Red Pepper Fermentation with Geothermal Mineral Water: Impact on Nutritional Profile and Quality Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fermentation, Pasteurization, and Storage Process
2.3. Analitycal Methods
2.4. The Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Fresh and Fermented Pepper Fruits
3.2. Bioactive Compounds of Fresh and Fermented Pepper Fruits
3.2.1. L-Ascorbic Acid
3.2.2. Total Carotenoids
3.2.3. Total Polyphenols
3.3. Mineral Composition
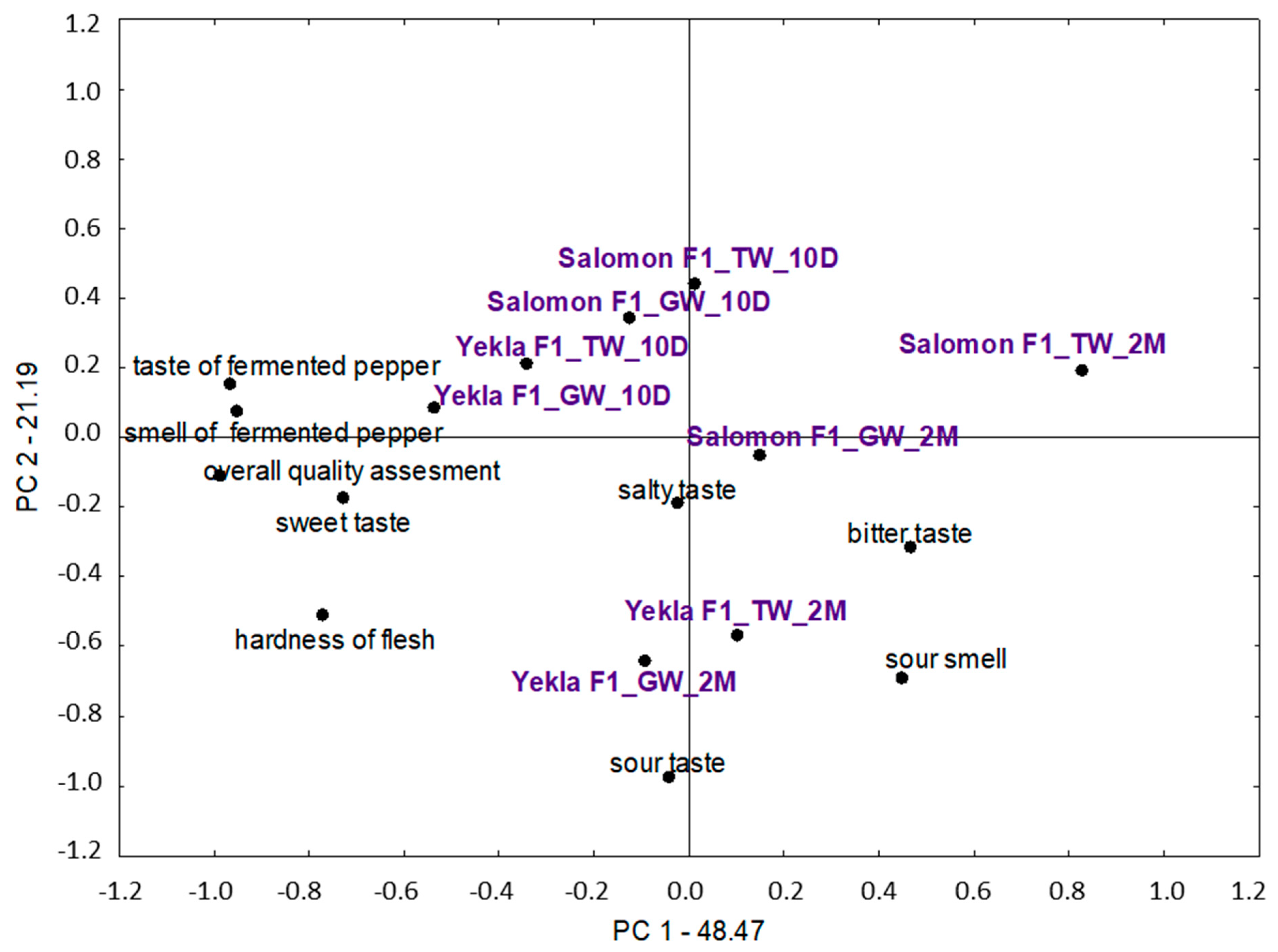
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateos, R.M.; León, A.M.; Sandalio, L.M.; Gómez, M.; del Río, L.A.; Palma, J.M. Peroxisomes from Pepper Fruits (Capsicum annuum L.): Purification, Characterisation and Antioxidant Activity. J. Plant Physiol. 2003, 160, 1507–1516. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant Constituents in Some Sweet Pepper (Capsicum annuum L.) Genotypes during Maturity. LWT-Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Garza-Juárez, A.; Pérez-Carrillo, E.; Arredondo-Espinoza, E.U.; Islas, J.F.; Benítez-Chao, D.F.; Escamilla-García, E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods 2023, 12, 3262. [Google Scholar] [CrossRef]
- Hu, X.; Saravanakumar, K.; Jin, T.; Wang, M.-H. Effects of Yellow and Red Bell Pepper (Paprika) Extracts on Pathogenic Microorganisms, Cancerous Cells and Inhibition of Survivin. J. Food Sci. Technol. 2021, 58, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Lucier, G.; Lin, B. Sweet Peppers: Saved by the Bell. Agric. Outlook 2001, 287, 12–15. [Google Scholar]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnol. Res. Inter. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Jiang, L.; Deng, F.; Zhou, H. Analysis of Bacterial Diversity during Fermentation of Chinese Traditional Fermented Chopped Pepper. Lett. Appl. Microbiol. 2019, 69, 346–352. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, M.; Rozos, G. Fermentative foods: Microbiology, biochemistry, potential human health benefits and public health issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Cui, F.; Wang, D.; Lv, X.; Li, X.; Li, J. Fermented Vegetables: Health Benefits, Defects, and Current Technological Solutions. Foods 2023, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef]
- Kiczorowski, P.; Kiczorowska, B.; Samolińska, W.; Szmigielski, M.; Winiarska-Mieczan, A. Effect of Fermentation of Chosen Vegetables on the Nutrient, Mineral, and Biocomponent Profile in Human and Animal Nutrition. Sci. Rep. 2022, 12, 13422. [Google Scholar] [CrossRef]
