Exogenous Application of IR-Specific dsRNA Inhibits Infection of Cucumber Green Mottle Mosaic Virus in Watermelon
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmid Construction and Bacterial Induction
2.3. Exogenous Application of dsRNAs on Watermelon Leaves
2.4. DAS-ELISA
2.5. qRT-PCR Analysis
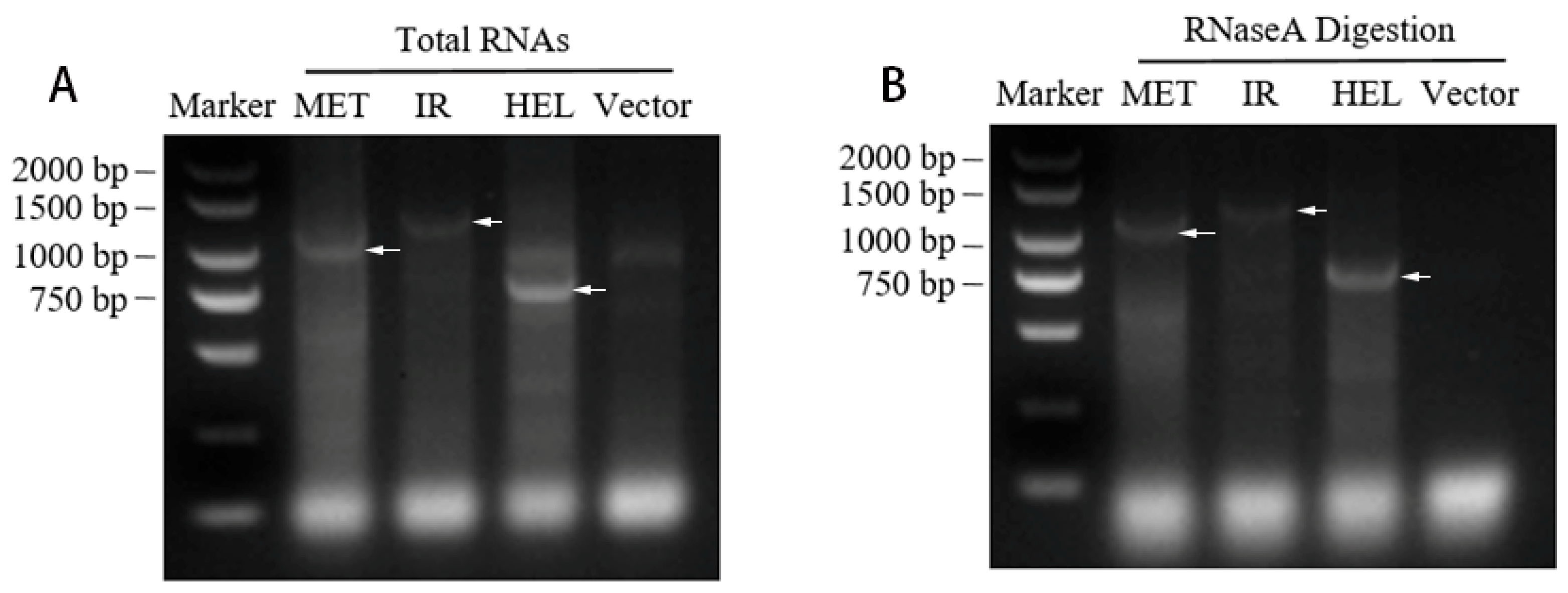
2.6. dsRNA Stability and Transport in Watermelon Leaves
2.7. Illumina Sequencing of Viral siRNAs
2.8. Statistical Analyses
3. Results
3.1. Generation of dsRNAs for the Different Regions of CGMMV
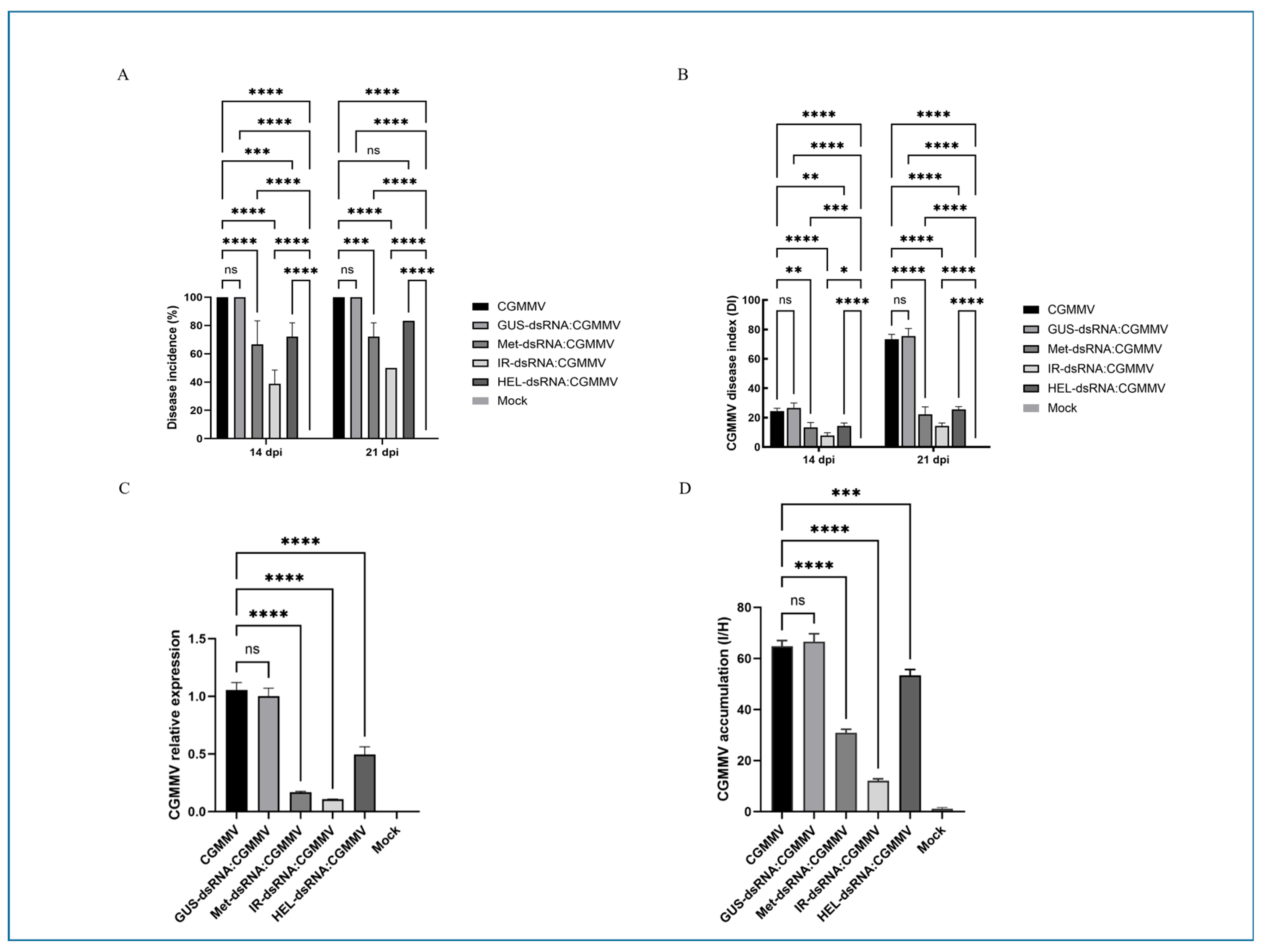
3.2. Exogenous Application of MET, IR and HEL-dsRNA
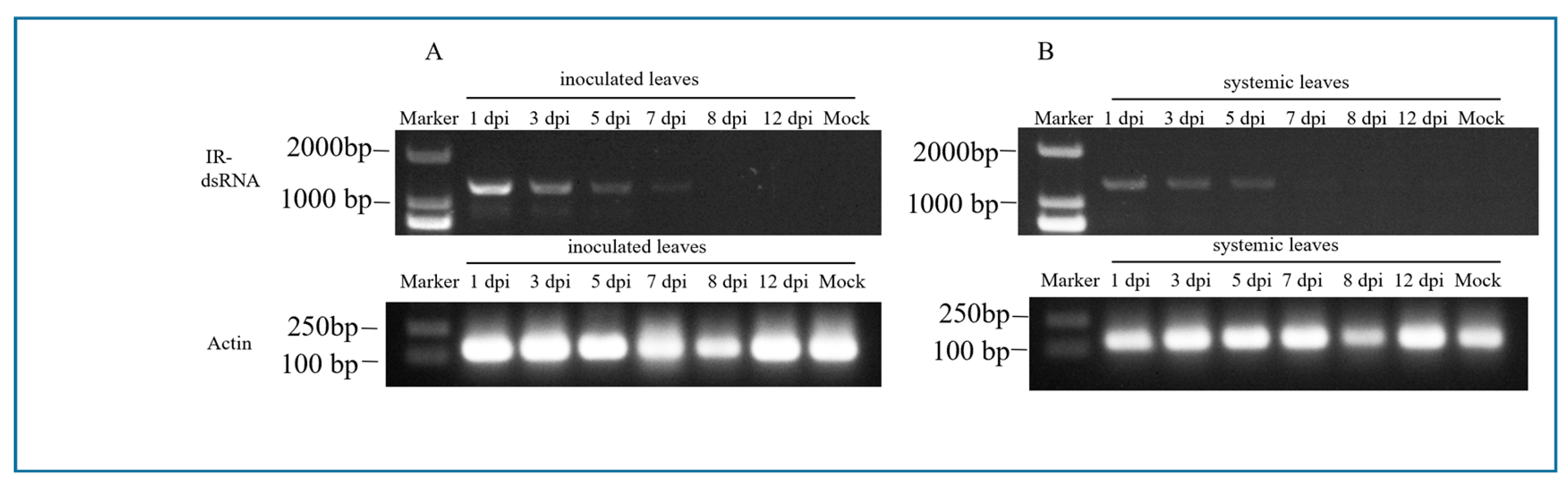
3.3. Systemic Movement of CGMMV IR-dsRNA
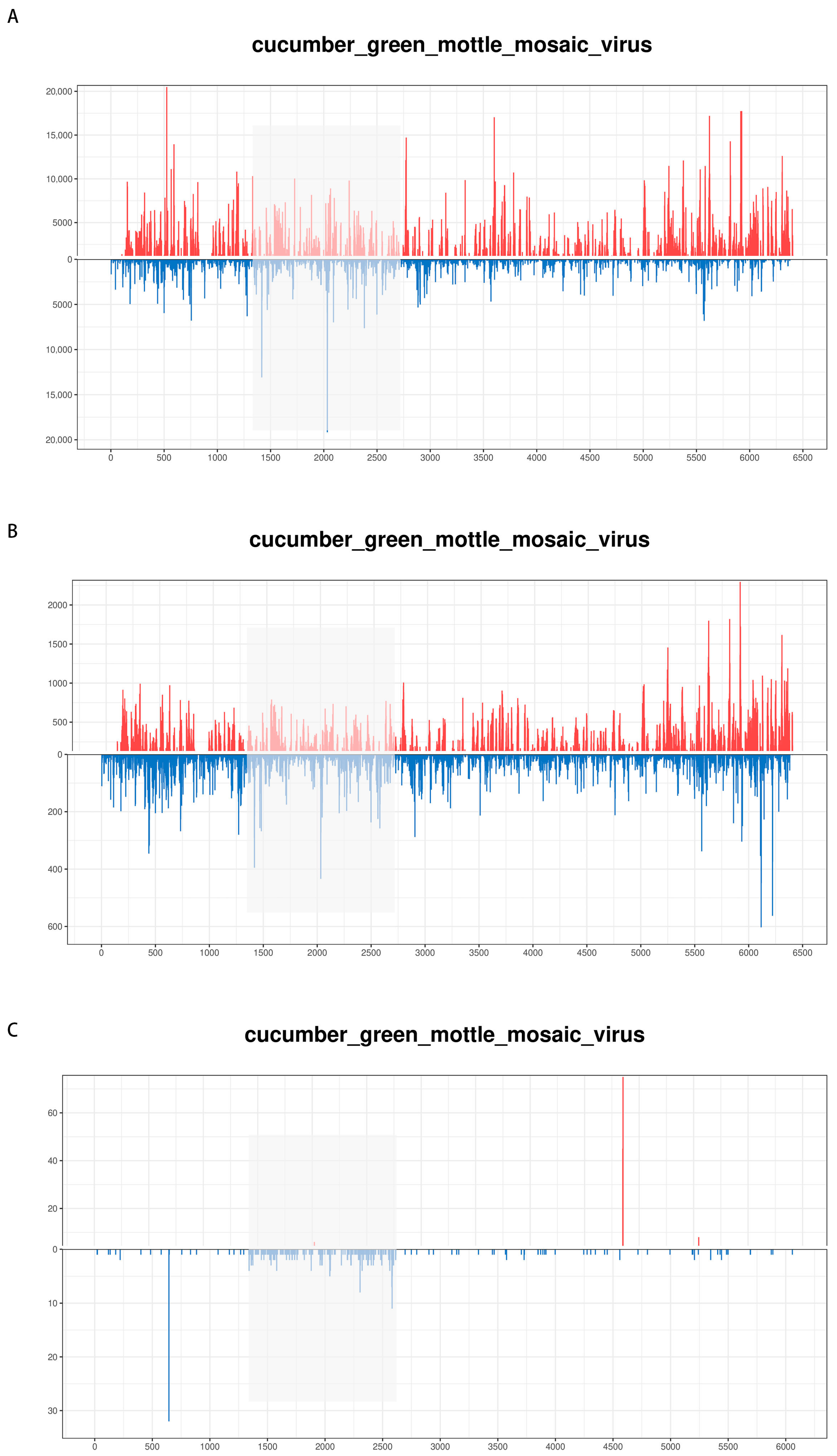
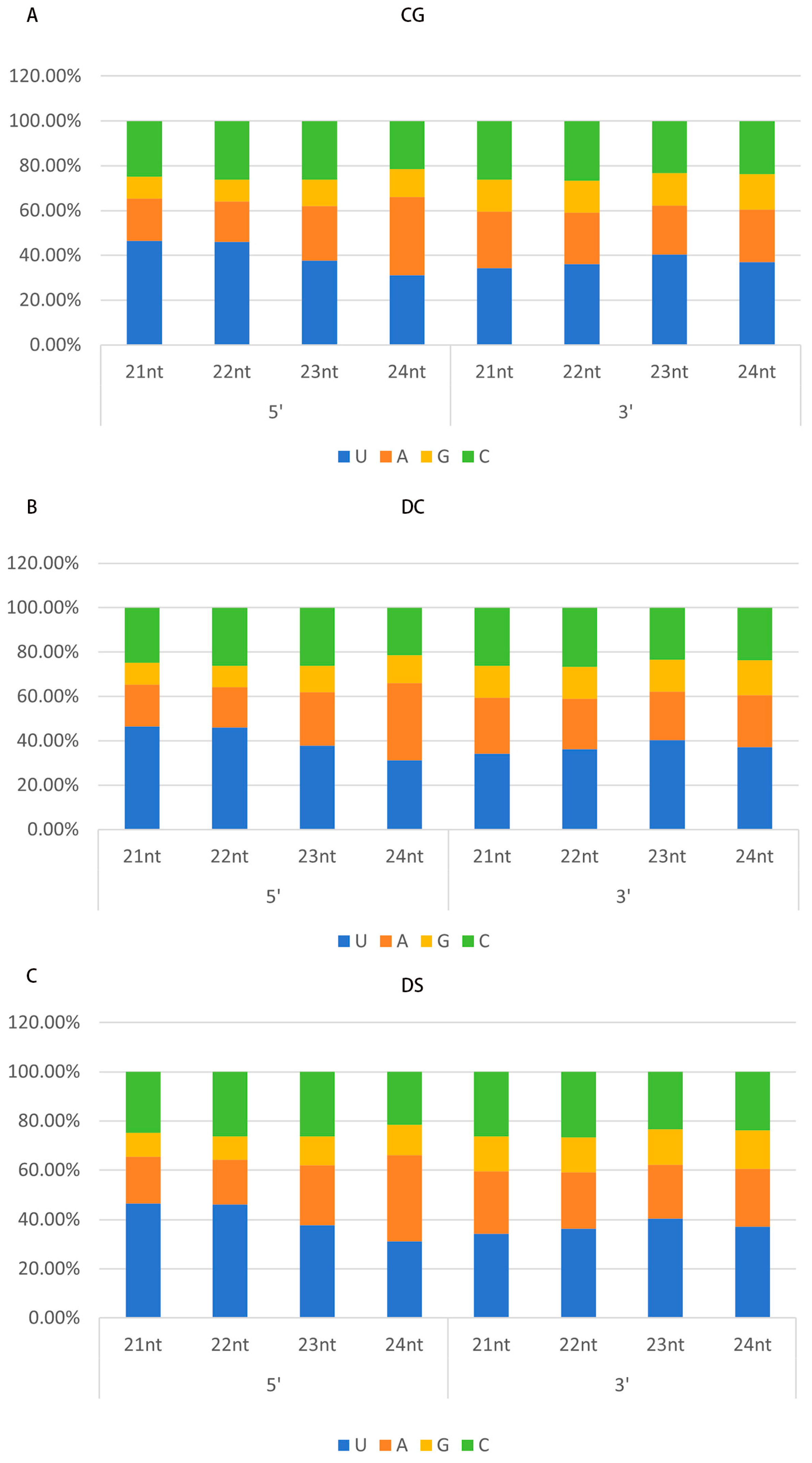
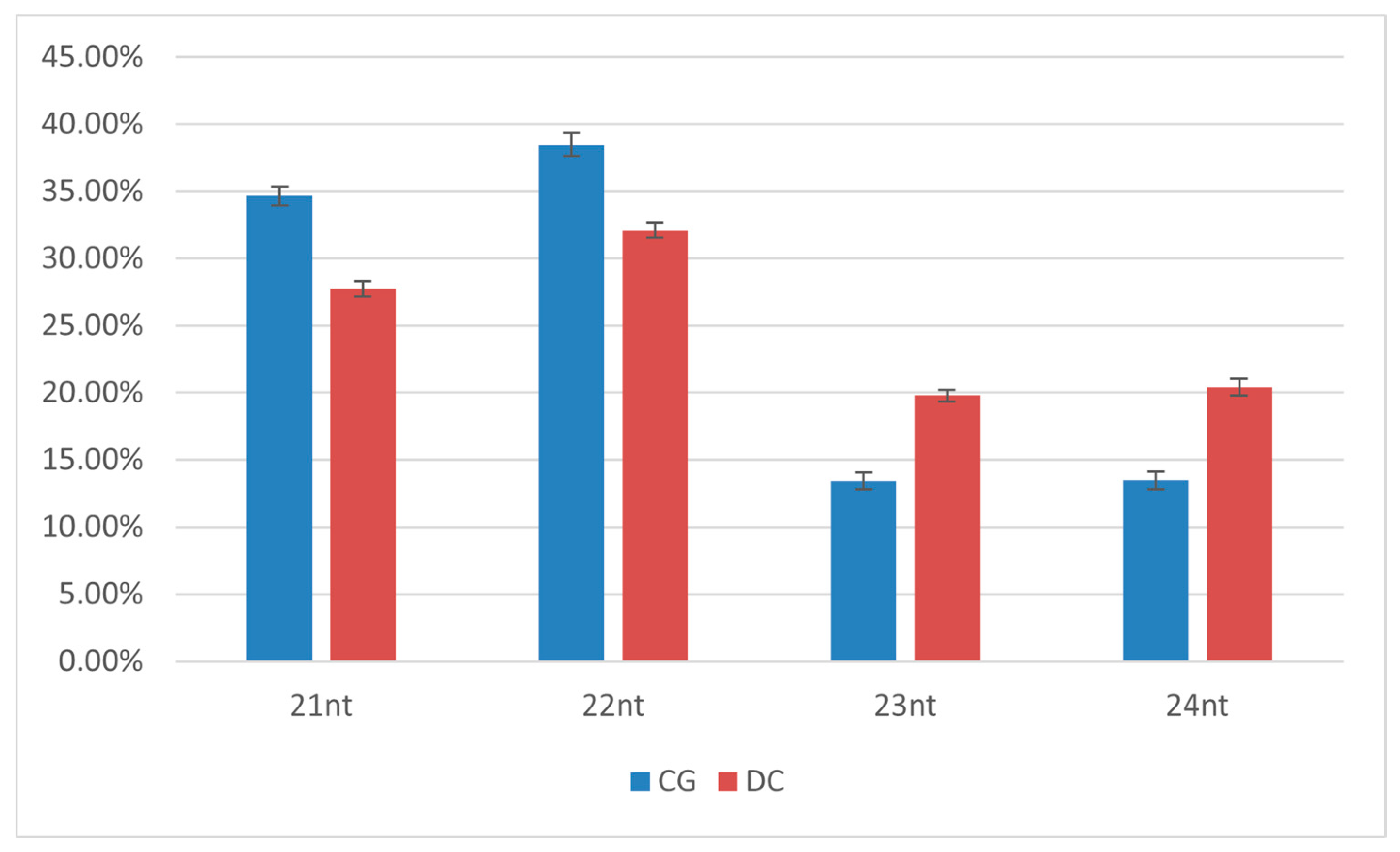
3.4. High-Throughput Sequencing (HTS) of vsiRNA in CGMMV-Infected Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisognin, D.A. Origin and evolution of cultivated cucurbits. Cienc. Rural 2002, 32, 715–723. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K. Flesh quality and lycopene stability of fresh-cut watermelon. Postharvest Biol. Technol. 2004, 31, 159–166. [Google Scholar] [CrossRef]
