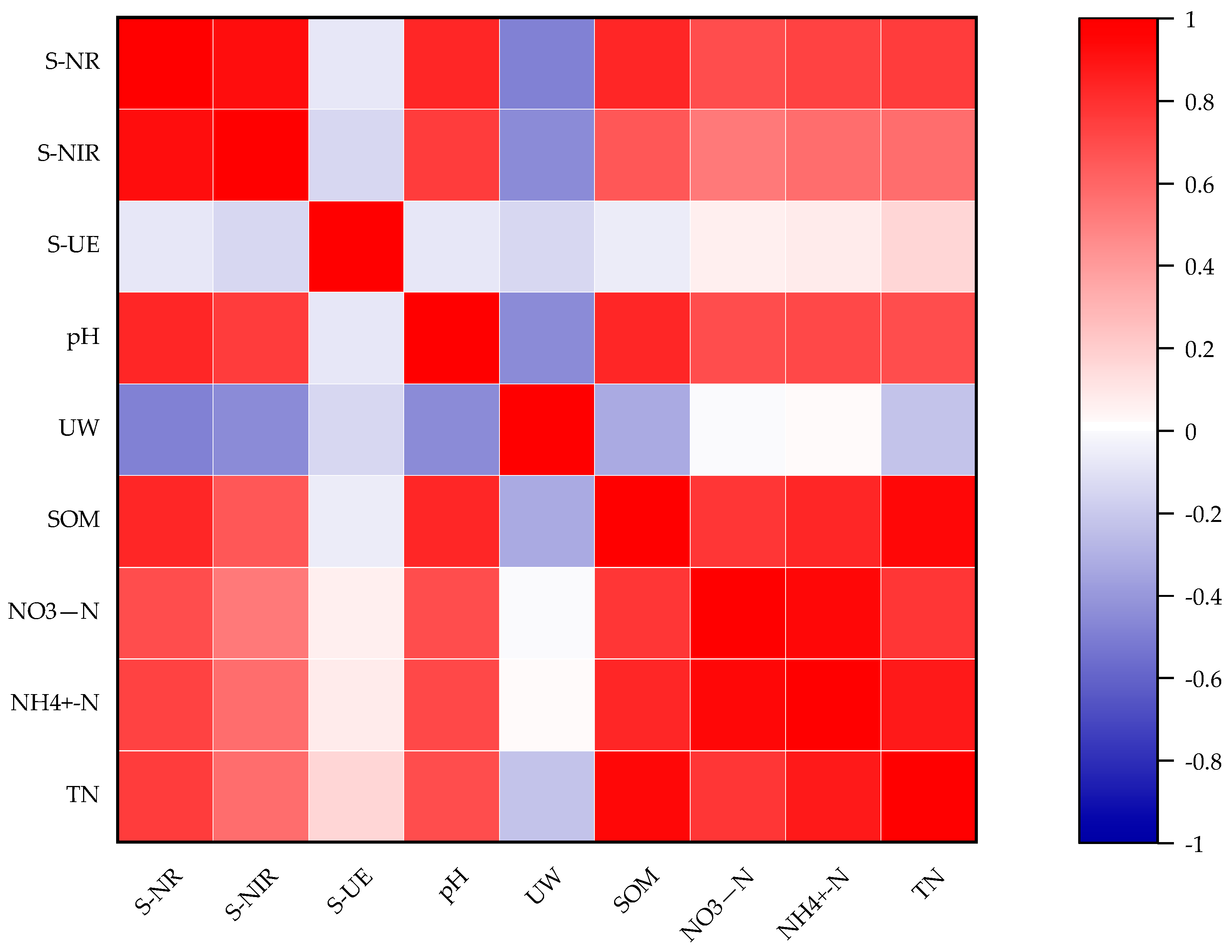
3.10. N-Cycling Enzymes and N-Use Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaman, M.; Saggar, S.; Blennerhassett, J.D. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol. Biochem. 2009, 41, 1270–1280. [Google Scholar] [CrossRef]
- Gang, H.; Wang, Z.H.; Li, F.; Dai, J.; Ma, X.; Li, Q. Soil nitrate–N residue, loss and accumulation affected by soil surface management and precipitation in a winter wheat-summer fallow system on dryland. Nutr. Cycl. Agroecosystems 2016, 106, 31–46. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, C.; Chen, X.; Hopkins, I.; Zhang, X.; Han, Z. The crucial factors of soil fertility and rapeseed yield—A five year field trial with biochar addition in upland red soil, China. Sci. Total Environ. 2019, 649, 1467–1480. [Google Scholar] [CrossRef]
- Wang, X.; Han, C.; Zhang, J.; Huang, Q.; Deng, H.; Deng, Y. Long-term fertilization effects on active ammonia oxidizers in an acidic upland soil in China. Soil Biol. Biochem. 2015, 84, 28–37. [Google Scholar] [CrossRef]
- Pan, X.G.; Li, L.Q.; Liu, X.Y. Industrialization of pyrolyzed biochar: A new pathway for straw prohibition and green agriculture. Sci. Technol. Rev. 2015, 33, 92–101. [Google Scholar]
- Tang, Y.; Wang, L.; Carswell, A.; Misselbrook, T.; Shen, J.; Han, J. Fate and transfer of heavy metals following repeated biogas slurry application in a rice-wheat crop rotation. J. Environ. Manag. 2020, 270, 110938. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, G.; Zhang, H.; Shen, Y.; Fei, H.; Cheng, W. Biogas slurry use as N fertilizer for two-season Zizania aquatica Turcz. in China. Nutr. Cycl. Agroecosystems 2017, 107, 303–320. [Google Scholar] [CrossRef]
- Dahiya, A.K.; Sudevan, P.V. Biogas Plant Slurry as an Alternative to Chemical Fertilizers. Biomass 1986, 9, 67–74. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, J.; Cui, J.; Wang, Y.; Zhou, J.; Ye, M. Effects of biogas slurry application on peanut yield, soil nutrients, carbon storage, and microbial activity in an Ultisol soil in southern China. J. Soils Sediments 2016, 16, 449–460. [Google Scholar] [CrossRef]
