Passivation Remediation of Cd-Contaminated Farmland in Yongkang, China by CaAl-LDH: A Mechanism and Application Study
Abstract
1. Introduction
2. Materials And Methods
2.1. Preparation
2.2. Adsorption Test of Cd by CaAl-LDH
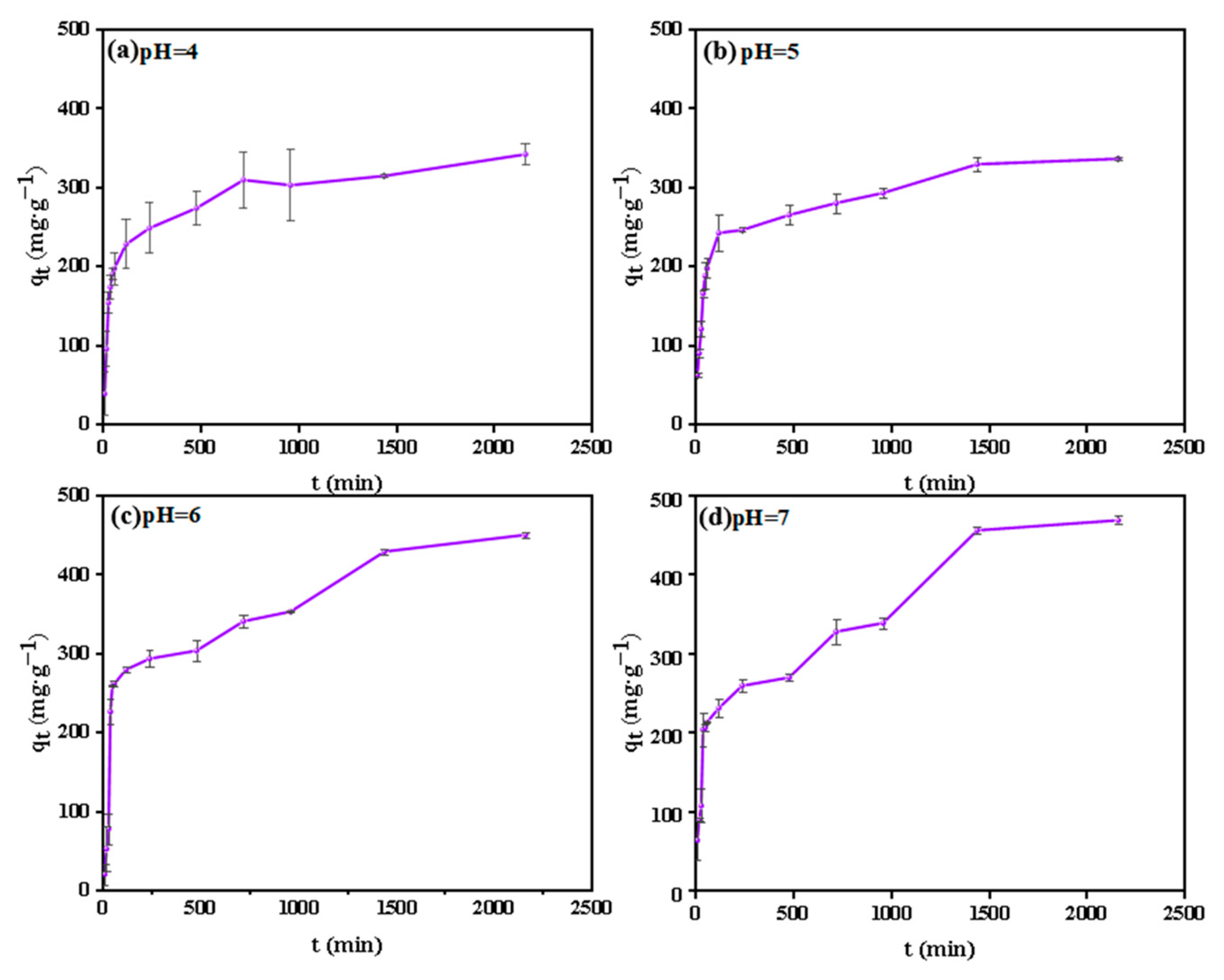
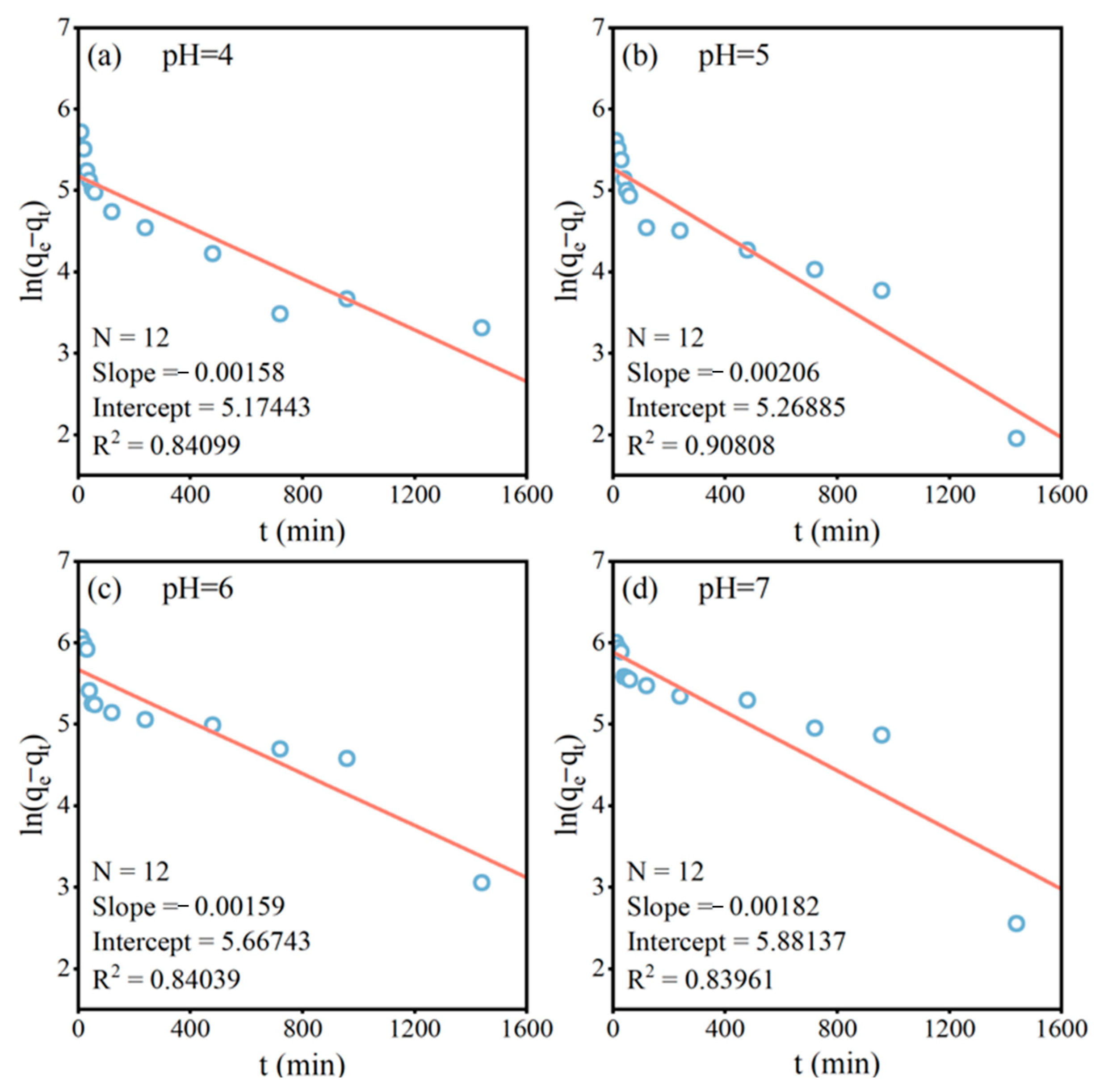
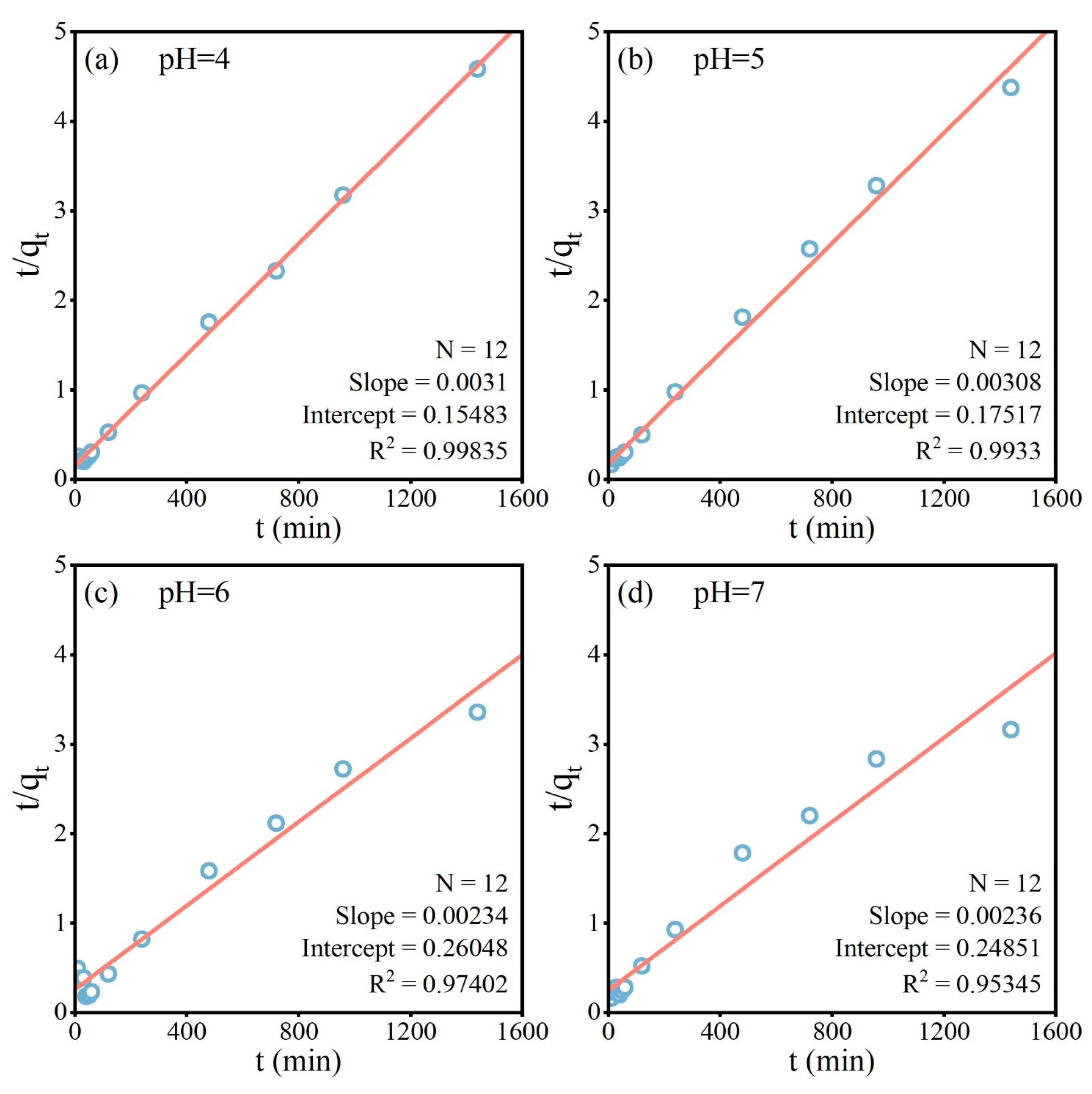
2.2.1. Adsorption Kinetics
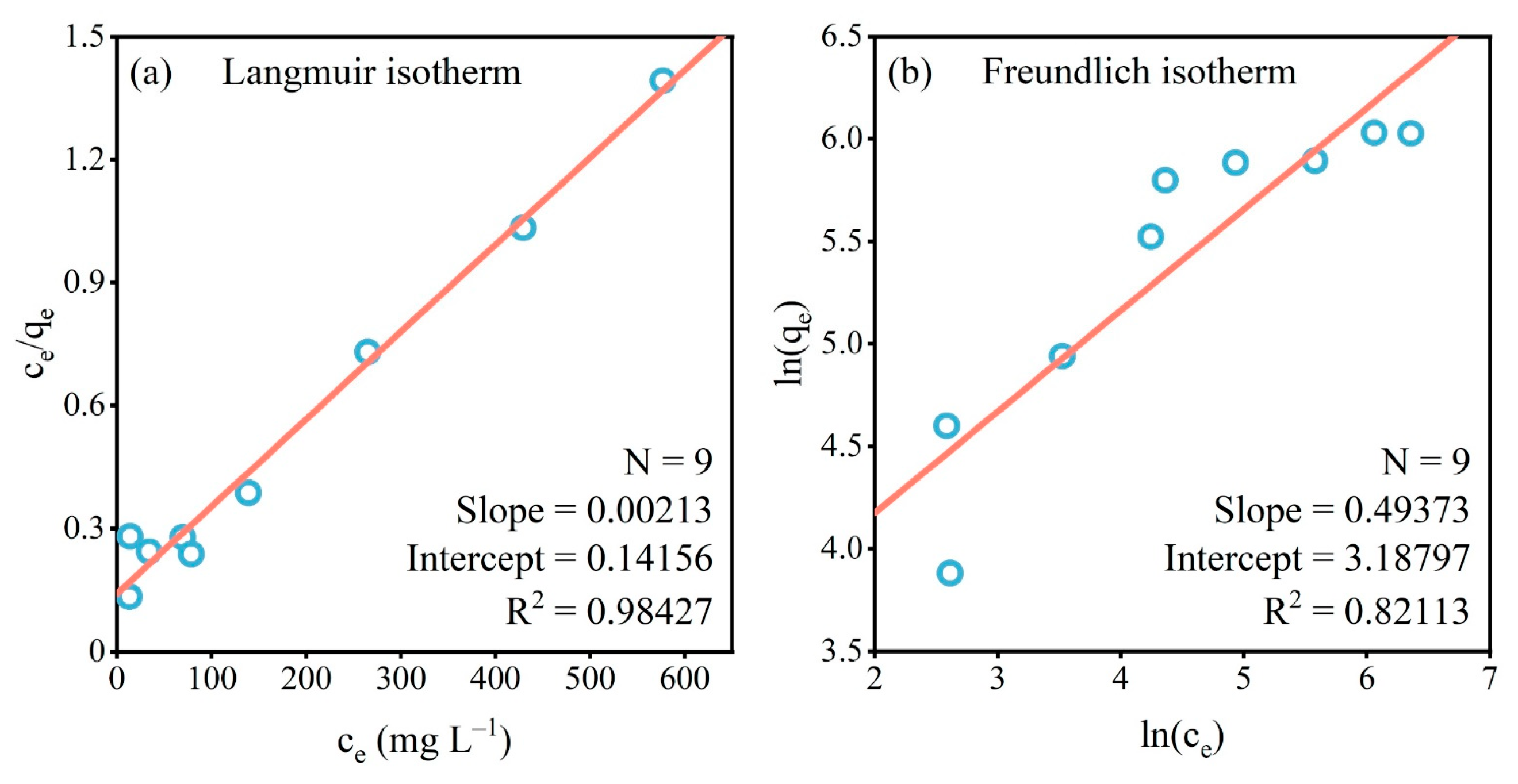
2.2.2. Adsorption Isotherms
2.2.3. Characterization of CaAl-LDH
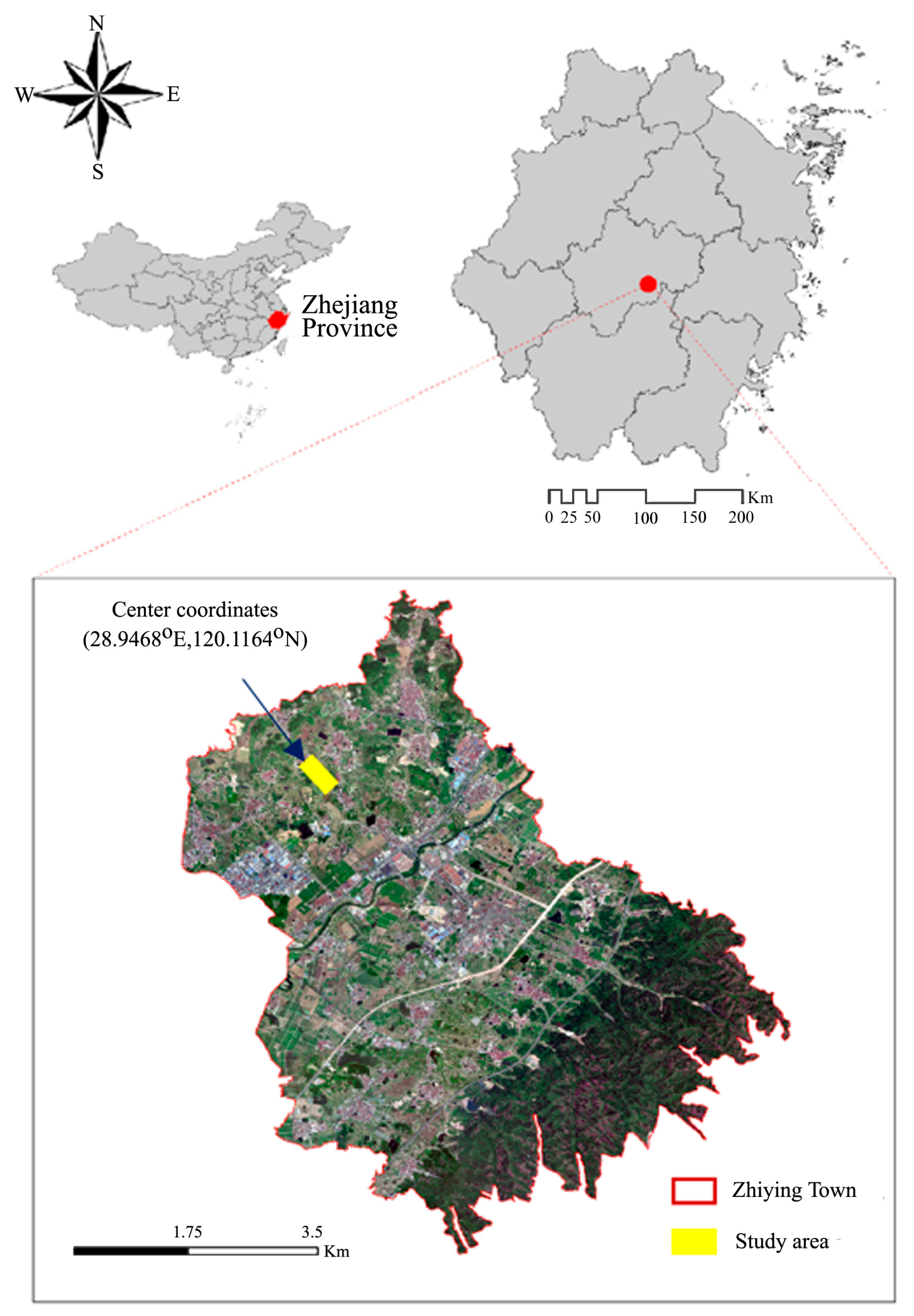
2.3. Experimental Site and Design
2.4. Sample Analysis and Quality Assurance
2.5. Data Processing
3. Results and Discussion
3.1. Characteristics of CaAl-LDH
3.1.1. Contents of Heavy Metals
3.1.2. SEM Analysis
3.2. Adsorption of Cd by CaAl-LDH
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
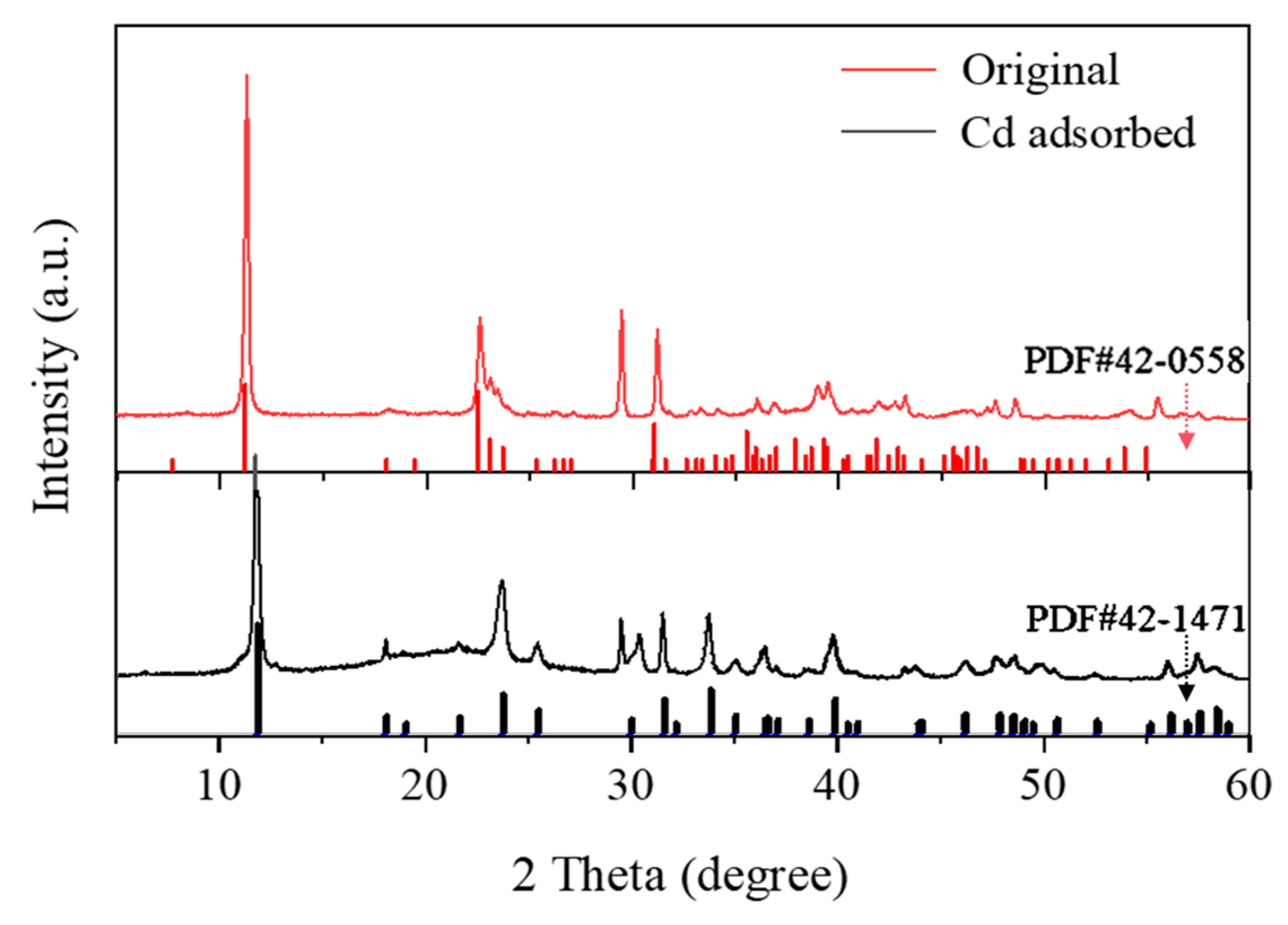
3.2.3. XRD Analysis
3.3. Effect of CaAl-LDH on Soil pH
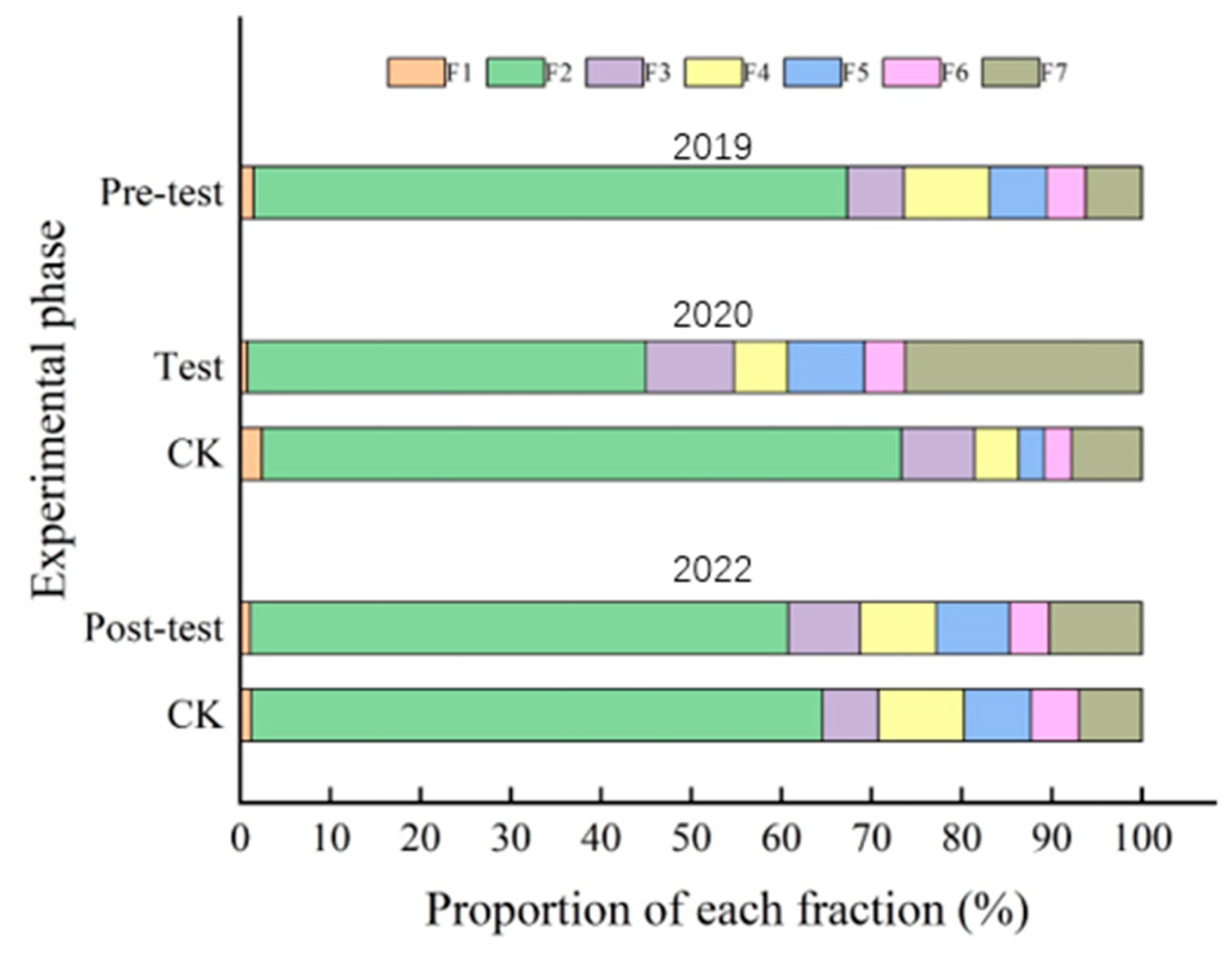
3.4. Effect of Passivation Remediation on Occurrence Forms of Soil Cd
3.5. Effect of Passivation Remediation on Cd Content in Rice Grains
3.6. Limitations and Prospect in Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, X.; Yang, Z.; Yu, T.; Zhang, C.; Hou, Q. Predicting Spatial and Temporal Variation of Cd Concentration in Rice Grains in the Lower Changjiang Plain during 2004–2014 Based on Soil Geochemical Survey Data with GIS. J. Geochem. Explor. 2019, 200, 276–283. [Google Scholar] [CrossRef]
- Chen, X.; Wang, A.; Wang, J.; Zhang, Z.; Yu, J.; Yan, Y.; Zhang, J.; Niu, J.; Cui, X.; Liu, X. Influences of Coexisting Aged Polystyrene Microplastics on the Ecological and Health Risks of Cadmium in Soils: A Leachability and Oral Bioaccessibility Based Study. J. Hazard. Mater. 2024, 469, 133884. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Deng, J.; Cao, J.; Meng, H.; Dong, J.; Zhang, H. Assessing Soil Cadmium Quality Standards for Different Land Use Types: A Global Synthesis. J. Hazard. Mater. 2024, 480, 136450. [Google Scholar] [CrossRef] [PubMed]
