Abstract
The degradation of soil structure in paddy fields is critical, and the application of organic amendments is an effective way to enhance soil structure and function. However, the mechanisms by which different organic amendments influence soil aggregate-associated humus carbon and nutrients remain unclear. Considering this, four treatments were employed in a randomized complete block design with three replications: (1) chemical fertilizer (CK); (2) chemical fertilizer plus organic amendment (MC); (3) chemical fertilizer plus organic amendment containing Bacillus subtilis (FT); and (4) Chemical fertilizer plus organic amendment containing polyacrylamide (PM). The results showed that all soil improvement measures significantly increased the proportion of macroaggregates (>2 mm and 2–0.25 mm), primarily the 2–0.25 mm fraction (34.53–48.46%), and the mean weight diameter (MWD), compared to CK. Soil organic carbon (SOC), humic acid carbon (HAC), fulvic acid carbon (FAC), humin carbon (HUC), total nitrogen (TN), and total phosphorus (TP) were predominantly concentrated within the macroaggregates. Relative to CK, the PM increased the HUC content in large aggregates (>2 mm) and significantly enhanced HAC by 19.53% within the same fraction, while the FT significantly boosted FAC by 31.78% in the >2 mm fraction. Furthermore, MC, FT, and PM treatments significantly enhanced SOC, TN, and TP contents within large macroaggregates compared to CK, with PM generally showing the highest SOC and TN levels, and FT being the highest in terms of TP in large aggregates (though differences among treatments were non-significant). Correlation analysis revealed that only in large aggregates did SOC show significant positive correlations with humus carbon fractions (except HAC), as well as with TN and TP. The amendments, particularly PM, effectively enhanced nutrient and humus carbon accumulation within large aggregates and improved aggregate stability. Notably, PM strengthened the direct pathways for the formation of SOC and humus carbon. In summary, the combined application of chemical fertilizer and organic amendments, containing polyacrylamide positively influenced aggregate stability and nutrient accumulation in paddy soil.
1. Introduction
Rice is one of the most important food crops in the world, and China, as the world’s largest rice producer, has steadily accounted for 30% of the global total annual output [1]. However, excessive chemical fertilizer use to boost rice yields has led to severe soil degradation, including acidification and crust formation [2]. In addition, studies have shown that rice yield growth will stagnate with the continuous input of chemical fertilizers [3]. Therefore, reducing the pressure of rice production on natural resources and the ecological environment while guaranteeing rice yield is a major difficulty in China’s agricultural production.
Soil aggregates are the basic units of soil structure and are important places for nutrient storage and microbial activities in soil [4,5], The composition and size distribution of soil aggregates play a crucial role in determining key soil characteristics, including nutrient availability, moisture retention, and pore network formation. These fundamental properties directly influence the soil’s overall functionality and productivity [6]. Currently, aggregates are usually classified into macroaggregates (>0.25 mm), microaggregates (0.25–0.053 mm), and powdery viscous fractions (<0.053 mm) by dry or wet sieving, or macroaggregates are further refined into large aggregates (>2 mm) and middle aggregates (2–0.25 mm) [7]. Soil aggregate stability refers to the ability of aggregates to resist external forces and maintain their morphology; as an important property of soil structure, it plays an important role in the retention of nutrients and water, and the maintenance of appropriate aeration and temperature in the soil [8]. Currently, commonly used parameters of soil aggregate stability include mean weight diameter (MWD), geometric mean diameter (GMD), and percentage of aggregates > 0.25 mm (R0.25) [9]. Most scholars believe that soil macroaggregates are formed by soil microaggregates through a series of physical, chemical, and biological effects, in which the quantity and quality of the cementing material in the soil play a crucial role in the formation and stabilization of soil aggregates [10]. Soil humus’s role in the assembly and alteration of aggregates is vital for ensuring the integrity of soil structure [11]. Among them, humic acid and fulvic acid are crucial elements of soil humus composition, and changes in their structure and properties are closely related to soil conditions [12]. The formation of organic–inorganic composite colloidal film occurs via the coordination of humic acid with clay minerals via carboxyl groups [13]; in contrast, fulvic acid enhances microaggregate stability by promoting Fe/Al oxide cementation [13,14].
In recent years, soil conditioners have emerged as an effective method for enhancing soil quality [15]. Soil amendments are specialized materials designed to enhance soil quality by optimizing its physical, chemical, and biological characteristics. These substances work by improving soil structure, boosting nutrient content, and stimulating microbial activity, ultimately fostering a healthier and more productive soil ecosystem. Through these mechanisms, they facilitate both the rehabilitation and long-term enrichment of degraded soils [16]. Soil amendments can be broadly categorized into natural amendments, synthetic amendments, and biological amendments [17], for example, organic amendments, which not only enrich soil with organic matter and nutrients but also improve granular structure, boosting water and nutrient retention [18]. However, the rapid rate of mineralization of organic materials and the uneven release of nutrients limit their long-term benefits [19]. Microbial amendments such as Bacillus subtilis can glue microaggregates together due to the secretion of extracellular polysaccharides and lipopeptides, etc. [20]. They also stimulate the humification process and optimize the distribution of humus fractions [21]. However, Bacillus subtilis still suffers from unstable enzyme activity and limited action efficiency in practical applications [22]. Polyacrylamide (PAM) is a linear polymer, with the chemical formula (C3H5NO). Due to its amide-containing structure, it readily forms hydrogen bonds, enhancing water solubility and chemical reactivity [23]. It maintains soil structure and suppresses internal water evaporation, thereby enhancing soil water retention and reducing evaporation-induced salt surface accumulation and nutrient loss. Consequently, it has gained widespread application in soil improvement in recent years [24]. It has been shown that the use of PAM as a soil conditioner reduces the formation of soil crusts, allows the soil to maintain good permeability, and increases infiltration rates [25]. Meanwhile, the study revealed that infiltrated water interacts with PAM, creating a hydrogel exhibiting robust adsorption properties, which adsorbs the surrounding soil particles and gradually forms a stable soil aggregate structure, enhancing the content of large aggregates in particular [26]. PAM also plays an important role in improving soil fertility and crop nutrient utilization efficiency, can adsorb and fix soil nutrients, and has a better ability to retain fertilizer and reduce soil nutrient loss [27]. In addition, PAM enhances plant development and improves harvests [28]. However, the effectiveness of its application varies depending on the application rate, soil, and nutrient type.
