Optimizing Winter Composting of Swine Manure Through Housefly Larva Bioconversion: Mechanisms of Protein Recovery and Enzymatic Nitrogen Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Materials
2.2. Experimental Design and Sampling
2.3. Measurement Methodology
2.4. Data Processing and Analysis
3. Results and Discussion
3.1. Larval Bioconversion Efficiency and Nutrient Recovery
3.2. Physicochemical Variations and Nitrogen Transformation
3.3. Microbial Enzyme Activity Dynamics and Regulatory Mechanisms
3.4. Evaluation of Compost Maturity and Quality
3.5. Correlation Analysis of the Compost Parameters and Enzymatic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ministry of Ecology and Environment of the People’s Republic of China. China Ecological and Environmental Statistical Yearbook 2023. 2023. Available online: https://www.mee.gov.cn/hjzl/sthjzk/sthjtjnb/202412/t20241231_1099687.shtml (accessed on 27 May 2025).
- Pan, D.; Chen, H.; Zhang, N.; Kong, F. Do livestock environmental regulations reduce water pollution in China? Ecol. Econ. 2023, 204, 107637. [Google Scholar] [CrossRef]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Convers. Biorefin. 2023, 13, 5445–5468. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient recovery from digestate of anaerobic digestion of livestock manure: A review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Pagliari, P.; Wilson, M.; He, Z. Animal Manure Production and Utilization: Impact of Modern Concentrated Animal Feeding Operations; Wiley: Hoboken, NJ, USA, 2020; pp. 1–14. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, W.; Hong, C.; Feng, M.; Xue, Z.; Chen, X.; Yao, Y.; Yu, M. Rapid production of maggots as feed supplement and organic fertilizer by the two-stage composting of pig manure. Bioresour. Technol. 2012, 116, 485–491. [Google Scholar] [CrossRef]
- Lalander, C.; Vinnerås, B. Actions needed before insects can contribute to a real closed-loop circular economy in the EU. J. Insects Food Feed 2022, 8, 337–342. [Google Scholar] [CrossRef]
- Qiao, C.; Penton, C.R.; Liu, C.; Shen, Z.; Ou, Y.; Liu, Z.; Xu, X.; Li, R.; Shen, Q. Key extracellular enzymes triggered high-efficiency composting associated with bacterial community succession. Bioresour. Technol. 2019, 288, 121576. [Google Scholar] [CrossRef]
