Depicting the Physiological, Biochemical and Metabolic Responses to the Removal of Adventitious Roots and Their Functions in Cucumis melo Under Waterlogging Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Stress Treatment
2.2. Detection of Chlorophyll, Proline, Flavonoids and ROS Contents
2.3. Detection of Enzyme Activity
2.4. Detection of Non-Enzymatic Antioxidants
2.5. Detection of Hormones Contents
2.6. Untargeted Metabolomic Analysis
2.7. Statistical Analysis
3. Results
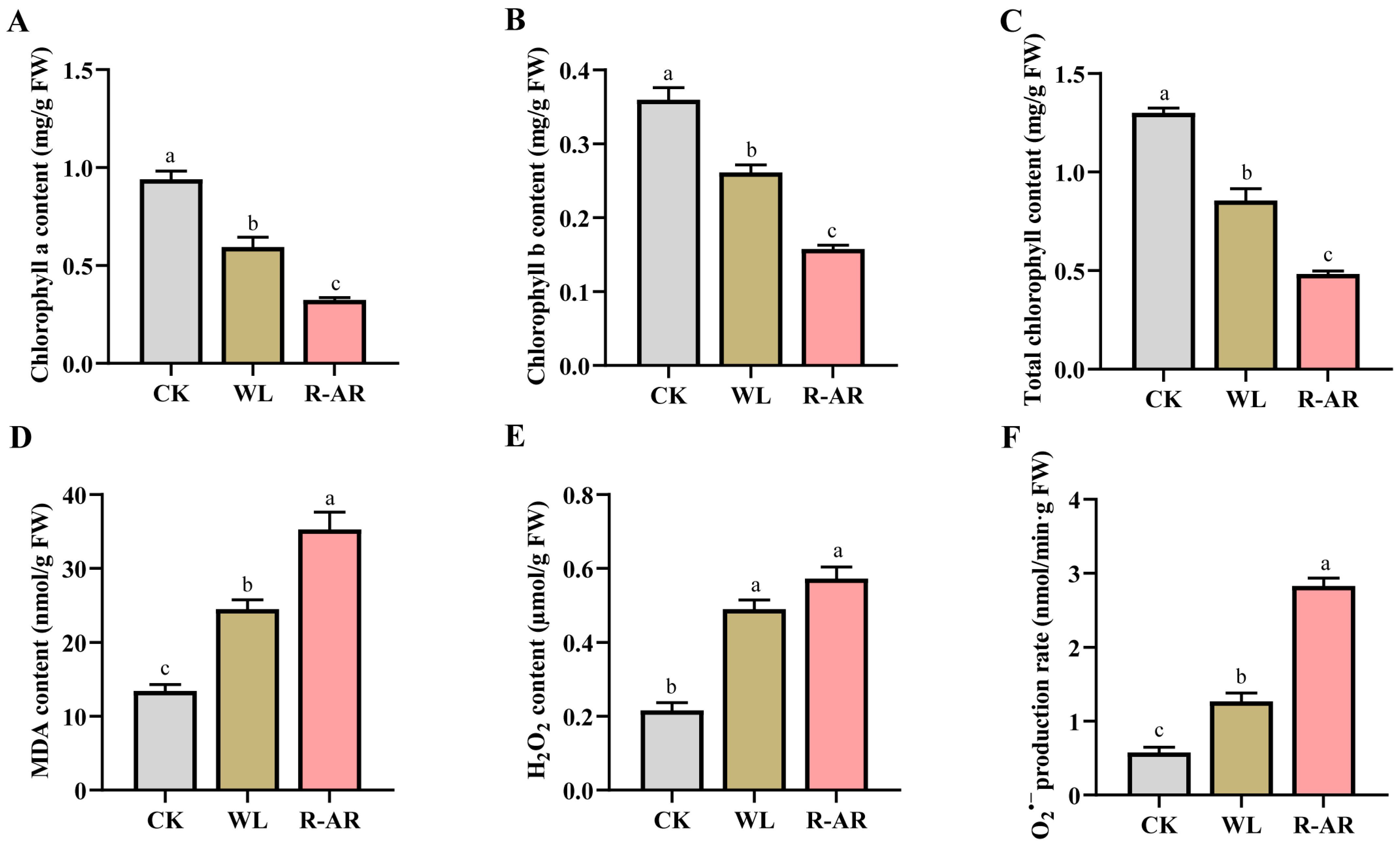
3.1. Phenotypic Observation and Chlorophyll Content Measurement
3.2. Changes in Contents of Malondialdehyde (MDA), H2O2 and O2•− Production Rate
3.3. Changes in Antioxidant Enzyme Activity
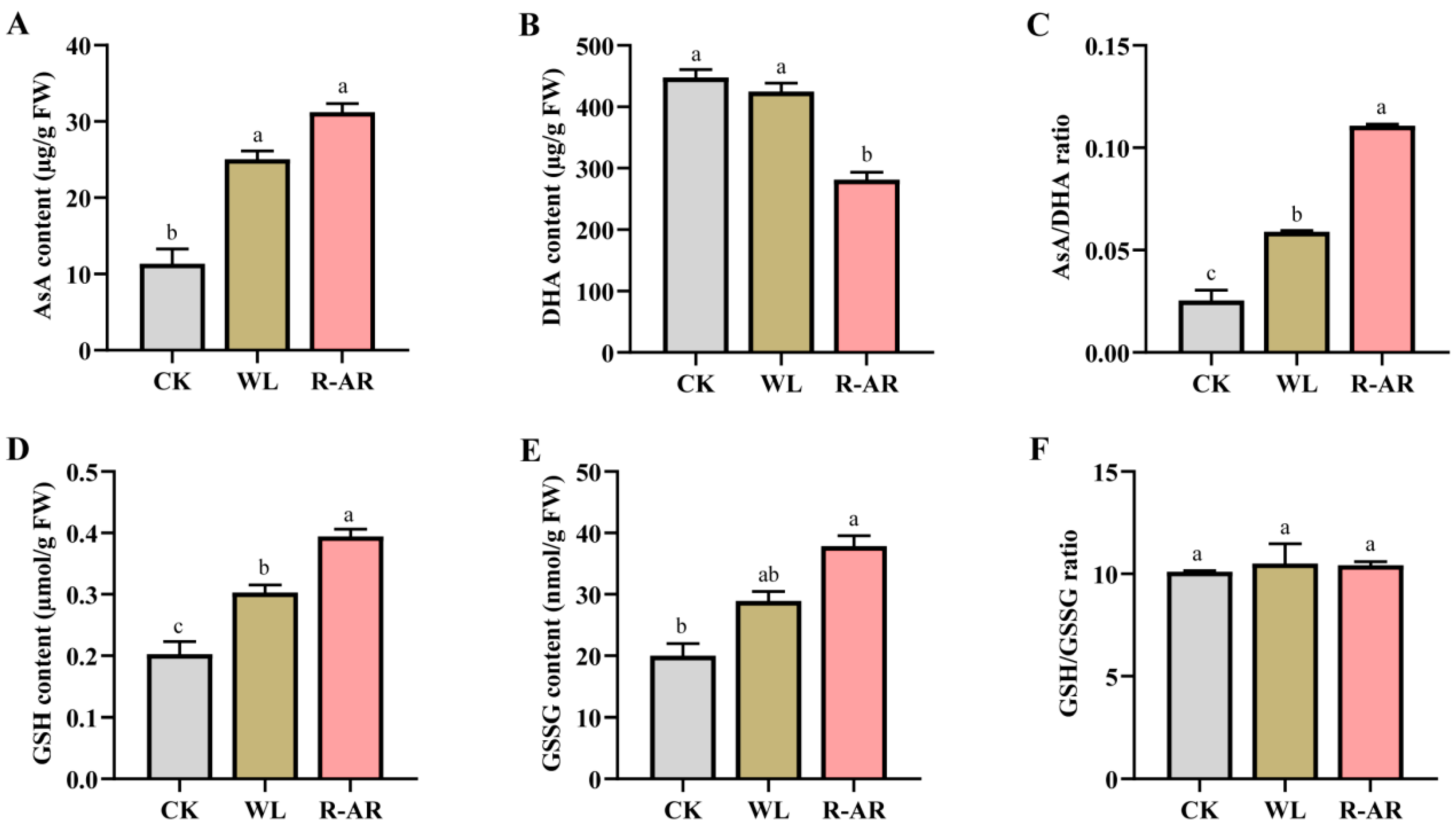
3.4. Changes in Non-Enzymatic Antioxidants
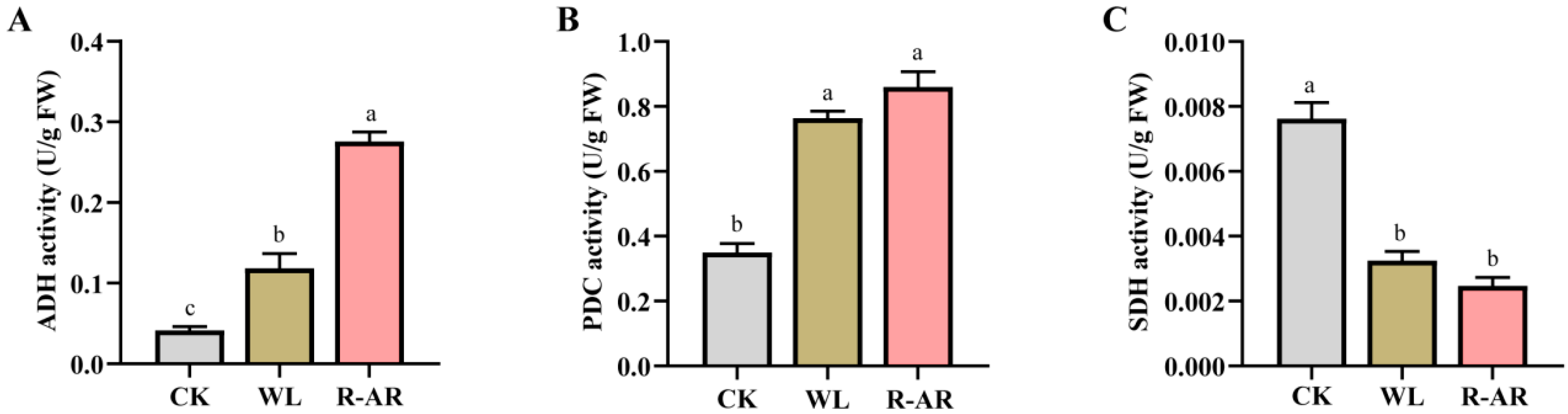
3.5. Changes in Enzyme Activities of Aerobic and Anaerobic Respiration
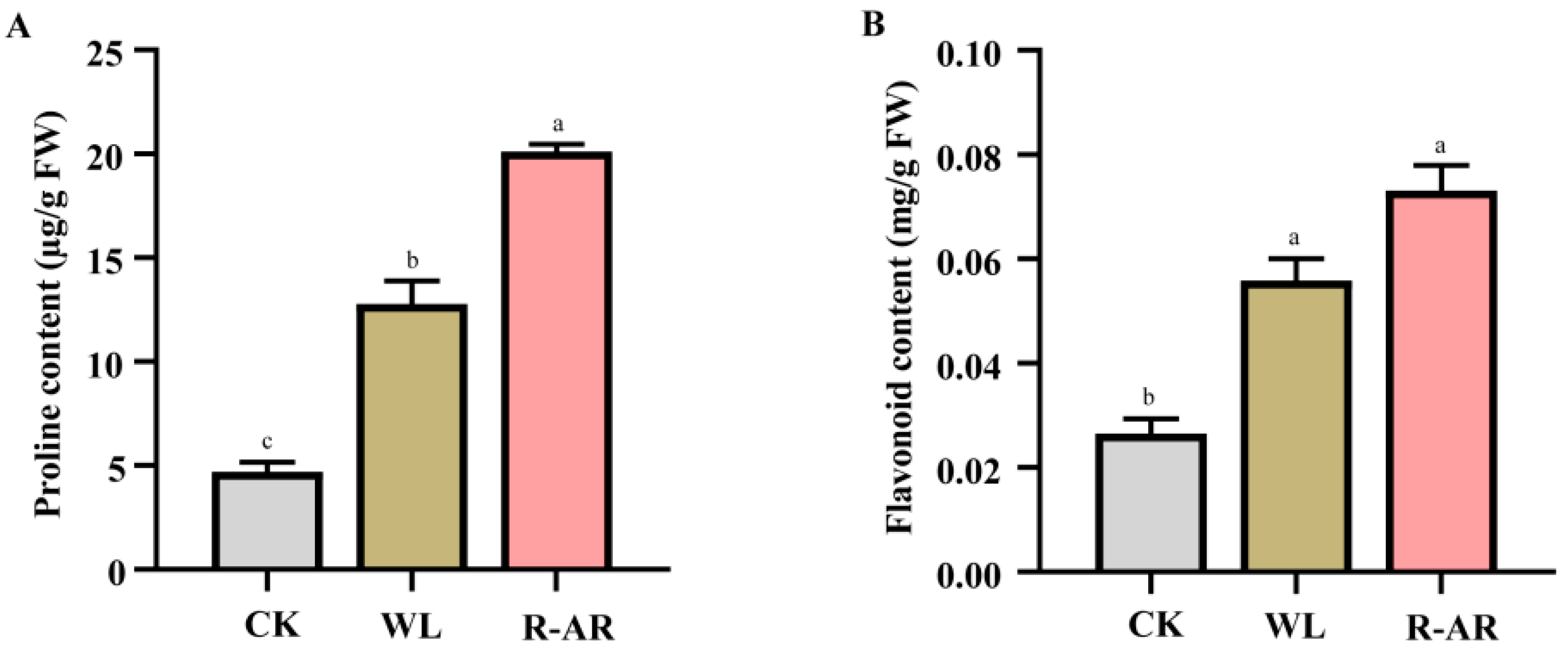
3.6. Changes in Proline and Flavonoids Contents
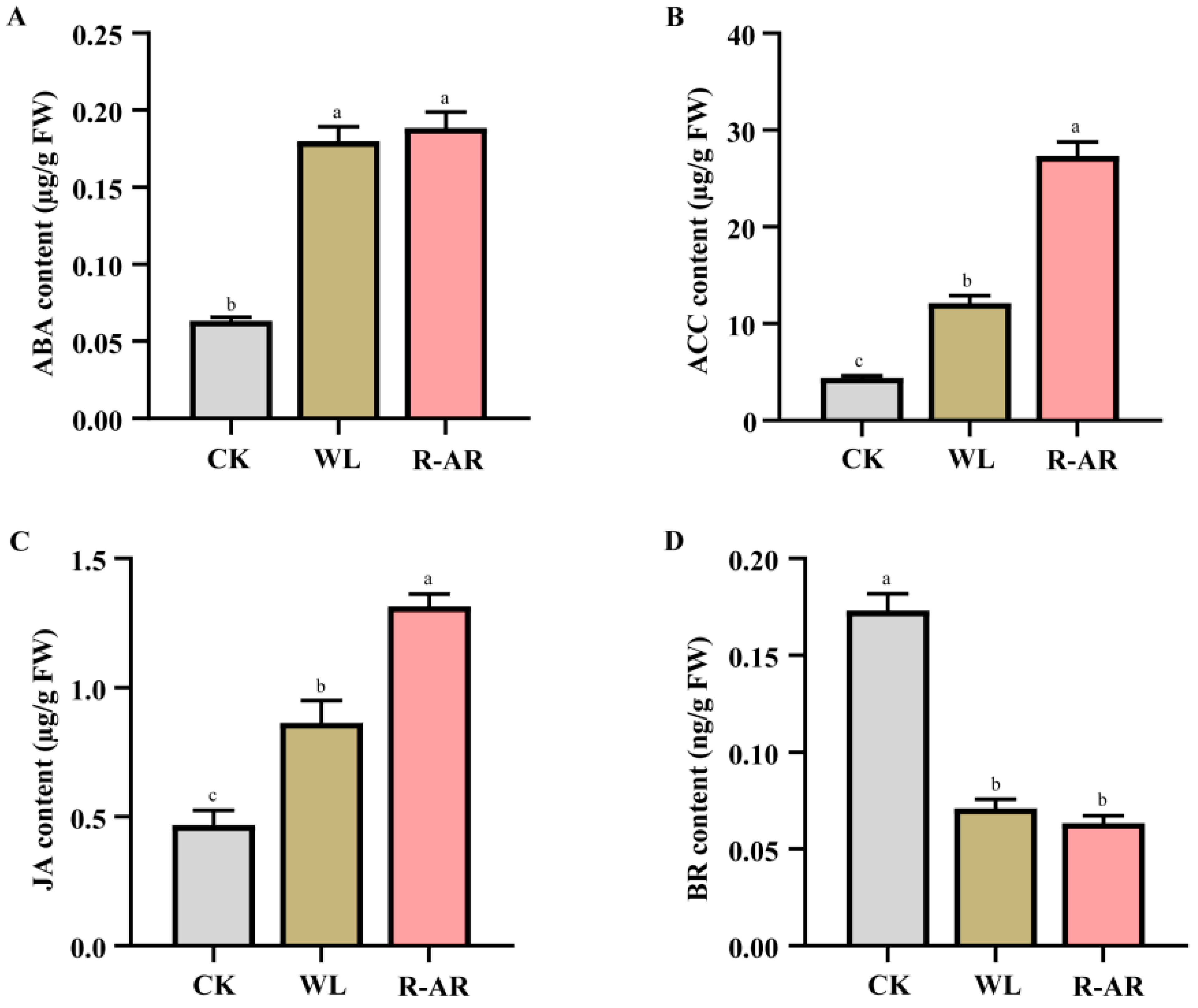
3.7. Changes in Plant Hormones Contents
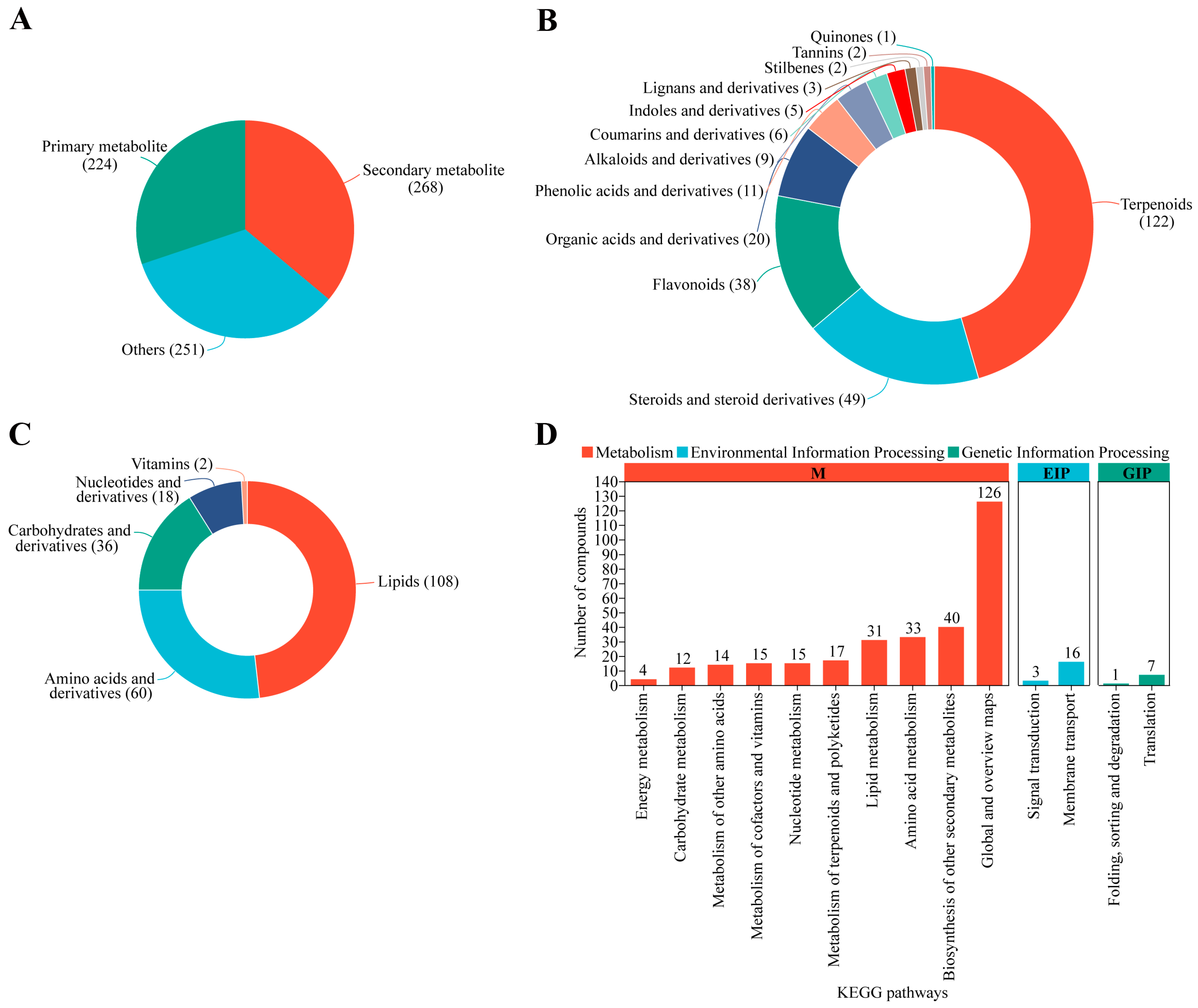
3.8. Overview of Detected Metabolites
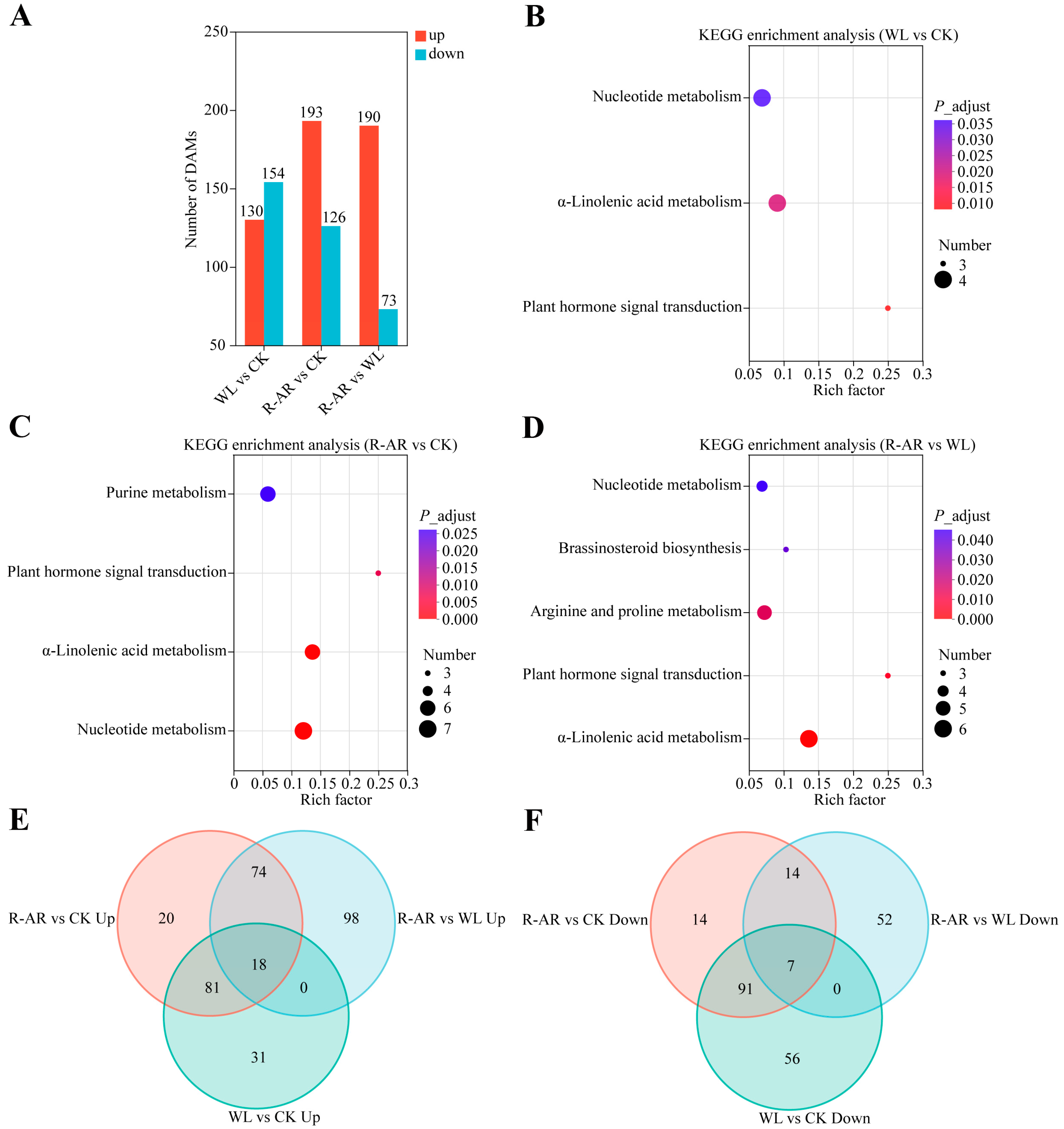
3.9. Identification of Differentially Accumulated Metabolites (DAMs)
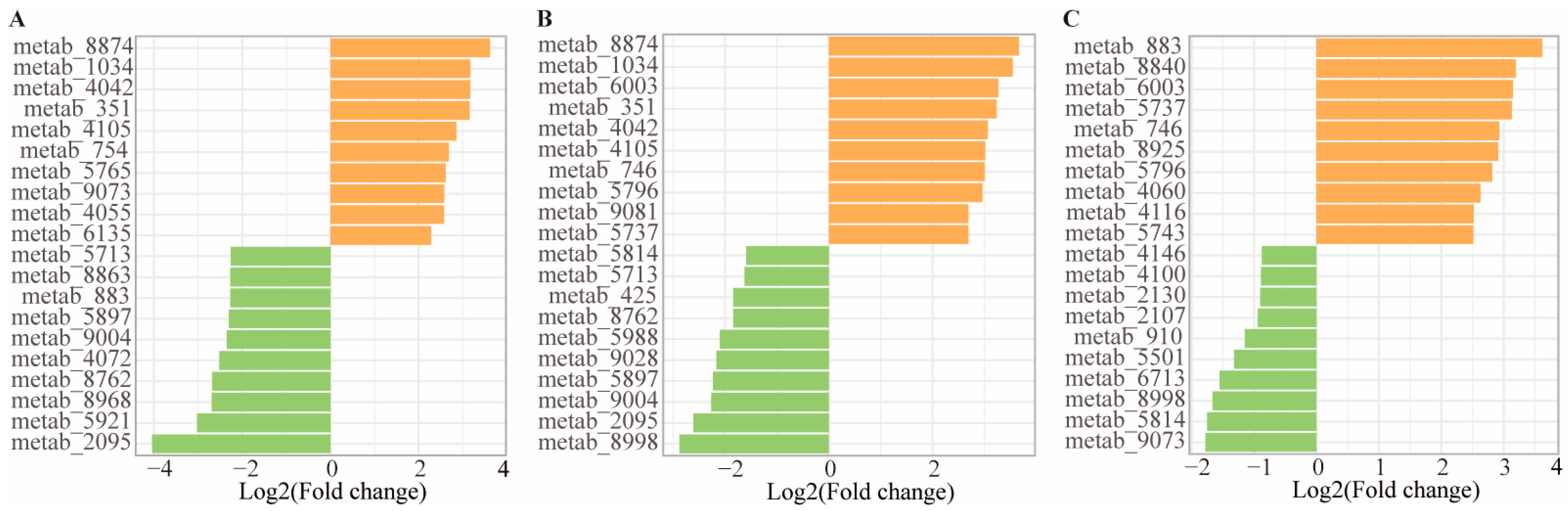
3.10. Identification of Metabolites with Top Fold Change
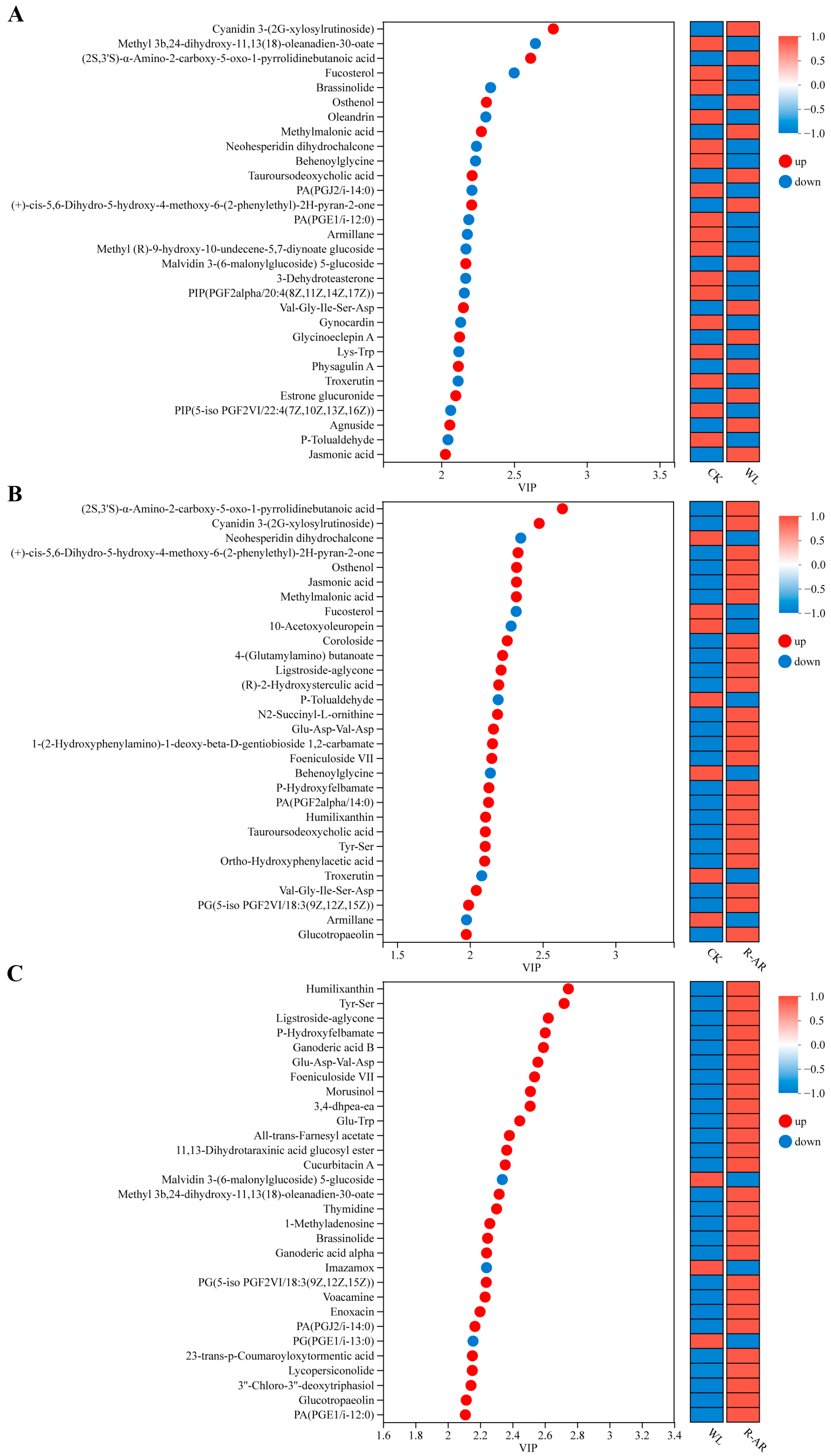
3.11. Identification of Metabolites with Top Variable Importance in Projection (VIP) Scores
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manghwar, H.; Hussain, A.; Alam, I.; Khoso, M.A.; Ali, Q.; Liu, F. Waterlogging stress in plants: Unraveling the mechanisms and impacts on growth, development, and productivity. Environ. Exp. Bot. 2024, 224, 105824. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, T.; Dong, H. Melatonin enhances waterlogging tolerance of field-grown cotton through quiescence adaptation and compensatory growth strategies. Field Crop. Res. 2024, 306, 109217. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Yan, C.; Zhang, X.; Cao, N.; Le, M.; Hu, X.; Zhu, F.; Liu, W. Elucidating the molecular responses to waterlogging stress in Cucumis melo by comparative transcriptome profiling. Horticulturae 2022, 8, 891. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Yan, C.; Cao, N.; Yang, H.; Le, M.; Zhu, F. Depicting the molecular responses of adventitious rooting to waterlogging in melon hypocotyls by transcriptome profiling. 3 Biotech 2021, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Song, J.; Sohail, H.; Sharif, R.; Yan, W.; Hu, Q.; Qi, X.; Yang, X.; Xu, X.; Chen, X. RNA-seq-based comparative transcriptome analysis reveals the role of CsPrx73 in waterlogging-triggered adventitious root formation in cucumber. Hortic. Res. 2024, 11, uhae062. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, J.; Xu, C.; Qi, S.; Sun, W.; Wu, S. AUREA maintains the balance between chlorophyll synthesis and adventitious root formation in tomato. Hortic. Res. 2020, 7, 166. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Señorans, J.; Zwiazek, J.J. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef]
- Li, S.-W.; Xue, L.; Xu, S.; Feng, H.; An, L. Mediators, genes and signaling in adventitious rooting. Bot. Rev. 2009, 75, 230–247. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Xu, Q.; Qi, X.; Chen, X. Inheritance and quantitative trail loci mapping of adventitious root numbers in cucumber seedlings under waterlogging conditions. Mol. Genet. Genom. 2017, 292, 353–364. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; Feng, N.; Xiang, H.; Liu, Y.; Wang, F.; Li, W.; Feng, S.; Liu, M.; Zheng, D. Physiological response of soybean leaves to uniconazole under waterlogging stress at R1 stage. J. Plant Physiol. 2022, 268, 153579. [Google Scholar] [CrossRef] [PubMed]
- Koramutla, M.K.; Tuan, P.A.; Ayele, B.T. Salicylic acid enhances adventitious root and aerenchyma formation in wheat under waterlogged conditions. Int. J. Mol. Sci. 2022, 23, 1243. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Zeeshan, M.; Zhou, X.-B. Melatonin and KNO3 application improves growth, physiological and biochemical characteristics of maize seedlings under waterlogging stress conditions. Biology 2022, 11, 99. [Google Scholar] [CrossRef]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.