Quality or Quantity? Increasing Legume Yield Using Traditional Inoculants and Rhizobial Nod Factors in the Context of Inter-Strain Competition
Abstract
1. Introduction—The Importance of Biological Dinitrogen Reduction
2. Legume–Bacterial Symbiosis: Its Importance and the Mechanisms Involved
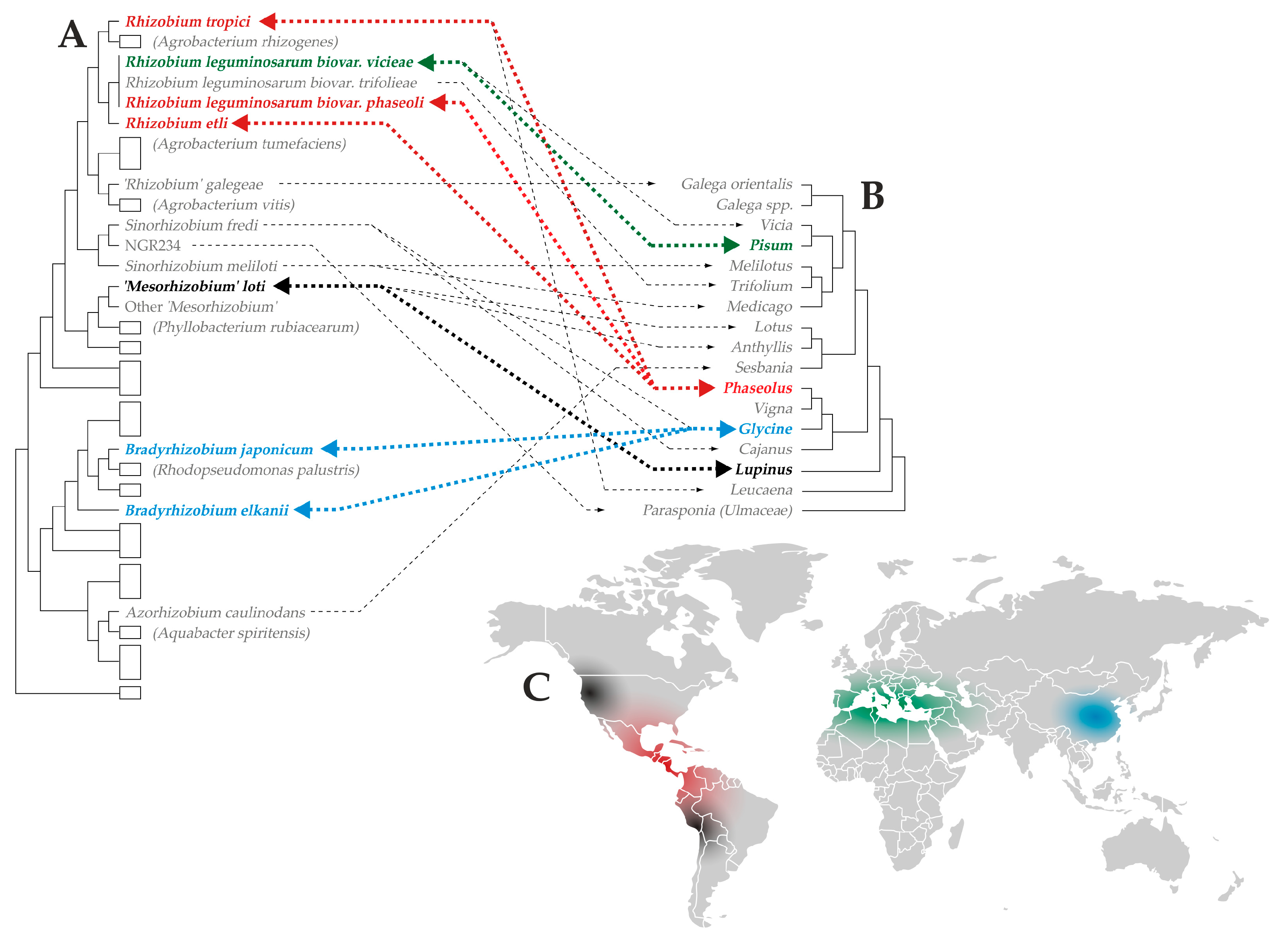
3. Rhizobial Populations: Abundance, Diversity, and Competition Between Strains
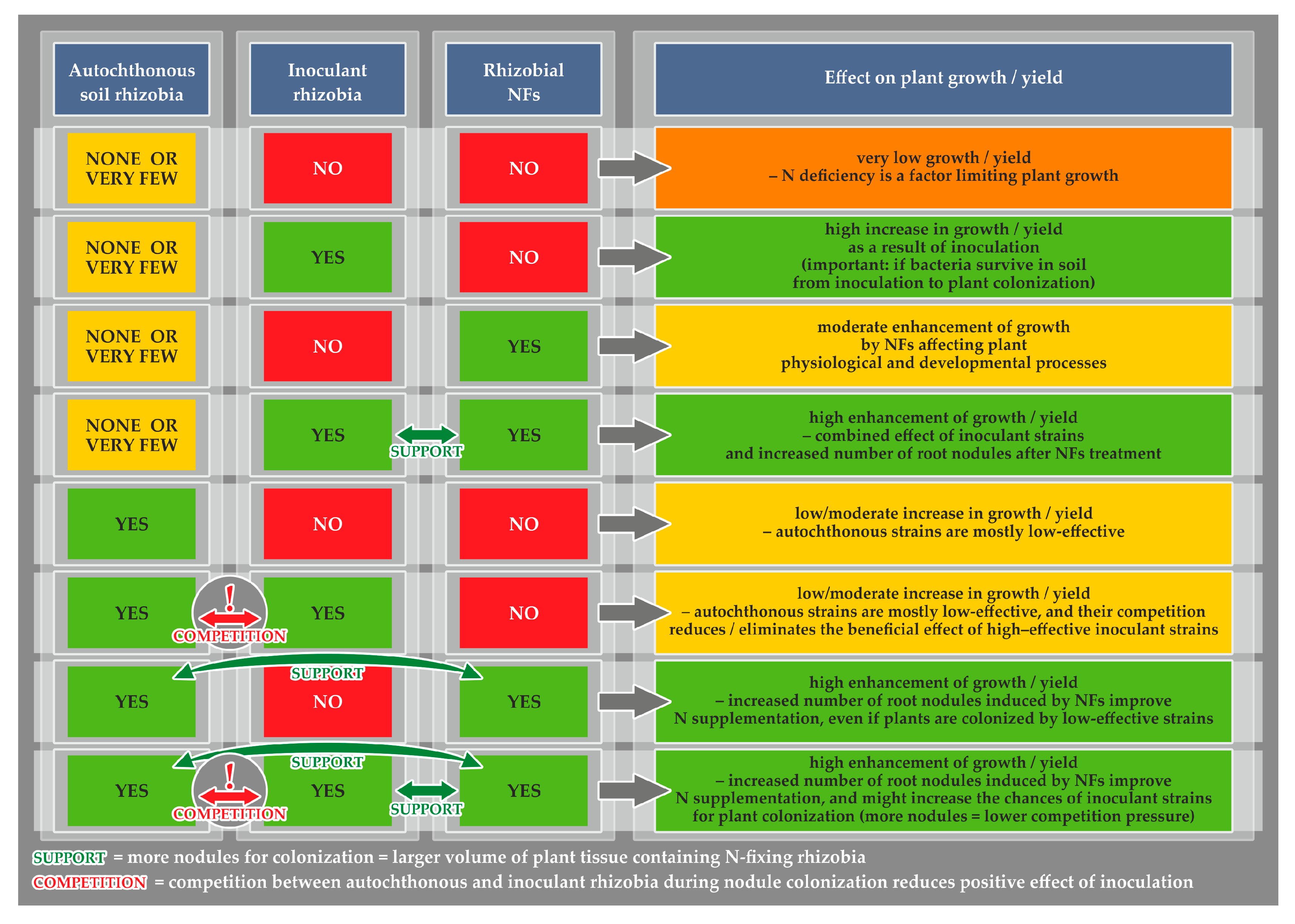
4. Traditional Rhizobial Inoculants and NF Preparations: Where, When, and How to Use Them to Increase the Yield of Legumes
5. Future Prospects—Extending NF Technology to Non-Legume Plants
6. Final Remarks
Funding
Conflicts of Interest
References
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Krug, E.C.; Winstanley, D. The Need for Comprehensive and Consistent Treatment of the Nitrogen Cycle in Nitrogen Cycling and Mass Balance Studies: I. Terrestrial Nitrogen Cycle. Sci. Total Environ. 2002, 293, 1–29. [Google Scholar] [CrossRef]
- Levine, J.S.; Augustsson, T.R.; Anderson, I.C.; Hoell, J.M. Tropospheric Sources of NOx: Lightning and Biology. Atmos. Environ. 1984, 18, 1797–1804. [Google Scholar] [CrossRef]
- Burris, R.H.; Roberts, G.P. Biological Nitrogen Fixation. Annu. Rev. Nutr. 1993, 13, 317–335. [Google Scholar] [CrossRef]
- Mancinelli, R.L. The Nature of Nitrogen: An Overview. Life Support. Biosph. Sci. 1996, 3, 17–24. [Google Scholar]
- Mancinelli, R.L.; McKay, C.P. The Evolution of Nitrogen Cycling. Orig. Life Evol. Biosph. 1988, 18, 311–325. [Google Scholar] [CrossRef]
- Navarro-González, R.; McKay, C.P.; Mvondo, D.N. A Possible Nitrogen Crisis for Archaean Life Due to Reduced Nitrogen Fixation by Lightning. Nature 2001, 412, 61–64. [Google Scholar] [CrossRef]
- Peoples, M.B.; Herridge, D.F.; Ladha, J.K. Biological Nitrogen Fixation: An Efficient Source of Nitrogen for Sustainable Agricultural Production? Plant Soil 1995, 174, 3–28. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Cheng, Q. Perspectives in Biological Nitrogen Fixation Research. J. Integr. Plant Biol. 2008, 50, 786–798. [Google Scholar] [CrossRef]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The Natural History of Nitrogen Fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Nitrogen Fixation and Diazotrophs—A Review. Rom. Biotechnol. Lett. 2021, 26, 2834–2845. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Baldock, J.; Peoples, M.B. Prospects and Problems of Simple Linear Models for Estimating Symbiotic N2 Fixation by Crop and Pasture Legumes. Plant Soil 2010, 329, 75–89. [Google Scholar] [CrossRef]
- Peoples, M.; Brockwell, J.; Herridge, D.; Rochester, I.; Alves, B.; Urquiaga, S.; Boddey, R.; Dakora, F.; Bhattarai, S.; Maskey, S.L.; et al. The Contributions of Nitrogen-Fixing Crop Legumes to the Productivity of Agricultural Systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Rajkumari, J.; Katiyar, P.; Dheeman, S.; Pandey, P.; Maheshwari, D.K. The Changing Paradigm of Rhizobial Taxonomy and Its Systematic Growth Upto Postgenomic Technologies. World J. Microbiol. Biotechnol. 2022, 38, 206. [Google Scholar] [CrossRef]
- Kuzmanović, N.; Fagorzi, C.; Mengoni, A.; Lassalle, F.; diCenzo, G.C. Taxonomy of Rhizobiaceae Revisited: Proposal of a New Framework for Genus Delimitation. Int. J. Syst. Evol. Microbiol. 2022, 72, 005243. [Google Scholar] [CrossRef]
- Ma, T.; Xue, H.; Piao, C.; Jiang, N.; Li, Y. Phylogenomic Reappraisal of the Family Rhizobiaceae at the Genus and Species Levels, Including the Description of Ectorhizobium quercum Gen. Nov., sp. Nov. Front. Microbiol. 2023, 14, 1207256. [Google Scholar] [CrossRef]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular Basis of Symbiotic Promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. Establishing Nitrogen-Fixing Symbiosis with Legumes: How Many Rhizobium Recipes? Trends Microbiol. 2009, 17, 458–466. [Google Scholar] [CrossRef]
- Rahimlou, S.; Bahram, M.; Tedersoo, L. Phylogenomics Reveals the Evolution of Root Nodulating Alpha- and Beta-Proteobacteria (Rhizobia). Microbiol. Res. 2021, 250, 126788. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Early Interactions between Legumes and Rhizobia: Disclosing Complexity in a Molecular Dialogue. J. Appl. Microbiol. 2007, 103, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Spaink, H.P. Root Nodulation and Infection Factors Produced by Rhizobial Bacteria. Annu. Rev. Microbiol. 2000, 54, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Fraysse, N.; Sharypova, L. Recent Advances in Studies on Structure and Symbiosis-Related Function of Rhizobial K-Antigens and Lipopolysaccharides. Mol. Plant Microbe Interact. 2005, 18, 899–905. [Google Scholar] [CrossRef]
- Fraysse, N.; Couderc, F.; Poinsot, V. Surface Polysaccharide Involvement in Establishing the Rhizobium-Legume Symbiosis. Eur. J. Biochem. 2003, 270, 1365–1380. [Google Scholar] [CrossRef]
- Skorupska, A.; Janczarek, M.; Marczak, M.; Mazur, A.; Król, J. Rhizobial Exopolysaccharides: Genetic Control and Symbiotic Functions. Microb. Cell Fact. 2006, 5, 7. [Google Scholar] [CrossRef]
- Veliz-Vallejos, D.F.; van Noorden, G.E.; Yuan, M.; Mathesius, U. A Sinorhizobium meliloti-Specific N-Acyl Homoserine Lactone Quorum-Sensing Signal Increases Nodule Numbers in Medicago truncatula Independent of Autoregulation. Front. Plant Sci. 2014, 5, 551. [Google Scholar] [CrossRef] [PubMed]
- Calatrava-Morales, N.; McIntosh, M.; Soto, M.J. Regulation Mediated by N-Acyl Homoserine Lactone Quorum Sensing Signals in the Rhizobium-Legume Symbiosis. Genes 2018, 9, 263. [Google Scholar] [CrossRef]
- Cooper, J.E. Multiple Responses of Rhizobia to Flavonoids During Legume Root Infection. Adv. Bot. Res. 2004, 41, 1–62. [Google Scholar] [CrossRef]
- Gutjahr, C.; Paszkowski, U. Weights in the Balance: Jasmonic Acid and Salicylic Acid Signaling in Root-Biotroph Interactions. Mol. Plant Microbe Interact. 2009, 22, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, H.; Ibrahim, R.K. Aldonic Acids: A Novel Family of Nod Gene Inducers of Mesorhizobium loti, Rhizobium lupini, and Sinorhizobium meliloti. MPMI 1998, 11, 988–998. [Google Scholar] [CrossRef]