- Vilela, R.N.d.S.; Orrico, A.C.A.; Junior, M.A.P.O.; Borquis, R.R.A.; Tomazi, M.; de Oliveira, J.D.; de Ávila, M.R.; dos Santos, F.T.; Leite, B.K.V. Effects of aeration and season on the composting of slaughterhouse waste. Environ. Technol. Innov. 2022, 27, 102505. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Lei, M. Positive impact of biochar amendment on thermal balance during swine manure composting at relatively low ambient temperature. Bioresour. Technol. 2019, 273, 25–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, F.; Su, X.; Su, H.; Li, D. Semi-permeable membrane-covered high-temperature aerobic composting: A review. J. Environ. Manag. 2024, 356, 120741. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Li, Y.; Liu, Y.; Jiang, H.; Li, H.; Yuan, Y.; Chen, Y.; Zou, B. Improving the humification by additives during composting: A review. Waste Manag. 2023, 158, 93–106. [Google Scholar] [CrossRef]
- Ge, M.; Zhou, H.; Shen, Y.; Meng, H.; Li, R.; Zhou, J.; Cheng, H.; Zhang, X.; Ding, J.; Wang, J.; et al. Effect of aeration rates on enzymatic activity and bacterial community succession during cattle manure composting. Bioresour. Technol. 2020, 304, 122928. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Ma, R.; Jiang, J.; Li, G.; Wang, J.; Wuyun, D.; Yuan, J. Thermophilic compost inoculating promoted the maturity and mature compost inoculating reduced the gaseous emissions during co-composting of kitchen waste and pig manure. Environ. Technol. Innov. 2023, 32, 103427. [Google Scholar] [CrossRef]
- Xiong, J.; Ma, S.; He, X.; Han, L.; Huang, G. Nitrogen transformation and dynamic changes in related functional genes during functional-membrane covered aerobic composting. Bioresour. Technol. 2021, 332, 125087. [Google Scholar] [CrossRef]
