Abstract
Polyploidization is a rapid breeding strategy for producing new varieties with superior agronomic traits. Kenaf (Hibiscus cannabinus L.), an important fiber crop, exhibits high adaptability to diverse stress conditions. However, comprehensive studies on polyploid induction, screening, and genetic identification in kenaf remain unreported. This study first established an optimal tetraploid induction system for diploid kenaf seeds using colchicine. The results showed that a 4-h treatment with 0.3% colchicine yielded the highest tetraploid induction rate of 37.59%. Compared with diploids, tetraploid plants displayed distinct phenotypic and physiological characteristics: dwarfism with shortened internodal distance, increased stem thickness, larger and thicker leaves with deeper green color and serration, as well as enlarged flowers, capsules, and seeds. Physiologically, tetraploid leaves featured increased chloroplast numbers in guard cells, reduced stomatal density, and larger pollen grains, elevated chlorophyll content. Further analyses revealed that tetraploid kenaf had elevated contents of various trace elements, enhanced photosynthetic efficiency, prolonged growth duration, and superior agronomic traits with higher biomass (54.54% higher fresh weight, 79.17% higher dry weight). These findings confirm the effectiveness of colchicine-induced polyploidization in kenaf, and the obtained tetraploid germplasm provides valuable resources for accelerating the breeding of elite kenaf varieties with improved yield and quality.
1. Introduction
Polyploid induction represents a pivotal breeding strategy for improving plant traits. Compared to diploid, polyploid exhibit pronounced morphological changes in roots, stems, and leaves [1]. Numerous studies have demonstrated that polyploid plants possess stronger growth vigor, prolonged growth periods, higher biomass accumulation, enhanced secondary metabolite synthesis, and improved tolerance to abiotic stresses [2,3]. For instance, in Odontarrhena bertolonii grown in serpentinite soil, tetraploid exhibited significantly higher biomass than diploid [4]. Under drought conditions, tetraploid accumulate higher levels of osmotic adjustment substances and exhibit stronger antioxidant enzyme activity, conferring enhanced drought adaptability [5]. In salt stress studies with diploid and tetraploid Lonicera japonica Thunb., tetraploid showed less biomass reduction and higher root Na+ exclusion rates, maintaining ion homeostasis and demonstrating superior salt tolerance [6].
Polyploidization can enhance the stress resistance of crops and improve the yield and quality of crops [7,8]. In medicinal plants, polyploid breeding increases the content of medicinal active ingredients [9]. For instance, colchicine-induced Pogostemon cablin octaploid contains higher patchouli alcohol [10], oryzalin-induced hexaploid Mentha spicata L. shows a 48.85% increase in essential oil content [11], and colchicine-induced tetraploid Echinacea purpurea (L.) accumulates 17% more biomass and bioactive substances (caffeic acid derivatives and alkylamides) than diploid [12]. Among fiber crops, cotton polyploidization occurred ~1 million years ago: the commercial cultivars Gossypium hirsutum and G. barbadense are allotetraploid, with G. hirsutum accounting for >90% of global cotton production due to its fiber yield and adaptability, while G. barbadense produces longer, finer fibers [13,14].
Polyploidization serves as a key driver of plant evolution and a powerful approach in breeding for novel varieties. Polyploid induction is a promising strategy for plant improvement, creating valuable commercial cultivars and effective pollen donors [15]. For example, induced tetraploid Gerbera hybrida retains 74% of its pollen vitality, enabling its use as a parent in crosses with diploid to produce triploids. These triploids are characterized by reduced pollen production, a trait that benefits consumers with pollen allergies [16]. In watermelon, colchicine-induced autotetraploid Citrullus lanatus derived from the diploid variety ‘Sugar Baby’ was subjected to four generations of artificial selection, resulting in the high-fertility autogamous line KAU-CL-TETRA-1. When used as the female parent in crosses with diploid males (CL-4 and CL-5), this line produces high-quality triploid seedless watermelons with red or yellow flesh, notable for their sweetness and seedlessness [17]. Additionally, the newly identified triploid Eriobotrya japonica strain Q24 exhibits male gamete sterility due to abnormal chromosome pairing during meiosis, while its female gametes remain fertile. Notably, 92.52% of its progeny are aneuploid, significantly enriching loquat germplasm resources [18].
Kenaf (Hiniscus cannabinus L.), a member of the Hibiscus genus in the Malvaceae family, is a significant fiber crop known for its rapid growth, high biomass production, abundant cellulose content, wide environmental adaptability, and strong resilience to stress [7]. Its versatile applications span multiple industries, including textile manufacturing, paper production, construction materials, adsorbents, animal feed, and biofuel production [19,20,21]. Its fiber possesses high tensile strength, good moisture absorption, rapid water dispersion, corrosion resistance, and durability, thereby playing a significant role in composite materials and various industrial applications, exhibiting considerable market potential [19,20]. The number of fiber bundles in the bast directly determines fiber yield, a key to high-quality production [22,23]. Thus, the stem bark is the main economically valuable organ of kenaf. In recent years, significant advancements have been achieved in the assessment and utilization of kenaf resources in China. However, the available resources of kenaf remain insufficient. There is still a considerable gap between research and innovation in germplasm resources and their utilization in production [24]. Therefore, strengthening innovation in kenaf germplasm resources becomes particularly crucial, especially regarding superior genetic resources.
Currently, approximately 80% of the world’s plants are polyploidy [25]. While polyploidy can naturally occur through spontaneous mutations, the mutation frequency is typically very low, around 0.3% [26]. Hence, artificial induction of polyploidy is adopted due to its rapid and efficient nature. In artificial methods, the most commonly employed approach involves the use of anti-mitotic agents to hinder the formation of the spindle apparatus, preventing the duplicated chromosomes in the cell from being pulled toward opposite poles, thereby inhibiting cell division and resulting in the doubling of cellular chromosomes [27,28]. Colchicine is the most commonly used anti-mitotic agent due to its widespread application, ease of operation, and high mutagenesis rate in the majority of studied plant species [27,29,30,31]. Colchicine is typically administered using methods such as soaking, injection, or smearing. The materials treated are generally tissues or organs exhibiting the most active growth and vigorous cell division in plants, such as tender stem segments [32], buds [33,34], callus tissue [35], pollens [36], and germinating seeds [37,38]. Determining the appropriate colchicine concentration and treatment duration based on the material type and plant species is crucial for successful induction of polyploidy. Additionally, the inclusion of dimethyl sulfoxide (DMSO) can facilitate the penetration of colchicine into cells, thereby enhancing induction efficiency.
The increase in the number of plant chromosomes often contributes to the improvement of stress resistance, the accumulation of endogenous substances and the enhancement of economic value. In this study, we systematically evaluated the optimal conditions for inducing tetraploid kenaf by testing various combinations of colchicine concentrations and exposure durations. Furthermore, we conducted a comprehensive comparative analysis of the biological characteristics between diploid and tetraploid kenaf, encompassing morphological traits, physiological parameters (photosynthetic efficiency, trace element content), and agronomic performance (biomass accumulation, fiber yield potential). The results of this study can provide an efficient method for obtaining tetraploid germplasm of kenaf and clarify its biological characteristics. The results are expected to offer valuable new genetic resources for promoting polyploid breeding of kenaf, thereby facilitating the cultivation of superior varieties with excellent fiber quality and yield of kenaf.
2. Materials and Methods
2.1. Plant Materials and Polyploid Induction
Kenaf seeds of the variety ‘CP065’ were conserved in the Plant Genetics and Breeding Laboratory, College of Agriculture, Guangxi University (Nanning, China). These seeds were air-dried and stored for further use. The experiment was conducted from March 2023 to December 2024 in the experimental fields of Guangxi University (22°51′7″ N and 108°17′28″ E), with supplementary controlled-environment treatments carried out in growth chambers.