- Yuenl, J.P.-Y.; Cassini, S.T.; Toledo, T.; Oliveira, D.; Nagem, T.J.; Staceyl, G. Xanthone Induction of Nod Gene Expression in Bradyrhizobium japonicum. Symbiosis 1995, 19, 131–140. [Google Scholar]
- Phillips, D.A.; Joseph, C.M.; Maxwell, C.A. Trigonelline and Stachydrine Released from Alfalfa Seeds Activate NodD2 Protein in Rhizobium meliloti. Plant Physiol. 1992, 99, 1526–1531. [Google Scholar] [CrossRef]
- Knee, E.M.; Gong, F.C.; Gao, M.; Teplitski, M.; Jones, A.R.; Foxworthy, A.; Mort, A.J.; Bauer, W.D. Root Mucilage from Pea and Its Utilization by Rhizosphere Bacteria as a Sole Carbon Source. Mol. Plant Microbe Interact. 2001, 14, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Yang, X.; Weston, L.A. The Role of Root Exudates and Allelochemicals in the Rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- D’Haeze, W.; Holsters, M. Nod Factor Structures, Responses, and Perception during Initiation of Nodule Development. Glycobiology 2002, 12, 79R–105R. [Google Scholar] [CrossRef]
- Spaink, H.P.; Sheeley, D.M.; van Brussel, A.A.; Glushka, J.; York, W.S.; Tak, T.; Geiger, O.; Kennedy, E.P.; Reinhold, V.N.; Lugtenberg, B.J. A Novel Highly Unsaturated Fatty Acid Moiety of Lipo-Oligosaccharide Signals Determines Host Specificity of Rhizobium. Nature 1991, 354, 125–130. [Google Scholar] [CrossRef]
- Spaink, H.P.; Bloemberg, G.V.; van Brussel, A.A.N.; Lugtenberg, B.J.J.; van der Drift, K.M.G.M.; Haverkamp, J.; Thomas-Oates, J.E. Host Specificity of Rhizobium leguminosarum Is Determined by the Hydrophobicity of Highly Unsaturated Fatty Acyl Moieties of the Nodulation Factors. Mol. Plant-Microbe Interact. 1995, 8, 155–164. [Google Scholar] [CrossRef]
- Mergaert, P.; Van Montagu, M.; Holsters, M. Molecular Mechanisms of Nod Factor Diversity. Mol. Microbiol. 1997, 25, 811–817. [Google Scholar] [CrossRef]
- Downie, J.A.; Walker, S.A. Plant Responses to Nodulation Factors. Curr. Opin. Plant Biol. 1999, 2, 483–489. [Google Scholar] [CrossRef]
- Walker, L.; Lagunas, B.; Gifford, M.L. Determinants of Host Range Specificity in Legume-Rhizobia Symbiosis. Front. Microbiol. 2020, 11, 585749. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Andrews, M.E. Specificity in Legume-Rhizobia Symbioses. Int. J. Mol. Sci. 2017, 18, 705. [Google Scholar] [CrossRef]
- Krönauer, C.; Radutoiu, S. Understanding Nod Factor Signalling Paves the Way for Targeted Engineering in Legumes and Non-Legumes. Curr. Opin. Plant Biol. 2021, 62, 102026. [Google Scholar] [CrossRef] [PubMed]
- Ghantasala, S.; Roy Choudhury, S. Nod Factor Perception: An Integrative View of Molecular Communication during Legume Symbiosis. Plant Mol. Biol. 2022, 110, 485–509. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Downie, J.A. Calcium, Kinases and Nodulation Signalling in Legumes. Nat. Rev. Mol. Cell Biol. 2004, 5, 566–576. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Downie, J.A. Nuclear Calcium Changes at the Core of Symbiosis Signalling. Curr. Opin. Plant Biol. 2006, 9, 351–357. [Google Scholar] [CrossRef]
- Lee, A.; Hirsch, A.M. Signals and Responses: Choreographing the Complex Interaction between Legumes and Alpha- and Beta-Rhizobia. Plant Signal. Behav. 2006, 1, 161–168. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.-H.; Lin, Y.-H.; Reid, D.E.; Gresshoff, P.M. Molecular Analysis of Legume Nodule Development and Autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef]
- Mathesius, U.; Schlaman, H.R.M.; Spaink, H.P.; Sautter, C.O.; Rolfe, B.G.; Djordjevic, M.A. Auxin Transport Inhibition Precedes Root Nodule Formation in White Clover Roots and Is Regulated by Flavonoids and Derivatives of Chitin Oligosaccharides. Plant J. 1998, 14, 23–24. [Google Scholar] [CrossRef]
- Breakspear, A.; Liu, C.; Roy, S.; Stacey, N.; Rogers, C.; Trick, M.; Morieri, G.; Mysore, K.S.; Wen, J.; Oldroyd, G.E.D.; et al. The Root Hair “Infectome” of Medicago truncatula Uncovers Changes in Cell Cycle Genes and Reveals a Requirement for Auxin Signaling in Rhizobial Infection. Plant Cell 2014, 26, 4680–4701. [Google Scholar] [CrossRef]
- Herrbach, V.; Chirinos, X.; Rengel, D.; Agbevenou, K.; Vincent, R.; Pateyron, S.; Huguet, S.; Balzergue, S.; Pasha, A.; Provart, N.; et al. Nod Factors Potentiate Auxin Signaling for Transcriptional Regulation and Lateral Root Formation in Medicago truncatula. J. Exp. Bot. 2017, 68, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Reid, D.E.; Lorenc, M.T.; Stiller, J.; Edwards, D.; Gresshoff, P.M.; Ferguson, B.J. Transient Nod Factor-Dependent Gene Expression in the Nodulation-Competent Zone of Soybean (Glycine max [L.] Merr.) Roots. Plant Biotechnol. J. 2012, 10, 995–1010. [Google Scholar] [CrossRef]
- van Zeijl, A.; Op den Camp, R.H.M.; Deinum, E.E.; Charnikhova, T.; Franssen, H.; Op den Camp, H.J.M.; Bouwmeester, H.; Kohlen, W.; Bisseling, T.; Geurts, R. Rhizobium Lipo-Chitooligosaccharide Signaling Triggers Accumulation of Cytokinins in Medicago truncatula Roots. Mol. Plant 2015, 8, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Jardinaud, M.-F.; Boivin, S.; Rodde, N.; Catrice, O.; Kisiala, A.; Lepage, A.; Moreau, S.; Roux, B.; Cottret, L.; Sallet, E.; et al. A Laser Dissection-RNAseq Analysis Highlights the Activation of Cytokinin Pathways by Nod Factors in the Medicago truncatula Root Epidermis. Plant Physiol. 2016, 171, 2256–2276. [Google Scholar] [CrossRef]
- Prudent, M.; Salon, C.; Smith, D.L.; Emery, R.J.N. Nod Factor Supply under Water Stress Conditions Modulates Cytokinin Biosynthesis and Enhances Nodule Formation and N Nutrition in Soybean. Plant Signal. Behav. 2016, 11, e1212799. [Google Scholar] [CrossRef]
- Larrainzar, E.; Riely, B.K.; Kim, S.C.; Carrasquilla-Garcia, N.; Yu, H.-J.; Hwang, H.-J.; Oh, M.; Kim, G.B.; Surendrarao, A.K.; Chasman, D.; et al. Deep Sequencing of the Medicago truncatula Root Transcriptome Reveals a Massive and Early Interaction between Nodulation Factor and Ethylene Signals. Plant Physiol. 2015, 169, 233–265. [Google Scholar] [CrossRef]