- Miranda, C.D.; Crippen, T.L.; Cammack, J.A.; Tomberlin, J.K. Black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), and house fly, Musca domestica L. (Diptera: Muscidae), larvae reduce livestock manure and possibly associated nutrients: An assessment at two scales. Environ. Pollut. 2021, 282, 116976. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hong, C.; Yao, Y.; Liu, L.; Wang, W.; Zhu, W.; Hong, L.; Weng, J.; Zhou, Y.; Zhu, F. The process of biotransformation can produce insect protein and promote the effective inactivation of heavy metals. Sci. Total Environ. 2021, 776, 145864. [Google Scholar] [CrossRef] [PubMed]
- Mertenat, A.; Diener, S.; Zurbrügg, C. Black Soldier Fly biowaste treatment—Assessment of global warming potential. Waste Manag. 2019, 84, 173–181. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Harahap, I.A.; Osei-Owusu, J.; Saikia, T.; Wu, Y.S.; Fernando, I.; Perestrelo, R.; Câmara, J.S. Bioconversion of organic waste by insects—A comprehensive review. Process Saf. Environ. Prot. 2024, 187, 1–25. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef]
- GB/T 7959-2012; Hygienic Requirements for Harmless Disposal of Night Soil. Standardization Administration of China, 2012. Standards Press of China: Beijing, China, 2012.
- NY/T 525-2021; Organic fertilizer. Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2021. Standards Press of China: Beijing, China, 2021.
- Cheng, Z.; Yu, L.; Li, H.; Xu, X.; Yang, Z. Use of housefly (Musca domestica L.) larvae to bioconversion food waste for animal nutrition and organic fertilizer. Environ. Sci. Pollut. Res. 2021, 28, 48921–48928. [Google Scholar] [CrossRef]
- Raksasat, R.; Lim, J.W.; Kiatkittipong, W.; Kiatkittipong, K.; Ho, Y.C.; Lam, M.K.; Font-Palma, C.; Mohd Zaid, H.F.; Cheng, C.K. A review of organic waste enrichment for inducing palatability of black soldier fly larvae: Wastes to valuable resources. Environ. Pollut. 2020, 267, 115488. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. FoodData Central, FDC ID: 1104766, Soybeans. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1104766/nutrients (accessed on 27 May 2025).
- GB/T 19164-2021; Feed Material—Fish Meal. Standardization Administration of China, 2021. Standards Press of China: Beijing, China, 2021.
- GB/T 13078-2017; Hygienical Standard for Feeds. Standardization Administration of China, 2017. Standards Press of China: Beijing, China, 2017.