Seed pretreatment and germination: Healthy, uniformly sized seeds were selected and subjected to the following procedures: (1) Washed thoroughly under running tap water for 30 min to remove surface impurities. (2) Sterilized by immersion in 3% (v/v) sodium hypochlorite solution for 10 min with gentle agitation, followed by 5 rinses with sterile tap water to eliminate residual disinfectant. (3) Soaked in sterile tap water for 24 h at room temperature to promote imbibition and germination. After pretreatment, seeds were evenly spread in germination boxes (27 cm × 18 cm × 9 cm) lined with two layers of moist filter paper, and incubated in a growth chamber under controlled conditions: 14-h light (30 °C, 300 μmol·m−2s−1 light intensity) and 10-h dark (25 °C) cycles, with relative humidity maintained at 62–66%. Seeds were watered daily with sterile tap water to keep the filter paper moist.
Colchicine-induced tetraploidy: When seedlings developed shoots 1–2 cm in length (1–2 days after initiation of germination), they were subjected to polyploid induction treatments. Colchicine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 2% (v/v) dimethyl sulfoxide (DMSO) to prepare solutions of 0.1%, 0.2%, and 0.3% (w/v). Seedlings were soaked in these solutions for 4, 8, or 12 h, with untreated seedlings (soaked in 2% DMSO without colchicine) serving as the control. The experiment employed a two-factor completely randomized design, with 4 colchicine concentrations (0%, 0.1%, 0.2%, 0.3%) and 3 treatment durations (4 h, 8 h, 12 h), resulting in 12 treatment combinations. Each treatment included 100 seeds, with 3 biological replicates.
Post-treatment cultivation: Following colchicine exposure, seedlings were removed from the solutions and rinsed 4 times with sterile tap water (5 min per rinse) to remove residual colchicine. They were then returned to germination boxes and maintained under the aforementioned growth chamber conditions. Once cotyledons were fully expanded (approximately 5–7 days post-treatment), seedlings were transplanted into seedling trays (48 mm upper diameter × 23 mm bottom diameter × 40 mm depth) filled with a 3:1 (v/v) mixture of peat soil and vermiculite, and grown in the greenhouse of Guangxi University at a temperature of 28 °C. Regular watering to maintain a relative humidity of 50–70%.
When seedlings developed 2 true leaves, the number of surviving plants and morphologically mutated plants (characterized by deformed true leaves, such as curled margins or asymmetric growth) was recorded. Survival rate and mutation rate were calculated as follows:
Survival rate (%) = (Number of surviving plants/Total number of treated seedlings) × 100; Mutation rate (%) = (Number of mutated plants/Total number of surviving seedlings) × 100.
All seedlings were subsequently transplanted into the experimental field (row spacing: 50 cm; plant spacing: 30 cm) and managed under standard agricultural practices, including weeding, irrigation, and pest control as needed. Seedlings derived directly from colchicine-treated seeds were designated as the T0 generation. At maturity (when 2/3 of capsules turned brown), all viable seeds from T0 plants were harvested, air-dried, and stored. Progeny grown from these seeds were prescreened for ploidy via flow cytometry, and those confirmed as tetraploid by chromosome counting were designated as the T1 generation. All comparative analyses between tetraploid and diploid were conducted using T1 tetraploid plants and diploid controls (untreated ‘CP065’ plants).
2.2. Ploidy Analysis by Flow Cytometry
For all mutant plants screened from the colchicine treatment combinations, the ploidy was analyzed via flow cytometry. Fresh leaf tissues (0.5 g) were collected during 09:00–10:00 AM and placed into a culture dish. Add 2 mL of nuclear isolation buffer (50 mmol/L glucose, 15 mmol/L KCl, 15 mmol/L NaCl, 5 mmol/L Na2EDTA, 50 mmol/L sodium citrate, 0.5% (V/V) Tween 20, 50 mmol/L HEPES, and 0.5% (V/V) β-mercaptoethanol, pH = 7.2), and the mixture was quickly chopped on ice using a blade. The homogenate was filtered through a 40 μm nylon mesh into a 2 mL centrifuge tube. Subsequently, added 1 mg/mL propidium iodide (PI) solution to a final concentration of 50 μg/mL, mixed gently, and incubated on ice in the dark for at least 20 min [39]. At least 10,000 nuclei per sample were analyzed using an Attune™ NxT Acoustic Focusing Flow Cytometer. Relative DNA content histograms were generated and evaluated using FlowJo software (version 10.0.7). Each sample was measured in triplicate, with more than 10,000 nuclei counted per replicate.
2.3. Chromosome Counting
Chromosome counting was conducted with slight modifications following the previously reported method [40]. Robust, freshly growing root tips were selected from diploid control plants and those previously determined to exhibit changes in ploidy levels through Flow Cytometry (FCM). After thorough rinsing, root tips were immersed in 2 mM 8-hydroxyquinoline at 4 °C for 4 h, then washed with distilled water and fixed in Carnoy’s solution containing absolute ethanol and glacial acetic acid (3:1) at 4 °C for 24 h.
Samples were rinsed with water, hydrolyzed in 1 mol/L HCl at 60 °C for 15 min, and stained with carbolfuchsin solution for 20 min. Chromosome spreads at metaphase were observed under an Olympus BX31 microscope (Olympus Optical Co., Ltd., Tokyo, Japan) at 1000× magnification, and images were captured. For each sample, an average of 30 cells were observed to count chromosome numbers.
2.4. Determination of Morphological and Agronomic Traits
Ten diploid plantlets and 10 T1 tetraploid plantlets were randomly selected for measurement. The assessed morphological growth parameters included leaf length, leaf width, leaf thickness, leaf color, petiole length, internode distance, flower diameter, and dimensions (length and width) of capsules and seeds. To ensure accuracy, we measured at least three leaves per plant, specifically the third and fourth fully expanded leaves from the apex. Leaf color was visually evaluated and described based on color intensity (e.g., deep green vs. green) and uniformity.
At the maturity stage of kenaf plants (defined as when 2/3 of the plants exhibit upper flowering and lower fruiting), 20 individuals were randomly selected from both diploid and tetraploid groups for agronomic trait assessment. The measurements included plant height, stem thickness, fresh peel thickness, plant fresh weight per plant, fresh peel weight per plant, dry weight per plant, dry stem weight per plant, dry peel weight per plant, along with calculations for stem peel ratio (%) and peel dryness ratio (%).
2.5. Stomata and Pollen Grain Observation
For stomatal and chloroplast observation: 10 diploid and 10 tetraploid kenaf plants were randomly selected, with three mature, healthy leaves (from the third to fourth node counting from the apex) collected per plant. Leaf surfaces were rinsed with distilled water to remove dust and debris, then gently blotted dry with filter paper. Using sharp forceps, small sections of the lower leaf epidermis (avoiding major veins) were peeled off and mounted on glass slides with distilled water (no staining required). Stomatal structure and chloroplasts in guard cells were observed under a light microscope (Olympus BX31, Tokyo, Japan) using a 100× objective lens. For each leaf, 10 non-overlapping random fields of view were captured. The following parameters including the stomatal density (number of stomata per mm2), stomatal size (length × width, μm2), and number of chloroplasts per guard cell were measured using Image J 2.3.0 software.
For pollen grain observation: 10 diploid and 10 tetraploid plants were randomly selected, with three fully opened flowers collected per plant. Anthers were gently detached from the flowers, and pollen grains were released onto glass slides with a drop of distilled water, then covered with a coverslip. Pollen grains were observed under an Olympus BX31 microscope (Olympus Optical Co., Ltd., Tokyo, Japan) at 1000× magnification (oil immersion lens). At least 50 pollen grains per flower were randomly selected, and their diameters were measured using Image J 2.3.0 software.