- Damiani, I.; Drain, A.; Guichard, M.; Balzergue, S.; Boscari, A.; Boyer, J.-C.; Brunaud, V.; Cottaz, S.; Rancurel, C.; Da Rocha, M.; et al. Nod Factor Effects on Root Hair-Specific Transcriptome of Medicago truncatula: Focus on Plasma Membrane Transport Systems and Reactive Oxygen Species Networks. Front. Plant Sci. 2016, 7, 794. [Google Scholar] [CrossRef]
- Fedorova, M.; van de Mortel, J.; Matsumoto, P.A.; Cho, J.; Town, C.D.; VandenBosch, K.A.; Gantt, J.S.; Vance, C.P. Genome-Wide Identification of Nodule-Specific Transcripts in the Model Legume Medicago truncatula. Plant Physiol. 2002, 130, 519–537. [Google Scholar] [CrossRef] [PubMed]
- El Yahyaoui, F.; Küster, H.; Ben Amor, B.; Hohnjec, N.; Pühler, A.; Becker, A.; Gouzy, J.; Vernié, T.; Gough, C.; Niebel, A.; et al. Expression Profiling in Medicago truncatula Identifies More than 750 Genes Differentially Expressed during Nodulation, Including Many Potential Regulators of the Symbiotic Program. Plant Physiol. 2004, 136, 3159–3176. [Google Scholar] [CrossRef]
- de Carvalho, G.A.B.; Batista, J.S.S.; Marcelino-Guimarães, F.C.; Costa do Nascimento, L.; Hungria, M. Transcriptional Analysis of Genes Involved in Nodulation in Soybean Roots Inoculated with Bradyrhizobium japonicum Strain CPAC 15. BMC Genom. 2013, 14, 153. [Google Scholar] [CrossRef]
- Wang, N.; Khan, W.; Smith, D.L. Changes in Soybean Global Gene Expression after Application of Lipo-Chitooligosaccharide from Bradyrhizobium japonicum under Sub-Optimal Temperature. PLoS ONE 2012, 7, e31571. [Google Scholar] [CrossRef]
- Subramanian, S.; Ricci, E.; Souleimanov, A.; Smith, D.L. A Proteomic Approach to Lipo-Chitooligosaccharide and Thuricin 17 Effects on Soybean GerminationUnstressed and Salt Stress. PLoS ONE 2016, 11, e0160660. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How Rhizobial Symbionts Invade Plants: The Sinorhizobium-Medicago Model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef]
- Martínez-Abarca, F.; Herrera-Cervera, J.A.; Bueno, P.; Sanjuan, J.; Bisseling, T.; Olivares, J. Involvement of Salicylic Acid in the Establishment of the Rhizobium meliloti-Alfalfa Symbiosis. MPMI 1998, 11, 153–155. [Google Scholar] [CrossRef]
- Hirsch, A.M. Developmental Biology of Legume Nodulation. New Phytol. 1992, 122, 211–237. [Google Scholar] [CrossRef]
- Brewin, N.J. Plant Cell Wall Remodelling in the Rhizobium–Legume Symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316. [Google Scholar] [CrossRef]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The Molecular Network Governing Nodule Organogenesis and Infection in the Model Legume Lotus japonicus. Nat. Commun. 2010, 1, 10. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume Nodulation: The Host Controls the Party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and Development of the Legume-Rhizobial Symbiotic Interface in Infection Threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszyński, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H.; et al. Receptor-Mediated Exopolysaccharide Perception Controls Bacterial Infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef]
- Quilbé, J.; Montiel, J.; Arrighi, J.-F.; Stougaard, J. Molecular Mechanisms of Intercellular Rhizobial Infection: Novel Findings of an Ancient Process. Front. Plant Sci. 2022, 13, 922982. [Google Scholar] [CrossRef]
- Ibáñez, F.; Wall, L.; Fabra, A. Starting Points in Plant-Bacteria Nitrogen-Fixing Symbioses: Intercellular Invasion of the Roots. J. Exp. Bot. 2017, 68, 1905–1918. [Google Scholar] [CrossRef]
- Sharma, V.; Bhattacharyya, S.; Kumar, R.; Kumar, A.; Ibañez, F.; Wang, J.; Guo, B.; Sudini, H.K.; Gopalakrishnan, S.; DasGupta, M.; et al. Molecular Basis of Root Nodule Symbiosis between Bradyrhizobium and “Crack-Entry” Legume Groundnut (Arachis hypogaea L.). Plants 2020, 9, 276. [Google Scholar] [CrossRef]
- Den Herder, G.; Parniske, M. The Unbearable Naivety of Legumes in Symbiosis. Curr. Opin. Plant Biol. 2009, 12, 491–499. [Google Scholar] [CrossRef]
- Popp, C.; Ott, T. Regulation of Signal Transduction and Bacterial Infection during Root Nodule Symbiosis. Curr. Opin. Plant Biol. 2011, 14, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Timmers, A.C.; Soupène, E.; Auriac, M.C.; de Billy, F.; Vasse, J.; Boistard, P.; Truchet, G. Saprophytic Intracellular Rhizobia in Alfalfa Nodules. Mol. Plant Microbe Interact. 2000, 13, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.R.; Golus, J.; Marek-Kozaczuk, M.M.; Skorupska, A. Symbiosis-Stage Associated Alterations in Quorum Sensing Autoinducer Molecules Biosynthesis in Sinorhizobium meliloti. Plant Soil 2010, 329, 399–410. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Hirsch, A.M. The Nodule Microbiome: N2-Fixing Rhizobia Do Not Live Alone. Phytobiomes J. 2017, 1, 70–82. [Google Scholar] [CrossRef]
- Silva, C.; Kan, F.L.; Martínez-Romero, E. Population Genetic Structure of Sinorhizobium meliloti and S. medicae Isolated from Nodules of Medicago spp. in Mexico. FEMS Microbiol. Ecol. 2007, 60, 477–489. [Google Scholar] [CrossRef]
- Duodu, S.; Carlsson, G.; Huss-Danell, K.; Svenning, M.M. Large Genotypic Variation but Small Variation in N2 Fixation among Rhizobia Nodulating Red Clover in Soils of Northern Scandinavia. J. Appl. Microbiol. 2007, 102, 1625–1635. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, E.-T.; Zhao, L.; Chen, W.-M.; Wei, G.-H. Diversity and Distribution of Rhizobia Nodulated with Phaseolus vulgaris in Two Ecoregions of China. Soil Biol. Biochem. 2014, 78, 128–137. [Google Scholar] [CrossRef]
- Drew, E.A.; Ballard, R.A. Improving N2 Fixation from the Plant down: Compatibility of Trifolium subterraneum L. Cultivars with Soil Rhizobia Can Influence Symbiotic Performance. Plant Soil 2010, 327, 261–277. [Google Scholar] [CrossRef]