-Q.; Jia, Y.; Li, C. Opportunities for improving waterlogging tolerance in cereal crops-physiological traits and genetic mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Zhu, F.-Y.; Chen, M.-X.; Chan, W.-L.; Yang, F.; Tian, Y.; Song, T.; Xie, L.-J.; Zhou, Y.; Xiao, S.; Zhang, J.; et al. SWATH-MS quantitative proteomic investigation of nitrogen starvation in Arabidopsis reveals new aspects of plant nitrogen stress responses. J. Proteom. 2018, 187, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, H.; Sun, C.; Ma, Q.; Bu, H.; Chong, K.; Xu, Y. A C2H2 zinc-finger protein OsZFP213 interacts with OsMAPK3 to enhance salt tolerance in rice. J. Plant Physiol. 2018, 229, 100–110. [Google Scholar] [CrossRef]
- Shao, P.; Wang, P.; Niu, B.; Kang, J. Environmental stress stability of pectin-stabilized resveratrol liposomes with different degree of esterification. Int. J. Biol. Macromol. 2018, 119, 53–59. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, C.; Li, G.; Zhang, X.; Cao, N.; Le, M.; Zhu, F.; Liu, W. Joint analysis of transcriptome and proteome revealed the molecular mechanism of melon (Cucumis melo L.) in response to waterlogging stress. J. Nucl. Agric. Sci. 2024, 38, 0662–0673. [Google Scholar] [CrossRef]
- Mo, Z.; Yang, X.; Hu, L.; Zhai, M.; Xuan, J. Histological, physio-biochemical, and transcriptomic analyses reveal the potential limiting factors for successful grafting of pecan. J. For. Res. 2024, 35, 35. [Google Scholar] [CrossRef]
- Si, J.; Fan, Z.; Wu, C.; Yang, Y.; Shan, W.; Kuang, J.; Lu, W.; Wei, W.; Chen, J. MaHsf24, a novel negative modulator, regulates cold tolerance in banana fruits by repressing the expression of HSPs and antioxidant enzyme genes. Plant Biotechnol. J. 2024, 22, 2873–2886. [Google Scholar] [CrossRef]
- Yan, S.; Si, Z.; Qi, G.; Zang, Y.; Xuan, L.; He, L.; Cao, Y.; Li, X.; Zhang, T.; Hu, Y. A CC-NB-ARC-LRR gene regulates bract morphology in cotton. Adv. Sci. 2024, 11, 2406111. [Google Scholar] [CrossRef]