- GB/T 1352-2023; Soya Bean. Standardization Administration of China, 2023. Standards Press of China: Beijing, China, 2023.
- Chang, R.; Guo, Q.; Chen, Q.; Bernal, M.P.; Wang, Q.; Li, Y. Effect of initial material bulk density and easily-degraded organic matter content on temperature changes during composting of cucumber stalk. J. Environ. Sci. 2019, 80, 306–315. [Google Scholar] [CrossRef]
- Ahmadinia, S.; Palviainen, M.; Kiuru, P.; Routa, J.; Sikanen, L.; Urzainki, I.; Laurén, A. Forest chip drying in self-heating piles during storage as affected by temperature and relative humidity conditions. Fuel 2022, 324, 124419. [Google Scholar] [CrossRef]
- Parodi, A.; De Boer, I.J.M.; Gerrits, W.J.J.; Van Loon, J.J.A.; Heetkamp, M.J.W.; Van Schelt, J.; Bolhuis, J.E.; Van Zanten, H.H.E. Bioconversion efficiencies, greenhouse gas and ammonia emissions during black soldier fly rearing—A mass balance approach. J. Clean. Prod. 2020, 271, 122488. [Google Scholar] [CrossRef]
- Wu, N.; Ma, Y.; Yu, X.; Wang, X.; Wang, Q.; Liu, X.; Xu, X. Black soldier fly larvae bioconversion and subsequent composting promote larval frass quality during pig and chicken manure transformation process. Bioresour. Technol. 2024, 402, 130777. [Google Scholar] [CrossRef] [PubMed]
- Troy, S.M.; Nolan, T.; Kwapinski, W.; Leahy, J.J.; Healy, M.G.; Lawlor, P.G. Effect of sawdust addition on composting of separated raw and anaerobically digested pig manure. J. Environ. Manag. 2012, 111, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wen, X.; Yang, H.; Lu, H.; Wang, A.; Liu, S.; Li, Q. Incorporating microbial inoculants to reduce nitrogen loss during sludge composting by suppressing denitrification and promoting ammonia assimilation. Sci. Total Environ. 2024, 915, 170000. [Google Scholar] [CrossRef]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Gao, Y.; Tan, W.; Xi, B. Additives for reducing nitrogen loss during composting: A review. J. Clean. Prod. 2021, 307, 127308. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Lin, Z.; Hu, D.; Liu, Y.; Pan, X. Evolution of enzyme activity, heavy metals bioavailability and microbial community in different temperature stages of the co-bioevaporation process. Waste Manag. 