- Ainsworth, G.C. Mosaic diseases of the Cucumber. Ann. Appl. Biol. 1935, 22, 55–67. [Google Scholar] [CrossRef]
- Crespo, O.; Robles, C.; Ruiz, L.; Janssen, D. Antagonism of Cucumber green mottle mosaic virus against Tomato leaf curl New Delhi virus in zucchini and cucumber. Ann. Appl. Biol. 2020, 176, 147–157. [Google Scholar] [CrossRef]
- Mackie, J.; Campbell, P.R.; Kehoe, M.A.; Tran-Nguyen, L.T.T.; Rodoni, B.C.; Constable, F.E. Genome Characterisation of the CGMMV Virus Population in Australia-Informing Plant Biosecurity Policy. Viruses 2023, 15, 743. [Google Scholar] [CrossRef]
- Miao, S.; Liang, C.Q.; Li, J.Q.; Baker, B.; Luo, L.X. Polycistronic Artificial microRNA-Mediated Resistance to Cucumber Green Mottle Mosaic Virus in Cucumber. Int. J. Mol. Sci. 2021, 22, 2237. [Google Scholar] [CrossRef]
- Ali, M.E.; Waliullah, S.; Nishiguchi, M. Molecular analysis of an attenuated strain of Cucumber green mottle mosaic virus using in vitro infectious cDNA clone: Pathogenicity and suppression of RNA silencing. J. Plant Biochem. Biotechnol. 2016, 25, 79–86. [Google Scholar] [CrossRef]
