Sustainable Phosphorus and Protein Recovery from Different Organic Wastes: Process Optimization and Struvite Precipitation Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Organic Sources Used
2.2. General Procedure for Phosphorus Extraction Through Fermentation
2.3. Optimization of Phosphorus Extraction Through Fermentation: Sugar Source, Ratio Sugar/Waste and Incubation Time
2.3.1. Optimization of the Sugar Source and Sugar-to-Waste Ratio
2.3.2. Incubation Assay to Optimize the Incubation Time
2.4. Alkaline Protein Extraction
2.5. Analytical Characterization of the Organic Wastes and Precipitates
2.6. Struvite Precipitation and Mineralogical Characterization
2.7. Statistical Analysis
3. Results and Discussion
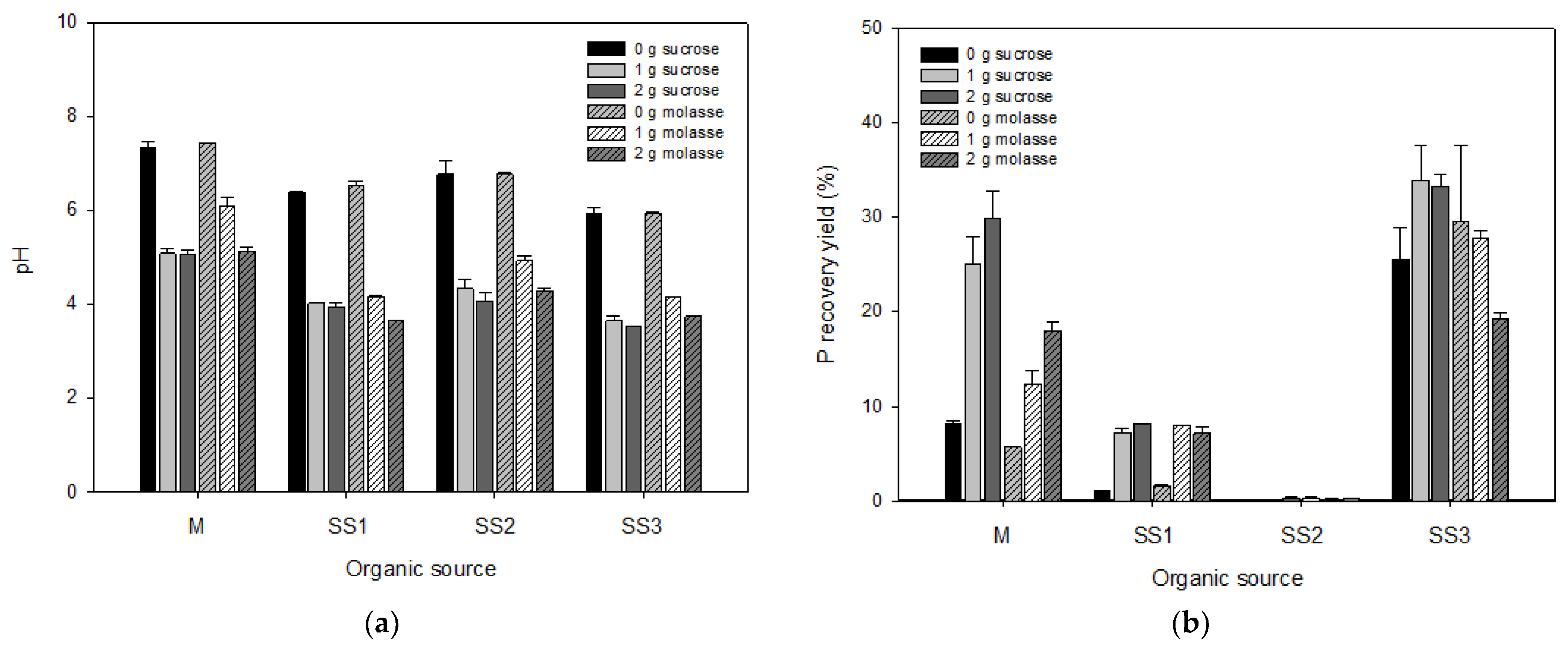
3.1. Optimization of the Phosphorus Extraction: Sugar Source and Ratio
3.2. Optimization of the Phosphorus Extraction: Incubation Time
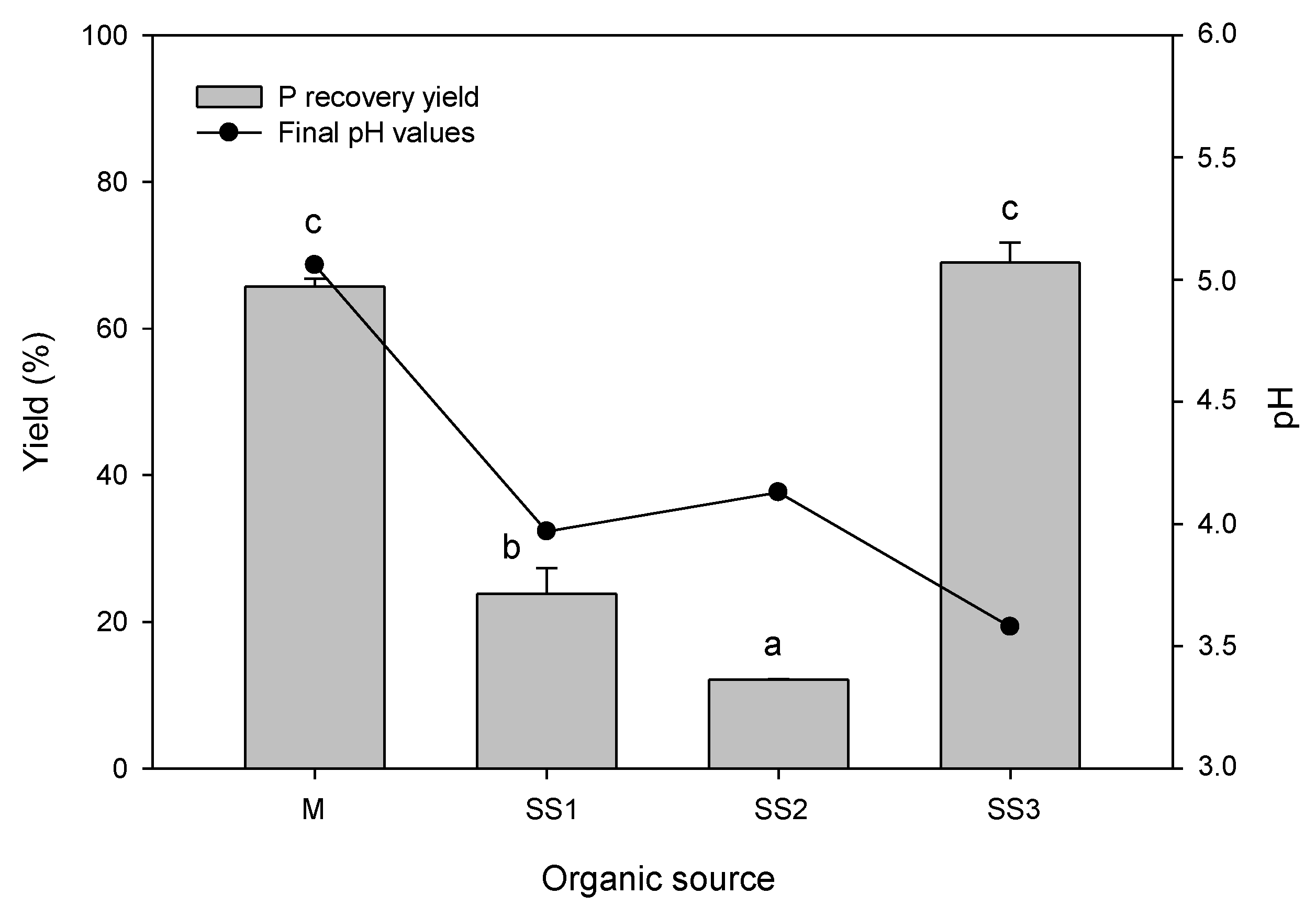
3.3. Phosphorus Extraction Efficiency with the Optimized Procedure
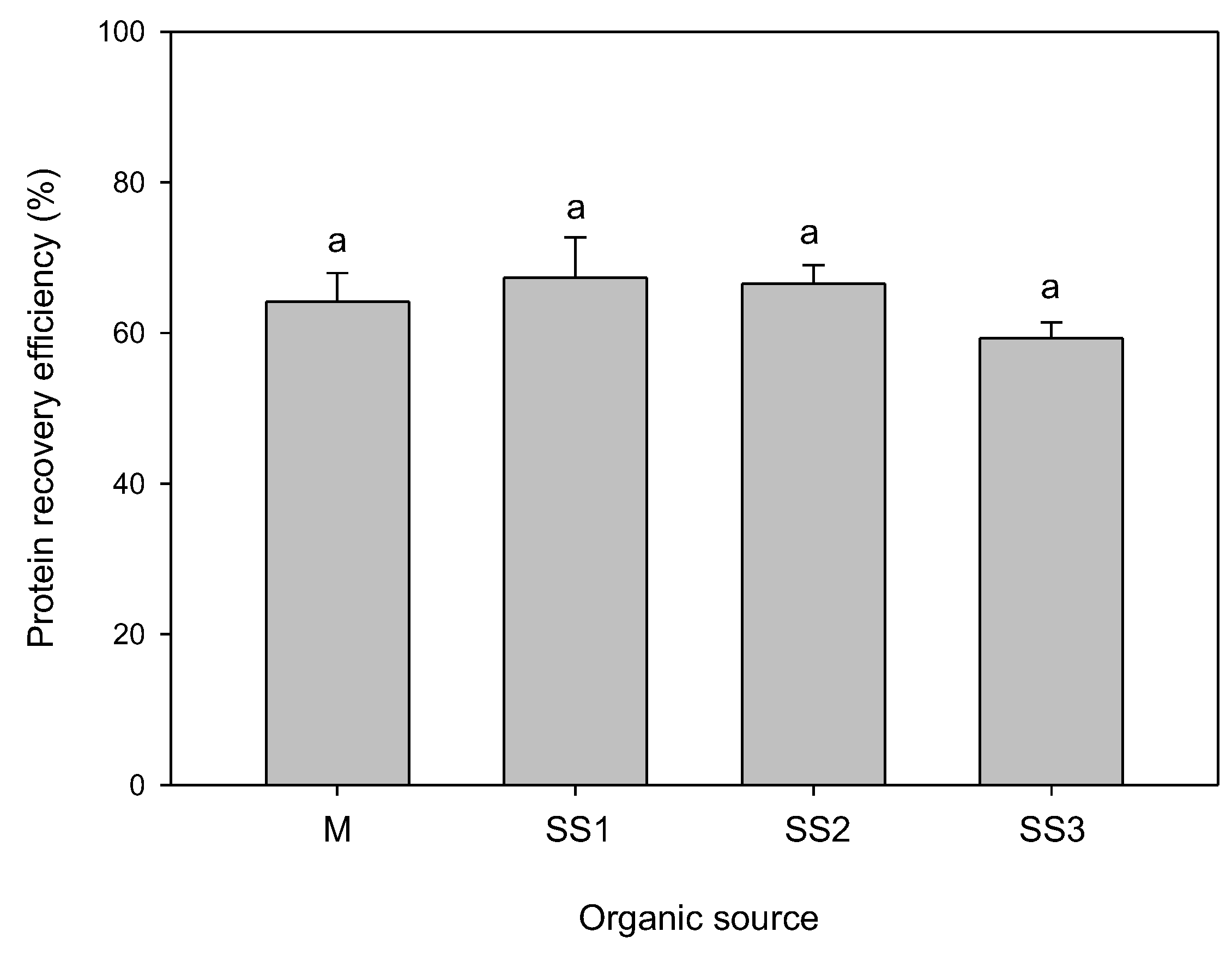
3.4. Protein Recovery Efficiency
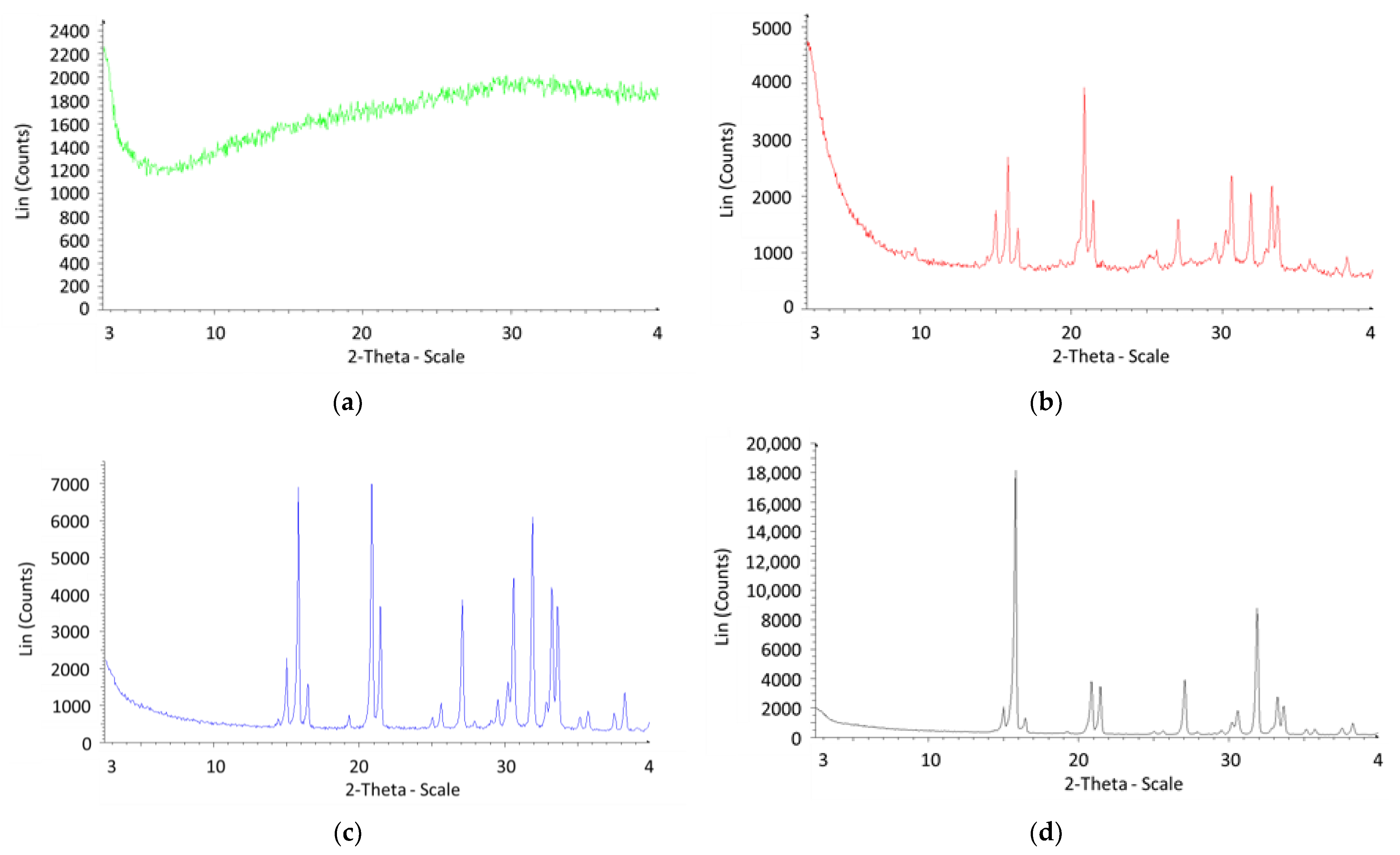
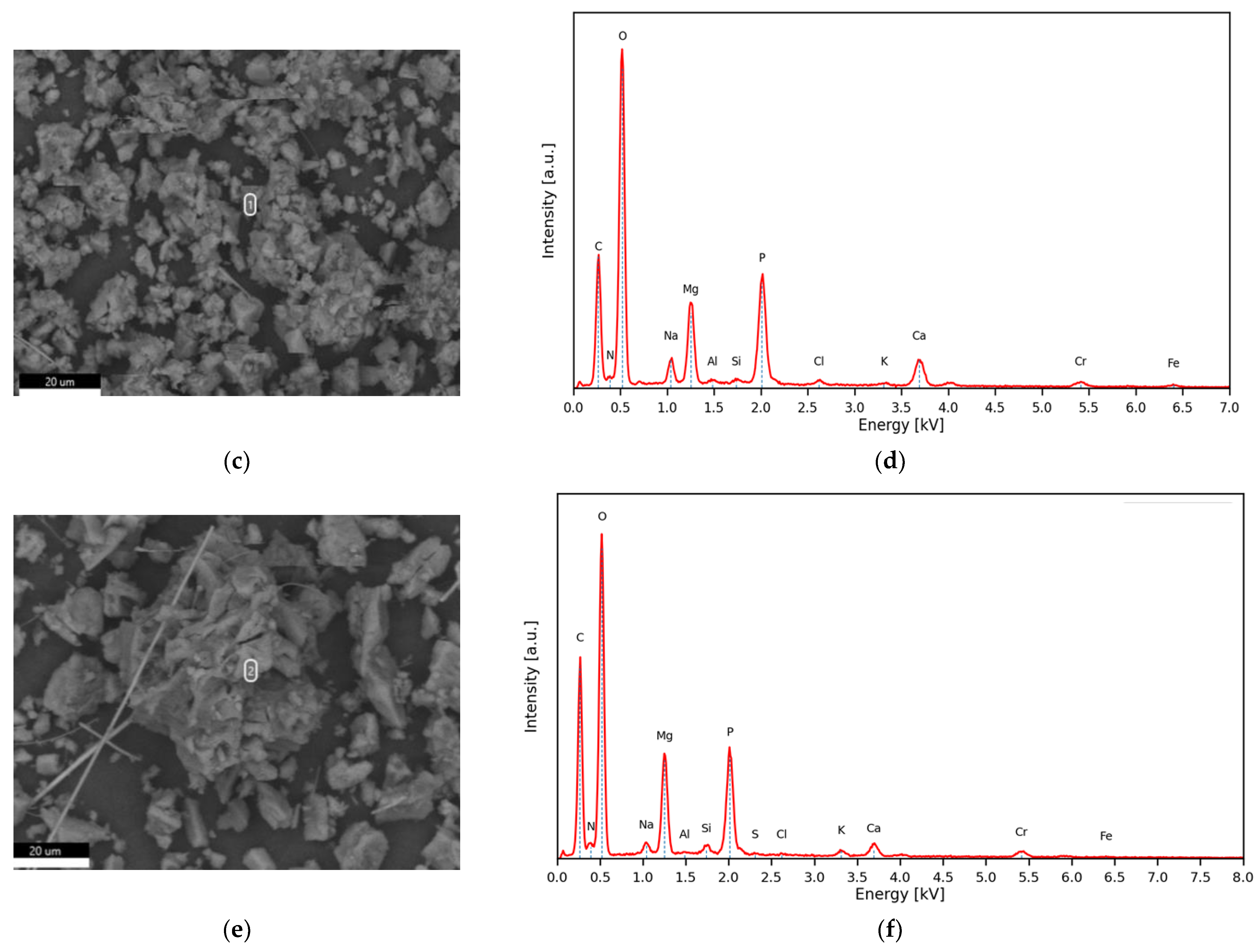
3.5. Struvite Production: Chemical and Mineralogical Characterization of the Precipitates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FESEM | Field emission scanning electron microscope |