- Cao, P.; Yang, J.; Xia, L.; Zhang, Z.; Wu, Z.; Hao, Y.; Liu, P.; Wang, C.; Li, C.; Yang, J.; et al. Two gene clusters and their positive regulator SlMYB13 that have undergone domestication-associated negative selection control phenolamide accumulation and drought tolerance in tomato. Mol. Plant 2024, 17, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Song, Y.; Liu, X.; Xiao, L.; Bu, C.; Liu, P.; Zhao, L.; Ingvarsson, P.K.; Wu, H.X.; El-Kassaby, Y.A.; et al. LncRNA PMAT–PtoMYB46 module represses PtoMATE and PtoARF2 promoting Pb2+ uptake and plant growth in poplar. J. Hazard. Mater. 2022, 433, 128769. [Google Scholar] [CrossRef]
- Zhao, H.; Qian, R.; Liang, X.; Ou, Y.; Sun, C.; Lin, X. Indium induces nitro-oxidative stress in roots of wheat (Triticum aestivum). J. Hazard. Mater. 2022, 428, 128260. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yang, Y.; Gong, D.; Yu, L.; Wang, Y.; Li, Y.; Prusky, D.; Bi, Y. Preharvest spraying of phenylalanine activates the sucrose and respiratory metabolism in muskmelon wounds during healing. Food Chem. 2024, 457, 140194. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Wu, Q.-Y.; Ni, Z.-X.; Hu, Q.-C.; Yang, Y.; Zheng, Y.-C.; Bi, W.-J.; Deng, H.-L.; Liu, Z.-Z.; Ye, N.-X.; et al. Metabolic flow of C6 volatile compounds from LOX-HPL pathway based on airflow during the post-harvest process of Oolong tea. Front. Plant Sci. 2021, 12, 738445. [Google Scholar] [CrossRef]
- Wang, T.; Zang, Z.; Wang, S.; Liu, Y.; Wang, H.; Wang, W.; Hu, X.; Sun, J.; Tai, F.; He, R. Quaternary ammonium iminofullerenes promote root growth and osmotic-stress tolerance in maize via ROS neutralization and improved energy status. Plant Physiol. Biochem. 2021, 164, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.; Wang, S.; Liang, B.; Li, Y.; Wu, J.; Fan, C.; Hu, X.; Wang, H.; He, R.; Wang, W. Quaternary ammonium iminofullerenes improve root growth of oxidative-stress maize through ASA-GSH cycle modulating redox homeostasis of roots and ROS-mediated root-hair elongation. J. Nanobiotechnol. 2022, 20, 15. [Google Scholar] [CrossRef]