- Ugaki, M.; Tomiyama, M.; Kakutani, T.; Hidaka, S.; Kiguchi, T.; Nagata, R.; Sato, T.; Motoyoshi, F.; Nishiguchi, M. The complete nucleotide sequence of cucumber green mottle mosaic virus (SH strain) genomic RNA. J. Gen. Virol. 1991, 72 Pt 7, 1487–1495. [Google Scholar] [CrossRef]
- Bronkhorst, A.W.; van Rij, R.P. The long and short of antiviral defense: Small RNA-based immunity in insects. Curr. Opin. Virol. 2014, 7, 19–28. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.Q.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Pugsley, C.E.; Isaac, R.E.; Warren, N.J.; Cayre, O.J. Recent Advances in Engineered Nanoparticles for RNAi-Mediated Crop Protection Against Insect Pests. Front. Agron. 2021, 3, 652981. [Google Scholar] [CrossRef]
- Vargason, J.M.; Szittya, G.; Burgyán, J.; Hall, T.M.T. Size selective recognition of siRNA by an RNA silencing suppressor. Cell 2003, 115, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.F.; Zhang, J.A.; Jiang, H.B.; Jiang, T.; Zhu, S.W.; Cheng, B.J. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 2010, 29, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Llave, C.; Díaz-Ruíz, J.R. RNA interference as a new biotechnological tool for the control of virus diseases in plants. Virus Res. 2004, 102, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Martínez-García, B.; Vargas, M.; Díaz-Ruíz, J.R. Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol. 2003, 3, 3. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Konakalla, N.C.; Kaldis, A.; Berbati, M.; Masarapu, H.; Voloudakis, A.E. Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 2016, 244, 961–969. [Google Scholar] [CrossRef]
- Liu, L.M.; Peng, B.; Zhang, Z.W.; Wu, Y.; Miras, M.; Aranda, M.A.; Gu, Q.S. Exploring Different Mutations at a Single Amino Acid Position of Cucumber green mottle mosaic virus Replicase to Attain Stable Symptom Attenuation. Phytopathology 2017, 107, 1080–1086. [Google Scholar] [CrossRef]
- Routhu, G.K.; Borah, M.; Siddappa, S.; Nath, P.D. Exogenous application of coat protein-specific dsRNA inhibits cognate cucumber mosaic virus (CMV) of ghost pepper. J. Plant Dis. Prot. 2022, 129, 293–300. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, T.T.; LI, M.Y.; Wu, H.L.; Guo, S.G.; Zhang, J.; Ren, Y.; Zhang, H.Y.; Gong, G.Y. Preliminary Study on the Prevention and Control Effect of Bacillus amyloliquefaciens Strain 5 on Cucumber Green Mottled Mosaic Virus Disease on Watermelon. Acta Hortic. Sin. 2024, 51, 2427–2438. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Konakalla, N.C.; Bag, S.; Deraniyagala, A.S.; Culbreath, A.K.; Pappu, H.R. Induction of Plant Resistance in Tobacco (Nicotiana tabacum) against Tomato Spotted Wilt Orthotospovirus through Foliar Application of dsRNA. Viruses 2021, 13, 662. [Google Scholar] [CrossRef] [PubMed]
- Rueda, A.; Barturen, G.; Lebrón, R.; Gómez-Martín, C.; Alganza, A.; Oliver, J.L.; Hackenberg, M. sRNAtoolbox: An integrated collection of small RNA research tools. Nucleic Acids Res. 2015, 43, W467–W473. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Kuo, Y.W.; Wuriyanghan, H.; Falk, B.W. RNA Interference Mechanisms and Applications in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef]
- Kaldis, A.; Berbati, M.; Melita, O.; Reppa, C.; Holeva, M.; Otten, P.; Voloudakis, A. Exogenously applied dsRNA molecules deriving from the Zucchini yellow mosaic virus (ZYMV) genome move systemically and protect cucurbits against ZYMV. Mol. Plant Pathol. 2018, 19, 883–895. [Google Scholar] [CrossRef]
- Tenllado, F.; Díaz-Ruíz, J.R. Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 2001, 75, 12288–12297. [Google Scholar] [CrossRef]