- Andrade, D.S.; Murphy, P.J.; Giller, K.E. The Diversity of Phaseolus-Nodulating Rhizobial Populations Is Altered by Liming of Acid Soils Planted with Phaseolus vulgaris L. in Brazil. Appl. Environ. Microbiol. 2002, 68, 4025–4034. [Google Scholar] [CrossRef]
- Palmer, K.M.; Young, J.P. Higher Diversity of Rhizobium leguminosarum Biovar Viciae Populations in Arable Soils than in Grass Soils. Appl. Environ. Microbiol. 2000, 66, 2445–2450. [Google Scholar] [CrossRef]
- Lakzian, A.; Murphy, P.; Turner, A.; Beynon, J.L.; Giller, K.E. Rhizobium leguminosarum Bv. Viciae Populations in Soils with Increasing Heavy Metal Contamination: Abundance, Plasmid Profiles, Diversity and Metal Tolerance. Soil Biol. Biochem. 2002, 34, 519–529. [Google Scholar] [CrossRef]
- Beyene, D.; Kassa, S.; Ampy, F.; Asseffa, A.; Gebremedhin, T.; van Berkum, P. Ethiopian Soils Harbor Natural Populations of Rhizobia That Form Symbioses with Common Bean (Phaseolus vulgaris L.). Arch. Microbiol. 2004, 181, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.; Marek-Kozaczuk, M.; Kubik-Komar, A.; Skorupska, A. Increased Metabolic Potential of Rhizobium spp. Is Associated with Bacterial Competitiveness. Can. J. Microbiol. 2007, 53, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.; Marek-Kozaczuk, M.; Mazur, A.; Kubik-Komar, A.; Skorupska, A. Genetic and Metabolic Divergence within a Rhizobium leguminosarum Bv. Trifolii Population Recovered from Clover Nodules. Appl. Environ. Microbiol. 2010, 76, 4593–4600. [Google Scholar] [CrossRef]
- Mazur, A.; Stasiak, G.; Wielbo, J.; Kubik-Komar, A.; Marek-Kozaczuk, M.; Skorupska, A. Intragenomic Diversity of Rhizobium leguminosarum Bv. Trifolii Clover Nodule Isolates. BMC Microbiol. 2011, 11, 123. [Google Scholar] [CrossRef]
- Mazur, A.; Stasiak, G.; Wielbo, J.; Koper, P.; Kubik-Komar, A.; Skorupska, A. Phenotype Profiling of Rhizobium leguminosarum Bv. Trifolii Clover Nodule Isolates Reveal Their Both Versatile and Specialized Metabolic Capabilities. Arch. Microbiol. 2013, 195, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Giraud, J.J.; Catroux, G. Genotypic Diversity of Sinorhizobium (Formerly Rhizobium) Meliloti Strains Isolated Directly from a Soil and from Nodules of Alfalfa (Medicago sativa) Grown in the Same Soil. FEMS Microbiol. Ecol. 1998, 25, 107–116. [Google Scholar] [CrossRef]
- Bottomley, P.J.; Cheng, H.H.; Strain, S.R. Genetic Structure and Symbiotic Characteristics of a Bradyrhizobium Population Recovered from a Pasture Soil. Appl. Environ. Microbiol. 1994, 60, 1754–1761. [Google Scholar] [CrossRef]
- Sachs, J.L.; Kembel, S.W.; Lau, A.H.; Simms, E.L. In Situ Phylogenetic Structure and Diversity of Wild Bradyrhizobium Communities. Appl. Environ. Microbiol. 2009, 75, 4727–4735. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.; Podleśna, A.; Kidaj, D.; Podleśny, J.; Skorupska, A. The Diversity of Pea Microsymbionts in Various Types of Soils and Their Effects on Plant Host Productivity. Microbes Environ. 2015, 30, 254–261. [Google Scholar] [CrossRef]
- Checcucci, A.; diCenzo, G.C.; Perrin, E.; Bazzicalupo, M.; Mengoni, A. Chapter 3—Genomic Diversity and Evolution of Rhizobia. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 37–46. ISBN 978-0-12-814849-5. [Google Scholar]
- Klepa, M.S.; Helene, L.C.F.; Hungria, M. Chapter 1.5—Understanding the Diversity and Evolution of Rhizobia from a Genomic Perspective. In Microbial Diversity in the Genomic Era (Second Edition); Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 89–115. ISBN 978-0-443-13320-6. [Google Scholar]
- Girvan, M.S.; Bullimore, J.; Pretty, J.N.; Osborn, A.M.; Ball, A.S. Soil Type Is the Primary Determinant of the Composition of the Total and Active Bacterial Communities in Arable Soils. Appl. Environ. Microbiol. 2003, 69, 1800–1809. [Google Scholar] [CrossRef]
- Wilson, R.A.; Handley, B.A.; Beringer, J.E. Bacteriocin Production and Resistance in a Field Population of Rhizobium leguminosarum Biovar Viciae. Soil Biol. Biochem. 1998, 30, 413–417. [Google Scholar] [CrossRef]
- Robleto, E.A.; Kmiecik, K.; Oplinger, E.S.; Nienhuis, J.; Triplett, E.W. Trifolitoxin Production Increases Nodulation Competitiveness of Rhizobium etli CE3 under Agricultural Conditions. Appl. Environ. Microbiol. 1998, 64, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Stuurman, N.; Pacios Bras, C.; Schlaman, H.R.; Wijfjes, A.H.; Bloemberg, G.; Spaink, H.P. Use of Green Fluorescent Protein Color Variants Expressed on Stable Broad-Host-Range Vectors to Visualize Rhizobia Interacting with Plants. Mol. Plant Microbe Interact. 2000, 13, 1163–1169. [Google Scholar] [CrossRef]
- Duodu, S.; Brophy, C.; Connolly, J.; Svenning, M.M. Competitiveness of a Native Rhizobium leguminosarum Biovar Trifolii Strain for Nodule Occupancy Is Manifested during Infection. Plant Soil 2009, 318, 117–126. [Google Scholar] [CrossRef]
- Wielbo, J.; Kuske, J.; Marek-Kozaczuk, M.; Skorupska, A. The Competition between Rhizobium leguminosarum Bv. Viciae Strains Progresses until Late Stages of Symbiosis. Plant Soil 2010, 337, 125–135. [Google Scholar] [CrossRef]
- Wielbo, J.; Marek-Kozaczuk, M.; Kidaj, D.; Skorupska, A. Competitiveness of Rhizobium leguminosarum Bv. Trifolii Strains in Mixed Inoculation of Clover (Trifolium pratense). Pol. J. Microbiol. 2011, 60, 43–49. [Google Scholar]
- Wielbo, J.; Marek-Kozaczuk, M.; Mazur, A.; Kubik-Komar, A.; Skorupska, A. The Structure and Metabolic Diversity of Population of Pea Microsymbionts Isolated from Root Nodules. Microbiol. Res. J. Int. 2011, 1, 55–69. [Google Scholar] [CrossRef]
- Bergersen, F.J.; Turner, G.L.; Appleby, C.A. Studies of the Physiological Role of Leghaemoglobin in Soybean Root Nodules. Biochim. Biophys. Acta 1973, 292, 271–282. [Google Scholar] [CrossRef]
- Simms, E.L.; Taylor, D.L. Partner Choice in Nitrogen-Fixation Mutualisms of Legumes and Rhizobia. Integr. Comp. Biol. 2002, 42, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.H.; Curnow, B.C.; Bergersen, F.J.; Brockwell, J.; Rominson, A.C. Studies of Field Populations of Rhizobium: Effectiveness of Strains of Rhizobium trifolii Associated with Trifolium subterraneum L. Pastures in South-Eastern Australia. Soil Biol. Biochem. 1975, 7, 95–102. [Google Scholar] [CrossRef]