- Tang, J.; Davy, A.J.; Wang, W.; Zhang, X.; Wu, D.; Hu, L. Effects of Biogas Slurry on Crop Yield, Physicochemical Properties and Aggregation Characteristics of Lime Concretion Soil in Wheat–Maize Rotation in the North China Plain. J. Soil Sci. Plant Nutr. 2022, 22, 2406–2417. [Google Scholar] [CrossRef]
- Yang, X.D.; Zeng, X.B.; Wen, T. Effects of pig manure application on physicochemical properties and enzyme activities of red soil upland. Acta Pedol. Sin. 2019, 56, 1–11. [Google Scholar]
- Wang, W.F.; Li, C.H.; Huang, S.W. Effects of different fertilization patterns on soil enzyme activities in greenhouse vegetable fields. Chin. J. Appl. Ecol. 2016, 27, 873–882. [Google Scholar]
- Yu, X.; Wu, C.; Fu, Y.; Brookes, P.C.; Lu, S. Three-dimensional pore structure and carbon distribution of macroaggregates in biochar-amended soil. Eur. J. Soil Sci. 2016, 67, 109–120. [Google Scholar] [CrossRef]
- Luo, P.; Chen, H.; Xiao, K.C. Effects of topography, tree species and soil properties on soil extracellular enzyme activity in a karst mountainous area. Environ. Sci. 2017, 38, 2577–2585. [Google Scholar]
- Qian, J.S.; Xie, W.Y.; He, Z.H. Effect of biochar amendment on orchard soil fertility and yellow peach yield and quality. J. Agric. Resour. Environ. 2023, 40, 680–688. [Google Scholar] [CrossRef]
- Lu, R.K. Methods for Soil Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Yang, X.; Lu, Y.; Ding, Y.; Yin, X.; Raza, S.; Tong, Y. Optimising nitrogen fertilisation: A key to improving nitrogen-use efficiency and minimising nitrate leaching losses in an intensive wheat/maize rotation (2008–2014). Field Crops Res. 2017, 206, 1–10. [Google Scholar] [CrossRef]
- Zhou, G.W.; Zhang, W.; Min, W. Effects of irrigation water salinity on ammonia volatilization in drip-irrigated cotton fields. J. Plant Nutr. Fertil. 2015, 21, 413–420. [Google Scholar]
- Liao, P.; Sui, F.; Tang, J. Effects of biochar application on comprehensive greenhouse effect and greenhouse gas emission intensity in double rice paddy fields. J. Nucl. Agric. Sci. 2018, 32, 1821–1830. [Google Scholar]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Y.S. Properties of biochar and its application in soil environment: A review. Chin. J. Soil Sci. Bull. 2013, 44, 1520–1525. [Google Scholar] [CrossRef]