2020, 102, 751–762. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Tang, D.K.H.; Zhang, X.; Zhang, X.; Liu, H.; Quan, F. Enhancing nitrogen transformation and humification in cow manure composting through psychrophilic and thermophilic nitrifying bacterial consortium inoculation. Bioresour. Technol. 2024, 413, 131507. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, F.; Hong, C.; Chen, H.; Wang, W.; Xue, Z.; Zhu, W.; Wang, G.; Tong, W. Utilization of gibberellin fermentation residues with swine manure by two-step composting mediated by housefly maggot bioconversion. Waste Manag. 2020, 105, 339–346. [Google Scholar] [CrossRef]
- Song, B.; Manu, M.K.; Li, D.; Wang, C.; Varjani, S.; Ladumor, N.; Michael, L.; Xu, Y.; Wong, J.W.C. Food waste digestate composting: Feedstock optimization with sawdust and mature compost. Bioresour. Technol. 2021, 341, 125759. [Google Scholar] [CrossRef]
- Zhu, F.; Hong, C.; Wang, W.; Lyu, H.; Zhu, W.; Xu, H.; Yao, Y. A microbial agent effectively reduces ammonia volatilization and ensures good maggot yield from pig manure composted via housefly larvae cultivation. J. Clean. Prod. 2020, 270, 122373. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.S.H.; Vo, D.-V.N.; Tran, H.T. Evaluate the role of biochar during the organic waste composting process: A critical review. Chemosphere 2022, 299, 134488. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J. Changes in mineral forms of nitrogen and sulfur and enzymatic activities during composting of lignocellulosic waste and chicken feathers. Environ. Sci. Pollut. Res. 2019, 26, 10333–10342. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guan, W.; Tai, X.; Qi, W.; Zhang, Y.; Ma, Y.; Sun, X.; Lu, Y.; Lin, D. Research progress on microbial nitrogen conservation technology and mechanism of microorganisms in aerobic composting. Microb. Ecol. 2025, 88, 19. [Google Scholar] [CrossRef]
- Mi, J.; Hou, L.; Hou, Y.; Song, C.; Pan, L.; Wei, Z. Enhancing compost quality: Biochar and zeolite’s role in nitrogen transformation and retention. Sci. Total Environ. 2025, 963, 178490. [Google Scholar] [CrossRef] [PubMed]
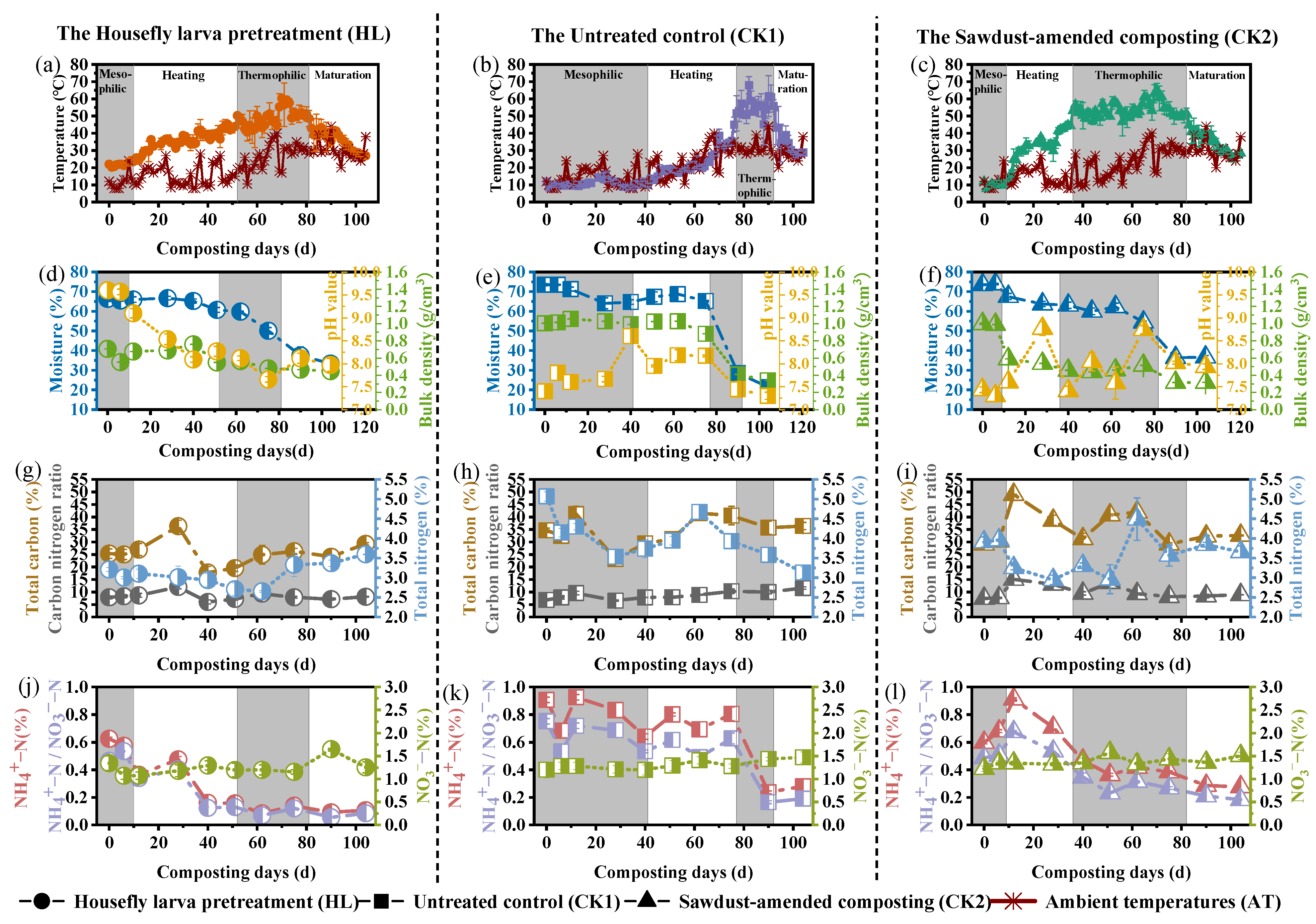