- Burdon, J.J.; Gibson, A.H.; Searle, S.D.; Woods, M.J.; Brockwell, J. Variation in the Effectiveness of Symbiotic Associations between Native Rhizobia and Temperate Australian Acacia: Within-Species Interactions. J. Appl. Ecol. 1999, 36, 398–408. [Google Scholar] [CrossRef]
- Denison, R.F.; Kiers, E.T. Lifestyle Alternatives for Rhizobia: Mutualism, Parasitism, and Forgoing Symbiosis. FEMS Microbiol. Lett. 2004, 237, 187–193. [Google Scholar] [CrossRef]
- Denison, R.F.; Toby Kiers, E. Why Are Most Rhizobia Beneficial to Their Plant Hosts, Rather than Parasitic? Microbes Infect. 2004, 6, 1235–1239. [Google Scholar] [CrossRef]
- Streeter, J.G. Failure of Inoculant Rhizobia to Overcome the Dominance of Indigenous Strains for Nodule Formation. Can. J. Microbiol. 1994, 40, 513–522. [Google Scholar] [CrossRef]
- Toro, A. Nodulation Competitiveness in the Rhizobium-Legume Symbiosis. World J. Microbiol. Biotechnol. 1996, 12, 157–162. [Google Scholar] [CrossRef]
- Mathu, S.; Herrmann, L.; Pypers, P.; Matiru, V.; Mwirichia, R.; Lesueur, D. Potential of Indigenous Bradyrhizobia versus Commercial Inoculants to Improve Cowpea (Vigna unguiculata L. Walp.) and Green Gram (Vigna radiata L. Wilczek.) Yields in Kenya. Soil Sci. Plant Nutr. 2012, 58, 750–763. [Google Scholar] [CrossRef]
- Mellor, H.Y.; Glenn, A.R.; Arwas, R.; Dilworth, M.J. Symbiotic and Competitive Properties of Motility Mutants of Rhizobium trifolii TA1. Arch. Microbiol. 1987, 148, 34–39. [Google Scholar] [CrossRef]
- Vinuesa, P.; Neumann-Silkow, F.; Pacios-Bras, C.; Spaink, H.P.; Martínez-Romero, E.; Werner, D. Genetic Analysis of a pH-Regulated Operon from Rhizobium tropici CIAT899 Involved in Acid Tolerance and Nodulation Competitiveness. Mol. Plant Microbe Interact. 2003, 16, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Oresnik, I.J.; Twelker, S.; Hynes, M.F. Cloning and Characterization of a Rhizobium leguminosarum Gene Encoding a Bacteriocin with Similarities to RTX Toxins. Appl. Environ. Microbiol. 1999, 65, 2833–2840. [Google Scholar] [CrossRef]
- Streit, W.R.; Joseph, C.M.; Phillips, D.A. Biotin and Other Water-Soluble Vitamins Are Key Growth Factors for Alfalfa Root Colonization by Rhizobium meliloti 1021. Mol. Plant Microbe Interact. 1996, 9, 330–338. [Google Scholar] [CrossRef]
- Mabood, F.; Jung, W.J.; Smith, D.L. Signals in the Underground: Microbial Signaling and Plant Productivity. In Molecular Mechanisms of Plant and Microbe Coexistence; Nautiyal, C.S., Dion, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 291–318. ISBN 978-3-540-75575-3. [Google Scholar]
- Maj, D.; Wielbo, J.; Marek-Kozaczuk, M.; Skorupska, A. Response to Flavonoids as a Factor Influencing Competitiveness and Symbiotic Activity of Rhizobium leguminosarum. Microbiol. Res. 2010, 165, 50–60. [Google Scholar] [CrossRef]
- Oresnik, I.J.; Pacarynuk, L.A.; O’Brien, S.A.P.; Yost, C.K.; Hynes, M.F. Plasmid-Encoded Catabolic Genes in Rhizobium leguminosarum Bv. Trifolii: Evidence for a Plant-Inducible Rhamnose Locus Involved in Competition for Nodulation. MPMI 1998, 11, 1175–1185. [Google Scholar] [CrossRef]
- Hynes, M.F.; O’Connell, M.P. Host Plant Effect on Competition among Strains of Rhizobium leguminosarum. Can. J. Microbiol. 1990, 36, 864–869. [Google Scholar] [CrossRef]
- López-García, S.L.; Vázquez, T.E.E.; Favelukes, G.; Lodeiro, A.R. Rhizobial Position as a Main Determinant in the Problem of Competition for Nodulation in Soybean. Environ. Microbiol. 2002, 4, 216–224. [Google Scholar] [CrossRef]
- Bhuvaneswari, T.V.; Bhagwat, A.A.; Bauer, W.D. Transient Susceptibility of Root Cells in Four Common Legumes to Nodulation by Rhizobia. Plant Physiol. 1981, 68, 1144–1149. [Google Scholar] [CrossRef]
- Mutch, L.A.; Young, J.P.W. Diversity and Specificity of Rhizobium leguminosarum Biovar Viciae on Wild and Cultivated Legumes. Mol. Ecol. 2004, 13, 2435–2444. [Google Scholar] [CrossRef]
- Kiers, E.T.; Rousseau, R.A.; Denison, R.F. Measured Sanctions: Legume Hosts Detect Quantitative Variation in Rhizobium Cooperation and Punish Accordingly. Evol. Ecol. Res. 2006, 8, 1077–1086. [Google Scholar]
- Rangin, C.; Brunel, B.; Cleyet-Marel, J.-C.; Perrineau, M.-M.; Béna, G. Effects of Medicago truncatula Genetic Diversity, Rhizobial Competition, and Strain Effectiveness on the Diversity of a Natural Sinorhizobium Species Community. Appl. Environ. Microbiol. 2008, 74, 5653–5661. [Google Scholar] [CrossRef]
- Depret, G.; Laguerre, G. Plant Phenology and Genetic Variability in Root and Nodule Development Strongly Influence Genetic Structuring of Rhizobium leguminosarum Biovar Viciae Populations Nodulating Pea. New Phytol. 2008, 179, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial Inoculants: Reviewing the Past, Discussing the Present and Previewing an Outstanding Future for the Use of Beneficial Bacteria in Agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Catroux, G.; Hartmann, A.; Revellin, C. Trends in Rhizobial Inoculant Production and Use. Plant Soil 2001, 230, 21–30. [Google Scholar] [CrossRef]
- Deaker, R.; Roughley, R.J.; Kennedy, I.R. Legume Seed Inoculation Technology—A Review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Martyniuk, S.; Wozniakowska, A.; Martyniuk, M. Effect of Agricultural Practices on Populations of Rhizobium in Some Field Experiments. Bot. Lith. 1999, 3, 99–102. [Google Scholar]
- Martyniuk, S.; Oroń, J.; Martyniuk, M. Diversity and Numbers of Root-Nodule Bacteria (Rhizobia) in Polish Soils. Acta Soc. Bot. Pol. 2005, 74, 83–86. [Google Scholar] [CrossRef]
- Stephens, J.H.G.; Rask, H.M. Inoculant Production and Formulation. Field Crops Res. 2000, 65, 249–258. [Google Scholar] [CrossRef]
- Santos, M.S.; Rodrigues, T.F.; Nogueira, M.A.; Hungria, M. The Challenge of Combining High Yields with Environmentally Friendly Bioproducts: A Review on the Compatibility of Pesticides with Microbial Inoculants. Agronomy 2021, 11, 870. [Google Scholar] [CrossRef]