- Shokr, M.S.; Abdellatif, M.A.; El Behairy, R.A.; Abdelhameed, H.H.; El Baroudy, A.A.; Mohamed, E.S.; Rebouh, N.Y.; Ding, Z.; Abuzaid, A.S. Assessment of Potential Heavy Metal Contamination Hazards Based on GIS and Multivariate Analysis in Some Mediterranean Zones. Agronomy 2022, 12, 3220. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, B.; Song, Y.; Yang, Y.; Cai, L.; Miao, Q.; Jiang, T. Soil Contamination in Contaminated Sites with Key Standards: A Global Analysis and Perspective. J. Hazard. Mater. 2025, 494, 138724. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Wang, Y.; Ye, M.; Xu, X.; Li, X. Remediation of Cd-Contaminated Soil by Electrokinetics Coupled with the Permeable Reactive Barrier from Immobilized Yeast. Sci. Total Environ. 2023, 882, 163451. [Google Scholar] [CrossRef]
- Sahoo, A.; Chhotaray, S.P.; Meher, I.; Behera, S.P.; Pal, A.; Meena, M.; Swapnil, P.; Yadav, A.; Bhardwaj, R. Phytoremediation for a Sustainable Future: Integrating Plant Based Strategies in Soil and Wastewater Remediation. Bioresour. Technol. Rep. 2025, 31, 102266. [Google Scholar] [CrossRef]
- Xia, X.; Kong, H.; Li, Y.; Qu, Z.; Yu, B. Research on Chemical Leaching Remediation of Cr, Ni, and Cu Composite Heavy Metal Contaminated Soil. Desalination Water Treat. 2025, 323, 101416. [Google Scholar] [CrossRef]
- Yan-bing, H.; Dao-You, H.; Qi-Hong, Z.; Shuai, W.; Shou-Long, L.; Hai-Bo, H.; Han-Hua, Z.; Chao, X. A Three-Season Field Study on the in-Situ Remediation of Cd-Contaminated Paddy Soil Using Lime, Two Industrial by-Products, and a Low-Cd-Accumulation Rice Cultivar. Ecotoxicol. Environ. Saf. 2017, 136, 135–141. [Google Scholar] [CrossRef]
- Li, C.; Tan, X.; Li, X.; Huang, Y.; Xiang, C.; Wu, C.; Guo, J.; Xue, S. Simultaneous Stabilization of Cadmium and Arsenic in Soil by Humic Acid and Mechanically Activated Phosphate Rock. J. Hazard. Mater. 2025, 489, 137628. [Google Scholar] [CrossRef]
- Huang, S.; Meng, Z.; Wu, J.; Xin, L.; Zhao, Q. Long-Term Cd Remediation Mechanisms and Potential Risks in Soil with Biochar Application under Dry-Wet Cycling at Different Soil Moisture Levels. Agric. Water Manag. 2025, 307, 109212. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhao, T.; Chen, G.; Wang, J.; Yuan, Z.; Zhang, J.; Yin, N.; Diao, G.; Xu, K. Influencing factors of Cd bioaccessibility in China’s representative soils and the human health risk assessment. Environ. Chem. 2021, 40, 3015–3023. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhao, T.; Zhang, F.; Zhao, Q.; Chen, Y.; Zhang, J.; Zhang, X.; Xiao, Y.; Yang, G. Comparative study between CaAl-Cl LDH and conventional immobilizing agents for reducing ecological and health risks of soil Cd. Trans. Chin. Soc. Agric. Eng. 2022, 38, 219–226. [Google Scholar] [CrossRef]
- Momin, Z.H.; Lingamdinne, L.P.; Kulkarni, R.; Pal, C.A.; Choi, Y.-L.; Yang, J.-K.; Kang, S.-H.; Chang, Y.-Y.; Koduru, J.R. Redefining Water Purification: gC3N4-CLDH’s Electrochemical SMX Eradication. Chemosphere 2024, 362, 142921. [Google Scholar] [CrossRef] [PubMed]
- Momin, Z.H.; Angaru, G.K.R.; Lingamdinne, L.P.; Koduru, J.R.; Chang, Y.-Y. Highly Efficient Cd(II) Removal from Groundwater Utilizing Layered Mixed Metal Oxides-Graphitic Carbon Nitride Composite with Improved Cycling Stability. J. Water Process Eng. 2023, 56, 104276. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of Metal Cations on Layered Double Hydroxides. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Zhu, Y.; Yan, H. DFT Study on MgAl-Layered Double Hydroxides with Different Interlayer Anions: Structure, Anion Exchange, Host–Guest Interaction and Basic Sites. Phys. Chem. Chem. Phys. 2020, 22, 2521–2529. [Google Scholar] [CrossRef]
- Forano, C. Environmental Remediation Involving Layered Double Hydroxides. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 425–458. ISBN 978-0-12-088439-1. [Google Scholar]
- Momin, Z.H.; Lingamdinne, L.P.; Kulkarni, R.; Pal, C.A.; Choi, Y.-L.; Chang, Y.-Y.; Koduru, J.R. Exploring Recyclable Alginate-Enhanced GCN-LDO Sponge for U(VI) and Cd(II) Removal: Insights from Batch and Column Studies. J. Hazard. Mater. 2024, 469, 134015. [Google Scholar] [CrossRef]