- Esmaeelnejad, L.; Shorafa, M.; Gorji, M.; Hosseini, S. Impacts of Woody Biochar Particle Size on Porosity and Hydraulic Conductivity of Biochar-Soil Mixtures: An Incubation Study. Commun. Soil Sci. Plant Anal. 2017, 48, 1710–1718. [Google Scholar] [CrossRef]
- Wang, R.F.; Zhao, L.X.; Shen, J.Y. Research progress on biochar preparation and its effects on soil physicochemical properties. J. Agric. Sci. Technol. 2015, 17, 126–133. [Google Scholar]
- Ma, N.; Zhang, L.; Zhang, Y.; Yang, L.; Yu, C.; Yin, G. Biochar Improves Soil Aggregate Stability and Water Availability in a Mollisol after Three Years of Field Application. PLoS ONE 2016, 11, e0154091. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.J.; Zhang, K.Q. Effects of different organic fertilizer substitution on soil physicochemical properties and rapeseed yield in Erhai Basin. J. Agro-Environ. Sci. 2021, 40, 2494–2502. [Google Scholar] [CrossRef]
- Peng, Y.Z.; Mao, D.W.; Lian, Y.Y.; Jia, T.X.; Huan, J.C. Optimization of water and nitrogen management in wheat cultivation affected by biochar application—Insights into resource utilization and economic benefits. Agric. Water Manag. 2024, 304. [Google Scholar] [CrossRef]
- He, D.W.; Zhao, Y.Z.; Gao, J.P. Effects of combined biochar and nitrogen fertilizer application on yield formation, ni-trogen use efficiency and residual effects of japonica rice. J. Plant Nutr. Fertil. 2021, 27, 2114–2124. [Google Scholar] [CrossRef]
- Huang, H.L.; Zong, N.; He, N.P. Stoichiometric characteristics of soil enzymes at different elevations in alpine meadows of the Qinghai-Tibet Plateau. Chin. J. Appl. Ecol. 2019, 30, 3689–3696. [Google Scholar]
- Liu, J.; Li, J.; Long, J. Altitudinal characteristics of soil ecological stoichiometry and enzyme activity in karst regions of Southwest China. J. For. Environ. 2022, 4, 113–122. [Google Scholar]
- Wang, Z.W.; Wang, S.Z.; Jiang, H.M. Soil enzyme activity and its influencing factors in different alpine grassland types on the Qinghai-Tibet Plateau. Acta Phytoecol. Sin. 2021, 45, 528–538. [Google Scholar]