- Hermann, K.; Meinhard, J.; Dobrev, P.; Linkies, A.; Pesek, B.; Hess, B.; Macháčková, I.; Fischer, U.; Leubner-Metzger, G. 1-Aminocyclopropane-1-carboxylic acid and abscisic acid during the germination of sugar beet (Beta vulgaris L.): A comparative study of fruits and seeds. J. Exp. Bot. 2007, 58, 3047–3060. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Guo, Q.; Li, B.; Cheng, S.; Yan, J.; Chu, J. A tailored high-efficiency sample pretreatment method for simultaneous quantification of 10 classes of known endogenous phytohormones. Plant Commun. 2020, 1, 100047. [Google Scholar] [CrossRef]
- Yang, R.; Yang, T.; Zhang, H.; Qi, Y.; Xing, Y.; Zhang, N.; Li, R.; Weeda, S.; Ren, S.; Ouyang, B.; et al. Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol. Biochem. 2014, 77, 23–34. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Lyu, X.; Chen, J.; Chen, X.; Guo, S. Untargeted metabolomics reveals multiple phytometabolites in the agricultural waste materials and medicinal materials of Codonopsis pilosula. Front. Plant Sci. 2022, 12, 814011. [Google Scholar] [CrossRef]
- Yue, C.; Peng, H.; Li, W.; Tong, Z.; Wang, Z.; Yang, P. Untargeted metabolomics and transcriptomics reveal the mechanism of metabolite differences in spring tender shoots of tea plants of different ages. Foods 2022, 11, 2303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef]
- Ma, S.; Gai, P.; Geng, B.; Wang, Y.; Ullah, N.; Zhang, W.; Zhang, H.; Fan, Y.; Huang, Z. Exogenous melatonin improves waterlogging tolerance in wheat through promoting antioxidant enzymatic activity and carbon assimilation. Agronomy 2022, 12, 2876. [Google Scholar] [CrossRef]
- Gu, X.; Xue, L.; Lu, L.; Xiao, J.; Song, G.; Xie, M.; Zhang, H. Melatonin enhances the waterlogging tolerance of Prunus persica by modulating antioxidant metabolism and anaerobic respiration. J. Plant Growth Regul. 2021, 40, 2178–2190. [Google Scholar] [CrossRef]
- Alizadeh-Vaskasi, F.; Pirdashti, H.; Araei, A.C.; Saadatmand, S. Waterlogging effects on some antioxidant enzymes activities and yield of three wheat promising lines. Acta Agric. Slov. 2018, 111, 621–631. [Google Scholar] [CrossRef]