- Doyle, J.J. Phylogenetic Perspectives on Nodulation: Evolving Views of Plants and Symbiotic Bacteria. Trends Plant Sci. 1998, 3, 473–478. [Google Scholar] [CrossRef]
- Kohlmeier, M.G.; O’Hara, G.W.; Ramsay, J.P.; Terpolilli, J.J. Closed Genomes of Commercial Inoculant Rhizobia Provide a Blueprint for Management of Legume Inoculation. Appl. Environ. Microbiol. 2025, 91, e0221324. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Mortier, V.; Holsters, M.; Goormachtig, S. Never Too Many? How Legumes Control Nodule Numbers. Plant Cell Environ. 2012, 35, 245–258. [Google Scholar] [CrossRef]
- Basile, L.A.; Lepek, V.C. Legume–Rhizobium Dance: An Agricultural Tool That Could Be Improved? Microb. Biotechnol. 2021, 14, 1897–1917. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.L.; Quides, K.W.; Wendlandt, C.E. Legumes versus Rhizobia: A Model for Ongoing Conflict in Symbiosis. New Phytol. 2018, 219, 1199–1206. [Google Scholar] [CrossRef]
- Mendoza-Suárez, M.; Akyol, T.Y.; Nadzieja, M.; Andersen, S.U. Increased Diversity of Beneficial Rhizobia Enhances Faba Bean Growth. Nat. Commun. 2024, 15, 10673. [Google Scholar] [CrossRef] [PubMed]
- Ovtsyna, A.O.; Schultze, M.; Tikhonovich, I.A.; Spaink, H.P.; Kondorosi, E.; Kondorosi, A.; Staehelin, C. Nod Factors of Rhizobium leguminosarum Bv. Viciae and Their Fucosylated Derivatives Stimulate a Nod Factor Cleaving Activity in Pea Roots and Are Hydrolyzed in Vitro by Plant Chitinases at Different Rates. Mol. Plant Microbe Interact. 2000, 13, 799–807. [Google Scholar] [CrossRef] [PubMed]
- D’Haeze, W.; Mergaert, P.; Promé, J.C.; Holsters, M. Nod Factor Requirements for Efficient Stem and Root Nodulation of the Tropical Legume Sesbania Rostrata. J. Biol. Chem. 2000, 275, 15676–15684. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Phillips, D.A. Effects of a Seed Color Mutation on Rhizobial nod-Gene-Inducing Flavonoids and Nodulation in Common Bean. MPMI 1993, 6, 418–422. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Joseph, C.M.; Phillips, D.A. Flavone Limitations to Root Nodulation and Symbiotic Nitrogen Fixation in Alfalfa. Plant Physiol. 1987, 84, 1193–1196. [Google Scholar] [CrossRef]
- Begum, A.A.; Leibovitch, S.; Migner, P.; Zhang, F. Specific Flavonoids Induced Nod Gene Expression and Pre-Activated Nod Genes of Rhizobium leguminosarum Increased Pea (Pisum sativum L.) and Lentil (Lens culinaris L.) Nodulation in Controlled Growth Chamber Environments. J. Exp. Bot. 2001, 52, 1537–1543. [Google Scholar] [CrossRef]
- Zhang, F.; Smith, D.L. Preincubation of Bradyrhizobium japonicum with Genistein Accelerates Nodule Development of Soybean at Suboptimal Root Zone Temperatures. Plant Physiol. 1995, 108, 961–968. [Google Scholar] [CrossRef]
- Zhang, F.; Smith, D.L. Inoculation of Soybean (Glycine max. (L.) Merr.) with Genistein-Preincubated Bradyrhizobium japonicum or Genistein Directly Applied into Soil Increases Soybean Protein and Dry Matter Yield under Short Season Conditions. Plant Soil 1996, 179, 233–241. [Google Scholar] [CrossRef]
- Podleśny, J.; Wielbo, J.; Podleśna, A.; Kidaj, D.; Perzyński, A. Search for ecological methods to enlarge symbiotic nitrogen fixation efficiency by pea (Pisum sativum L.). J. Res. Appl. Agric. Eng. 2015, 60, 67–70. [Google Scholar]
- Leibovitch, S.; Migner, P.; Zhang, F.; Smith, D.L. Evaluation of the Effect of SoyaSignal Technology on Soybean Yield [Glycine max (L.) Merr.] under Field Conditions Over 6 Years in Eastern Canada and the Northern United States. J. Agron. Crop Sci. 2001, 187, 281–292. [Google Scholar] [CrossRef]
- Souleimanov, A.; Prithiviraj, B.; Smith, D.L. The Major Nod Factor of Bradyrhizobium japonicum Promotes Early Growth of Soybean and Corn. J. Exp. Bot. 2002, 53, 1929–1934. [Google Scholar] [CrossRef]
- Prithiviraj, B.; Zhou, X.; Souleimanov, A.; Khan, W.M.; Smith, D.L. A Host-Specific Bacteria-to-Plant Signal Molecule (Nod Factor) Enhances Germination and Early Growth of Diverse Crop Plants. Planta 2003, 216, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Maj, D.; Wielbo, J.; Marek-Kozaczuk, M.; Martyniuk, S.; Skorupska, A. Pretreatment of Clover Seeds with Nod Factors Improves Growth and Nodulation of Trifolium pratense. J. Chem. Ecol. 2009, 35, 479–487. [Google Scholar] [CrossRef]
- Kidaj, D.; Wielbo, J.; Skorupska, A. Nod Factors Stimulate Seed Germination and Promote Growth and Nodulation of Pea and Vetch under Competitive Conditions. Microbiol. Res. 2012, 167, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, D.U.; Somasundaram, E.; Amanullah, M.M. Effect of Rhizobial Nod Factors (Lipo chitooligosaccharide) on Seedling Growth of Blackgram under Salt Stress. Legume Res. 2017, 41, 159–162. [Google Scholar]
- Oláh, B.; Brière, C.; Bécard, G.; Dénarié, J.; Gough, C. Nod Factors and a Diffusible Factor from Arbuscular Mycorrhizal Fungi Stimulate Lateral Root Formation in Medicago truncatula via the DMI1/DMI2 Signalling Pathway. Plant J. 2005, 44, 195–207. [Google Scholar] [CrossRef]
- Macchiavelli, R.E.; Brelles-Mariño, G. Nod Factor-Treated Medicago truncatula Roots and Seeds Show an Increased Number of Nodules When Inoculated with a Limiting Population of Sinorhizobium meliloti. J. Exp. Bot. 2004, 55, 2635–2640. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Nod Factor [Nod Bj V (C(18:1), MeFuc)] and Lumichrome Enhance Photosynthesis and Growth of Corn and Soybean. J. Plant Physiol. 2008, 165, 1342–1351. [Google Scholar] [CrossRef]
- Podleśny, J.; Wielbo, J.; Podleśna, A.; Kidaj, D. The Pleiotropic Effects of Extract Containing Rhizobial Nod Factors on Pea Growth and Yield. Cent. Eur. J. Biol. 2014, 9, 396–409. [Google Scholar] [CrossRef]
- Siczek, A.; Lipiec, J.; Wielbo, J.; Szarlip, P.; Kidaj, D. Pea Growth and Symbiotic Activity Response to Nod Factors (Lipo-Chitooligosaccharides) and Soil Compaction. Appl. Soil Ecol. 2013, 72, 181–186. [Google Scholar] [CrossRef]