2.6. Flowering and Fruit Set Characteristics
Flowering characteristics were determined according to the guidelines for kenaf germplasm resource description. Seeds used were T0 generation tetraploid seeds, and diploid seeds harvested in the same year. Sowing date: the date of sowing; Emergence date: when 50% of the seedlings have completely unfolded two cotyledons; Bud present date: the date when 50% of the plants have visible flower buds (2 mm in diameter) forming; Flowering date: the date when 50% of the plants are in full bloom; Technological maturity date: the date when 2/3 or more of the plants have flowers at the top and capsules below. Days to emergence: the number of days from sowing to emergence; Days to bud present: the number of days from emergence to the bud present stage; Days to flowering: the number of days from emergence to flowering; Days of growth: the number of days from emergence to red flower technological maturity. Under natural pollination conditions, recorded the number of flowers and capsules for each plant.
2.7. Chlorophyll Determination
Chlorophyll content was determined following a previously reported protocol [41] with slight modifications. Leaves from ten diploid and ten tetraploid plants were rinsed, dried, and prepared. Fresh leaves of mature diploid control and tetraploid plants were obtained avoiding veins by a 6 mm diameter puncher to create circular leaf discs. Thirty leaf discs were selected and placed in test tubes containing 10 mL of a chlorophyll extraction solution (acetone: absolute ethanol: water at a volume ratio of 4.5:4.5:1), with three replicates for each group. The tubes were kept in darkness at room temperature overnight. Subsequently, a spectrophotometer was used to measure chlorophyll a (663 nm) and b (645 nm) absorbance, and their concentrations were calculated for diploid and tetraploid plants.
2.8. Photosynthetic Parameter Measurement
Photosynthetic measurements were conducted with slight modifications following a previous protocol [39]. On sunny, windless days, the Li-6400XT portable photosynthesis system (LI-COR, Lincoln, NE, USA) was used to measure gaseous exchange in kenaf leaves. The diurnal variation in gaseous exchange such as net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were determined hourly between 06:00 and 19:00.
2.9. Element Content Determination
The materials were diploid and tetraploid kenaf grown for two months under the same hydroponic conditions. The whole dried kenaf was ground and broken, finely ground and sieved through a 0.5 mm sieve, and 0.2000 g of the sieved plant sample was weighed for digestion of H2NO3-H2O2. The elements Ca, Mg, Fe, Mn, Cu, Ni, and Zn were analyzed using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) [42]. Three biological replications and three technical replications were carried out on diploid and tetraploid kenaf.
2.10. Statistical Analysis
Measurements of stomata, pollen grains, capsules, and seed size were conducted using Image J 2.3.0 software (Bethesda, Rockville, MD, USA). Statistical analysis, including diploid and T1 tetraploid kenaf survival rate, mutation rate, plant height, stem thickness, stomatal size, chloroplast count, and other data, was performed using the statistical software SPSS 25.0 through one-way analysis of variance (ANOVA). Then Tukey and Duncan were used for significance analysis. If p < 0.05, the statistical difference was assumed to be significant. All graphs and charts were generated using GraphPad Prism 8.
3. Results
3.1. Survival Rate and Polyploid Induction
Seed survival rate varied significantly across treatments, ranging from 8% to 100% (Table 1). The highest survival rate (100%) was observed in the control group (0% colchicine, 4 h), where seeds were treated with 2% DMSO alone, indicating no toxic effects of DMSO on seed viability. In contrast, survival rates decreased with increasing colchicine concentration and treatment duration. For example, at the highest concentration (0.3%), survival rates dropped from 37.83% (4 h) to 8.00% (12 h), with the 12 h treatment showing the lowest survival rate overall. This trend suggests that prolonged exposure to high colchicine concentrations exacerbates cytotoxicity, likely due to the compound’s ability to disrupt microtubule formation and inhibit cell division [43], leading to increased seed mortality. Notably, when treatment duration exceeded 8 h, survival rates for all colchicine concentrations fell below 50% (half-lethal threshold), indicating severe damage to seedling viability under extended exposure.

Table 1.
Survival rate and tetraploid induction rate of kenaf seeds under different colchicine treatments.
Tetraploid induction rate (previously referred to as “mutation rate”) also exhibited distinct patterns in response to colchicine concentration and treatment duration (Table 1). At low concentrations (0.1%), induction rates decreased with prolonged treatment, from 30.51% (4 h) to 18.78% (12 h), suggesting that short-term exposure to low colchicine levels is more effective for inducing polyploidy. Furthermore, at high concentrations (0.3%), induction rates peaked at 37.59% under 4 h treatment but declined sharply to 17.72% (8 h) and 8.00% (12 h), likely due to excessive toxicity overriding induction effects at longer durations. The 0.2% colchicine treatment showed an intermediate trend, with the highest induction rate (35.48%) at 4 h, followed by a gradual decrease with longer exposure.
Statistical analysis via Duncan’s multiple range test confirmed significant differences (p < 0.05) between most treatments. The 0.3% colchicine treatment for 4 h emerged as the optimal combination, achieving the highest tetraploid induction rate (37.59%) while maintaining a moderate survival rate (37.83%). This balance between induction efficiency and seed viability highlights the importance of optimizing both concentration and duration: sufficient colchicine is required to induce chromosome doubling, but excessive exposure leads to lethal effects that negate induction success.
3.2. Flow Cytometry (FCM) Analysis
The FCM histograms revealed distinct differences in DNA content between diploid and induced tetraploid plants (Figure 1). The diploid control exhibited a single prominent peak in the fluorescence channel corresponding to a relative DNA content of approximately 2000 (Figure 1A), consistent with the 2n = 2x = 36 chromosome complement. In contrast, the colchicine-induced tetraploid plants displayed a peak at approximately 4000 (Figure 1B), indicating a doubling of nuclear DNA content, which aligns with the 2n = 4x = 72 chromosome count confirmed by subsequent cytological analysis.
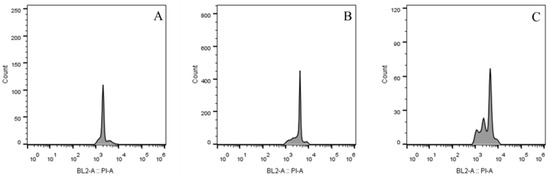
Figure 1.
Histogram of flow cytometry analysis. (A) diploid (peak fluorescence at 2000); (B) tetraploid (peak fluorescence at 4000); (C) Diploid-tetraploid mixoploids (exhibiting peaks at both 2000 and 4000 fluorescence values). The x-axis represents the DNA content (PI fluorescence intensity), and the y-axis represents the number of nuclei counted.
In addition, a small proportion of treated plants (approximately 5% of mutant individuals) showed dual peaks in their FCM profiles, with fluorescence values at both 2000 and 4000 (Figure 1C). This bimodal pattern indicates the presence of both diploid and tetraploid cells within the same plant, identifying them as diploid-tetraploid mixoploids. Such chimeric individuals likely arise from incomplete chromosome doubling during cell division, where only a subset of meristematic cells undergoes polyploidization.
3.3. Chromosome Numbers and Ploidy Identification
In diploid plants, metaphase cells consistently exhibited a chromosome number of 2n = 2x = 36 (Figure 2A), with chromosomes displaying typical small-to-medium size and uniform staining, arranged in a relatively compact configuration. In contrast, tetraploid plants showed metaphase cells with 2n = 4x = 72 chromosomes (Figure 2B), with chromosomes morphologically similar to those of diploid but distributed across a larger spread area due to the increased number, confirming successful chromosome doubling. In addition, no abnormal chromosome structures (e.g., breaks, translocations) or numerical variations (other than 36 or 72) were observed in the majority of cells, indicating stable chromosome inheritance in both diploid and tetraploid plants. This result is consistent with previous reports on kenaf cytology, where the diploid chromosome number was documented as 2n = 36 [44], further supporting the reliability of our findings.