- Li, S.; Gao, H.; Xu, R.; Guo, Z. Mg-Al Layered Double Hydroxides Derived from Secondary Aluminum Ash for Soil Remediation Contaminated with Cd(II) and Cr(VI). J. Hazard. Mater. 2025, 497, 139585. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Wang, A.; Wang, X.; Chen, X.; Delgado, J.J.; Zhang, T. A Noble-Metal-Free Catalyst Derived from Ni-Al Hydrotalcite for Hydrogen Generation from N2 H4 ⋅H2 O Decomposition. Angew. Chem. Int. Ed. 2012, 51, 6191–6194. [Google Scholar] [CrossRef]
- Chen, X.; Yao, C.; Zhao, T.; Xu, J.; Bu, Y.; Zhang, W.; Liu, Y.; Diao, G.; Zhang, J. Immobilization remediation of Cr-contaminated soils by hydrocalumite and the relevant risk assessment. China Environ. Sci. 2021, 41, 1790–1798. [Google Scholar] [CrossRef]
- Kong, X.; Ge, R.; Liu, T.; Xu, S.; Hao, P.; Zhao, X.; Li, Z.; Lei, X.; Duan, H. Super-Stable Mineralization of Cadmium by Calcium-Aluminum Layered Double Hydroxide and Its Large-Scale Application in Agriculture Soil Remediation. Chem. Eng. J. 2021, 407, 127178. [Google Scholar] [CrossRef]
- Richardson, M.C.; Braterman, P.S. Cation Exchange by Anion-Exchanging Clays: The Effects of Particle Aging. J. Mater. Chem. 2009, 19, 7965. [Google Scholar] [CrossRef]
- Tarasov, K.A.; O’Hare, D.; Isupov, V.P. Solid-State Chelation of Metal Ions by Ethylenediaminetetraacetate Intercalated in a Layered Double Hydroxide. Inorg. Chem. 2003, 42, 1919–1927. [Google Scholar] [CrossRef]
- Choy, J.; Choi, S.; Oh, J.; Park, T. Clay Minerals and Layered Double Hydroxides for Novel Biological Applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of Layered Double Hydroxides for Removal of Oxyanions: A Review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, F.; Wu, J.; Lu, Y.; Zhang, J.; Yao, Z.; Xiao, J.; Zhong, Q.; Li, J. Spent Graphite/Mg-Al Nanosheets Composite for Ultra-Efficient Removal of Cu2+: Adsorption Behavior, Mechanism, and DFT Study. Sep. Purif. Technol. 2025, 361, 131609. [Google Scholar] [CrossRef]
- Zang, Y.; Hou, W.; Xu, J. Removal of Cu(II) from CuSO4 Aqueous Solution by Mg-Al Hydrotalcite-like Compounds. Chin. J. Chem. 2011, 29, 847–852. [Google Scholar] [CrossRef]
- Lehmann, M.; Zouboulis, A.I.; Matis, K.A. Removal of Metal Ions from Dilute Aqueous Solutions: A Comparative Study of Inorganic Sorbent Materials. Chemosphere 1999, 39, 881–892. [Google Scholar] [CrossRef]