Current research on PAM still focuses on the physical properties of the soil, as well as the effects on infiltration rates, soil slope erosion, and runoff [29,30]. Research on PAM’s effects on soil aggregates and their nutrient contents is limited. Accordingly, this study investigated the impacts of PAM versus other soil amendments on aggregate humus carbon and soil nutrients in paddy soil, and our objectives were (1) to determine the impact of varied amendments on soil aggregates’ dispersal and structural integrity, along with the levels of humic carbon, total nitrogen (TN), and total phosphorus (TP) within the aggregates, and (2) to analyze the characteristics of aggregates related to nutrients and the intrinsic relationship between humus carbon and soil nutrient content in soil aggregates. We hypothesize that (1) different amendments will affect the distribution of soil aggregates and increase their stability, and the effect of exogenously added organic amendments on improving the stability of soil aggregates is better than that of chemical fertilizer applied alone; (2) the decomposition processes of soil amendments directly influence organic carbon distribution, humus composition, and nutrient levels within soil aggregates; and (3) the distribution of soil aggregates will be influenced by the decomposition and transformation of different amendments, components and nutrient content, thus changing the pathway of soil aggregate formation. This study aims to reveal the important roles of different amendments in regulating soil aggregate formation, promoting soil carbon sequestration, and enhancing nutrient and fertility, and to provide theories and bases for the effective enhancement of soil productivity and sustainable rice cultivation.
2. Materials and Methods
2.1. Experimental Site
The experiments were conducted between May and October 2024 at the test site of the Water-saving Park (118°45′ N, 31°54′ E) of Jiangning Campus of Hohai University in Nanjing, Jiangsu Province. The test area is located in the lower reaches of the Yangtze River and has a humid subtropical climate. The region receives an average of 1021.3 millimeters of rainfall annually, while evaporation averages around 900 millimeters per year. With a mean annual temperature of 15.7 °C, summers peak at 28.1 °C during the warmest month. The area enjoys a generous frost-free season lasting approximately 237 days each year. The experimental site had been under a consistent rice–wheat rotation system for two consecutive years prior to the initiation of this study, and the field was maintained under standard local practices for both crops, including seasonal flooding during rice cultivation and drainage for wheat production. Field trials started in May 2024. The soil classification was Anthrosols (World Reference Base for Soil Resources system), and the soil texture was clay loam. The soil’s physicochemical traits were as follows: pH 7.63, bulk density1.32 g cm−3, soil organic carbon (SOC) 11.34 g kg−1, available phosphorus 31.3 mg kg−1, available potassium 149.26 mg kg−1, ammonium nitrogen 42.3 mg kg−1, and nitrate nitrogen 68.9 mg kg−1.
2.2. Experimental Design
Four experimental treatments were established as follows: chemical fertilizer (CK, control); chemical fertilizer + organic amendment (MC); chemical fertilizer + organic amendment containing Bacillus subtilis (FT); and chemical fertilizer + organic amendment containing polyacrylamide (PM). The amount of N, P, and K as pure nutrients was the same for all treatments: N (260 kg ha−1), P2O5 (100 kg ha−1), and K2O (150 kg ha−1). Eight thousand mL ha−1 of Bacillus subtilis, and 0.5 t ha−1 of PAM were applied. The experiment was conducted in a randomized block design with three replications for each treatment group, the experimental area of each plot was 12 m2, and the water intake and drainage of each plot were designed separately to prevent mutual interference. Organic amendment and polypropylene amide were applied at one time during land preparation and tilling 7 days before rice planting, respectively. Microbial inoculant diluted 500 times with water was sprayed according to the instructions on the soil surface to make the material mix well with the soil. Nitrogen fertilizer was applied as urea (46% N) in split applications: 40% as basal fertilizer, 40% as tillering fertilizer, and 20% as panicle fertilizer. Phosphorus fertilizer (as calcium superphosphate, 15% P2O5) and potassium fertilizer (as potassium chloride, 60% K2O) were applied in a single application as basal fertilizer. Irrigation was carried out before rice planting, and a 5 cm flooded layer was maintained; field management was conducted according to local habits. The organic amendment used in the experiment was produced by Stanley Fertilizer Co., Ltd., Shandong, China and was mainly made of brown-rotted coal mine meal, soybean meal residue, and highly fermented sheep manure, with an organic matter content of 50%, and the contents of N, P2O5, and K2O were 3%, 1%, and 1%, respectively. The urea, calcium superphosphate, and potassium chloride used were produced by Shuangchang Chemical Fertilizer Co., Ltd., Yanchen, China, with a nitrogen (N) content of 46%, a phosphorus (P2O5) content of 12%, and a potassium (K2O) content of 60%. Bacillus subtilis—effective live bacterial number ≥ 2 × 103 million g−1—was purchased from Nanhua Qianmu Company, Henan, China. Anionic polyacrylamide (Polyacrylamide, PAM), water-soluble polymer, molecular weight of 20 million, was purchased from Dachuan Environmental Protection Technology Co., Ltd., Changzhou, China.
2.3. Sample Collection
In October 2024 (post-harvest), topsoil (0–20 cm depth) was collected from 12 experimental plots using a 5 cm diameter corer. Composite samples were created by homogenizing soil from five randomly selected cores per plot, with careful removal of plant debris prior to gentle fragmentation. These samples were transported to the laboratory in rigid plastic containers to preserve physical integrity. For comparative baseline data, pre-experimental soil sampling was conducted in May 2024 to assess initial physicochemical properties. All samples were subdivided: one portion underwent aggregate fraction analysis, while the air-dried counterpart was reserved for physicochemical characterization.
2.4. Aggregate Separation and Stability Calculation
Undisturbed in situ soil samples were collected from the 0–20 cm layer and subjected to natural air-drying before being sieved to remove large particles. Afterwards, the wet sieving method was utilized, where the soil samples were immersed in water and aggregates of different particle sizes were separated by oscillation or sieving. Four size aggregate fractions including large aggregates (>2 mm), middle aggregates (2–0.25 mm), micro aggregates (0.25–0.053) mm, and caly and salt (<0.053 mm) were separated. Macroaggregates are defined as >0.25 mm. The separated aggregates were dried and weighed, the mass of each class of aggregates was recorded, and the average diameter of each class of aggregates was determined.
The percentage of soil aggregates (Wi) was calculated for each grain level according to Equation (1). MWD, GMD, and R0.25 were used to determine soil aggregate stability [31], calculated using Equations (2)–(4).
Mi represents the mass of agglomerates of different particle sizes (i), g; MT represents the total mass of soil used for sieving of agglomerates, g; Xi is the average diameter of agglomerates of each particle size, mm; and MX<0.25 denotes the mass of agglomerates < 0.25 mm, g.