- Basu, S.; Kumari, S.; Kumar, P.; Kumar, G.; Rajwanshi, R. Redox imbalance impedes photosynthetic activity in rice by disrupting cellular membrane integrity and induces programmed cell death under submergence. Physiol. Plant. 2021, 172, 1764–1778. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, J.; Tan, D.-X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; López-Climent, M.F.; Arbona, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J. Plant Physiol. 2009, 166, 1391–1404. [Google Scholar] [CrossRef]
- Cheng, X.-X.; Yu, M.; Zhang, N.; Zhou, Z.-Q.; Xu, Q.-T.; Mei, F.-Z.; Qu, L.-H. Reactive oxygen species regulate programmed cell death progress of endosperm in winter wheat (Triticum aestivum L.) under waterlogging. Protoplasma 2016, 253, 311–327. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Mahmood, U.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zamir, S.; Al-Robai, S.A.; Alzahrani, F.O.; Hano, C.; El-Esawi, M.A. Morpho-physio-biochemical and molecular responses of maize hybrids to salinity and waterlogging during stress and recovery phase. Plants 2021, 10, 1345. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Xu, J.; Liang, B. Melatonin and dopamine enhance waterlogging tolerance by modulating ROS scavenging, nitrogen uptake, and the rhizosphere microbial community in Malus hupehensis. Plant Soil 2023, 483, 475–493. [Google Scholar] [CrossRef]
- Huo, L.; Wang, H.; Wang, Q.; Gao, Y.; Xu, K.; Sun, X. Exogenous treatment with melatonin enhances waterlogging tolerance of kiwifruit plants. Front. Plant Sci. 2022, 13, 1081787. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2006, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Umer, M.J.; Yuan, P.; Wang, W.; Zhu, H.; Lu, X.; Xing, Y.; Gong, C.; Batool, R.; Sun, X.; et al. Physiological, biochemical, and metabolic changes in diploid and triploid watermelon leaves during flooding. Front. Plant Sci. 2023, 14, 1108795. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef]
- Lothier, J.; Diab, H.; Cukier, C.; Limami, A.M.; Tcherkez, G. Metabolic responses to waterlogging differ between roots and shoots and reflect phloem transport alteration in Medicago truncatula. Plants 2020, 9, 1373. [Google Scholar] [CrossRef]
- Jackson, M.B. Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 1985, 36, 145–174. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Najeeb, U.; Bange, M.P.; Tan, D.K.Y.; Atwell, B.J. Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 2015, 7, plv080. [Google Scholar] [CrossRef]