- Rojas, R. Copper, Lead and Cadmium Removal by Ca Al Layered Double Hydroxides. Appl. Clay Sci. 2014, 87, 254–259. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, K.; Hao, Y.; Du, B. Adsorption of Cd(II) by Mg–Al–CO3- and Magnetic Fe3O4/Mg–Al–CO3-Layered Double Hydroxides: Kinetic, Isothermal, Thermodynamic and Mechanistic Studies. J. Hazard. Mater. 2015, 299, 42–49. [Google Scholar] [CrossRef]
- Simmons, R.W.; Pongsakul, P.; Saiyasitpanich, D.; Klinphoklap, S. Elevated Levels of Cadmium and Zinc in Paddy Soils and Elevated Levels of Cadmium in Rice Grain Downstream of a Zinc Mineralized Area in Thailand: Implications for Public Health. Environ. Geochem. Health 2005, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.W.; Lan, C.Y.; Wang, H.B.; Zhuang, P.; Shu, W.S. Cadmium in Soil–Rice System and Health Risk Associated with the Use of Untreated Mining Wastewater for Irrigation in Lechang, China. Agric. Water Manag. 2006, 84, 147–152. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Yang, Z.; Zhang, Q.; Ji, J. Enrichment and Source Identification of Cd and Other Heavy Metals in Soils with High Geochemical Background in the Karst Region, Southwestern China. Chemosphere 2020, 245, 125620. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A Review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Akakuru, O.U.; Xu, X.; Wu, A. Research Progress and Mechanism of Nanomaterials-Mediated in-Situ Remediation of Cadmium-Contaminated Soil: A Critical Review. J. Environ. Sci. 2021, 104, 351–364. [Google Scholar] [CrossRef]
- Zhao, H.; Du, L.; Wu, Y.; Wu, X.; Han, W. Numerical Assessment of the Passivator Effectiveness for Cd-Contaminated Soil Remediation. Sci. Total Environ. 2021, 779, 146485. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, Q.; Ma, L.Q. Availability and Assessment of Fixing Additives for The in Situ Remediation of Heavy Metal Contaminated Soils: A Review. Environ. Monit. Assess. 2006, 116, 513–528. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Yang, Z.; Zhuo, X.; Guan, D.-X.; Song, Y.; Guo, C.; Ji, J. Evaluation of Various Approaches to Predict Cadmium Bioavailability to Rice Grown in Soils with High Geochemical Background in the Karst Region, Southwestern China. Environ. Pollut. 2020, 258, 113645. [Google Scholar] [CrossRef]