2.5. SOC and Its Humus Carbon Fractions Measurements
Humus fraction extraction was performed using a NaOH + Na4P2O7 mixed solution method [31]. Briefly, the residue in the centrifuge tube was treated with 30 mL of a 0.1 mol·L−1 NaOH and 0.1 mol L−1 Na4P2O7 mixed solution. The mixture was homogenized using a glass rod and then shaken in a constant-temperature water bath (70 ± 2 °C) for 1 h. After removal, the mixture was centrifuged at 4000 rpm for 15 min. The supernatant was adjusted to pH 1.0 to separate fulvic acid (FA) and humic acid (HA). The precipitate was washed 2–3 times with distilled water to obtain humin (HU). Organic carbon determination for the humus fractions (HA, FA, HU) was conducted using the potassium dichromate oxidation method (external heating) [32]. SOC was analyzed using an elemental analyzer (EA 3000, Euro Vector, Rome, Italy) and the content of TN and TP in the soil aggregates were assessed using the Kjeldahl and ammonium molybdate colorimetric method, respectively [32].
2.6. Data Analysis
In this study, the distribution and stability of soil aggregates were quantified and analyzed using IBM SPSS Statistic 28.0 software. Soil samples were classified into aggregates of different particle sizes using the wet sieving method, and the metrics such as MWD, GMD, and R0.25 were computed to assess soil aggregate stability. Significant differences were analyzed using the SPSS software. One-way ANOVA was used to compare the differences in the indicators between different treatment groups, multiple comparisons were made using the least significant difference (LSD) method, and Pearson correlation analysis was performed to analyze correlations among SOC, humus carbon fraction, TN, and TP, and plotted based on R 6.3 software. Path analysis was carried out based on AMOS 21.0 to further investigate the effects of different amendments on soil aggregate formation, and the pictures were beautified based on the Origin graphic tool.
3. Results
3.1. Soil Aggregate Size Distribution and Stability
Soil aggregate size distribution and stability are listed in Table 1. Compared with CK, the MC, FT, and PM treatments showed significant increases in MWD, GMD, and R0.25 (p < 0.05). The MC, FT, and PM treatments showed significant increases in mass ratios: 19.61%, 48.84%, and 37.24% in >2 mm aggregates, and 18.21%, 41.46%, and 65.90% in 2–0.25 mm aggregates, respectively. The PM treatment resulted in a significant increase compared to the other treatments (p < 0.05), indicating that exogenous application of added polyacrylamide in fertilizers can significantly improve soil aggregate stabilization.

Table 1.
Mass percentage and stability of aggregates under different treatments.
3.2. Contents of Humus Carbon in Soil Aggregates
The contents of humus carbon fractions within soil aggregates are presented in Figure 1. Compared to the CK in bulk soil, MC, FT, and PM treatments increased humic acid carbon (HAC) content by 12.94%, 31.99%, and 72.99% on average, respectively (Figure 1a). Within the >2 mm aggregate fraction, FT and PM treatments significantly increased HAC by 13.02% and 19.53%, respectively, whereas the MC treatment significantly decreased it by 23.98%. In the 2–0.25 mm fraction, no significant difference (p > 0.05) was observed between PM and CK; however, MC and FT treatments increased HAC by 31.78% and 26.76%, respectively. Within the 0.25–0.053 mm fraction, MC and FT treatments increased HAC by 31.78% and 26.76%, respectively (compared to CK), while PM showed no significant difference from CK. For the <0.053 mm aggregate fraction, MC, FT, and PM treatments significantly increased HAC by 20.55%, 35.15% and 14.15%, respectively. For fulvic acid carbon (FAC) in bulk soil, MC, FT, and PM treatments increased the content by 17.89%, 39.47%, and 58.76%, respectively, relative to CK (Figure 1b). Compared to bulk soil, the FT treatment resulted in significantly higher FAC content within >2 mm aggregates compared to all other treatments. FAC content showed no significant variation across treatments in the 2–0.25 mm or <0.053 mm aggregate fractions. However, within the 0.25–0.053 mm fraction, FT and PM treatments significantly increased FAC content by 23.05% and 27.32%, respectively, compared to CK. Humin carbon (HUC) content in bulk soil was higher under all amendment treatments (MC, FT, PM) than under CK, with increases of 15.49%, 23.18%, and 29.91% on average, respectively (Figure 1c). PM exhibited the most significant improvement in HUC content within macroaggregate sizes. Notably, across all treatments, the peak measurements of FAC, HAC, and HUC were predominantly found within macroaggregates.
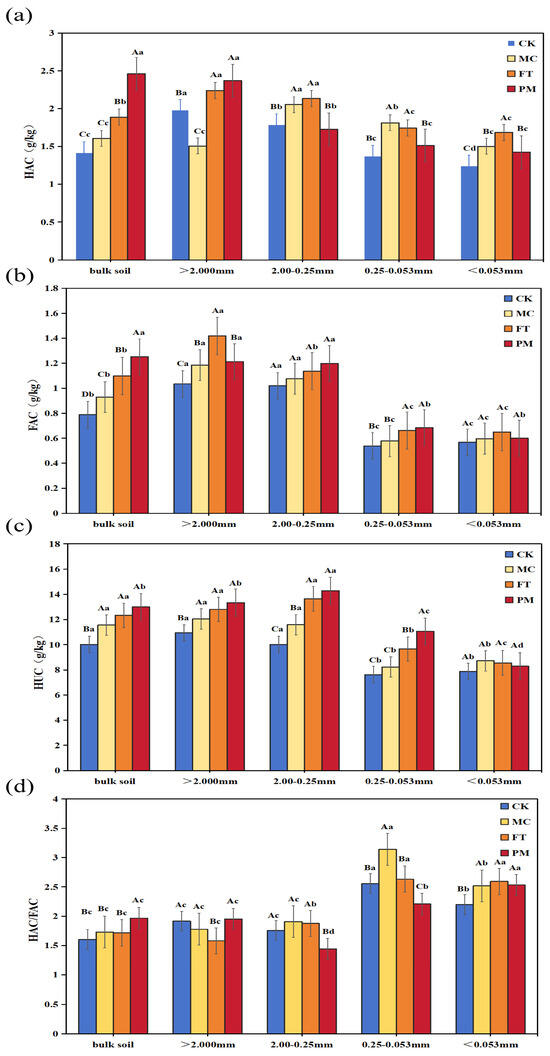
Figure 1.
Contents of HAC (a), FAC (b), HUC (c), and HAC/FAC (d) in soil aggregates under different treatments. Note: Uppercase letters denote significant differences (p < 0.05) between treatments for the same aggregate fraction; lowercase letters denote significant differences (p < 0.05) between aggregate fractions within the same treatment.