- Gibbs, J.; Greenway, H. Mechanisms of anoxia tolerance in plants. I. growth, survival and anaerobic catabolism. Funct. Plant Biol. 2003, 30, 1–47. [Google Scholar] [CrossRef]
- Narsai, R.; Rocha, M.; Geigenberger, P.; Whelan, J.; van Dongen, J.T. Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. New Phytol. 2011, 190, 472–487. [Google Scholar] [CrossRef]
- Li, C.; Bai, T.; Ma, F.; Han, M. Hypoxia tolerance and adaptation of anaerobic respiration to hypoxia stress in two Malus species. Sci. Hortic. 2010, 124, 274–279. [Google Scholar] [CrossRef]
- Shukla, V.; Lombardi, L.; Pencik, A.; Novak, O.; Weits, D.A.; Loreti, E.; Perata, P.; Giuntoli, B.; Licausi, F. Jasmonate signalling contributes to primary root inhibition upon oxygen deficiency in Arabidopsis thaliana. Plants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Sreeratree, J.; Butsayawarapat, P.; Chaisan, T.; Somta, P.; Juntawong, P. RNA-seq reveals waterlogging-triggered root plasticity in mungbean associated with ethylene and jasmonic acid signal integrators for root regeneration. Plants 2022, 11, 930. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, L.-C.; Yang, J.; Qian, Z.-H.; He, Y.-X.; Li, M.-W. Physiological and transcriptional changes provide insights into the effect of root waterlogging on the aboveground part of Pterocarya stenoptera. Genomics 2021, 113, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Zhang, Y.; Gao, Y.; Yu, J.; Wei, X.; Zhang, X. Tolerant and susceptible sesame genotypes reveal waterlogging stress response patterns. PLoS ONE 2016, 11, e0149912. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Jiu, S.; Zhang, K.; Wang, C.; Fang, J. Analysis of the regulation networks in grapevine reveals response to waterlogging stress and candidate gene-marker selection for damage severity. R. Soc. Open Sci. 2018, 5, 172253. [Google Scholar] [CrossRef]