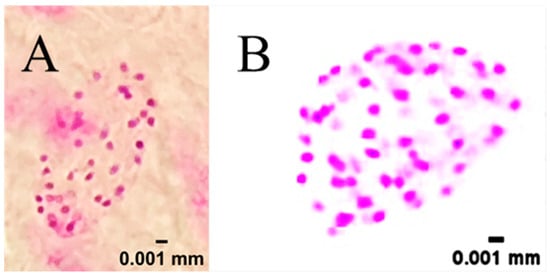
Figure 2.
Chromosome count in root-tip metaphase cells. (A) Diploid control kenaf, showing 36 chromosomes (2n = 2x = 36); (B) Induced tetraploid kenaf, showing 72 chromosomes (2n = 4x = 72).
The consistency between chromosome counting (a direct measure of ploidy) and FCM analysis (a rapid DNA content-based method) provides robust evidence for the successful induction of tetraploid kenaf. This dual validation addresses the limitations of FCM alone (which relies on relative DNA content) and confirms that the induced plants are stable autotetraploid with a complete set of doubled chromosomes.
3.4. Tetraploid Leaves Exhibit Enhanced Size, Thickness, and Chlorophyll Accumulation
Morphological analysis of leaf traits revealed distinct differences between tetraploid and diploid kenaf plants (Table 2 and Figure 3). While no significant variation was observed in leaf length, tetraploid displayed a 9.29% increase in leaf width, contributing to a larger overall leaf area. This expansion in leaf width, coupled with a 46.41% increase in leaf thickness, suggests a Gigas effect in leaf development, consistent with typical polyploidy-induced organ enlargement.

Table 2.
Comparison of leaf morphological characteristics and chlorophyll content between diploid and tetraploid plants.

Figure 3.
Leaf morphology of diploid (A) and tetraploid (B) kenaf plants, showing differences in size and coloration. Bar = 1 cm.
Notable changes in leaf architecture were also observed: tetraploid showed a 26.75% reduction in petiole length and a 20.85% shortening of internode distance. These traits—shorter petioles and denser leaf arrangement—may enhance light capture efficiency by reducing self-shading, a valuable adaptation for optimizing photosynthetic output in tetraploid.
Physiologically, tetraploid leaves exhibited significant increases in photosynthetic pigment content. Chlorophyll b, which plays a key role in light absorption and energy transfer, showed the most dramatic change, with a 49.00% increase. Chlorophyll a content increased by 3.65%, leading to a 9.26% rise in total chlorophyll (28.18 ± 0.38 μg/cm2 vs. 30.79 ± 0.23 μg/cm2). This elevated pigment content, combined with thicker leaves, likely underpins the deeper green coloration of tetraploid foliage, visually distinguishing them from diploid (Figure 3).
These leaf-associated traits, including increased width, greater thickness, enhanced chlorophyll accumulation, and more compact internode spacing, act as dependable morphological and physiological indicators for the preliminary identification of tetraploid kenaf.
3.5. Tetraploid Plants Exhibit Increased Stomatal Size, Reduced Stomatal Density, and More Chloroplasts in Guard Cells
Microscopic observations of leaf epidermis revealed significant differences in stomatal morphology and guard cell characteristics between tetraploid and diploid kenaf (Table 3, Figure 4). These variations further support the distinct ploidy-induced phenotypic changes in tetraploid. Stomatal density, quantified as the average number of stomata per 1000× field of view, was markedly lower in tetraploid (4.4 ± 0.5) compared to diploid (14.4 ± 1.8), representing a 69.44% reduction (Table 3). Conversely, stomatal size showed the opposite trend: the average stomatal area of tetraploid (70.09 ± 12.59 μm2) was 82.2% larger than that of diploid (38.47 ± 10.59 μm2), with a significant difference (p < 0.05) confirmed by statistical analysis (Table 3). Notably, the number of chloroplasts in guard cells—a key indicator of photosynthetic potential in stomatal apparatus—was also significantly higher in tetraploid (19.65 ± 2.12) than in diploid (12.50 ± 1.65), an increase of 57.2% (Table 3, Figure 4C,D). This enhancement in chloroplast abundance within guard cells aligns with the elevated chlorophyll content observed in tetraploid leaves (Section 3.4), suggesting a coordinated improvement in photosynthetic machinery at both cellular and subcellular levels.

Table 3.
Comparison of Epidermal Stomatal Characteristics between Diploid and Tetraploid.
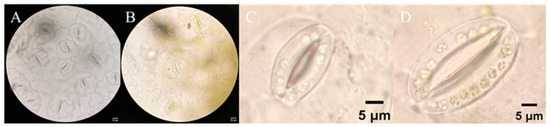
Figure 4.
Stomatal characteristics of leaves in diploid (A) and tetraploid (B) kenaf. Chloroplast count in guard cells of diploid (C) and tetraploid (D) kenaf.
3.6. Tetraploid Plants Possess Enhanced Photosynthetic Capacity
Diurnal variations in photosynthetic parameters revealed consistent patterns between tetraploid and diploid kenaf, with net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) all following similar daily trends (Figure 5). However, tetraploid showed significantly superior performance in key indicators, reflecting their stronger photosynthetic capacity.
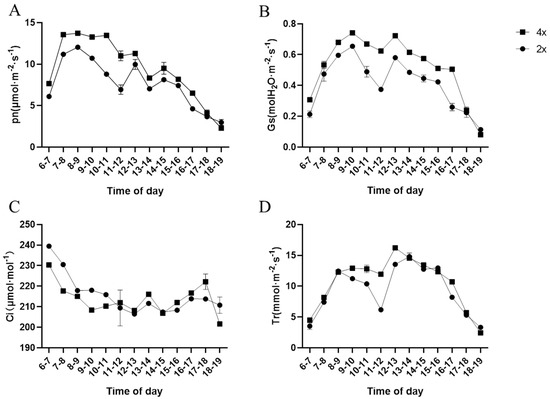
Figure 5.
Diurnal variations in gaseous exchange of diploid and tetraploid kenaf. The data presented are mean ± SD (n = 3). (A) Net photosynthetic rate (Pn); (B) Stomatal conductance (Gs); (C) Intercellular CO2 concentration (Ci); (D) Transpiration rate (Tr).
From 6:00 to 18:00, tetraploid maintained higher Pn and Gs compared to diploid (Figure 5A,B). The elevated Gs in tetraploid—consistent with their larger stomatal size (Section 3.5)—likely facilitated more efficient CO2 uptake, supporting the higher Pn. Especially, Pn peaks in tetraploid occurred between 9:00–11:00, reaching values approximately 15–20% higher than those of diploid during the same period, indicating enhanced light utilization under optimal irradiance.
Transpiration rate (Tr) displayed more nuanced differences: while Tr was comparable between the two ploidies during early morning (6:00–8:00) and late afternoon (16:00–18:00) when light intensity was lower, tetraploid exhibited significantly higher Tr from 9:00 to 14:00 (Figure 5D). This midday increase in Tr aligns with the higher Gs, suggesting more active water transport to support intensified photosynthesis under increased light conditions.
Intercellular CO2 concentration (Ci) showed an inverse pattern relative to Pn: tetraploid had lower Ci than diploid between 6:00–11:00 (Figure 5C), reflecting more efficient CO2 assimilation during peak photosynthesis. After 12:00, Ci in tetraploid slightly exceeded that in diploid, potentially due to sustained CO2 uptake via larger stomata even as photosynthetic rates gradually declined.