- Mao, F.; Hao, P.; Zhu, Y.; Kong, X.; Duan, X. Layered Double Hydroxides: Scale Production and Application in Soil Remediation as Super-Stable Mineralizer. Chin. J. Chem. Eng. 2022, 41, 42–48. [Google Scholar] [CrossRef]
- Chen, H.; Chen, M.; Zhang, W.; Yang, L.; Feng, Y.; Jia, Y.; Li, J. Dual Regulation Mechanisms of LDHs on Cadmium and Arsenic Availability in Paddy Soils under Alternating Wet-Dry Conditions: Chemical Immobilization and Microbial Responses. Chem. Eng. J. 2025, 523, 168484. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Chen, B.; Xu, Y.; Wang, H.; Jin, F.; Shen, Z.; Hou, D. Green Synthesis of Layered Double Hydroxides (LDH) for the Remediation of As and Cd in Water and Soil. Appl. Clay Sci. 2024, 249, 107262. [Google Scholar] [CrossRef]
- Guan, X.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption Behaviors and Mechanisms of Fe/Mg Layered Double Hydroxide Loaded on Bentonite on Cd (II) and Pb (II) Removal. J. Colloid Interface Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Senthil, R.A.; Pan, J.; Sun, Y. A Facile Preparation of 3D Flower-Shaped Ni/Al-LDHs Covered by β-Ni(OH)2 Nanoplates as Superior Material for High Power Application. Chin. J. Chem. Eng. 2019, 27, 2526–2534. [Google Scholar] [CrossRef]
- NY/T 2272-2012; Soil Amendment—Determination of Calcium, Magnesium and Silicon Content. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2012.
- NY/T 2273-2012; Soil Amendment—Determination of Phosphorus Content. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2012.
- NY/T 3035-2016; Soil Amendment—Determination of Moisture Content, pH, Bulk Density, Water Holding Capacity and Total Porosity. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2016.
- NY/T 3034-2016; Soil Amendment—General Requirements. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2016.
- Tao, Y.; Carta, G.; Ferreira, G.; Robbins, D. Adsorption of Deamidated Antibody Variants on Macroporous and Dextran-Grafted Cation Exchangers: II. Adsorption Kinetics. J. Chromatogr. A 2011, 1218, 1530–1537. [Google Scholar] [CrossRef]