- Holeva, M.C.; Sklavounos, A.; Rajeswaran, R.; Pooggin, M.M.; Voloudakis, A.E. Topical Application of Double-Stranded RNA Targeting 2b and CP Genes of Cucumber mosaic virus Protects Plants against Local and Systemic Viral Infection. Plants 2021, 10, 963. [Google Scholar] [CrossRef]
- Hagiwara, K.; Ichiki, T.U.; Ogawa, Y.; Omura, T.; Tsuda, S. A single amino acid substitution in 126-kDa protein of Pepper mild mottle virus associates with symptom attenuation in pepper: The complete nucleotide sequence of an attenuated strain, C-1421. Arch. Virol. 2002, 147, 833–840. [Google Scholar] [CrossRef]
- Nakazono-Nagaoka, E.; Omura, T.; Uehara-Ichiki, T. A single amino acid substitution in the 126-kDa protein of pepper mild mottle virus controls replication and systemic movement into upper non-inoculated leaves of bell pepper plants. Arch. Virol. 2011, 156, 897–901. [Google Scholar] [CrossRef]
- Delgado-Martin, J.; Ruiz, L.; Janssen, D.; Velasco, L. Exogenous Application of dsRNA for the Control of Viruses in Cucurbits. Front. Plant Sci. 2022, 13, 895953. [Google Scholar] [CrossRef]
- Namgial, T.; Kaldis, A.; Chakraborty, S.; Voloudakis, A. Topical application of double-stranded RNA molecules containing sequences of Tomato leaf curl virus and Cucumber mosaic virus confers protection against the cognate viruses. Physiol. Mol. Plant Pathol. 2019, 108, 101432. [Google Scholar] [CrossRef]