- Smytkiewicz, K.; Podleśny, J.; Wielbo, J.; Podleśna, A. The Effect of a Preparation Containing Rhizobial Nod Factors on Pea Morphological Traits and Physiology. Agronomy 2021, 11, 1457. [Google Scholar] [CrossRef]
- Podleśna, A.; Wielbo, J.; Podleśny, J.; Kidaj, D. Effect of Sulfur and Nod Factors (LCOs) on Some Physiological Features and Yield of Pea (Pisum sativum L.). In Molecular Physiology and Ecophysiology of Sulfur; De Kok, L.J., Hawkesford, M.J., Rennenberg, H., Saito, K., Schnug, E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 221–226. ISBN 978-3-319-20137-5. [Google Scholar]
- Podleśny, J.; Wielbo, J.; Podleśna, A.; Kidaj, D. Usefulness of Nod Preparation (LCOs) Use to Presowable Dressing of Pea Seeds. J. Res. Appl. Agric. Eng. 2013, 58, 124–129. [Google Scholar]
- Siczek, A.; Wielbo, J.; Lipiec, J.; Kalembasa, S.; Kalembasa, D.; Kidaj, D.; Szarlip, P. Nod Factors Improve the Nitrogen Content and Rhizobial Diversity of Faba Bean and Alter Soil Dehydrogenase, Protease, and Acid Phosphomonoesterase Activities. Int. Agrophys. 2020, 34, 9–15. [Google Scholar] [CrossRef]
- Siczek, A.; Lipiec, J.; Wielbo, J.; Kidaj, D.; Szarlip, P. Symbiotic Activity of Pea (Pisum sativum) after Application of Nod Factors under Field Conditions. Int. J. Mol. Sci. 2014, 15, 7344–7351. [Google Scholar] [CrossRef]
- Almaraz, J.J.; Mabood, F.; Zhou, X.; Souleimanov, A.; Smith, D.L. Effect of Nod Factor Sprays on Soybean Growth and Productivity under Field Conditions. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 228–234. [Google Scholar] [CrossRef]
- Gautam, K.; Schwinghamer, T.D.; Smith, D.L. The Response of Soybean to Nod Factors and a Bacteriocin. Plant Signal. Behav. 2016, 11, e1241934. [Google Scholar] [CrossRef]
- Capoen, W.; Goormachtig, S.; Holsters, M. Water-Tolerant Legume Nodulation. J. Exp. Bot. 2010, 61, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Novonesis NodPro® LCO. Available online: https://www.novonesis.com/en/campaigns/nodpro-lco (accessed on 23 September 2025).
- Novonesis Product Page: Torque® IF. Available online: https://www.novonesis.com/en/campaigns/are-you-getting-most-out-every-acre/torque-if (accessed on 23 September 2025).
- Novonesis Product Page: Ratchet®. Available online: https://www.novonesis.com/en/campaigns/are-you-getting-most-out-every-acre/ratchet (accessed on 23 September 2025).
- Optimize® LV. Available online: https://ag.fmc.com/ca/en/biologicals/optimize-lv (accessed on 23 September 2025).
- TagTeam® BioniQ®. Available online: https://ag.fmc.com/ca/en/biologicals/tagteam-bioniq (accessed on 23 September 2025).
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef] [PubMed]
- Atti, S.; Bonnell, R.; Prasher, S.; Smith, D.L. Response of Soybean Glycine max (L.) Merr. under Chronic Water Deficit to LCO Application during Flowering and Pod Filling. Irrig. Drain. 2005, 54, 15–30. [Google Scholar] [CrossRef]
- Duzan, H.M.; Zhou, X.; Souleimanov, A.; Smith, D.L. Perception of Bradyrhizobium japonicum Nod Factor by Soybean [Glycine max (L.) Merr.] Root Hairs under Abiotic Stress Conditions. J. Exp. Bot. 2004, 55, 2641–2646. [Google Scholar] [CrossRef]
- Duzan, H.M.; Mabood, F.; Zhou, X.; Souleimanov, A.; Smith, D.L. Nod Factor Induces Soybean Resistance to Powdery Mildew. Plant Physiol. Biochem. 2005, 43, 1022–1030. [Google Scholar] [CrossRef]
- Rey, T.; André, O.; Nars, A.; Dumas, B.; Gough, C.; Bottin, A.; Jacquet, C. Lipo-Chitooligosaccharide Signalling Blocks a Rapid Pathogen-Induced ROS Burst without Impeding Immunity. New Phytol. 2019, 221, 743–749. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kaku, H.; Shimoda, Y.; Sugiyama, A.; Shimamura, M.; Takanashi, K.; Yazaki, K.; Aoki, T.; Shibuya, N.; Kouchi, H. From Defense to Symbiosis: Limited Alterations in the Kinase Domain of LysM Receptor-like Kinases Are Crucial for Evolution of Legume-Rhizobium Symbiosis. Plant J. 2011, 65, 169–180. [Google Scholar] [CrossRef]
- Grundy, E.B.; Gresshoff, P.M.; Su, H.; Ferguson, B.J. Legumes Regulate Symbiosis with Rhizobia via Their Innate Immune System. Int. J. Mol. Sci. 2023, 24, 2800. [Google Scholar] [CrossRef]
- Stokkermans, T.J.; Ikeshita, S.; Cohn, J.; Carlson, R.W.; Stacey, G.; Ogawa, T.; Peters, N.K. Structural Requirements of Synthetic and Natural Product Lipo-Chitin Oligosaccharides for Induction of Nodule Primordia on Glycine soja. Plant Physiol. 1995, 108, 1587–1595. [Google Scholar] [CrossRef][Green Version]
- Yang, W.C.; de Blank, C.; Meskiene, I.; Hirt, H.; Bakker, J.; van Kammen, A.; Franssen, H.; Bisseling, T. Rhizobium Nod Factors Reactivate the Cell Cycle during Infection and Nodule Primordium Formation, but the Cycle Is Only Completed in Primordium Formation. Plant Cell 1994, 6, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-P.; Müller, J.; Wiemken, A.; Broughton, W.J.; Boller, T. Nod Factors and Tri-Iodobenzoic Acid Stimulate Mycorrhizal Colonization and Affect Carbohydrate Partitioning in Mycorrhizal Roots of Lablab purpureus. New Phytol. 1998, 139, 361–366. [Google Scholar] [CrossRef]
- Maillet, F.; Poinsot, V.; André, O.; Puech-Pagès, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal Lipochitooligosaccharide Symbiotic Signals in Arbuscular Mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef]
- Cope, K.R.; Bascaules, A.; Irving, T.B.; Venkateshwaran, M.; Maeda, J.; Garcia, K.; Rush, T.A.; Ma, C.; Labbé, J.; Jawdy, S.; et al. The Ectomycorrhizal Fungus Laccaria bicolor Produces Lipochitooligosaccharides and Uses the Common Symbiosis Pathway to Colonize Populus Roots. Plant Cell 2019, 31, 2386–2410. [Google Scholar] [CrossRef]
- Rush, T.A.; Puech-Pagès, V.; Bascaules, A.; Jargeat, P.; Maillet, F.; Haouy, A.; Maës, A.Q.; Carriel, C.C.; Khokhani, D.; Keller-Pearson, M.; et al. Lipo-Chitooligosaccharides as Regulatory Signals of Fungal Growth and Development. Nat. Commun. 2020, 11, 3897. [Google Scholar] [CrossRef]