| EDX | Energy dispersive X-ray system |
| XRD | X-ray diffraction |
| SS | Sewage sludge |
| M | Solid fraction of pig manure |
| PR | Phosphate rock |
| N | Nitrogen |
| P | Phosphorus |
References
- Neset, T.S.S.; Cordell, D. Global phosphorus scarcity: Identifying synergies for a sustainable future. J. Sci. Food Agric. 2012, 92, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resources. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Chtouki, M.; Naciri, R.; Oukarroum, A. A review on phosphorus drip fertigation in the Mediterranean region: Fundamentals. current situation. challenges. and perspectives. Heliyon 2024, 10, e25543. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Schenk, G.; Robinson, N.; John, S.; Dayananda, B.; Krishnan, V.; Adam, C.; Hermann, L.; Schmidt, S. The Circular Phosphorus Economy: Agronomic Performance of Recycled Fertilizers and Target Crops. J. Plant Nutr. Soil Sci. 2025, 188, 408–421. [Google Scholar] [CrossRef]
- Tan, M.; Hou, Y.; Zhang, T.; Ma, Y.; Long, W.; Gao, C.; Liu, P.; Fan, Q.; Dai, G.; Shi, S.; et al. Relationships between livestock density and soil phosphorus contents–County and farm level analyses. Catena 2023, 222, 106817. [Google Scholar] [CrossRef]