- Garcia, C.; Remize, F. Lactic Acid Fermentation of Fruit and Vegetable Juices and Smoothies: Innovation and Health Aspects. In Lactic Acid Bacteria in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–46. ISBN 978-0-323-89875-1. [Google Scholar]
- Mustafa, S.M.; Chua, L.S. 13-Green Technological Fermentation for Probioticated Beverages for Health Enhancement. In Biotechnological Progress and Beverage Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 407–434. ISBN 978-0-12-816678-9. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Zhang, Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting Phytate-Element Interactions: Implications for Iron, Zinc and Calcium Bioavailability, with Emphasis on Legumes. Crit. Rev. Food Sci. Nutr. 2020, 62, 1696–1712. [Google Scholar] [CrossRef]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Tamang, J.P. Health Benefits of Fermented Foods and Beverages, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-0-429-16834-5. [Google Scholar]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Production of Probiotic Cabbage Juice by Lactic Acid Bacteria. Bioresour. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules 2022, 27, 1008. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa, J.Y.; Guan, Y.; Feng, K. Microbial Dynamics and Volatilome Profiles during the Fermentation of Chinese Northeast Sauerkraut by Leuconostoc Mesenteroides ORC 2 and Lactobacillus Plantarum HBUAS 51041 under Different Salt Concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Removal of Heavy Metals by Waste Tea Leaves from Aqueous Solution. Eng. Life Sci. 2005, 5, 158–162. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for Remediation of Heavy Metals: Current Status and Their Future Prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Derbalah, A.; Moghanm, F. Removal of Heavy Metals from Aqueous Solution by Zeolite in Competitive Sorption System. Int. J. Environ. Sci. Dev. 2012, 3, 362–367. [Google Scholar] [CrossRef]
- Montet, D.; Ray, R.C.; Zakhia-Rozis, N. Lactic Acid Fermentation of Vegetables and Fruits. Microorg. Ferment. Tradit. Foods 2014, 21, 108–140. [Google Scholar]
- PN-A-77701:1997; Vegetable products—Fermented Cucumbers and Fermented Cucumbers Puree. Polish Committee for Normalisation: Warsaw, Poland, 1997.