These results demonstrate that tetraploid kenaf possesses stronger photosynthetic efficiency, which can be attributed to coordinated improvements in stomatal morphology (larger stomata, Section 3.5) and chlorophyll content (higher pigment levels, Section 3.4).
3.7. Floral, Capsule, and Seed Traits, and Fruiting Characteristics of Tetraploid Plants
In the second growing season, all T1 generation tetraploid plants survived, grew, and flowered normally, exhibiting robust vitality despite delayed developmental stages compared to diploid. Specifically, tetraploid showed a 1-day delay in seedling emergence, an 8-day delay in bud appearance, a 12-day delay in flowering, and a 13-day extension in the maturity period (Table 4). This prolonged growth cycle may contribute to the enhanced biomass accumulation observed in tetraploid (Section 3.8).

Table 4.
Comparison of floral, capsule, seed traits, and fruiting characteristics between diploid and tetraploid kenaf.
Morphologically, tetraploid floral organs displayed a significant “Gigas effect” with substantial enlargement compared to diploid. The flower diameter of tetraploid was 9.87% larger than that of diploid. Petals of tetraploid were both longer and wider than those of diploid. Pollen grains, a key indicator of ploidy, were notably larger in tetraploid than in diploid, representing a 28.07% increase (Table 4, Figure 6). This enlargement of pollen grains aligns with the increased cell size observed in tetraploid stomata (Section 3.5) and likely reflects the overall genomic doubling effect.
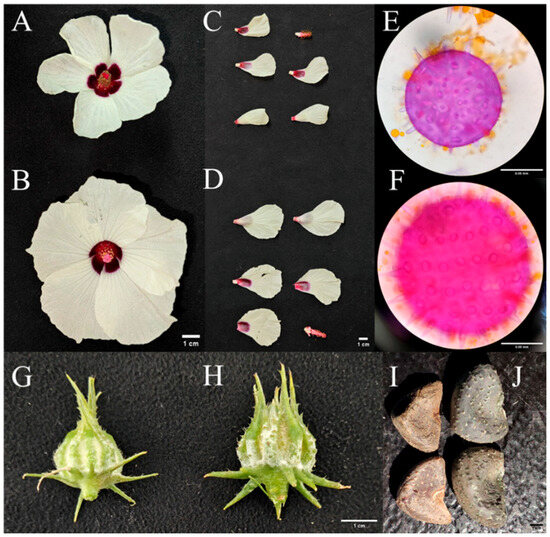
Figure 6.
Morphological comparison of floral organs between diploid and tetraploid plants. Diploid structures (A,C,E,G,I) are consistently smaller than their tetraploid counterparts (B,D,F,H,J), demonstrating the “Gigas effect”.
Tetraploid reproductive organs (capsules and seeds) also exhibited distinct morphological advantages. The capsules of tetraploid were longer and wider than those of diploid, with a higher length-width ratio. Seeds of tetraploid were significantly larger in both length and width and had a 33.54% higher 100-seed weight compared to diploid (Table 4; Figure 6). These traits suggest improved seed quality and potential storage capacity, which may benefit seedling establishment in subsequent generations.
However, tetraploid showed reduced reproductive efficiency. The fruit setting percentage of tetraploid (40.91%) was less than half that of diploid (80.52%), and the number of seeds per capsule was significantly lower (5.00 vs. 15.10) (Table 4). This reduction in fertility is a common phenomenon in autotetraploid, often attributed to abnormal chromosome pairing during meiosis, which impairs gamete formation and fertilization success.
3.8. Tetraploid Kenaf Shows Superior Agronomic Traits
During the maturity stage of kenaf plants, agronomic trait analysis was conducted on diploid and tetraploid kenaf. In comparison to diploid, tetraploid showed an increase of 12.88% in stem thickness, 44.63% increase in fresh peel thickness, 54.54% increase in individual plant fresh weight, 46.34% increase in individual plant fresh stem weight, 38.64% increase in individual plant fresh peel weight, 79.17% increase in individual plant dry weight, 59.10% increase in individual plant dry stem weight, 54.55% increase in individual plant dry peel weight, 28.16% increase in fresh stem dry peel ratio, and 13.41% increase in fresh peel dry ratio (Table 5). The results of the present study showed that except for the height of tetraploid plants, which was slightly shorter than that of diploid plants (4.96 m for diploid and 4.59 m for tetraploid), tetraploid plants showed superior performance in agronomic traits. Tetraploid kenaf has higher biomass and higher fiber production potential.

Table 5.
Comparison of Agronomic Traits between Diploid and Tetraploid Kenaf Plants.
3.9. Tetraploid Shows Elevated Levels of Multiple Elements
The analysis of macronutrient and micronutrient contents in diploid and tetraploid kenaf plants revealed significant differences in elemental accumulation (Table 6). Compared to diploid plants, tetraploid kenaf showed distinct variations in the concentrations of key elements: Calcium (Ca) content in tetraploid was 18.21% lower than in diploid, and magnesium (Mg) content was reduced by 9.72%. However, most micronutrients and essential macronutrients were significantly accumulated in tetraploid. Manganese (Mn) showed the most dramatic increase, with a 114.88% higher content in tetraploid. Sodium (Na) and copper (Cu) also exhibited substantial elevations, with increases of 76.23% and 69.21%, respectively. Additionally, iron (Fe), potassium (K), nickel (Ni), phosphorus (P), and zinc (Zn) contents in tetraploid were 35.83%, 4.75%, 20.79%, 12.23%, and 14.16% higher than in diploid, respectively.

Table 6.
Differential elemental content between diploid and tetraploid kenaf.
Mn and Cu element are critical for photosynthesis, and Zn and Ni are critical for stress resistance [45,46,47]. These results indicate that tetraploid kenaf has a modified elemental uptake or accumulation mechanism, with a notable enrichment of micronutrients Mn, Cu, Zn, and Ni. Such changes may contribute to the enhanced physiological performance and biomass production observed in tetraploid.
4. Discussion
Kenaf is a high-value industrial crop with versatile applications spanning textiles, papermaking, construction materials, and bioenergy production [20,22]. However, its cultivation and industrial potential are constrained by limited germplasm diversity and the narrow genetic base of existing varieties in China [24]. Polyploidization is a powerful breeding strategy that enhances biomass, stress tolerance, and metabolite production in plants [7,8]. However, systematic investigations into polyploid induction, characterization, and trait improvement in kenaf remain limited, highlighting the novelty and practical significance of the present study.
4.1. Optimal Colchicine Treatment for Tetraploid Induction in Kenaf
Colchicine, a classical mitotic inhibitor, induces polyploidy by binding to β-tubulin subunits, disrupting microtubule assembly, and blocking chromosome segregation during mitosis. This mechanism results in the retention of duplicated chromosomes within a single nucleus, leading to chromosome doubling [29,43]. The efficacy of colchicine-induced polyploidization is highly dependent on species-specific factors, including concentration, treatment duration, and the developmental stage of the target tissue [27,48].
In our study, we systematically evaluated combinations of colchicine concentrations (0.1%, 0.2%, 0.3%) and treatment durations (4, 8, 12 h) to identify optimal induction conditions for kenaf. The highest tetraploid induction rate (37.59%) was achieved with 0.3% colchicine applied for 4 h, accompanied by a moderate survival rate (37.83%; Table 1). This result aligns with the short-term high-concentration principle observed in other crops, where excessive exposure to high colchicine concentrations (e.g., 0.3% for 12 h) drastically reduces survival due to cytotoxicity (8.00% survival in our study), while low concentrations or insufficient durations (e.g., 0.1% for 4 h) result in suboptimal induction efficiency (30.51%).