- Günther, S.; Grunert, M.; Müller, S. Overview of recent advances in phosphorus recovery for fertilizer production. Eng. Life Sci. 2018, 18, 434–439. [Google Scholar] [CrossRef]
- Gell, K.; Ruijter, F.J.; Kuntke, P.; Graaff, M.; Smit, A.L. Safety and effectiveness of struvite from black water and urine as a phosphorus fertilizer. J. Agric. Sci. 2011, 3, 67–80. [Google Scholar] [CrossRef]
- Rahman, Z.A.; Gikonyo, E.; Silek, B.; Goh, K.; Soltangheisi, A. Evaluation of phosphate rock sources and rate of application on oil palm yield grown on peat soils of Sarawak, Malaysia. J. Agron. 2014, 13, 12–22. [Google Scholar] [CrossRef]
- Iftikhar, A.; Tao, W. Recovery of struvite for organic production: Mineral-based magnesium supplementation and pH elevation. J. Clean. Prod. 2024, 452, 142244. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2023/121 of 17 January 2023 amending and correcting Implementing Regulation (EU) 2021/1165 authorising certain products and substances for use in organic production and establishing their lists. Off. J. Eur. Union 2023, 16, 24–31. [Google Scholar]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite from farm, municipal and industrial waste: Feedstock suitability, methods and pre-treatments. Waste Manag. 2016, 49, 437–454. [Google Scholar] [CrossRef]