- Strnad, S.W.; Satora, P. Utilization of Brine Created during the Process of White Cabbage (Brassica Oleracea Var. Capitata f. Alba) Fermentation. Ecol. Eng. Environ. Technol. 2018, 19, 77–83. [Google Scholar] [CrossRef]
- Wrzodak, A.; Szwejda-Grzybowska, J. Application of Mineral Water from Geothermal Source for Fermentation of Beetroot. J. Hort. Res. 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Drobnik, M.; Latour, T.; Sziwa, D. Bioaktywne składniki mineralne w polskich naturalnych wodach mineralnych udostępnianych do spożycia w opakowaniach jednostkowych. Bromat. Chem. Toksykol. 2016, 3, 266–271. (In Polish) [Google Scholar]
- PN 90/A-75101/03; Fruit and Vegetable Products. Preparation of Samples for Physico-Chemical Studies. Determination of Dry Matter Content by Gravimetric Method. Polish Committee for Standardization: Warsaw, Poland, 1990.
- PN-EN 12147:2000; Fruit and Vegetable Juices—Determination of Titrable Acidity. Polish Committee for Standardization: Warsaw, Poland, 2000.
- EN 12630:1999; Fruit and Vegetable Juices. Determination of glucose, fructose, sorbitol and sucrose contents. Method using high-performance liquid chromatography. British Standards Institution: London, UK, 1999.
- Bohoyo-Gil, D.; Dominguez-Valhondo, D.; García-Parra, J.J.; González-Gómez, D. UHPLC as a suitable methodology for the analysis of carotenoids in food matrix. Eur. Food Res. Technol. 2012, 235, 1055–1061. [Google Scholar] [CrossRef]
- Umiel, N.; Gabelman, W.H. Analytical procedures for detecting carotenoids of carrot (Daucus carota L.) roots and tomato (Lycopersicon esculentum) fruits. J. Am. Soc. Hort. Sci. 1971, 96, 702–704. [Google Scholar] [CrossRef]
- Sluis, A.; Dekker, M.; Skrede, G.; Jongen, W. Activity and concentration of polyphenolic antioxidants in apple juice. Effect of existing production methods. J. Agric. Food Chem. 2002, 50, 7211–7214. [Google Scholar] [CrossRef]
- EN 16943:2017; Foodstuffs—Determination of Calcium, Copper, Iron, Magnesium, Manganese, Phosphorus, Potassium, Sodium, Sulfur and Zinc by ICP-OES. European Committee for Normalisation: Brussels, Belgium, 2017.
- EN 15763:2009; Foodstuffs—Determination of Trace Elements-Determination of Arsenic, Cadmium, Mercury and Lead in Foodstuffs by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) After Pressure Digestion. European Committee for Normalisation: Brussels, Belgium, 2009.
- EN 12014-2:2017; Foodstuffs—Determination of Nitrate and/or Nitrite Content—Part 2: HPLC/IC Method for the Determination of Nitrate Content of Vegetables and Vegetable Products. European Committee for Normalisation: Brussels, Belgium, 2017.
- EN ISO 11885:2009; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). European Committee for Normalisation: Brussels, Belgium, 2009.