In bulk soil, PM exhibited significantly higher HAC/FAC compared to CK, MC, and FT, which showed no notable differences (Figure 1d). The highest values of HAC/FAC were found in the 0.25–0.053 mm aggregates under the MC treatment.
3.3. Nutrient Contents in Soil Aggregates
In bulk soil, SOC content followed the order PM > FT > MC > CK (Figure 2a). The PM treatment exhibited higher SOC content than the other treatments across all aggregate size fractions. Compared to CK, the MC, FT, and PM treatments significantly increased SOC content in >2 mm aggregates by 17.31%, 29.92%, and 41.61%, respectively. For 2–0.25 mm aggregates, FT and PM increased SOC by 31.72% and 43.76%, respectively, while MC decreased it by 8.76%. In 0.25–0.053 mm aggregates, MC, FT, and PM significantly increased SOC by 18.36%, 33.84%, and 56.40%, respectively. Similarly, in <0.053 mm aggregates, the PM treatment maintained significantly higher SOC than the other treatments, with MC, FT, and PM increasing SOC content by 26.06%, 8.88%, and 15.17%, respectively.
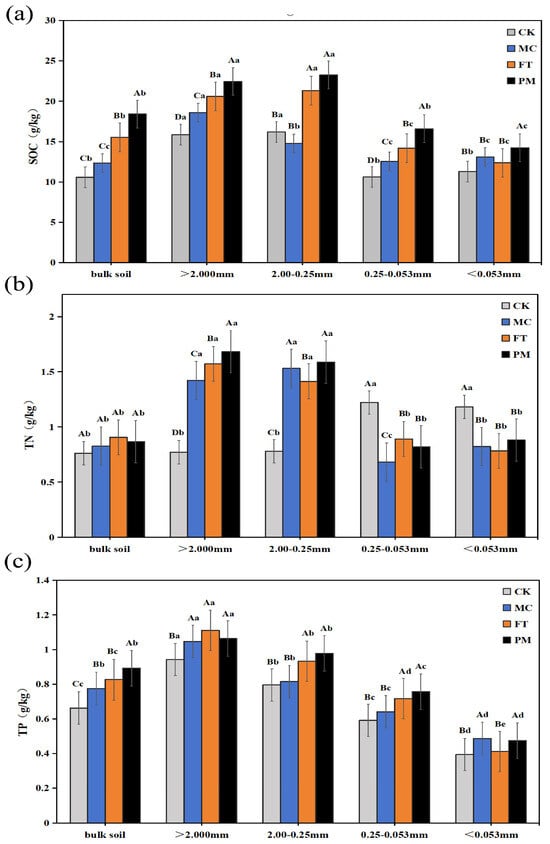
Figure 2.
Contents of SOC (a), TN (b), and TP (c) in soil aggregates under different treatments. Note: Uppercase letters denote significant differences (p < 0.05) between treatments for the same aggregate fraction; lowercase letters denote significant differences (p < 0.05) between aggregate fractions within the same treatment.
Compared to CK, TN content in bulk soil increased by 8.39%, 18.87% and 13.63% under MC, FT, and PM treatments, respectively (Figure 2b). In >2 mm aggregates, the TN content under MC, FT, and PM increased by 84.20%, 103.63%, and 117.88%, respectively. For the 2–0.25 mm aggregates, the TN content under MC, FT, and PM increased by 96.03%, 81.05%, and 103.33%, respectively. Interestingly, the TN content in the 0.25–0.053 mm aggregate was significantly increased under CK, with MC, FT, and PM decreasing by 44.24%, 27.15%, and 32.87%, respectively, compared to CK. Similarly to the phenomenon in 0.25–0.053 mm aggregates, the TN content under the CK treatment was significantly higher than the other treatments in <0.053 mm aggregates, and MC, FT and PM were reduced by 30.43%, 33.73%, and 25.53%, respectively, compared to CK. There was no significant TN change among the three treatments (p > 0.05). The highest TN values were found in >2 mm aggregates in the PM treatment.
The different amendments significantly increased the TP content in the bulk soil compared to CK (Figure 2c). The PM treatment increased by 34.59%, which was significantly higher than the other treatments (p < 0.05). Additionally, MC, FT, and PM treatment significantly increased the TP content in the >2 mm aggregates by 11.03%, 17.82%, and 12.73%, respectively. The TP content of 2–0.25 mm fraction was significantly increased by 2.39%, 17.09%, and 22.86% in MC, FT, and PM treatment, respectively. Similarly, TP content in the 0.25–0.053 mm aggregates increased by 8.28%, 21.28%, and 27.70% under MC, FT, and PM treatments, respectively. In <0.053 mm aggregates, MC and PM treatments significantly increased by 23.60% and 20.56%, while FT treatment showed no significant difference compared to CK. The highest TP content was found in aggregates in the >2 mm aggregate fraction, followed by aggregates with 2–0.25 mm particle size in each of the improvement measures.
3.4. Correlation Analysis
Relationships among various parameters (SOC, HAC, FAC, HUC, HAC/FAC, TN, and TP) across aggregate size fractions were analyzed using the Pearson correlation (Figure 3). The analysis indicated that HAC, FAC, HUC, TN, and TP were positively correlated with SOC content within >2 mm aggregates (p < 0.05). Additionally, SOC showed a significant positive correlation with FAC, HUC, and TP in both the 2–0.25 mm and 0.25–0.053 mm fractions (p < 0.05). Notably, within the <0.053 mm fraction, SOC exhibited a positive correlation with TP and a negative correlation with TN.
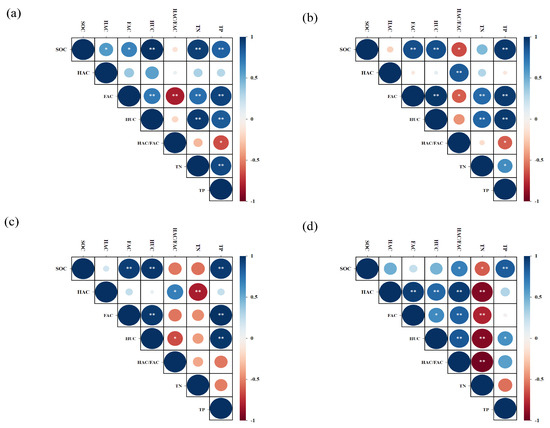
Figure 3.
Correlation between the parameters in soil aggregates. Note: (a), (b), (c) and (d) represent the aggregate distribution of >2 mm, 2–0.25 mm, 0.25–0.053 mm and <0.053 mm, respectively. The significance level is indicated as follows: *: p < 0.05, **: p < 0.01.