- Guo, Q.; Reardon, E.J. Fluoride Removal from Water by Meixnerite and Its Calcination Product. Appl. Clay Sci. 2012, 56, 7–15. [Google Scholar] [CrossRef]
- Yan, L.; Yang, K.; Shan, R.; Yan, T.; Wei, J.; Yu, S.; Yu, H.; Du, B. Kinetic, Isotherm and Thermodynamic Investigations of Phosphate Adsorption onto Core–Shell Fe3O4@LDHs Composites with Easy Magnetic Separation Assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef]
- Ling, F.; Fang, L.; Lu, Y.; Gao, J.; Wu, F.; Zhou, M.; Hu, B. A Novel CoFe Layered Double Hydroxides Adsorbent: High Adsorption Amount for Methyl Orange Dye and Fast Removal of Cr(VI). Microporous Mesoporous Mater. 2016, 234, 230–238. [Google Scholar] [CrossRef]
- Abreu, F.O.M.D.S.; Silva, N.A.D.; Sipauba, M.D.S.; Pires, T.F.M.; Bomfim, T.A.; Monteiro Junior, O.A.D.C.; Forte, M.M.D.C. Chitosan and Gum Arabic Nanoparticles for Heavy Metal Adsorption. Polímeros 2018, 28, 231–238. [Google Scholar] [CrossRef]
- GB2762-2022; National Food Safety Standard—Maximum Levels of Contaminants in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2022.
- Dold, B. Speciation of the Most Soluble Phases in a Sequential Extraction Procedure Adapted for Geochemical Studies of Copper Sulfide Mine Waste. J. Geochem. Explor. 2003, 80, 55–68. [Google Scholar] [CrossRef]
- Lu, M.; Wang, B.; Li, J.; Li, W.; Yang, X.; Song, J. Research of Heavy Metals Determination in Cereals by Inductively Coupled Plasma Mass Spec-trometry. Spectrosc. Spectr. Anal. 2012, 32, 2234–2237. [Google Scholar] [CrossRef]
- Bi, Z.; Sun, J.; Xie, Y.; Gu, Y.; Zhang, H.; Zheng, B.; Ou, R.; Liu, G.; Li, L.; Peng, X.; et al. Machine learning-driven source identification and ecological risk prediction of heavy metal pollution in cultivated soils. J. Hazard. Mater. 2024, 476, 135109. [Google Scholar] [CrossRef] [PubMed]
- DZ/T 0279.5-2016; Analysis Methods for Regional Geochemical Sample—Part 5: Determination of Arsenic, Bismuth, Antimony, Mercury and Selenium Contents. Ministry of Land and Resources of the People’s Republic of China: Beijing, China, 2016.