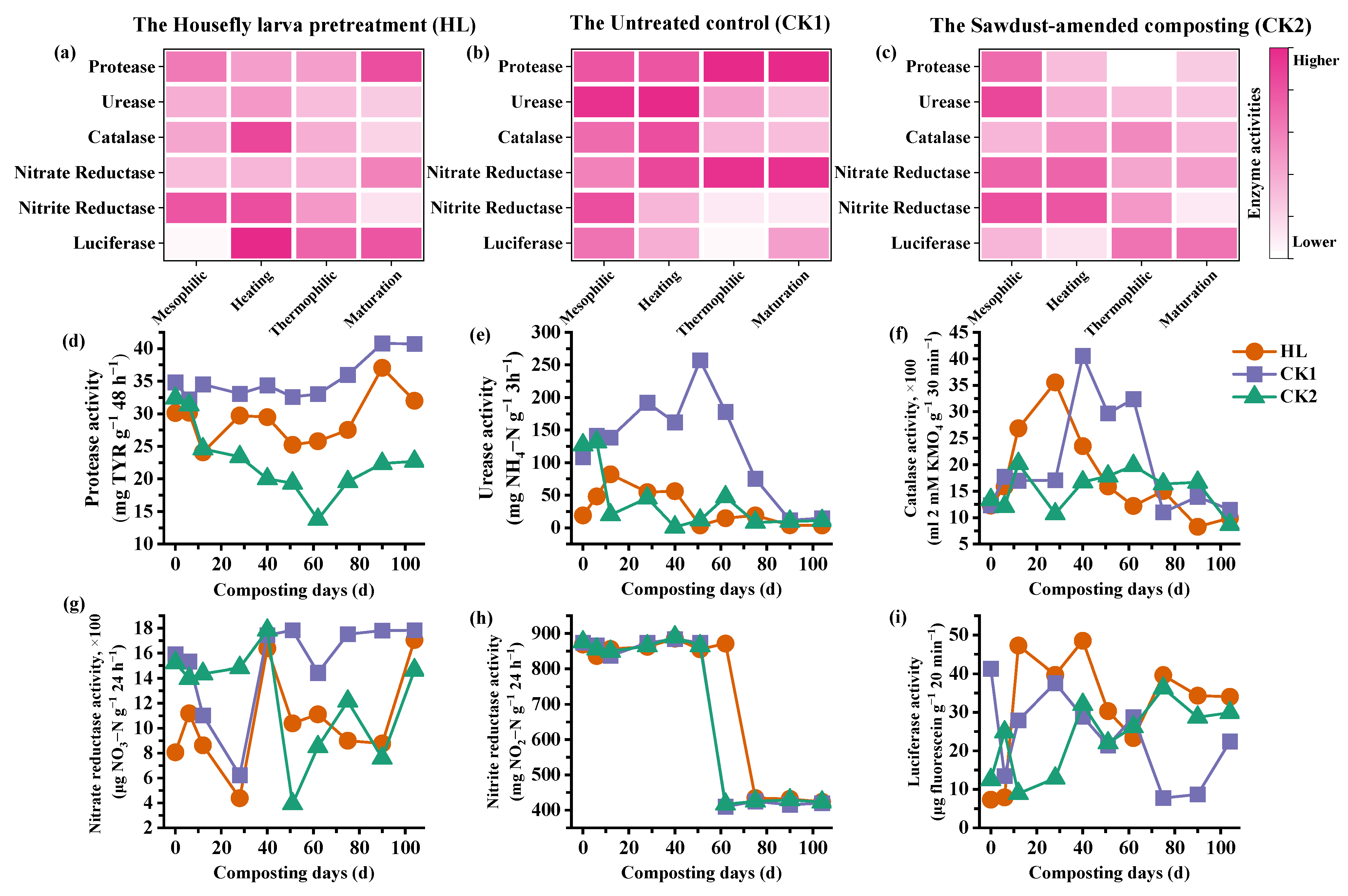
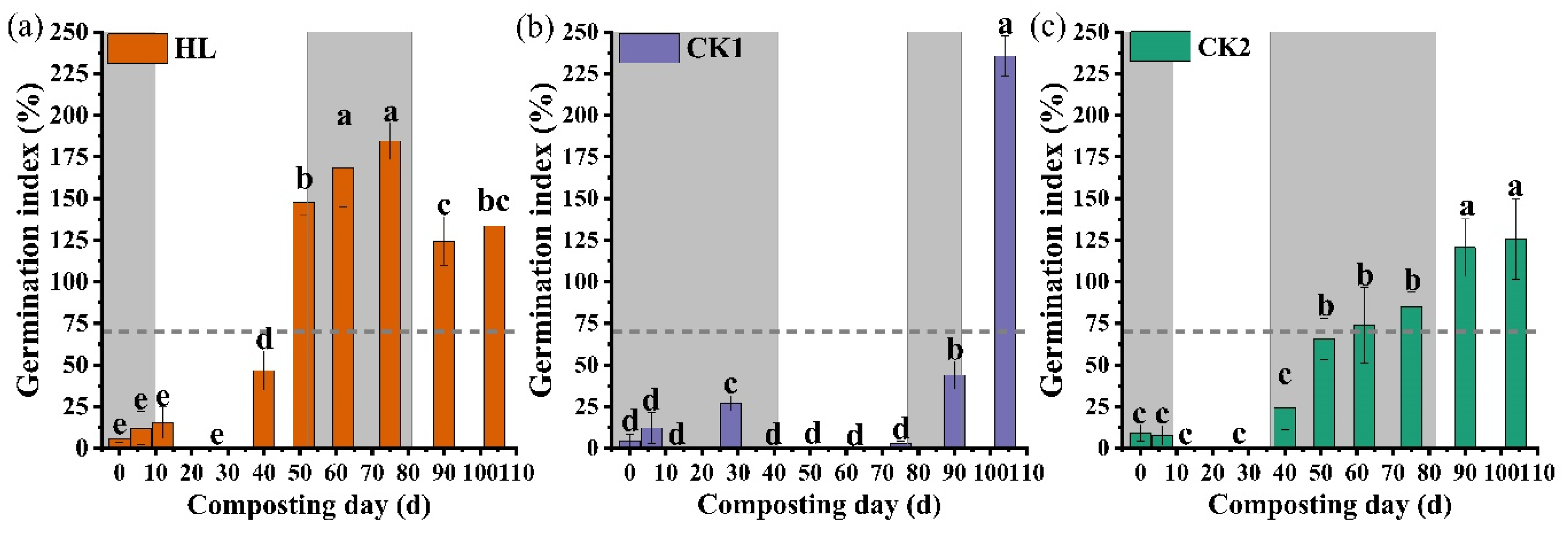

| Material | Moisture (%) | Total N (%) | Organic C (%) | C/N | Total K (%) | Total P (%) |
|---|---|---|---|---|---|---|
| Pig manure | 73.53 | 2.42 | 30.8 | 12.73 | 3.235 | 1.857 |
| Sawdust | 15.56 | 0.14 | 49.1 | 350.71 | 0.361 | 0.03 |
| Extruded pig manure | 56.52 | 1.02 | 43.9 | 43.4 | 0.28 | 1.14 |
| Parameter | Value (Mean ± SD) | Unit |
|---|---|---|
| Initial pig manure | 20.06 ± 0.04 | kg (FW) |
| Larval inoculation density | 2079 | individuals·g−1 (FW) |
| Larval inoculation amount | 150.00 ± 0.05 | g (FW) |
| Larval biomass harvested | 2.55 ± 0.02 | kg (FW) |
| Larval harvest rate | 12.71 ± 0.07 | % (FW) |
| C conversion rate | 10.69 ± 0.06 | % (DW) |
| N conversion rate | 30.55 ± 0.39 | % (DW) |
| P conversion rate | 8.54 ± 0.12 | % (DW) |
| K conversion rate | 11.53 ± 0.50 | % (DW) |
| Category | Parameter | Value | Standard/Reference Value |
|---|---|---|---|
| Heavy Metals | As (mg·kg−1) | 0.134 ± 0.000 | ≤2 [27] |
| Pb (mg·kg−1) | 0.821 ± 0.037 | ≤10 [27] | |
| Hg (mg·kg−1) | 0.005 ± 0.002 | ≤ 0.1 [27] | |
| Cd (mg·kg−1) | 0.149 ± 0.000 | ≤10 [27] | |
| Cr (mg·kg−1) | 1.231 ± 0.037 | ≤5 [27] | |
| Nutritional Composition | Crude protein (%) | 53.35 ± 0.69 | ≥50.0 [26] |
| ≥44.0 [28] | |||
| Crude fat (%) | 27.6 ± 1.18 | ≥22.0 [28] | |
| Moisture (%) | 73.2 ± 0.0 | 7.85 [25] | |
| Amino Acid Profile | ASP | 4.68 ± 0.19 | 3.76 [25] |
| THR | 2.16 ± 0.07 | 1.48 [25] | |
| SER | 2.16 ± 0.07 | 2.07 [25] | |
| GLU | 6.37 ± 0.23 | 6.80 [25] | |
| PRO | 2.14 ± 0.11 | 2.13 [25] | |
| LYS | 3.91 ± 0.11 | 2.14 [25] | |
| ≥3.0 [26] | |||
| GLY | 2.13 ± 0.07 | 1.35 [25] | |
| ALA | 3.07 ± 0.09 | 2.14 [25] | |
| CYS | ND | 0.77 [25] | |
| VAL | 2.43 ± 0.11 | 1.74 [25] | |
| MET | 1.11 ± 0.06 | 0.49 [25] | |
| ILE | 1.89 ± 0.09 | 1.74 [25] | |
| LEU | 3.18 ± 0.11 | 3.06 [25] | |
| TYR | 2.39 ± 0.13 | 1.40 [25] | |
| PHE | 3.21 ± 0.11 | 2.15 [25] | |
| HIS | 4.49 ± 0.09 | 0.95 [25] | |
| ARG | 2.24 ± 0.13 | 2.94 [25] | |
| Total (%) | 47.55 ± 1.77 | 37.10 [25] | |
| 17 AA/crude protein (%) | 89.1 | ≥87 [26] | |
| 96.11 [25] | |||
| GLY/total AA (%) | 4.47 | ≤8.0 [26] | |