Notably, the optimal induction conditions for kenaf differ from those reported for other fiber crops. For instance, allotetraploid cotton (Gossypium hirsutum) evolved naturally over millions of years [14], whereas hexaploid induction in mint (Mentha spicata) requires oryzalin, a different microtubule inhibitor, to enhance essential oil production [11]. These species-specific differences highlight the need for tailored induction protocols, and our findings provide a reliable framework for future polyploid breeding in kenaf.
4.2. Synergistic Use of Flow Cytometry and Chromosome Counting for Ploidy Validation
Accurate ploidy determination is critical for validating polyploid induction and ensuring the reliability of subsequent phenotypic analyses. Flow cytometry (FCM) offers a rapid, high-throughput method to assess relative nuclear DNA content, as demonstrated by distinct peaks at 2000 (diploid) and 4000 (tetraploid) fluorescence units in our study (Figure 1). This technique has been widely used for ploidy screening in plants, including taxus [49] and mulberry [50], due to its efficiency and reproducibility.
However, FCM has limitations: it relies on relative DNA content and may misidentify chimeras or aneuploids in species with variable genome sizes [51]. For example, deviations in nuclear DNA content have been reported in banana [52] and asparagus [53], emphasizing the need for complementary methods. To address this, we confirmed ploidy via chromosome counting, a direct and definitive approach. Diploid kenaf exhibited 2n = 2x = 36 chromosomes, consistent with previous reports [44], while tetraploid showed 2n = 4x = 72 chromosomes (Figure 2). This dual validation confirmed the stability of our induced tetraploid, ruling out chimerism and ensuring the accuracy of our phenotypic analyses.
4.3. Morphological Changes in Tetraploid Kenaf and Their Implications for Fiber Production
Polyploidization often triggers the gigas effect, characterized by enlarged organs and altered plant architecture, which can directly enhance crop productivity [8]. In tetraploid kenaf, we observed several morpho-agronomic traits with significant implications for fiber production. For stem and bark traits: Tetraploid exhibited a 12.88% increase in stem thickness and a 44.63% increase in fresh bark thickness (Table 5). Since kenaf fiber is primarily derived from bast fiber bundles in the stem bark, these changes directly translate to improved fiber yield potential. For leaf characteristics: Tetraploid leaves were wider (+9.29%), thicker (+46.41%), and darker green than diploid, with shorter petioles (−26.75%) and reduced internodal distance (−20.85%; Table 2). These traits optimize light capture by reducing self-shading and increasing photosynthetic surface area, supporting greater biomass accumulation. For reproductive organs: Tetraploid displayed larger flowers, capsules, and seeds (Table 4), consistent with the gigas effect reported in tetraploid irises [37] and pears [26]. While larger seeds may enhance seedling vigor, the reduced fruit-set percentage (40.91% vs. 80.52% in diploid) and fewer seeds per capsule (5.00 vs. 15.10) reflect typical fertility reductions in autotetraploid, likely due to abnormal chromosome pairing during meiosis [16]. These changes are important for the production and cultivation of kenaf because tetraploid kenaf can produce greater biomass per unit of land area, thus greatly increasing the total yield and improving economic income. These morphological changes, particularly enhanced stem and bark traits, are important for improving biomass, fiber yield, and quality by using tetraploid kenaf.
4.4. Synergistic Improvements in Microstructure, Photosynthetic Physiology, and Element Accumulation
Polyploidy-induced modifications in cellular architecture frequently underpin enhancements in physiological performance, with elemental accumulation patterns further reinforcing these adaptive traits. In tetraploid kenaf, stomatal characteristics followed a typical polyploidy associated pattern: a substantial increase in stomatal area (+82.2%) and a marked reduction in stomatal density (−69.44%), accompanied by a significant rise in chloroplast numbers within guard cells (+57.2%; Table 3). These structural alterations combined with elevated chlorophyll content, including a 9.26% increase in total chlorophyll and a most pronounced 49.00% increase in chlorophyll b (Table 2), contributed to improved photosynthetic efficiency.
Diurnal assessments of photosynthetic parameters revealed that tetraploid sustained higher net photosynthetic rates (Pn) and stomatal conductance (Gs) compared to diploid, with the most notable differences observed during peak light intensity (9:00–11:00; Figure 5). This enhanced performance can be attributed to two key factors: first, the increased chloroplast abundance and chlorophyll content in tetraploid leaves, which facilitate more efficient light absorption and subsequent energy conversion; second, the larger stomatal size, which promotes greater CO2 uptake (reflected in higher Gs) to support enhanced carbon fixation and biomass accumulation.
Such coordinated interactions between stomatal traits and photosynthetic capacity have been documented in other tetraploid species, including chrysanthemums [54] and jujubes [55]. However, our study uniquely links these physiological improvements to kenaf’s specific agricultural value, as evidenced by the significant increase in dry bark weight (+54.55%; Table 5), which is a key determinant of fiber yield in this crop.
Additionally, these physiological enhancements are further supported by distinct elemental accumulation patterns in tetraploid. Compared to diploid, tetraploid exhibited reduced calcium (Ca, −18.21%) and magnesium (Mg, −9.72%) but significant increases in most other elements, particularly micronutrients closely tied to photosynthesis and stress tolerance (Table 6). Manganese (Mn) showed the highest increase (114.88%), followed by copper (Cu, 69.21%) and sodium (Na, 76.23%). Manganese is a critical cofactor in the oxygen-evolving complex of photosystem II [45], directly supporting the light-dependent reactions that underpin the elevated Pn in tetraploid. Copper, essential for chlorophyll biosynthesis and antioxidant enzyme function [46], reinforces both photosynthetic capacity and oxidative stress resilience traits that support tetraploid’ prolonged growth period.
Furthermore, elevated iron (Fe), zinc (Zn), and nickel (Ni) levels enhance adaptive potential: Fe facilitates electron transport in photosynthesis [47], Zn strengthens stress signaling [56], and Ni optimizes nitrogen metabolism [57]. Together, these elements synergistically boost photosynthetic efficiency, antioxidant defenses, and metabolic pathways, directly contributing to tetraploid higher biomass and fiber yield potential.
This integration of structural, physiological, and elemental traits highlights a coordinated adaptive strategy in tetraploid kenaf, where polyploidy-induced changes in cell morphology, photosynthetic machinery, and nutrient homeostasis collectively drive superior agronomic performance.
5. Conclusions
In this study, we successfully induced tetraploid kenaf from diploid seeds using colchicine and identified the optimal induction conditions: treatment with 0.3% colchicine for 4 h yielded the highest tetraploid induction rate of 37.59%, with a moderate survival rate that balanced induction efficiency and seedling viability.
Comprehensive characterization of the induced tetraploid revealed significant advantages over diploid. Morphologically, they exhibited distinct Gigas effects: wider (+9.29%) and thicker (+46.41%) leaves, shortened internodal distance (−20.85%), increased stem thickness (+12.88%), and enlarged flowers, capsules, and seeds (33.54% higher 100-seed weight). Agronomically, tetraploid showed enhanced fiber production potential, with thicker stem bark (+44.63%) and higher biomass (54.54% higher fresh weight, 79.17% higher dry weight per plant). Physiologically, tetraploid displayed superior photosynthetic capacity, supported by more chloroplasts in guard cells (+57.2%), larger stomata (+82.2%), reduced stomatal density (−69.44%), and elevated chlorophyll (9.26% higher total, with 49.00% more chlorophyll b). They also accumulated higher levels of key micronutrients (e.g., Mn +114.88%, Cu +69.21%), reinforcing stress tolerance. The overall performance of tetraploid confirms colchicine-induced polyploidization as an effective strategy for improving kenaf traits.