- Catoira, R.; Galera, C.; de Billy, F.; Penmetsa, R.V.; Journet, E.P.; Maillet, F.; Rosenberg, C.; Cook, D.; Gough, C.; Dénarié, J. Four Genes of Medicago truncatula Controlling Components of a Nod Factor Transduction Pathway. Plant Cell 2000, 12, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.; Cullimore, J. Lipo-Chitooligosaccharide Signaling in Endosymbiotic Plant-Microbe Interactions. Mol. Plant Microbe Interact. 2011, 24, 867–878. [Google Scholar] [CrossRef]
- Camps, C.; Jardinaud, M.-F.; Rengel, D.; Carrère, S.; Hervé, C.; Debellé, F.; Gamas, P.; Bensmihen, S.; Gough, C. Combined Genetic and Transcriptomic Analysis Reveals Three Major Signalling Pathways Activated by Myc-LCOs in Medicago truncatula. New Phytol. 2015, 208, 224–240. [Google Scholar] [CrossRef]
- Bonhomme, M.; Bensmihen, S.; André, O.; Amblard, E.; Garcia, M.; Maillet, F.; Puech-Pagès, V.; Gough, C.; Fort, S.; Cottaz, S.; et al. Distinct Genetic Basis for Root Responses to Lipo-Chitooligosaccharide Signal Molecules from Different Microbial Origins. J. Exp. Bot. 2021, 72, 3821–3834. [Google Scholar] [CrossRef]
- De Jong, A.J.; Heidstra, R.; Spaink, H.P.; Hartog, M.V.; Meijer, E.A.; Hendriks, T.; Schiavo, F.L.; Terzi, M.; Bisseling, T.; Van Kammen, A.; et al. Rhizobium Lipooligosaccharides Rescue a Carrot Somatic Embryo Mutant. Plant Cell 1993, 5, 615–620. [Google Scholar] [CrossRef]
- Dyachok, J.V.; Tobin, A.E.; Price, N.P.J.; von Arnold, S. Rhizobial Nod Factors Stimulate Somatic Embryo Development in Picea abies. Plant Cell Rep. 2000, 19, 290–297. [Google Scholar] [CrossRef]
- Khan, W.; Costa, C.; Souleimanov, A.; Prithiviraj, B.; Smith, D.L. Response of Arabidopsis thaliana Roots to Lipo-Chitooligosaccharide from Bradyrhizobium japonicum and Other Chitin-like Compounds. Plant Growth Regul. 2011, 63, 243–249. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, Y.; Tanaka, K.; Thibivilliers, S.; Wan, J.; Choi, J.; Kang, C.h.; Qiu, J.; Stacey, G. Nonlegumes Respond to Rhizobial Nod Factors by Suppressing the Innate Immune Response. Science 2013, 341, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Souleimanov, A.; Smith, D.L. Proteomic Studies on the Effects of Lipo-Chitooligosaccharide and Thuricin 17 under Unstressed and Salt Stressed Conditions in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1314. [Google Scholar] [CrossRef]
- Tanaka, K.; Cho, S.-H.; Lee, H.; Pham, A.Q.; Batek, J.M.; Cui, S.; Qiu, J.; Khan, S.M.; Joshi, T.; Zhang, Z.J.; et al. Effect of Lipo-Chitooligosaccharide on Early Growth of C4 Grass Seedlings. J. Exp. Bot. 2015, 66, 5727–5738. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Mitkus, E.; Souleimanov, A.; Smith, D.L. Lipo-Chitooligosaccharide and Thuricin 17 Act as Plant Growth Promoters and Alleviate Drought Stress in Arabidopsis Thaliana. Front. Microbiol. 2023, 14, 1184158. [Google Scholar] [CrossRef] [PubMed]
- Buendia, L.; Maillet, F.; O’Connor, D.; van de-Kerkhove, Q.; Danoun, S.; Gough, C.; Lefebvre, B.; Bensmihen, S. Lipo-Chitooligosaccharides Promote Lateral Root Formation and Modify Auxin Homeostasis in Brachypodium distachyon. New Phytol. 2019, 221, 2190–2202. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Rhizobial Lipo-Chitooligosaccharides and Gibberellins Enhance Barley (Hordeum vulgare L.) Seed Germination. Biotechnology 2009, 8, 270–275. [Google Scholar] [CrossRef]
- Schwinghamer, T.; Souleimanov, A.; Dutilleul, P.; Smith, D. Supplementation with Solutions of Lipo-Chitooligosacharide Nod Bj V (C18:1, MeFuc) and Thuricin 17 Regulates Leaf Arrangement, Biomass, and Root Development of Canola (Brassica napus [L.]). Plant Growth Regul. 2016, 78, 31–41. [Google Scholar] [CrossRef]
- John McIver, C.C.; Yuming Bai, Y.Y.; Schultz, B.; McIver, A. Foliar Application of Lipo-Chitooligosaccharides (Nod Factors) to Tomato (Lycopersicon Esculentum) Enhances Flowering and Fruit Production. Can. J. Plant Sci. 2007, 87, 365–372. [Google Scholar] [CrossRef]
- Kidaj, D.; Zamlynska, K.; Swatek, A.; Komaniecka, I. The Influence of Rhizobial Nod Factors on the Synthesis of Flavonoids in Common Buckwheat (Fagopyrum esculentum Moench). Molecules 2024, 29, 4546. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, D.U.; Somasundaram, E. Effects of Rhizobial Nod Factors (Lipo Chitooligosaccharide) on Seedling Growth of Maize (Zea mays L.) under Salt Stress. Bangladesh J. Bot. 2018, 47, 831–837. [Google Scholar] [CrossRef]
- Chen, C.; Cholewa, E.M.; McIver, J.D.; Schultz, B.C.; Yang, Y. Use of Lipo Chitooligosacchardes to Nitate Early Flowering and Fruit Development in Plants and Related Methods and Compositions. U.S. Patent US20160309714A1, 27 October 2016. [Google Scholar]
- Lipo-Chitooligosaccharide Compositions for Enhancing Plant Growth. U.S. Patent US020240292838A1, 5 September 2024.
- Aqueous Compositions Comprising Solubilzed Lipo—Chitooligosaccharides. U.S. Patent US020200375179A1, 3 December 2020.
- Engineered Bacteria and Methods of Producing Sustainable Biomolecules. U.S. Patent US020230126375A1, 27 April 2023.
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome Sequence of the Bioplastic-Producing “Knallgas” Bacterium Ralstonia Eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielbo, J. Quality or Quantity? Increasing Legume Yield Using Traditional Inoculants and Rhizobial Nod Factors in the Context of Inter-Strain Competition. Agronomy 2025, 15, 2303. https://doi.org/10.3390/agronomy15102303
Wielbo J. Quality or Quantity? Increasing Legume Yield Using Traditional Inoculants and Rhizobial Nod Factors in the Context of Inter-Strain Competition. Agronomy. 2025; 15(10):2303. https://doi.org/10.3390/agronomy15102303
Chicago/Turabian StyleWielbo, Jerzy. 2025. "Quality or Quantity? Increasing Legume Yield Using Traditional Inoculants and Rhizobial Nod Factors in the Context of Inter-Strain Competition" Agronomy 15, no. 10: 2303. https://doi.org/10.3390/agronomy15102303
APA StyleWielbo, J. (2025). Quality or Quantity? Increasing Legume Yield Using Traditional Inoculants and Rhizobial Nod Factors in the Context of Inter-Strain Competition. Agronomy, 15(10), 2303. https://doi.org/10.3390/agronomy15102303