| 3.64 [25] |
| Parameter | HL | CK1 | CK2 | Compost Quality Threshold | |||
|---|---|---|---|---|---|---|---|
| Before (0 Day) | After (104 Day) | Before (0 Day) | After (104 Day) | Before (0 Day) | After (104 Day) | ||
| Moisture (%) | 66.3 ± 1.3 | 33.3 ± 0.2 | 73.5 ± 0.5 | 21.5 ± 1.1 | 73.5 ± 0.4 | 36.7 ± 0.9 | ≤30 [22] |
| Bulk density (g·cm−3) | 0.71 | 0.45 | 1.00 | 0.34 | 0.998 | 0.32 | |
| pH | 9.60 ± 0.00 | 7.97 ± 0.14 | 7.40 ± 0.00 | 7.30 ± 0.08 | 7.43 ± 0.06 | 7.94 ± 0.06 | 5.5~8.5 [22] |
| Organic matter (%) | 43.7 ± 0.88 | 50.5 ± 0.49 | 59.7 ± 0.88 | 62.7 ± 1.4 | 50.1 ± 1.0 | 56.3 ± 0.16 | ≥30 [22] |
| Total N (%) | 3.21 ± 0.05 | 3.60 ± 0.01 | 5.07 ± 0.12 | 3.13 ± 0.15 | 3.91 ± 0.13 | 3.66 ± 0.15 | |
| C/N ratio | 7.90 ± 0.58 | 8.14 ± 0.14 | 6.83 ± 0.17 | 11.61 ± 0.46 | 7.44 ± 0.27 | 8.92 ± 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Yao, Y.; Hong, C.; Zhu, W.; Hong, L.; Zhang, T.; Guo, R.; Ding, C.; Zhou, Y.; Zhu, F. Optimizing Winter Composting of Swine Manure Through Housefly Larva Bioconversion: Mechanisms of Protein Recovery and Enzymatic Nitrogen Regulation. Agronomy 2025, 15, 2324. https://doi.org/10.3390/agronomy15102324
Lu N, Yao Y, Hong C, Zhu W, Hong L, Zhang T, Guo R, Ding C, Zhou Y, Zhu F. Optimizing Winter Composting of Swine Manure Through Housefly Larva Bioconversion: Mechanisms of Protein Recovery and Enzymatic Nitrogen Regulation. Agronomy. 2025; 15(10):2324. https://doi.org/10.3390/agronomy15102324
Chicago/Turabian StyleLu, Nanyang, Yanlai Yao, Chunlai Hong, Weijing Zhu, Leidong Hong, Tao Zhang, Rui Guo, Chengrong Ding, Ying Zhou, and Fengxiang Zhu. 2025. "Optimizing Winter Composting of Swine Manure Through Housefly Larva Bioconversion: Mechanisms of Protein Recovery and Enzymatic Nitrogen Regulation" Agronomy 15, no. 10: 2324. https://doi.org/10.3390/agronomy15102324
APA StyleLu, N., Yao, Y., Hong, C., Zhu, W., Hong, L., Zhang, T., Guo, R., Ding, C., Zhou, Y., & Zhu, F. (2025). Optimizing Winter Composting of Swine Manure Through Housefly Larva Bioconversion: Mechanisms of Protein Recovery and Enzymatic Nitrogen Regulation. Agronomy, 15(10), 2324. https://doi.org/10.3390/agronomy15102324