Our findings confirm that colchicine-induced polyploidization is an effective strategy to enhance kenaf traits. The tetraploid germplasm developed in this study provides a promising resource for accelerating kenaf breeding programs, with potential to improve yield and quality.
Author Contributions
P.C.: Conceptualization, Supervision, Visualization, Writing—review & editing. T.C. and X.L.: Methodology, Validation, Investigation, Writing—original draft. D.L. and J.P.: Visualization, writing—review & editing. M.R.: Validation, Investigation, Software. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 32460499) and the National Natural Science Foundation of Guangxi Province (No. 2024GXNSFAA999039).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Tadeo, F.; Froelicher, Y.; Talon, M.; Navarro, L.; Ollitrault, P.; Morillon, R. Large changes in anatomy and physiology between diploid Rangpur lime (Citrus limonia) and its autotetraploid are not associated with large changes in leaf gene expression. J. Exp. Bot. 2011, 62, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Ahlem, A.; Lobna, M.F.; Mohamed, C. Soil water leaf gas exchange and biomass production of Buffelgrass (Cenchrus ciliaris L.) with two ploidy levels under arid zone. Acta Ecol. Sin. 2023, 43, 506–512. [Google Scholar] [CrossRef]
- Touchell, D.H.; Palmer, I.E.; Ranney, T.G. In Vitro Ploidy Manipulation for Crop Improvement. Front. Plant Sci. 2020, 11, 722. [Google Scholar] [CrossRef]
- Colzi, I.; Gonnelli, C.; Bettarini, I.; Selvi, F. Polyploidy affects responses to Nickel in Ni-hyperaccumulating plants: Evidence from the model species Odontarrhena bertolonii (Brassicaceae). Environ. Exp. Bot. 2023, 213, 105403. [Google Scholar] [CrossRef]
- Fakhrzad, F.; Jowkar, A. Water stress and increased ploidy level enhance antioxidant enzymes, phytohormones, phytochemicals and polyphenol accumulation of tetraploid induced wallflower. Ind. Crops Prod. 2023, 206, 117612. [Google Scholar] [CrossRef]
- Yan, K.; Xu, H.; Zhao, S.; Shan, J.; Chen, X. Saline soil desalination by honeysuckle (Lonicera japonica Thunb.) depends on salt resistance mechanism. Ecol. Eng. 2016, 88, 226–231. [Google Scholar] [CrossRef]
- Chen, P.; Chen, T.; Li, Z.; Jia, R.; Luo, D.; Tang, M.; Lu, H.; Hu, Y.; Yue, J.; Huang, Z. Transcriptome analysis revealed key genes and pathways related to cadmium-stress tolerance in Kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 158, 112970. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2015, 243, 281–296. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Chen, P.Y.; To, K.Y. Induction of polyploidy and metabolic profiling in the medicinal herb Wedelia chinensis. Plants 2021, 10, 1232. [Google Scholar] [CrossRef]
- Yan, H.-J.; Xiong, Y.; Zhang, H.-Y.; He, M.-L. In Vitro induction and morphological characteristics of octoploid plants in Pogostemon cablin. Breed. Sci. 2016, 66, 169–174. [Google Scholar] [CrossRef]
- Bharati, R.; Fernández-Cusimamani, E.; Gupta, A.; Novy, P.; Moses, O.; Severová, L.; Svoboda, R.; Šrédl, K. Oryzalin induces polyploids with superior morphology and increased levels of essential oil production in Mentha spicata L. Ind. Crops Prod. 2023, 198, 116683. [Google Scholar] [CrossRef]
- Xu, C.-g.; Tang, T.-x.; Chen, R.; Liang, C.-h.; Liu, X.-y.; Wu, C.-l.; Yang, Y.-s.; Yang, D.-p.; Wu, H. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tissue Organ Cult. 2013, 116, 323–332. [Google Scholar] [CrossRef]
- Guan, X.; Song, Q.; Chen, Z.J. Polyploidy and small RNA regulation of cotton fiber development. Trends Plant Sci. 2014, 19, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Wendel, J.F.; Cronn, R.C. Polyploidy and the evolutionary history of cotton. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2003; pp. 139–186. [Google Scholar] [CrossRef]
- Yuan, Y.; Scheben, A.; Edwards, D.; Chan, T.-F. Toward haplotype studies in polyploid plants to assist breeding. Mol. Plant 2021, 14, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.; Kareem, A.; Deng, Z. In Vivo induction and characterization of polyploids in gerbera daisy. Sci. Hortic. 2021, 282, 110054. [Google Scholar] [CrossRef]
- Thayyil, P.; Remani, S.; Raman, G.T. Potential of a tetraploid line as female parent for developing yellow- andred-fleshed seedless watermelon. Turk. J. Agric. For. 2016, 40, 75–82. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Lei, C.; Xia, Q.; Wu, D.; He, Q.; Jing, D.; Guo, Q.; Liang, G.; Dang, J. A female fertile triploid loquat line produces fruits with less seed and aneuploid germplasm. Sci. Hortic. 2023, 319, 112141. [Google Scholar] [CrossRef]
- Noor Abbas, A.-G.; Nora Aznieta Abdul Aziz, F.; Abdan, K.; Azline Mohd Nasir, N.; Fahim Huseien, G. Experimental study on durability properties of kenaf fibre-reinforced geopolymer concrete. Constr. Build. Mater. 2023, 396, 132160. [Google Scholar] [CrossRef]
- Ramesh, M. Kenaf (Hibiscus cannabinus L.) fibre based bio-materials: A review on processing and properties. Prog. Mater. Sci. 2016, 78–79, 1–92. [Google Scholar] [CrossRef]
- Szulczyk, K.R.; Badeeb, R.A. Nontraditional sources for biodiesel production in Malaysia: The economic evaluation of hemp, jatropha, and kenaf biodiesel. Renew. Energy 2022, 192, 759–768. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yusra, A.F.I.; Bhat, A.H.; Jawaid, M. Cell wall ultrastructure, anatomy, lignin distribution, and chemical composition of Malaysian cultivated kenaf fiber. Ind. Crops Prod. 2010, 31, 113–121. [Google Scholar] [CrossRef]
- Nishimura, A.; Katayama, H.; Kawahara, Y.; Sugimura, Y. Characterization of kenaf phloem fibers in relation to stem growth. Ind. Crops Prod. 2012, 37, 547–552. [Google Scholar] [CrossRef]
- Morris, J.B.; Dierig, D.; Heinitz, C.; Hellier, B.; Bradley, V.; Marek, L. Vulnerability of U.S. new and industrial crop genetic resources. Ind. Crops Prod. 2023, 206, 117364. [Google Scholar] [CrossRef]
- Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Current Strategies of Polyploid Plant Genome Sequence Assembly. Front. Plant Sci. 2018, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, C.; Jin, J.; Wang, Y.; Jia, X.; Ma, H.; Zhang, Y.; Zhang, H.; Qi, B.; Xu, J. Induction and identification of tetraploids of pear plants (Pyrus bretschneideri and Pyrus betulaefolia). Sci. Hortic. 2022, 304, 111322. [Google Scholar] [CrossRef]
- Taratima, W.; Rohmah, K.N.; Plaikhuntod, K.; Maneerattanarungroj, P.; Trunjaruen, A. Optimal protocol for In Vitro polyploid induction of Cymbidium aloifolium (L.) Sw. BMC Plant Biol. 2023, 23, 295. [Google Scholar] [CrossRef]