- Ramaswamy, J.; Solaiappan, V.; Albasher, G.; Alamri, O.; Alsultan, N.; Sathiasivan, K. Process optimization of struvite recovered from slaughterhouse wastewater and its fertilizing efficacy in amendment of biofertilizer. Environ. Res. 2022, 211, 113011. [Google Scholar] [CrossRef] [PubMed]
- Turker, M.; Celen, I. Chemical equilibrium model of struvite precipitation from anaerobic digester effluents. Turk. J. Eng. Environ. Sci. 2010, 34, 39–48. [Google Scholar]
- Huang, H.; Song, Q.; Wang, W.; Wu, S.; Dai, J. Treatment of anaerobic digester effluents of nylon wastewater through chemical precipitation and a sequencing batch reactor process. J. Environ. Manage. 2012, 30, 68–74. [Google Scholar] [CrossRef]
- Ryu, H.D.; Lee, S.I. Application of struvite precipitation as a pre-treatment in treating swine wastewater. Process Biochem. 2010, 45, 563–572. [Google Scholar] [CrossRef]
- Liu, Y.H.; Kwag, J.H.; Kim, J.H.; Ra, C.S. Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination 2011, 277, 364–369. [Google Scholar] [CrossRef]
- Huang, H.M.; Xiao, X.M.; Yang, L.P.; Yan, B. Removal of ammonium from rare-earth wastewater using natural brucite as a magnesium source of struvite precipitation. Water Sci. Technol. 2011, 63, 468–474. [Google Scholar] [CrossRef]