- Song, Y.; Li, Y.; Cai, Y.; Fu, S.; Luo, Y.; Wang, H. Biochar decreases soil N2O emissions in Moso bamboo plantations through decreasing labile N concentrations, N-cycling enzyme activities and nitrification/denitrification rates. Geoderma 2019, 348, 135–145. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Shi, W.; Zhou, M.; Ma, X. Effect of biochar on nitrogen use efficiency, grain yield and amino acid content of wheat cultivated on saline soil. Plant Soil Environ. 2019, 65, 83–89. [Google Scholar] [CrossRef]
- Xu, Y.X.; He, L.L.; Liu, Y.X. Effects of biochar application after six years on soil enzyme activity and fertility in paddy fields. Chin. J. Appl. Ecol. 2019, 30, 1110–1118. [Google Scholar]
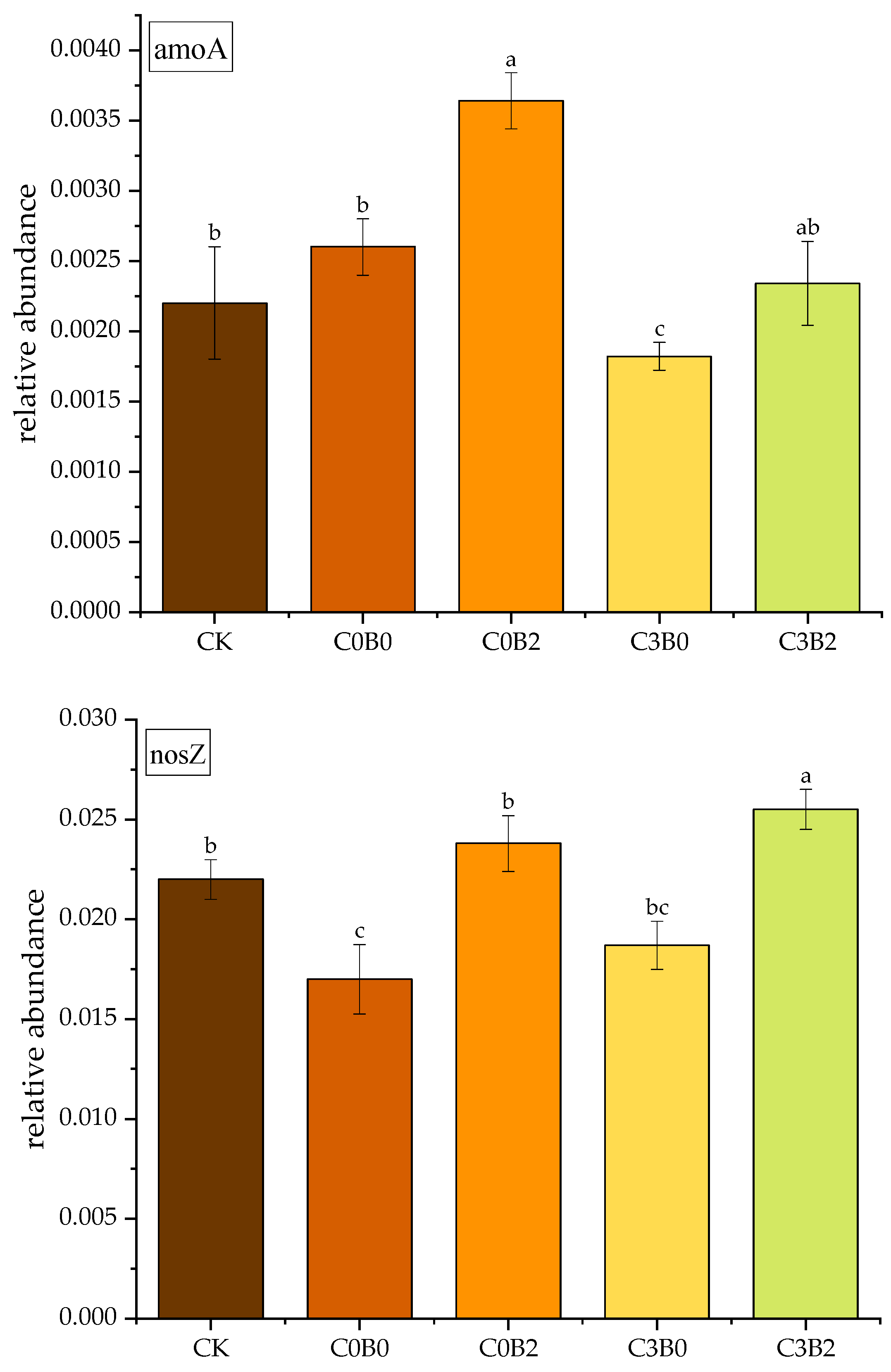
| Treatment | Biochar Level (%) | Biogas Slurry Level (%) | Treatment | Biochar Level (%) | Biogas Slurry Level (%) | Treatment | Biochar Level (%) | Biogas Slurry Level (%) |
|---|---|---|---|---|---|---|---|---|
| C0B0 | 0 | 0 | C0B1 | 0 | 50 | C0B2 | 0 | 100 |
| C1B0 | 0.5 | 0 | C1B1 | 0.5 | 50 | C1B2 | 0.5 | 100 |
| C2B0 | 1 | 0 | C2B1 | 1 | 50 | C2B2 | 1 | 100 |
| C3B0 | 2 | 0 | C3B1 | 2 | 50 | C3B2 | 2 | 100 |
| Treatment | pH | UW/ (g·cm−3) | NO3N/ (mg·kg−1) | NH4+N/ (mg·kg−1) | TN/ (g·kg−1) | SOM/ (g·kg−1) | |
|---|---|---|---|---|---|---|---|
| CK | 5.31 ± 0.16c | 1.45 ± 0.01a | 3.54 ± 0.74c | 0.79 ± 0.10d | 0.6 ± 0.01c | 8.31 ± 0.97d | |
| C0 | B0 | 5.15 ± 0.17c | 1.43 ± 0.01ab | 4.13 ± 1.37c | 2.37 ± 0.52c | 0.5 ± 0.01c | 7.46 ± 0.64d |
| B1 | 5.31 ± 0.07c | 1.49 ± 0.01a | 4.58 ± 0.07c | 3.39 ± 0.10c | 0.5 ± 0.01c | 7.48 ± 0.33d | |
| B2 | 5.17 ± 0.09b | 1.47 ± 0.02a | 4.37 ± 0.93d | 4.04 ± 0.81c | 0.9 ± 0.06cd | 7.17 ± 0.19d | |
| C1 | B0 | 6.28 ± 2.8b | 1.25 ± 0.01c | 8.68 ± 1.33b | 3.40 ± 0.58c | 1.4 ± 0.03b | 19.78 ± 1.18c |
| B1 | 7.14 ± 0.20b | 1.28 ± 0.02b | 3.51 ± 0.02c | 4.11 ± 0.24c | 1.3 ± 0.00b | 20.55 ± 1.15c | |
| B2 | 7.38 ± 0.04a | 1.29 ± 0.01c | 8.02 ± 0.86c | 4.88 ± 0.46c | 1.7 ± 0.05bc | 17.29 ± 1.77c | |
| C2 | B0 | 7.15 ± 0.34b | 1.41 ± 0.02ab | 14.78 ± 2.07a | 7.44 ± 0.97b | 1.9 ± 0.06b | 27.35 ± 2.08b |