HAC in macroaggregates showed no significant correlation with TN and TP. However, HAC exhibited highly significant negative correlation with TN in both the 0.25–0.053 mm and <0.053 mm fractions (p < 0.01). In contrast, FAC and HUC exhibited strong positive correlations with both TN and TP in the macroaggregates (p < 0.01). Within the 0.25–0.053 mm fraction, FAC and HUC were highly significantly positively correlated with TP but showed no significant correlation with TN. In the <0.053 mm fraction, both FAC and HUC were highly significantly negatively correlated with TN. Furthermore, significant negative correlations were observed between the HAC/FAC ratio and TP in macroaggregates, and with TN in the <0.053 mm fraction.
3.5. Pathway Analysis
Different treatments exhibited variations in both the pathways of aggregate formation and the complexity of aggregates formed (Figure 4). In the CK treatment, the model including SOC, HAC, HUC, and FAC explained nearly all of the variance in aggregate formation (R2 = 0.999), with HAC exhibiting the strongest direct effect. Among all treatments, the PM treatment showed stronger direct effects on aggregate formation than the other treatments. Specifically, under the MC treatment, the variance in aggregate formation was explained by a model containing SOC, HUC, TN, and TP (R2 = 0.999), among which TP showed the strongest direct effect (p < 0.01). For the FT treatment, a model comprising FAC, HUC, TN, and TP explained all of the variance in aggregate formation (R2 = 1.00); TN and TP showed significant positive effects (p < 0.05), whereas FAC and HUC showed significant negative effects (p < 0.05). Furthermore, for the PM treatment, the model including HAC, FAC, HUC, TN, and TP accounted for all variance in aggregate formation (R2 = 1.00), with HAC, TN, and TP displaying significant positive direct effects (p < 0.01), while FAC and HUC had significant negative direct effects (p < 0.05). The above results show that SOC, humic carbon fraction, TN and TP directly promote aggregate formation, and this formation pathway is enhanced following PAM addition.
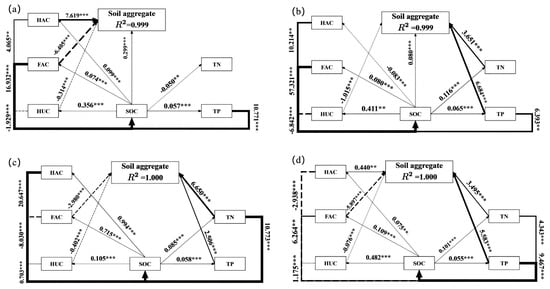
Figure 4.
Pathway analysis exploring the direct and indirect effects of SOC, humus carbons, TN and TP on soil aggregate formation. Note: (a), (b), (c), and (d) indicate CK, MC, FT, and PM. R2 is the proportion of variance explained by the corresponding variable in the path analysis. Numbers above the arrows indicate standardized path coefficients. The width of the arrows indicates the strength of the neighboring path coefficients. Solid lines indicate positive correlation, while dashed lines indicate negative correlation. ** and *** indicate significance at the p < 0.05 and p < 0.01 levels, respectively.
4. Discussion
The application of different amendments influences soil structure and aggregate stability [33,34]. The results indicate that soil MWD and GMD followed comparable trends with the order of PM > FT > MC > CK, which indicates that exogenous addition of PAM is more conducive to the stabilization of soil aggregates, mainly because the anionic PAM, on the one hand, can enhance the adhesion of dispersed particles in the soil through its own cementing effect, so as to form a larger aggregated structure [35]. On the other hand, its active moieties may engage with surface functionalities or ions of soil constituents, facilitating the formation of ionic, covalent, and hydrogen bonds, thereby enhancing intermolecular attraction, including electrostatic and van der Waals bonds, potentially aggregating diffused soil particles [36]. Similar studies report that PAM addition promotes water-stable aggregate formation, enhancing soil structural stability [37]. Qiang et al. [38] carried out a soil improvement experiment with a PAM agent in Loess Plateau soil and found that PAM application boosted MWD by 33.70% and GMD by 29.80%. The proportion of middle aggregates (2–0.25 mm) in PAM-treated soils increased significantly by 52.90% compared to the control (CK) treatment, which is in accordance with the results of this experiment.
In this study, we hypothesized that changes in soil aggregate distribution would influence the distribution of soil nutrients and humus carbon fractions. Our results confirmed that all organic amendments increased humus carbon content in bulk soil, with humus carbon fractions predominantly accumulating in macroaggregates under FT and PM treatments. Significant differences were also observed in the humic carbon fractions across various aggregate size fractions. Compared to CK, the PM treatment significantly enhanced the HUC content in aggregates > 0.25 mm and notably increased the HAC content within >2 mm aggregates. In contrast, the FT treatment only increased the HAC content in the 2–0.25 mm fraction and the FAC content in >2 mm aggregates. This phenomenon likely stems from hierarchical protection mechanisms [39]. As a long-chain polymer, PAM facilitates macroaggregates formation via microaggregate bridging [40], generating occluded microdomains that create physical constraints on microbial access. These periodically anoxic zones specifically inhibit aerobic decomposers and limit enzymatic hydrolysis [41], thereby providing critical protection for hydrophobic HUC fractions through physical encapsulation [42]. Concurrently, PAM’s carboxyl groups (-COOH) coordinate with dissolved Fe3+/Al3+ under flooding conditions to form ternary complexes with carboxyl/phenolic moieties in HAC [43], while amide groups facilitate hydrogen bonding with mineral surfaces [44]. Regarding the FT treatment, the introduction of Bacillus subtilis significantly influenced the differential distribution of HAC and FAC across aggregate fractions. The significant increase in HAC within the 2–0.25 mm aggregates and FAC within the >2 mm aggregates suggests that Bacillus subtilis mediates divergent stabilization pathways for these fractions, primarily driven by microbially induced aggregation processes [45]. Bacillus subtilis, a prolific producer of extracellular polymeric substances (EPS) [46], facilitates the binding of microaggregates into larger structures. Within the 2–0.25 mm fraction, microbial processing and EPS likely promoted the chemical stabilization of HAC. Conversely, the accumulation of the more hydrophilic FAC fraction within the >2 mm macroaggregates points towards physical occlusion as the dominant protective mechanism. This spatial isolation limits accessibility to decomposers, effectively shielding FAC, despite the relatively more aerobic conditions typical of large aggregates compared to occluded microsites. Thus, the FT treatment influenced humus carbon fractions through microbially mediated aggregation: HAC was predominantly stabilized chemically within the 2–0.25 mm aggregates via enhanced organomineral interactions, while FAC was primarily protected physically within the >2 mm aggregates through occlusion [47]. In the <0.053 mm aggregates, FAC and HUC contents were not significantly different between treatments, which may be attributed to the physicochemical protection of organic carbon by small aggregates (especially the clay-powder grades) through organic–inorganic complexes (e.g., adsorption on the surface of clay minerals, and co-precipitation of Fe/Al oxides) [48]. In paddy soils with high clay contents, such protective mechanisms significantly enhance the stability of organic carbon, leading to a relatively insensitive response of FAC and HUC within the microaggregates to exogenous perturbations such as amendment additions. The HAC/FAC ratio serves as a reliable indicator of soil humification, with higher values denoting an elevated degree of humification [49]. Relative to the CK, the HAC/FAC values in soils amended with PAM were significantly higher than those in the other treatments (p < 0.01). This suggests that the application of PAM promotes the accumulation of soil humus.