- Huang, H.M.; Xu, C.; Zhang, W. Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour. Technol. 2011, 102, 2523–2528. [Google Scholar] [CrossRef]
- Zhang, D.M.; Chen, Y.X.; Jilani, G.; Wu, W.X.; Liu, W.; Hen, J. Optimization of struvite crystallization protocol for pretreating the swine wastewater and its impact on subsequent anaerobic biodegradation of pollutants. Bioresour. Technol. 2012, 116, 386–395. [Google Scholar] [CrossRef]
- Nagarajan, A.; Goyette, B.; Raghavan, V.; Bhaskar, A.; Rajagopal, R. Nutrient recovery via struvite production from livestock manure-digestate streams: Towards closed loop bio-economy. Process Saf. Environ. Prot. 2023, 171, 273–288. [Google Scholar] [CrossRef]
- Nagarajan, A.; Chen, Y.; Raghavan, V.; Goyette, B.; Rajagopal, R. Sustainable nutrient recovery through struvite precipitation from poultry and multi-substrate agricultural waste digestates. Bioresour. Technol. Rep. 2024, 27, 101924. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, T.; Frear, C.; Bowers, K.; Harrison, J.; Chen, S. Phosphorus Recovery Technology in Conjunction with Dairy Anaerobic Digestion. Washington State University Centre for Sustaining Agriculture and Natural Resources. Research Report 2010-001. Available online: https://wpcdn.web.wsu.edu/cahnrs/uploads/sites/44/CSANR2010-001.Ch07.pdf (accessed on 23 September 2025).