- Ding, L.-N.; Liu, R.; Li, T.; Li, M.; Liu, X.-Y.; Wang, W.-J.; Yu, Y.-K.; Cao, J.; Tan, X.-L. Physiological and comparative transcriptome analyses reveal the mechanisms underlying waterlogging tolerance in a rapeseed anthocyanin-more mutant. Biotechnol. Biofuels Bioprod. 2022, 15, 55. [Google Scholar] [CrossRef]
- Liu, S.; Ju, J.; Xia, G. Identification of the flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase genes from Antarctic moss and their regulation during abiotic stress. Gene 2014, 543, 145–152. [Google Scholar] [CrossRef]
- Ueyama, Y.; Suzuki, K.-I.; Fukuchi-Mizutani, M.; Fukui, Y.; Miyazaki, K.; Ohkawa, H.; Kusumi, T.; Tanaka, Y. Molecular and biochemical characterization of torenia flavonoid 3′-hydroxylase and flavone synthase II and modification of flower color by modulating the expression of these genes. Plant Sci. 2002, 163, 253–263. [Google Scholar] [CrossRef]
- He, N.; Umer, M.J.; Yuan, P.; Wang, W.; Zhu, H.; Zhao, S.; Lu, X.; Xing, Y.; Gong, C.; Liu, W.; et al. Expression dynamics of metabolites in diploid and triploid watermelon in response to flooding. PeerJ 2022, 10, e13814. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yan, C.; Chen, Q.; Li, G. Depicting the Physiological, Biochemical and Metabolic Responses to the Removal of Adventitious Roots and Their Functions in Cucumis melo Under Waterlogging Stress. Agronomy 2025, 15, 2281. https://doi.org/10.3390/agronomy15102281
Zhang H, Yan C, Chen Q, Li G. Depicting the Physiological, Biochemical and Metabolic Responses to the Removal of Adventitious Roots and Their Functions in Cucumis melo Under Waterlogging Stress. Agronomy. 2025; 15(10):2281. https://doi.org/10.3390/agronomy15102281
Chicago/Turabian StyleZhang, Huanxin, Chengpu Yan, Qian Chen, and Guoquan Li. 2025. "Depicting the Physiological, Biochemical and Metabolic Responses to the Removal of Adventitious Roots and Their Functions in Cucumis melo Under Waterlogging Stress" Agronomy 15, no. 10: 2281. https://doi.org/10.3390/agronomy15102281
APA StyleZhang, H., Yan, C., Chen, Q., & Li, G. (2025). Depicting the Physiological, Biochemical and Metabolic Responses to the Removal of Adventitious Roots and Their Functions in Cucumis melo Under Waterlogging Stress. Agronomy, 15(10), 2281. https://doi.org/10.3390/agronomy15102281