Soil aggregates are crucial locations for the retention and conversion of nutrients (e.g., carbon, nitrogen, phosphorus, etc.) in the soil, as well as for the survival of microorganisms [50]. Changes in the particle size of soil aggregates were strongly correlated with soil nutrient content and significantly affected soil fertility and sustainability [51]. The PM treatment in this experiment had higher SOC content in all aggregates than the other treatments. Abulaiti et al. [52] observed enhanced soil organic matter levels due to PAM application over a five-year rice crop study. The results of this experiment showed that the application of different organic amendments increased soil TN and TP content. These nutrients were primarily stored in >2 mm and 2–0.25 mmaggregates, likely due to the physical protection offered by large aggregates. This protection shields encapsulated organic matter from rapid microbial decomposition and mineralization, thereby delaying the release of organic carbon, nitrogen, and phosphorus [47,53]. The highest TN values were found in >2 mm aggregates under PM treatment, presumably because PAM, as a polymer, possesses long chains that entangle soil particles via hydrogen bonding and van der Waals forces [54]. Promoting the cementation of microaggregates (<0.25 mm) to form >2 mm macroaggregates, which have a well-developed internal pore structure, will provide space for the physical encapsulation of nitrogen (especially NH4+-N) and reduce the risk of leaching. Hong et al. [55] also found that the application of PAM in combination with nitrogen fertilizer significantly enhanced plant height, yield, and nitrogen use efficiency in drip-irrigated wheat. Within the 0.25–0.053 mm and <0.053 mm aggregate size classes, TN was significantly higher under CK than under other treatments, which is mainly due to the fact that fertilizers provide mainly water-soluble, fast-acting mineral nitrogen [56]. Small particle-size aggregates (especially microaggregates or silt-clay particles) possess a large specific surface area and abundant negative charge sites. Under the CK treatment, the positively charged NH4+ was readily fixed through electrostatic interactions onto the surfaces of these small aggregates and their internal negatively charged clay minerals. This process led to a relative enrichment of nitrogen content within the small particle-size fractions [39].
Correlation analysis revealed significant positive correlations (p < 0.05) in macroaggregates between SOC, FAC, HUC and both TN and TP, but only with TP in the 0.25–0.053 mm fraction HAC, FAC, HUC were negatively correlated with TN in < 0.053 mm aggregates. The above results also verified our previous hypothesis that organic carbon fractions are more closely associated with nutrients in large aggregates. We hypothesize that this phenomenon is due to the fact that macroaggregates are mainly composed of smaller microaggregates and mineral particles bonded by plant roots, fungal hyphae, and organic binders such as FAC and HUC [47]. In this process, plant residues, microbial residues and their metabolites (rich in TN and TP) are encapsulated or embedded in the pores and structure of the macroaggregates. TN is mainly found in organic matter (e.g., proteins, amino sugars) [57]. TP also often binds to organic matter or inorganic phosphorus complexed with organic matter [58]. Therefore, the organic carbon fractions (FAC, HUC) and nutrients (TN, TP) coexist closely and co-accumulate spatially during the formation and stabilization of macroaggregates, leading to a positive correlation. The powder and sticky particles have huge specific surface area and rich charge (especially sticky particles). The organic matter encapsulated within these aggregates is highly stable and tightly bound to minerals, particularly Fe/Al oxides. These mineral organic complexes co-adsorb or encapsulate organic/inorganic phosphorus. In contrast, soil nitrogen, due to its water solubility, is not readily adsorbed stably onto mineral surfaces. It is therefore susceptible to microbial decomposition or leaching loss [43], resulting in a negative correlation with humus carbon fractions.
Furthermore, the results of the pathway analysis (Figure 4) revealed significant direct effects of SOC, humic carbon fractions, TN, and TP on soil aggregates across all treatments, with both positive and negative correlations observed. Specifically, the path coefficients associated with soil aggregate formation were highest under PM treatment. This suggests that the addition of PAM was more effective in promoting soil aggregate formation compared to the other amelioration measures tested.
5. Conclusions
Analysis of soil aggregate distribution, stability, humic carbon, and nutrient contents in paddy soils amended with PAM and other organic amendments revealed significant improvements. Specifically, PAM application resulted in the most pronounced enhancement in MWD and macroaggregate proportion, demonstrating its superior effect on improving aggregate stability. Moreover, all organic amendments effectively increased the content of nutrients and humic carbons across various aggregate size fractions. It is noteworthy that both soil nutrients and humic carbon were predominantly concentrated within macroaggregates, with PM treatment demonstrating the most significant enrichment effect within these larger aggregates. Further correlation and path analysis revealed that the relationships among soil nutrients and humic carbons were significantly stronger and more positively correlated within macroaggregates than within 0.25–0.053 and <0.053 mm fractions. Importantly, the addition of PAM was identified as particularly conducive to promoting these synergistic relationships. Overall, this study revealed the efficacy of PAM, along with other soil amendments, in improving structural stability, sequestering carbon, and storing nutrients. The results of this study provide valuable insights into the development of effective soil improvement strategies aimed at achieving long-term soil health and carbon sequestration in intensive rice cropping systems.