- Shen, Y.; Ogejo, J.A.; Bowers, K.E. Abating the effects of calcium on struvite precipitation in liquid dairy manure. Trans. ASABE 2011, 54, 325–336. [Google Scholar] [CrossRef]
- Munir, M.T.; Li, B.; Mardon, I.; Young, B.R.; Baroutian, S. Integrating wet oxidation and struvite precipitation for sewage sludge treatment and phosphorus recovery. J. Clean. Prod. 2019, 232, 1043–1052. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Kruse, A.; Becker, G.C. Valorization of byproducts from hydrothermal liquefaction of sewage sludge and manure: The development of a struvite-producing unit for nutrient recovery. Energy Fuels 2021, 35, 9408–9423. [Google Scholar] [CrossRef]
- Cañas, J.; Álvarez-Torrellas, S.; Hermana, B.; García, J. Phosphorus Recovery from Sewage Sludge as Struvite. Water 2023, 15, 2382. [Google Scholar] [CrossRef]
- Piveteau, S.; Picard, S.; Dabert, P.; Daumer, M.L. Dissolution of particulate phosphorus in pig slurry through biological acidification: A critical step for maximum phosphorus recovery as struvite. Water Res. 2017, 124, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Capdevielle, A.; Sýkorová, E.; Béline, F.; Daumer, M.L. Effects of organic matter on crystallization of struvite in biologically treated swine wastewater. Environ. Technol. 2016, 37, 880–892. [Google Scholar] [CrossRef]
- Daumer, M.L.; Picard, S.; Saint-Cast, P.; Dabert, P. Technical and economical assessment of formic acid to recycle phosphorus from pig slurry by a combined acidification–precipitation process. J. Hazard. Mater. 2010, 180, 361–365. [Google Scholar] [CrossRef]
- Szögi, A.A.; Vanotti, M.B.; Hunt, P.G. Phosphorus recovery from pig manure solids prior to land 492 application. J. Environ. Manag. 2015, 157, 1–7. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R.; Garrido-Baserba, M. Economic Feasibility Study for Phosphorus Recovery Processes. AMBIO 2011, 40, 408–416. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Zessner, M. Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour. Conserv. Recycl. 2015, 105, 325–346. [Google Scholar] [CrossRef]
- Nykänen, A.M.; Hämäläinen, N.; Kostia, S.; Mikola, J.; Romantschuk, M. Reduction of odorants in swine manure by carbohydrate and bacterial amendments. J. Environ. Qual. 2010, 39, 678–685. [Google Scholar] [CrossRef]
- Regueiro, I.; Gómez-Muñoz, B.; Lübeck, M.; Hjorth, M.; Jensen, L.S. Bio-acidification of animal slurry: Efficiency, stability and the mechanisms involved. Bioresour. Technol. Rep. 2022, 19, 101135. [Google Scholar] [CrossRef]
- Bareha, Y.; Saoudi, M.; Santellani, A.C.; Le Bihan, A.; Picard, S.; Mebarki, C.; Cunha, M.; Daumer, M.L. Use of fermentation processes for improving the dissolution of phosphorus and its recovery from waste activated sludge. Environ. Technol. 2022, 43, 1307–1317. [Google Scholar] [CrossRef]
- Braak, E.; Auby, S.; Piveteau, S.; Guilayn, F.; Daumer, M.-L. Phosphorus recycling potential assessment by a biological test applied to wastewater sludge. Environ. Technol. 2016, 37, 1398–1407. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, J.; Zhang, T.; Zhao, J.; Xu, R.; Zhang, Q.; Fang, F.; Luo, J. A novel approach of synchronously recovering phosphorus as vivianite and volatile fatty acids during waste activated sludge and food waste co-fermentation: Performance and mechanisms. Bioresour. Technol. 2020, 305, 123078. [Google Scholar] [CrossRef] [PubMed]
- Piveteau, S.; Daumer, M.L.; Dabert, P. Phosphorus recovery from pig slurry through biological acidification and re-crystallization as struvite. In Proceedings of the Orbit 2016, Organic Resources and Biological Treatment, 10 th International Conference on “Circular Economy and Organic Waste”, Heraklion, Greece, 25–28 May 2016; p. 11. [Google Scholar]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in Struvite Precipitation Technologies for Nutrients Removal and Recovery from Aqueous Waste and Wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Weeks, J.J.; Hettiarachchi, G.M. A Review of the Latest in Phosphorus Fertilizer Technology: Possibilities and Pragmatism. J. Environ. Qual. 2019, 48, 1300–1313. [Google Scholar] [CrossRef]
- Yan, Y.; Kallikazarou, N.I.; Nisiforou, O.; Shang, Q.; Fu, D.; Antoniou, M.G.; Fotidis, I.A. Phosphorus recovery through struvite crystallization from real wastewater: Bridging gaps from lab to market. Bioresour. Technol. 2025, 427, 132408. [Google Scholar] [CrossRef]