- Wang, X.B.; Jovel, J.; Udomporn, P.; Wang, Y.; Wu, Q.F.; Li, W.X.; Gasciolli, V.; Vaucheret, H.; Ding, S.W. The 21-Nucleotide, but Not 22-Nucleotide, Viral Secondary Small Interfering RNAs Direct Potent Antiviral Defense by Two Cooperative Argonautes in Arabidopsis thaliana. Plant Cell 2011, 23, 1625–1638. [Google Scholar] [CrossRef]
- Wang, X.B.; Wu, Q.F.; Ito, T.; Cillo, F.; Li, W.X.; Chen, X.M.; Yu, J.L.; Ding, S.W. RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef]
- Pooggin, M.M. Small RNA-Omics for Plant Virus Identification, Virome Reconstruction, and Antiviral Defense Characterization. Front. Microbiol. 2018, 9, 2779. [Google Scholar] [CrossRef] [PubMed]
- Donaire, L.; Wang, Y.; Gonzalez-Ibeas, D.; Mayer, K.F.; Aranda, M.A.; Llave, C. Deep-sequencing of plant viral small RNAs reveals effective and widespread targeting of viral genomes. Virology 2009, 392, 203–214. [Google Scholar] [CrossRef]
- Mi, S.J.; Cai, T.; Hu, Y.G.; Chen, Y.; Hodges, E.; Ni, F.R.; Wu, L.; Li, S.; Zhou, H.; Long, C.Z.; et al. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 2008, 133, 116–127. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, L.; Fan, Y.; Han, Y.; Liang, Z.; Geng, Y.; Liu, F.; Gu, Q.; Kang, B.; Luo, C. Exogenous Application of IR-Specific dsRNA Inhibits Infection of Cucumber Green Mottle Mosaic Virus in Watermelon. Agronomy 2025, 15, 2332. https://doi.org/10.3390/agronomy15102332
Wang Y, Liu L, Fan Y, Han Y, Liang Z, Geng Y, Liu F, Gu Q, Kang B, Luo C. Exogenous Application of IR-Specific dsRNA Inhibits Infection of Cucumber Green Mottle Mosaic Virus in Watermelon. Agronomy. 2025; 15(10):2332. https://doi.org/10.3390/agronomy15102332
Chicago/Turabian StyleWang, Yanhui, Liming Liu, Yongqiang Fan, Yanli Han, Zhiling Liang, Yanfei Geng, Fengnan Liu, Qinsheng Gu, Baoshan Kang, and Chaoxi Luo. 2025. "Exogenous Application of IR-Specific dsRNA Inhibits Infection of Cucumber Green Mottle Mosaic Virus in Watermelon" Agronomy 15, no. 10: 2332. https://doi.org/10.3390/agronomy15102332
APA StyleWang, Y., Liu, L., Fan, Y., Han, Y., Liang, Z., Geng, Y., Liu, F., Gu, Q., Kang, B., & Luo, C. (2025). Exogenous Application of IR-Specific dsRNA Inhibits Infection of Cucumber Green Mottle Mosaic Virus in Watermelon. Agronomy, 15(10), 2332. https://doi.org/10.3390/agronomy15102332