| B1 | 7.35 ± 0.06ab | 1.43 ± 0.06b | 16.24 ± 5.75b | 11.16 ± 1.46b | 2.3 ± 0.006a | 30.65 ± 1.05a | |
| B2 | 7.37 ± 0.16a | 1.45 ± 0.04ab | 15 ± 0.51b | 13.54 ± 1.22b | 2.7 ± 0.04b | 23.62 ± 1.20b | |
| C3 | B0 | 7.49 ± 0.1a | 1.36 ± 0.01b | 15.02 ± 1.25a | 15.72 ± 1.13a | 4.5 ± 0.04a | 38.67 ± 0.90a |
| B1 | 7.39 ± 0.23a | 1.42 ± 0.06b | 28.34 ± 1.19a | 18.66 ± 0.61a | 2.2 ± 0.016a | 24.51 ± 0.59b | |
| B2 | 7.46 ± 0.05a | 1.36 ± 0.02bc | 27.9 ± 1.8a | 19.46 ± 1.00a | 5.2 ± 0.09a | 39.75 ± 0.28a | |
| pH | UW | SOM | NO3—N | NH4+-N | TN | |
|---|---|---|---|---|---|---|
| C | 0.052 | 0.035 | 0.021 | 0.025 | 0.047 | 0.008 |
| B | 0.29 | 0.15 | 0.34 | 0.02 | 0.26 | 0.1 |
| B × C | 0.005 | 0.012 | 0.04 | 0.04 | 0.01 | 0.046 |
| NH3 | N2O | NR | NiR | Urea | amoA | nosZ | COP | NUE | |
|---|---|---|---|---|---|---|---|---|---|
| B | 0.006 ** | 0.013 * | 0.04 * | 0.08 ns | 0.02 * | 0.03 * | 0.25 ns | 0.0016 ** | 0.012 * |
| C | 0.48 ns | 0.81 ns | 0.01 * | 0.42 ns | 0.07 ns | 0.05 ns | 0.003 ** | 0.049 * | 0.007 ** |
| B × C | 0.005 ** | 0.05 ns | 0.47 ns | 0.33 ns | 0.35 ns | 0.01 * | 0.60 ns | 0.41 ns | 0.005 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Jin, Z.; Ping, L. Study of the Effects of Co-Application of Biochar and Biogas Slurry on Nitrogen Cycling Enzyme Activity and Nitrogen Use Efficiency. Agronomy 2025, 15, 2408. https://doi.org/10.3390/agronomy15102408
Xu T, Jin Z, Ping L. Study of the Effects of Co-Application of Biochar and Biogas Slurry on Nitrogen Cycling Enzyme Activity and Nitrogen Use Efficiency. Agronomy. 2025; 15(10):2408. https://doi.org/10.3390/agronomy15102408
Chicago/Turabian StyleXu, Tianxiu, Zewen Jin, and Lifeng Ping. 2025. "Study of the Effects of Co-Application of Biochar and Biogas Slurry on Nitrogen Cycling Enzyme Activity and Nitrogen Use Efficiency" Agronomy 15, no. 10: 2408. https://doi.org/10.3390/agronomy15102408
APA StyleXu, T., Jin, Z., & Ping, L. (2025). Study of the Effects of Co-Application of Biochar and Biogas Slurry on Nitrogen Cycling Enzyme Activity and Nitrogen Use Efficiency. Agronomy, 15(10), 2408. https://doi.org/10.3390/agronomy15102408