Author Contributions
Conceptualization, Q.W.; methodology, Q.W.; software, W.L.; validation, Z.W.; formal analysis, Q.W.; investigation, Z.W.; resources, X.S.; data curation, Q.W.; writing—original draft preparation, Q.W.; writing—review and editing, Z.W. and X.S.; visualization, Q.W.; supervision, X.S.; project administration, Q.W. and X.S.; funding acquisition, X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the Key Science and Technology Project of Water Resources Ministry (SKR-2022070), the National Key Research and Development Plan (2020YFD0900705), and the Key Science and Technology Project for Nanjing Water Conservancy Bureau (2019-1).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the help of Liangkai Wang for the research guidance, and Zhong Wang for improving an earlier version of this manuscript. During the preparation of this manuscript/study, the author(s) used Deepseek and DeepL for the purposes of language polishing and refinement. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PAM | polyacrylamide |
| GMD | Geometric Mean Diameter |
| MWD | mean weight diameter |
| SOC | Soil organic carbon |
| TN | total nitrogen |
| TP | total phosphorus |
| HAC | humic acid carbon |
| FAC | fulvic acid carbon |
| HUC | humin carbon |
References
- Yu, F.; Lin, Q.; Chen, X. Contents and distributions of cadmium and lead in rice from main rice cultivation areas in China. J. Ecol. Rural. Environ. 2013, 29, 24–28. [Google Scholar] [CrossRef]
- Song, W.; Shu, A.; Liu, J.; Shi, W.; Li, M.; Li, Z.; Liu, G.; Yuan, F.; Liu, Z.; Gao, Z. Effects of long-term fertilization with different substitution ratios of organic fertilizer on paddy soil. Pedosphere 2022, 32, 637–648. [Google Scholar] [CrossRef]
- Cao, X.; Liu, L.; Ma, Q.; Lu, R.; Kong, H.; Kong, Y.; Zhu, L.; Zhu, C.; Tian, W.; Jin, Q. Optimum organic fertilization enhances rice productivity and ecological multifunctionality via regulating soil microbial diversity in a double rice cropping system. Field Crops Res. 2024, 318, 109569. [Google Scholar] [CrossRef]
- Jasinska, E.; Wetzel, H.; Baumgartl, T.; Horn, R. Heterogeneity of physico-chemical properties in structured soils and its consequences. Pedosphere 2006, 16, 284–296. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Wang, J. Formation and stability mechanism of soil aggregates: Progress and prospect. Acta Pedol. Sin. 2023, 60, 627–643. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Zhang, H.; Schroder, J.; Li, C.; Zhou, J. Impact of land use and soil fertility on distributions of soil aggregate fractions and some nutrients. Pedosphere 2010, 20, 666–673. [Google Scholar] [CrossRef]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil. Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Duan, J.; Liu, G.; Nie, X.; Li, Z. Leguminous cover orchard improves soil quality, nutrient preservation capacity, and aggregate stoichiometric balance: A 22-year homogeneous experimental site. Agric. Ecosyst. Environ. 2024, 363, 108876. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Yao, R.; Wang, X.; Xie, W.; Xiao, P. Interactive effects of salinity and straw on the soil aggregate stability and organic carbon sequestration in saline soils in the Hetao area, China. Land. Degrad. Dev. 2024, 35, 1685–1698. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Czachor, H. Impact of organic matter, iron oxides, alumina, silica and drying on mechanical and water stability of artificial soil aggregates. Assessment of new method to study water stability. Geoderma 2014, 221, 1–10. [Google Scholar] [CrossRef]
- Yu, X.; Fu, Y.; Lu, S. Characterization of the pore structure and cementing substances of soil aggregates by a combination of synchrotron radiation X-ray micro-computed tomography and scanning electron microscopy. Eur. J. Soil. Sci. 2017, 68, 66–79. [Google Scholar] [CrossRef]
- Luo, L.; Lu, J.T.; Xu, C.; Guo, Z.; Zhang, S.Z. Study on C-Functional Groups of Soil Humus Fractions Affected by Phosphate Using C 1s Near-edge X-ray Absorption Fine Structure Spectroscopy. Chin. J. Anal. Chem. 2013, 41, 1279–1282. [Google Scholar] [CrossRef]
- Laird, D.; Martens, D.; Kingery, W. Nature of clay-humic complexes in an agricultural soil: I. Chemical, biochemical, and spectroscopic analyses. Soil. Sci. Soc. Am. J. 2001, 65, 1413–1418. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Hu, Y.; Xue, S.; Zhang, Y.; Wang, X. Soil carbon sequestration and aggregate stability improvement by tillage methods in China’s Loess Plateau. Arch. Agron. Soil. Sci. 2023, 69, 1718–1733. [Google Scholar] [CrossRef]
- Matisic, M.; Dugan, I.; Bogunovic, I. Challenges in sustainable agriculture—The role of organic amendments. Agriculture 2024, 14, 643. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.-H.; Kim, H.-U.; Owens, G.; Kim, K.-R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil. Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Kim, M.-S.; Min, H.-G.; Lee, S.-H.; Kim, J.-G. The effects of various amendments on trace element stabilization in acidic, neutral, and alkali soil with similar pollution index. PLoS ONE 2016, 11, e0166335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wei, H.; Chai, Q.; Li, L.; Wang, Y.; Sun, J. Biological soil conditioner with reduced rates of chemical fertilization improves soil functionality and enhances rice production in vegetable-rice rotation. Appl. Soil. Ecol. 2024, 195, 105242. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Duanis-Assaf, T.; Zeng, G.; Meyer, R.L.; Reches, M.; Steinberg, D.; Shemesh, M. Cell wall associated protein TasA provides an initial binding component to extracellular polysaccharides in dual-species biofilm. Sci. Rep. 2018, 8, 9350. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure–straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Sousa-Ortega, C.; Recena, R.; Perea-Torres, F.; Delgado, A. Microbial inoculants improve nutrients uptake and yield of durum wheat in calcareous soils under drought stress in the Mediterranean region. Arch. Agron. Soil. Sci. 2023, 69, 2233–2247. [Google Scholar] [CrossRef]
- Lu, S.; Chen, F.; Ngo, H.H.; Guo, W.; Feng, C.; Wu, J.; Zheng, B. Effect of straw and polyacrylamide on the stability of land/water ecotone soil and the field implementation. Ecol. Eng. 2016, 94, 12–21. [Google Scholar] [CrossRef]
- Mamedov, A.I.; Huang, C.-h.; Aliev, F.A.; Levy, G.J. Aggregate stability and water retention near saturation characteristics as affected by soil texture, aggregate size and polyacrylamide application. Land. Degrad. Dev. 2017, 28, 543–552. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Ao, C. Polyacrylamide and rill flow rate effects on erosion and ammonium nitrogen losses. Water Air Soil. Poll. 2019, 230, 11. [Google Scholar] [CrossRef]