- Kim, D.; Min, K.J.; Yu, M.S.; Lee, K.; Kweon, J.; Park, K.Y. Use of concentrate water from seawater desalination plant as magnesium sources for struvite formation by using anaerobically digested effluent of swine wastewater. Desalination Water Treat. 2016, 57, 26751–26757. [Google Scholar] [CrossRef]
- Cichy, B.; Kużdżał, E.; Krztoń, H. Phosphorus recovery from acidic wastewater by hydroxyapatite precipitation. J. Environ. Manag. 2019, 232, 421–427. [Google Scholar] [CrossRef]
- García Becerra, F.Y.; Acosta, E.J.; Grant Allen, D. Alkaline extraction of wastewater activated sludge biosolids. Bioresour. Technol. 2010, 101, 6972–6980. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szogi, A.A. Extraction of Amino Acids and Phosphorus from Biological Materials. US Patent 10,150,711, 11 December 2018. [Google Scholar]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Morales, A.B.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Moral, R.; Ros, M.; Pascual, J.A. Agri-food sludge management using different co-composting strategies: Study of the added value of the composts obtained. J. Clean. Prod. 2016, 121, 186–197. [Google Scholar] [CrossRef]
- Coelho, C.; Roqueiro, N.; Hotza, D. Rational mineralogical analysis of ceramics. Mater. Lett. 2002, 52, 394–398. [Google Scholar] [CrossRef]
- Su, C.; Wang, S.; Meng, J.; Zhan, X. Enhanced phosphorus release from pig manure by co-fermentation with food waste. Bioprocess Biosyst. Eng. 2025, 48, 427–435. [Google Scholar] [CrossRef]
- Wilfert, P.; Suresh Kumar, P.; Korving, L.; Witkamp, G.J.; van Loosdrecht, M.C.M. The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: A review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef]
- Hernández, D.; Molinuevo-Salces, B.; Riaño, B.; Larrán-García, A.M.; Tomás-Almenar, C.; García-González, M.C. Recovery of protein concentrates from microalgal biomass grown in manure for fish feed and valorization of the by-products through anaerobic digestion. Front. Sustain. Food Syst. 2018, 2, 28. [Google Scholar] [CrossRef]
- Lorenzo-Hernando, A.; Ruiz-Vegas, J.; Vega-Alegre, M.; Bolado-Rodríguez, S. Recovery of proteins from biomass grown in pig manure microalgae-based treatment plants by alkaline hydrolysis and acidic precipitation. Bioresour. Technol. 2019, 273, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Calvo-de Diego, P.; García-González, M.C.; Riaño, B.; Vanotti, M.B.; Sánchez-Bascones, M.; Molinuevo-Salces, B. A biorefinery approach to recover nutrients, proteins, and methane from raw swine manure. J. Environ. Manag. 2025, 389, 126254. [Google Scholar] [CrossRef]
- Guilayn, F.; Braak, E.; Piveteau, S.; Daumer, M.L. Sequencing biological acidification of waste-activated sludge aiming to optimize phosphorus dissolution and recovery. Environ. Technol. 2017, 38, 1399–1407. [Google Scholar] [CrossRef]
- Shih, Y.J.; Abarca, R.R.M.; de Luna, M.D.G.; Huang, Y.H.; Lu, M.C. Recovery of phosphorus from synthetic wastewaters by struvite crystallization in a fluidized-bed reactor: Effects of pH, phosphate concentration and coexisting ions. Chemosphere 2017, 173, 466–473. [Google Scholar] [CrossRef]
- Korving, L.; Van Loosdrecht, M.; Wilfert, P. Effect of Iron on Phosphate Recovery from Sewage Sludge. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019. [Google Scholar]
- EU. Commission Delegated Regulation (EU) 2021/2086 of 5 July 2021 amending Annexes II and IV to Regulation (EU) 2019/1009 of the European Parliament and of the Council for the purpose of adding precipitated phosphate salts and derivates as a component material category in EU fertilising products. Off. J. Eur. Union 2021. Available online: http://data.europa.eu/eli/reg_del/2021/2086/oj (accessed on 23 September 2025).
- EU. Regulation (EU) 2019/1009, 2019. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertiliser products, amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, L170, 1. [Google Scholar]
- Xu, H.; Guo, L.; Zhao, Y.; Gao, M.; Jin, C.; Ji, J.; She, Z. Accelerating phosphorus release from waste activated sludge by nitrilotriacetic acid addition during anaerobic fermentation process and struvite recovery. Process Saf. Environ. Prot. 2021, 147, 1066–1076. [Google Scholar] [CrossRef]