- Yang, J.-w.; Liu, Z.-h.; Qu, Y.-z.; Zhang, Y.-z.; Li, H.-c. Cytological study on haploid male fertility in maize. J. Integr. Agric. 2022, 21, 3158–3168. [Google Scholar] [CrossRef]
- Eng, W.-H.; Ho, W.-S. Polyploidization using colchicine in horticultural plants: A review. Sci. Hortic. 2019, 246, 604–617. [Google Scholar] [CrossRef]
- Farhadi, N.; Panahandeh, J.; Motallebi-Azar, A.; Mokhtarzadeh, S. Production of autotetraploid plants by In Vitro chromosome engineering in Allium hirtifolium. Hortic. Plant J. 2023, 9, 986–998. [Google Scholar] [CrossRef]
- Sabooni, N.; Gharaghani, A.; Jowkar, A.; Eshghi, S. Successful polyploidy induction and detection in blackberry species by using an In Vitro protocol. Sci. Hortic. 2022, 295, 110850. [Google Scholar] [CrossRef]
- Glowacka, K.; Jeżowski, S.; Kaczmarek, Z. In Vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind. Crops Prod. 2010, 32, 88–96. [Google Scholar] [CrossRef]
- Huy, N.P.; Tam, D.T.T.; Luan, V.Q.; Tung, H.T.; Hien, V.T.; Ngan, H.T.M.; Duy, P.N.; Nhut, D.T. In Vitro polyploid induction of Paphiopedilum villosum using colchicine. Sci. Hortic. 2019, 252, 283–290. [Google Scholar] [CrossRef]
- Prasath, D.; Nair, R.R.; Babu, P.A. Effect of colchicine induced tetraploids of ginger (Zingiber officinale Roscoe) on cytology, rhizome morphology, and essential oil content. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100422. [Google Scholar] [CrossRef]
- Diem, L.T.; Phong, T.H.; Tung, H.T.; Khai, H.D.; Anh, T.T.L.; Mai, N.T.N.; Cuong, D.M.; Luan, V.Q.; Que, T.; Phuong, H.T.N.; et al. Tetraploid induction through somatic embryogenesis in Panax vietnamensis Ha et Grushv by colchicine treatment. Scientia Horticulturae 2022, 303, 111254. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, Z.; Chen, X.; Cao, J.; Zhang, W.; Sun, R.; Teixeira da Silva, J.A.; Yu, X. Induction of 2n pollen by colchicine during microsporogenesis to produce polyploids in herbaceous peony (Paeonia lactiflora Pall.). Sci. Hortic. 2022, 304, 111264. [Google Scholar] [CrossRef]
- Ding, L.; Liu, R.; Gao, Y.; Xiao, J.; Lv, Y.; Zhou, J.; Zhang, Q. Effect of tetraploidization on morphological and fertility characteristics in Iris × norrisii Lenz. Sci. Hortic. 2023, 322, 112403. [Google Scholar] [CrossRef]
- Mo, L.; Chen, J.-h.; Chen, F.; Xu, Q.-w.; Tong, Z.-k.; Huang, H.-h.; Dong, R.-h.; Lou, X.-z.; Lin, E.-p. Induction and characterization of polyploids from seeds of Rhododendron fortunei Lindl. J. Integr. Agric. 2020, 19, 2016–2026. [Google Scholar] [CrossRef]
- Wang, L.-J.; Cao, Q.-Z.; Zhang, X.-Q.; Jia, G.-X. Effects of polyploidization on photosynthetic characteristics in three Lilium species. Sci. Hortic. 2021, 284, 110098. [Google Scholar] [CrossRef]
- Beranová, K.; Bharati, R.; Žiarovská, J.; Bilčíková, J.; Hamouzová, K.; Klíma, M.; Fernández-Cusimamani, E. Morphological, Cytological, and Molecular Comparison between Diploid and Induced Autotetraploids of Callisia fragrans (Lindl.) Woodson. Agronomy 2022, 12, 2520. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef]
- Bilgin, A.K.; Cengiz, M.F.; Karakaş-Budak, B.; Gümüş, C.; Kılıç, S.A.; Perinçek, F.; Basançelebi, O.; Sezik, E.; Certel, M. Elemental compositions and stable isotope signatures for determining the geographical origin of salep orchids collected from different regions of Turkey. J. Appl. Res. Med. Aromat. Plants 2023, 37, 100505. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-x.; Wei, C.-l.; Qi, J.-m.; Chen, X.-b.; Su, J.-g.; Li, A.-Q.; Tao, A.-f.; Wu, W.-r. Genetic linkage map construction for kenaf using SRAP, ISSR and RAPD markers. Plant Breed. 2011, 130, 679–687. [Google Scholar] [CrossRef]
- Lanquar, V.; Ramos, M.S.; Lelièvre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Krämer, U.; Thomine, S. Export of Vacuolar Manganese by AtNRAMP3 and AtNRAMP4 Is Required for Optimal Photosynthesis and Growth under Manganese Deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Barros, L.; Soares, M.E.; Bastos, M.L.; Pereira, J.A. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem. 2007, 103, 188–195. [Google Scholar] [CrossRef]
- Herlihy, J.H.; Long, T.A.; McDowell, J.M. Iron homeostasis and plant immune responses: Recent insights and translational implications. J. Biol. Chem. 2020, 295, 13444–13457. [Google Scholar] [CrossRef]
- Aqafarini, A.; Lotfi, M.; Norouzi, M.; Karimzadeh, G. Induction of tetraploidy in garden cress: Morphological and cytological changes. Plant Cell Tissue Organ Cult. 2019, 137, 627–635. [Google Scholar] [CrossRef]
- Escrich, A.; Hidalgo, D.; Bonfill, M.; Palazon, J.; Sanchez-Muñoz, R.; Moyano, E. Polyploidy as a strategy to increase taxane production in yew cell cultures: Obtaining and characterizing a Taxus baccata tetraploid cell line. Plant Sci. 2023, 334, 111776. [Google Scholar] [CrossRef]
- Kruthika, H.S.; Rukmangada, M.S.; Naik, V.G. Genome size, chromosome number variation and its correlation with stomatal characters for assessment of ploidy levels in a core subset of mulberry (Morus spp.) germplasm. Gene 2023, 881, 147637. [Google Scholar] [CrossRef]
- Ochatt, S.J. Flow cytometry in plant breeding. Cytom. Part A 2008, 73, 581–598. [Google Scholar] [CrossRef]
- Roux, N.; Toloza, A.; Radecki, Z.; Zapata-Arias, F.J.; Dolezel, J. Rapid detection of aneuploidy in Musa using flow cytometry. Plant Cell Rep. 2002, 21, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Narikiyo, K.; Fujita, C.; Okubo, H. Ploidy variation of progenies from intra- and inter-ploidy crosses with regard to trisomic production in asparagus (Asparagus officinalis L.). Sex. Plant Reprod. 2004, 17, 157–164. [Google Scholar] [CrossRef]
- Dong, B.; Wang, H.; Liu, T.; Cheng, P.; Chen, Y.; Chen, S.; Guan, Z.; Fang, W.; Jiang, J.; Chen, F. Whole genome duplication enhances the photosynthetic capacity of Chrysanthemum nankingense. Mol. Genet. Genom. 2017, 292, 1247–1256. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Wang, L.; Deng, W.; Wei, H.; Liu, P.; Liu, M. Morphological, cytological and nutritional changes of autotetraploid compared to its diploid counterpart in Chinese jujube (Ziziphus jujuba Mill.). Sci. Hortic. 2019, 249, 263–270. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef]
- Gomes-Junior, R.A.; Moldes, C.A.; Delite, F.S.; Gratão, P.L.; Mazzafera, P.; Lea, P.J.; Azevedo, R.A. Nickel elicits a fast antioxidant response in Coffea arabica cells. Plant Physiol. Biochem. 2006, 44, 420–429. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).