- GB15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Cao, L.; Guo, J.; Tian, J.; Xu, Y.; Hu, M.; Wang, M.; Fan, J. Preparation of Ca/Al-Layered Double Hydroxide and the Influence of Their Structure on Early Strength of Cement. Constr. Build. Mater. 2018, 184, 203–214. [Google Scholar] [CrossRef]
- Liu, T.; Yang, J.; Ji, K.; Zheng, M.; Yang, X.; Shao, M.; Duan, H.; Kong, X. Multiple Anchor Sites of CaFe-LDH Enhanced the Capture Capacity to Cadmium, Arsenite, and Lead Simultaneously in Contaminated Water/Soil: Scalable Synthesis, Mechanism, and Validation. ACS EST Eng. 2024, 4, 550–561. [Google Scholar] [CrossRef]
- Dai, C.; Wu, X.; Wang, Q.; Bai, Y.; Zhao, D.; Fu, J.; Fu, B.; Ding, H. Layered Double Hydroxides for Efficient Treatment of Heavy Metals and Organic Pollutants: Recent Progress and Future Perspectives. Sep. Purif. Technol. 2025, 352, 128277. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Tan, L.; Chen, Y.; Hayat, T.; Hu, J.; Alsaedi, A.; Ahmad, B.; Guo, W.; Wang, X. Performances and Mechanisms of Mg/Al and Ca/Al Layered Double Hydroxides for Graphene Oxide Removal from Aqueous Solution. Chem. Eng. J. 2016, 297, 106–115. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Y.; Hu, W.; Wu, Q.; Zhong, X.; Huang, B.; Hu, X.; Wu, C. Health Management of Cd-Contaminated Soil Using Ca-Al Layered Double Hydroxide: Response of Different Vegetables. Agric. Ecosyst. Environ. 2023, 356, 108631. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Zhao, J.; Cheng, J.; Yu, Q.; Zhang, S.; Zhao, J.; Qiu, X. Remediation of Chromium-Contaminated Soil Using Delaminated Layered Double Hydroxides with Different Divalent Metals. Chemosphere 2020, 254, 126879. [Google Scholar] [CrossRef]
- Bhattacharjee, P.R. Discovery of the Lack of Success of Bragg’s Law in Respect of Perfect Quantification of the X-Ray Diffraction Phenomenon. Next Res. 2025, 2, 100312. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, G.; Wang, H.; Hu, W.; Huang, B. Hydrocalumite Passivation Effect and Mechanism on Heavy Metals in Different Cd-Contaminated Farmland Soils. Environ. Sci. 2019, 40, 352–361. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Cao, H.; Li, H.; Li, Z. Removal of Cd2+ from Water by Friedel’s Salt (FS: 3CaO·A12O3·CaCl2·10H2O): Sorption Characteristics and Mechanisms. J. Environ. Sci. 2013, 25, 1719–1725. [Google Scholar] [CrossRef]
- Kosolsaksakul, P.; Farmer, J.G.; Oliver, I.W.; Graham, M.C. Geochemical Associations and Availability of Cadmium (Cd) in a Paddy Field System, Northwestern Thailand. Environ. Pollut. 2014, 187, 153–161. [Google Scholar] [CrossRef]
- Liu, B.; Ai, S.; Zhang, W.; Huang, D.; Zhang, Y. Assessment of the Bioavailability, Bioaccessibility and Transfer of Heavy Metals in the Soil-Grain-Human Systems near a Mining and Smelting Area in NW China. Sci. Total Environ. 2017, 609, 822–829. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Shi, H.; Liu, Y. Redox Dependence of Manganese Controls Cadmium Isotope Fractionation in a Paddy Soil-Rice System under Unsteady Pe + pH Conditions. Sci. Total Environ. 2022, 806, 150675. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Zhang, C.; Liu, X.; Liang, X.; Liu, R.; Zhao, Y. Mechanisms of S Cooperating with Fe and Mn to Regulate the Conversion of Cd and Cu during Soil Redox Process Revealed by LDHs-DGT Technology. Sci. Total Environ. 2023, 867, 161431. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Wang, A.; Yu, J.; Zhang, J.; Xiao, Z.; Cui, X.; Liu, X.; Yin, N.; Cui, Y. Immobilisation Remediation of Arsenic-Contaminated Soils with Promising CaAl-Layered Double Hydroxide and Bioavailability, Bioaccessibility, and Speciation-Based Health Risk Assessment. J. Hazard. Mater. 2024, 469, 134096. [Google Scholar] [CrossRef]

| Year | Testing | Control Group | Test Group |
|---|---|---|---|
| 2020 | Test | Base fertilizer | CaAl-LDH+Base fertilizer |
| 2021 | Post-test | Base fertilizer | Base fertilizer |
| 2022 | Post-test | Base fertilizer | Base fertilizer |
| Base fertilizer: N, P, K compound fertilizer 630 kg·hm–2, urea 270 kg·hm–2 | |||
| CaAl-LDH: 1500 kg hm–2 | |||
| Year | Testing | Control Group | Experimental Group |
|---|---|---|---|
| 2019 | Pre-test | 5.04 ± 0.24 B | 5.04 ± 0.24 A |
| 2020 | Test | 5.75 ± 0.48 A a | 5.04 ± 0.09 A b |
| 2021 | Post-test | 5.38 ± 0.61 AB a | 5.19 ± 0.16 A a |
| 2022 | Post-test | 5.07 ± 0.06 B a | 5.14 ± 0.18 A a |
| Year | Testing | Cd Content in Rice (mg/kg) | |
|---|---|---|---|
| Control Group | Experimental Group | ||
| 2020 | Test | 0.19 ± 0.10 A a | 0.06 ± 0.02 B a |
| 2021 | Post-test | 0.16 ± 0.03 A a | 0.10 ± 0.02 AB b |
| 2022 | Post-test | 0.23 ± 0.04 A a | 0.14 ± 0.03 A b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Wei, N.; Fang, H.; Hu, F.; Cheng, J.; Sun, R.; Chen, Y.; Zhang, J.; Chen, Y.; Zhang, X.; et al. Passivation Remediation of Cd-Contaminated Farmland in Yongkang, China by CaAl-LDH: A Mechanism and Application Study. Agronomy 2025, 15, 2354. https://doi.org/10.3390/agronomy15102354
Lu X, Wei N, Fang H, Hu F, Cheng J, Sun R, Chen Y, Zhang J, Chen Y, Zhang X, et al. Passivation Remediation of Cd-Contaminated Farmland in Yongkang, China by CaAl-LDH: A Mechanism and Application Study. Agronomy. 2025; 15(10):2354. https://doi.org/10.3390/agronomy15102354
Chicago/Turabian StyleLu, Xinzhe, Nan Wei, Haochen Fang, Feng Hu, Jianjun Cheng, Rui Sun, Yining Chen, Jianyu Zhang, Yanfang Chen, Xuchuan Zhang, and et al. 2025. "Passivation Remediation of Cd-Contaminated Farmland in Yongkang, China by CaAl-LDH: A Mechanism and Application Study" Agronomy 15, no. 10: 2354. https://doi.org/10.3390/agronomy15102354
APA StyleLu, X., Wei, N., Fang, H., Hu, F., Cheng, J., Sun, R., Chen, Y., Zhang, J., Chen, Y., Zhang, X., Oh, K., Yonekura, T., Chen, X., Niu, J., & Wang, X. (2025). Passivation Remediation of Cd-Contaminated Farmland in Yongkang, China by CaAl-LDH: A Mechanism and Application Study. Agronomy, 15(10), 2354. https://doi.org/10.3390/agronomy15102354