- Zeng, F.; Zhao, Q.; Jin, W.; Liu, Y.; Wang, K.; Lee, D. Struvite precipitation fromanaerobic sludge supernatant and mixed fresh/stale human urine. Chem. Eng. J. 2018, 344, 254–261. [Google Scholar] [CrossRef]
- Oliveira, V.; Labrincha, J.; Dias-Ferreira, C. Extraction of phosphorus and struvite production from the anaerobically digested organic fraction of municipal solid waste. J. Environ. Chem. Eng. 2018, 6, 2837–2845. [Google Scholar] [CrossRef]

| M | SS1 | SS2 | SS3 | |
|---|---|---|---|---|
| Dry weight (%) | 27.6 ± 0.3 | 19.8 ± 5.9 | 22.2 ± 0.1 | 24.7 ± 0.3 |
| pH | 8.9 ± 0.4 | 6.2 ± 0.3 | 7.9 ± 0.0 | 7.2 ± 0.2 |
| EC (dS m−1) | 2.3 ± 0.0 | 1.0 ± 0.2 | 1.7 ± 0.4 | 1.4 ± 0.7 |
| TC (%) | 29.6 ± 0.4 | 32.7 ± 0.5 | 30.0 ± 0.4 | 38.4 ± 0.3 |
| TN (%) | 2.3 ± 0.8 | 6.1 ± 0.2 | 5.1 ± 0.2 | 7.1 ± 0.0 |
| NH4 (g kg−1) | 0.9 ± 0.1 | 2.3 ± 0.7 | 4.0 ± 0.7 | 2.1 ± 0.5 |
| NO3 (mg kg−1) | 185 ± 35 | 599 ± 20 | 175 ± 21 | 109 ± 16 |
| P (%) | 3.9 ± 0.2 | 3.3 ± 0.4 | 3.3 ± 0.5 | 2.5 ± 0.5 |
| K (%) | 1.6 ± 0.5 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.2 |
| Ca (%) | 5.9 ± 0.1 | 4.7 ± 1.7 | 6.1 ± 0.3 | 3.2 ± 0.3 |
| Mg (%) | 3.1 ± 0.8 | 0.7 ± 0.3 | 0.7 ± 0.1 | 0.8 ± 0.3 |
| Na (%) | 0.5 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Fe (g kg−1) | 3.67 ± 0.01 | 18.4 ± 0.1 | 45.7 ± 0.2 | 4.65 ± 0.01 |
| Cu (mg kg−1) | 317 ± 1 | 172 ± 2 | 222 ± 0 | 183 ± 0 |
| Mn (mg kg−1) | 1198 ± 1 | 108 ± 0 | 177 ± 1 | 59 ± 2 |
| Zn (mg kg−1) | 1355 ± 0 | 2209 ± 0 | 1006 ± 1 | 1206 ± 0 |
| Cd (mg kg−1) | 0.31 ± 0.04 | 2.71 ± 0.80 | 1.35 ± 0.38 | 1.00 ± 0.25 |
| Cr (mg kg−1) | 13.2 ± 2.9 | 196 ± 2 | 97.3 ± 3.5 | 33.9 ± 2.1 |
| Hg (mg kg−1) | 0.66 ± 0.02 | 2.99 ± 0.03 | 1.81 ± 0.78 | 0.94 ± 0.31 |
| Ni (mg kg−1) | 19.9 ± 1.5 | 118 ± 2 | 145 ± 5 | 35.8 ± 0.3 |
| Pb (mg kg−1) | < 0.01 ± 0.00 | 67.9 ± 1.7 | 156 ± 0 | 42.7 ± 1.2 |
| Organic Waste | Initial pH | pH After 6 h | pH After 12 h | pH After 24 h | pH After 48 h |
|---|---|---|---|---|---|
| M1 | 8.73 | 7.51 | 6.49 | 5.24 | 5.06 |
| SS1 | 6.13 | 6.00 | 5.49 | 4.43 | 3.97 |
| SS2 | 7.10 | 6.32 | 5.60 | 4.87 | 4.13 |
| SS3 | 5.90 | 5.38 | 4.88 | 4.12 | 3.58 |
| P-M | P-SS1 | P-SS2 | P-SS3 | |
|---|---|---|---|---|
| TC (%) | 5.50 | 19.4 | 19.0 | 10.4 |
| TN (%) | 4.05 | 0.44 | 1.19 | 2.54 |
| P2O5 (%) | 17.1 | 13.2 | 10.7 | 16.6 |
| K (%) | 1.28 | 0.38 | 0.074 | 0.057 |
| Ca (%) | 2.5 | 10.3 | 5.3 | 5.1 |
| Mg (%) | 8.97 | 1.77 | 0.49 | 6.28 |
| Na (%) | 0.63 | 2.03 | 1.31 | 1.94 |
| Fe (g kg−1) | 0.73 | 28.5 | 17.6 | 12.0 |
| Cu (mg kg−1) | 47.0 | 7.9 | 20.0 | 3.6 |
| Mn (mg kg−1) | 1420 | 330 | 884 | 363 |
| Zn (mg kg−1) | 490 | 1079 | 137 | 86 |
| Cd (mg kg−1) | <0.01 | 1.64 | 3.93 | <0.01 |
| Cr (mg kg−1) | 3.76 | 37.8 | 108 | 22.3 |
| Ni (mg kg−1) | 4.73 | 103 | 5.50 | 7.68 |
| Pb (mg kg−1) | 2.11 | 11.6 | 44.6 | 6.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valverde-Vozmediano, L.; Martínez-Sabater, E.; Jordán, M.M.; Santateresa, E.; Sáez-Tovar, J.A.; Vanotti, M.B.; Bustamante, M.Á.; Moral, R. Sustainable Phosphorus and Protein Recovery from Different Organic Wastes: Process Optimization and Struvite Precipitation Potential. Agronomy 2025, 15, 2305. https://doi.org/10.3390/agronomy15102305
Valverde-Vozmediano L, Martínez-Sabater E, Jordán MM, Santateresa E, Sáez-Tovar JA, Vanotti MB, Bustamante MÁ, Moral R. Sustainable Phosphorus and Protein Recovery from Different Organic Wastes: Process Optimization and Struvite Precipitation Potential. Agronomy. 2025; 15(10):2305. https://doi.org/10.3390/agronomy15102305
Chicago/Turabian StyleValverde-Vozmediano, Lucía, Encarnación Martínez-Sabater, Manuel M. Jordán, Ernesto Santateresa, José Antonio Sáez-Tovar, Matias B. Vanotti, María Ángeles Bustamante, and Raúl Moral. 2025. "Sustainable Phosphorus and Protein Recovery from Different Organic Wastes: Process Optimization and Struvite Precipitation Potential" Agronomy 15, no. 10: 2305. https://doi.org/10.3390/agronomy15102305
APA StyleValverde-Vozmediano, L., Martínez-Sabater, E., Jordán, M. M., Santateresa, E., Sáez-Tovar, J. A., Vanotti, M. B., Bustamante, M. Á., & Moral, R. (2025). Sustainable Phosphorus and Protein Recovery from Different Organic Wastes: Process Optimization and Struvite Precipitation Potential. Agronomy, 15(10), 2305. https://doi.org/10.3390/agronomy15102305