- Ji, L.; Li, L.; Si, H.; Yang, Y. The effects of polyacrylamide amendment on ability of gravelled soil to retain water and fertilizer and its consequence for yield and quality of wine grape. J. Irrig. Drain. 2020, 39, 7–15. [Google Scholar]
- Li, F.; Wang, A. Interaction effects of polyacrylamide application and slope gradient on potassium and nitrogen losses under simulated rainfall. Catena 2016, 136, 162–174. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Zheng, H.; Hong, M.; Hu, Y.; Zhao, B.; De, H. Effects of three types of soil amendments on yield and soil nitrogen balance of maize-wheat rotation system in the Hetao Irrigation Area, China. J. Arid. Land. 2019, 11, 904–915. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Zhao, Z.; Telyatnikova, N.; Maxim, M. Model test study on the rainfall erosion mechanisms and reclamation potential of open-pit coal mine dump soil improved by fly ash and polyacrylamide. Eng. Geol. 2025, 344, 107837. [Google Scholar] [CrossRef]
- Kebede, B.; Tsunekawa, A.; Haregeweyn, N.; Tsubo, M.; Mulualem, T.; Mamedov, A.I.; Meshesha, D.T.; Adgo, E.; Fenta, A.A.; Ebabu, K. Effect of Polyacrylamide integrated with other soil amendments on runoff and soil loss: Case study from northwest Ethiopia. Int. Soil. Water Conserv. Res. 2022, 10, 487–496. [Google Scholar] [CrossRef]
- Meng, F.; Dou, S.; Yin, X.; Zhang, G.; Zhong, S. Effects of maize stalk biochar on humus composition and humic acid structure in black soil. J. Agro Environ. Sci. 2016, 35, 122–128. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Gu, W.; Wang, Y.; Sun, Y.; Liu, Z.; Wang, W.; Wu, D.; Zhang, Y.; Sun, W.; Wang, X.; Feng, Z. Assessing the formation and stability of paddy soil aggregate driven by organic carbon and Fe/Al oxides in rice straw cyclic utilization strategies: Insight from a six-year field trial. Sci. Total Environ. 2024, 951, 175607. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Sun, Y.; Li, X.; Wang, N.; Wang, X.; Meng, T. Interaction force mechanism for the improvement of reclaimed soil aggregate stability in abandoned homestead by different organic-inorganic soil conditioners. Front. Environ. Sci. 2023, 11, 1207887. [Google Scholar] [CrossRef]
- Mamedov, A.; Wagner, L.; Huang, C.; Norton, L.; Levy, G. Polyacrylamide effects on aggregate and structure stability of soils with different clay mineralogy. Soil. Sci. Soc. Am. J. 2010, 74, 1720–1732. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Du, J.; Pei, X.; Du, P.; Zhou, L. Effects of several polymeric materials on the improvement of the sandy soil under rainfall simulation. J. Environ. Manag. 2023, 345, 118847. [Google Scholar] [CrossRef]
- Nadler, A.; Perfect, E.; Kay, B. Effect of polyacrylamide application on the stability of dry and wet aggregates. Soil. Sci. Soc. Am. J. 1996, 60, 555–561. [Google Scholar] [CrossRef]
- Qiang, M.; Zhang, X.; Zhuang, X.; Zhang, H. Effect of Organic Amendment and Mineral Fertilizer on Soil Aggregate Stability and Maize Yield on the Loess Plateau of China. Pol. J. Environ. Stud. 2024, 33, 2255–2265. [Google Scholar] [CrossRef]
- Lützow, M.v.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions–a review. Eur. J. Soil. Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Kang, X.; Bate, B.; Chen, R.-P.; Yang, W.; Wang, F. Physicochemical and mechanical properties of polymer-amended kaolinite and fly ash–kaolinite mixtures. J. Mater. Civ. Civil. Eng. 2019, 31, 04019064. [Google Scholar] [CrossRef]
- Keiluweit, M.; Gee, K.; Denney, A.; Fendorf, S. Anoxic microsites in upland soils dominantly controlled by clay content. Soil. Biol. Biochem. 2018, 118, 42–50. [Google Scholar] [CrossRef]
- Li, C.; Cao, Z.; Chang, J.; Zhang, Y.; Zhu, G.; Zong, N.; He, Y.; Zhang, J.; He, N. Elevational gradient affect functional fractions of soil organic carbon and aggregates stability in a Tibetan alpine meadow. Catena 2017, 156, 139–148. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Ahmad, A.; Martsinovich, N. Atomic-scale modelling of organic matter in soil: Adsorption of organic molecules and biopolymers on the hydroxylated α-Al2O3 (0001) surface. Philos. Trans. Math. Phys. Eng. Sci. 2023, 381, 20220254. [Google Scholar] [CrossRef]
- Kravchenko, A.; Guber, A.; Razavi, B.; Koestel, J.; Quigley, M.; Robertson, G.; Kuzyakov, Y. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 2019, 10, 3121. [Google Scholar] [CrossRef]
- Yin, X.; Weitzel, F.; Jimenez-Lopez, C.; Griesshaber, E.; Fernandez-Diaz, L.; Rodríguez-Navarro, A.; Ziegler, A.; Schmahl, W.W. Directing effect of bacterial extracellular polymeric substances (EPS) on calcite organization and EPS–carbonate composite aggregate formation. Cryst. Growth Des. 2020, 20, 1467–1484. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil. Till. Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil. Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Zheng, S.; Dou, S.; Duan, H. Effects of straw enrichment and deep incorporation on humus composition and humic acid structure of black soil profile in Northeast China. Appl. Ecol. Environ. Res. 2022, 20, 1051–1063. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil. Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Abulaiti, A.; She, D.; Liu, Z.; Sun, X.; Wang, H. Application of biochar and polyacrylamide to revitalize coastal saline soil quality to improve rice growth. Environ. Sci. Pollut. Res. 2023, 30, 18731–18747. [Google Scholar] [CrossRef]
- An, S.; Mentler, A.; Mayer, H.; Blum, W.E. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the Loess Plateau, China. Catena 2010, 81, 226–233. [Google Scholar] [CrossRef]
- Aoyama, Y.; Sato, N.; Toyotama, A.; Okuzono, T.; Yamanaka, J. Particle adsorption on polymer gel surface driven by van der waals attraction. Bull. Chem. Soc. Jpn. 2022, 95, 314–324. [Google Scholar] [CrossRef]
- Hong, D.; Chang, D.; Shao, C.; Cui, W.; Lu, X.; Dong, W.; Fan, H.; Wang, K.; Liu, Y. Effects of polymer conditioner and nitrogen fertilizer application on nitrogen absorption and utilization of drip-irrigated wheat in arid areas. Agronomy 2024, 14, 232. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Mengel, K. Turnover of organic nitrogen in soils and its availability to crops. Plant Soil 1996, 181, 83–93. [Google Scholar] [CrossRef]
- Gavelaki, F.; Favaretto, N.; de Albuquerque, C.G.; Motta, A.C.V.; da Rocha, G.; Pauletti, V. Phosphorus adsorption in subtropical Histosol and Inceptisol with contrasting organic matter contents and clay mineralogy. Catena 2025, 249, 108682. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).