- Gajc-Wolska, J.; Zielony, T.; Łyszkowska, M. The effect of Goteo, BM86 on yield, fruit quality of sweet pepper (Capsicum annuum L.) in the field production. In Progress in Research on Capsicum & Eggplant, 1st ed.; Niemirowicz-Szczytt, K., Ed.; Warsaw University of Life Sciences Press: Warsaw, Poland, 2007; pp. 267–274. [Google Scholar]
- Jadczak, D.; Grzeszczuk, M. The estimation of yielding and biological value of some cultivars of sweet pepper grown in the climatic conditions of Western Pomeranian region of Poland. Acta Hortic. 2009, 830, 369–376. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper. Molecules 2020, 25, 4287. [Google Scholar] [CrossRef] [PubMed]
- Ryznar-Luty, A.; Szymański, M. Evaluation of selected properties of brine marinade and pickled cucumber juice. Eng. Sci. Technol. 2020, 36, 160–170. [Google Scholar] [CrossRef]
- Ratajczak, K.; Piotrowska-Cyplik, A.; Myszka, K. Analysis of metapopulation of selected fermented foods of plant origin. Postępy Nauk. Technol. Prz. Rol.-Spoż. 2017, 72, 26–38. [Google Scholar]
- Breidt, F.; McFeeters, R.F.; Pérez-Díaz, I.; Lee, C.-H. Fermented vegetables. In Food Microbiology: Fundamentals and Frontiers, 4th ed.; Doyle, M.P., Buchanan, R.L., Eds.; ASM Press: Washington, DC, USA, 2013; pp. 841–855. [Google Scholar]
- Chen, M.; Wang, X.; Liu, Y.; Li, P.; Wang, R.; Jiang, L. Discoloration investigations of yellow lantern pepper sauce (Capsicum chinense Jacq.) fermented by Lactobacillus plantarum: Effect of carotenoids and physiochemical indices. Molecules 2022, 27, 7139. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Block, D.E.; Shoemaker, S.P.; Mills, D.A. Conversion of rice straw to bio-based chemicals: An integrated process using Lactobacillus brevis. Appl. Microbiol. Biotechnol. 2010, 86, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Ropelewska, E.; Szwejda-Grzybowska, J.; Wrzodak, A.; Mieszczakowska-Frąc, M. Determination of the correlation between image parameters and chemical properties of red sweet bell pepper during fermentation. Acta Univ. Cibiniensis Ser. E Food Technol. 2024, 28, 145–158. [Google Scholar] [CrossRef]
- Kantar, M.B.; Anderson, J.E.; Lucht, S.A.; Mercer, K.; Bernau, V.; Case, K.A.; Le, N.C.; Frederiksen, M.K.; DeKeyser, H.C.; Wong, Z.Z. Vitamin variation in Capsicum spp. provides opportunities to improve nutritional value of human diets. PLoS ONE 2016, 11, e0161464. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Mullor, R.; Ceccanti, C.; Padilla, Y.G.; López-Galarza, S.; Calatayud, A.; Conte, G.; Guidi, L. Effect of grafting on the production, physico-chemical characteristics and nutritional quality of fruit from pepper landraces. Antioxidants 2020, 9, 501. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Adalid-Martínez, A.M.; Pires, C.K.; Ribes-Moya, A.M.; Fita, A.; Rodríguez-Burruezo, A. The Effect of the Varietal Type, Ripening Stage, and Growing Conditions on the Content and Profile of Sugars and Capsaicinoids in Capsicum Peppers. Plants 2023, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- de Jesús Ornelas-Paz, J.; Cira-Chávez, L.A.; Gardea-Béjar, A.A.; Guevara-Arauza, J.C.; Sepúlveda, D.R.; Reyes-Hernández, J.; Ruiz-Cruz, S. Effect of Heat Treatment on the Content of Some Bioactive Compounds and Free Radical-Scavenging Activity in Pungent and Non-Pungent Peppers. Food Res. Int. 2013, 50, 519–525. [Google Scholar] [CrossRef]
- Nerdy, N. Determination of Vitamin C in Various Colours of Bell Pepper (Capsicum annuum L.) by Titration Method. Alchemy J. Penelit. Kim. 2018, 14, 164–177. [Google Scholar] [CrossRef]
- Medina-Juárez, L.Á.; Molina-Quijada, D.M.A.; Toro-Sánchez, C.L.D.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Interciencia 2012, 37, 588–593. [Google Scholar]
- Campos, M.; Gómez, K.; Moguel Ordóñez, Y.; Betancur, D. Polyphenols, Ascorbic Acid and Carotenoids Contents and Antioxidant Properties of Habanero Pepper (Capsicum chinense) Fruit. Food Nutr. Sci. 2013, 4, 47–54. [Google Scholar] [CrossRef]
- Herbig, A.L.; Renard, M.G.C. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–445. [Google Scholar] [CrossRef]
- Migut, D.; Gorzelany, J.; Wołowiec, A. Evaluation of selected chemical properties of fresh and pickled ground cucumbers. Inż. Przetw. Spoż. 2018, 3/4, 33–39. (In Polish) [Google Scholar]
- Franco, W.; Perez-Diaz, I.; Johanningsmeier, S.; McFeeters, R. Characteristic of spoilage-associated econdary cucumber fermentation. Appl. Environ. Microbiol. 2012, 78, 1273–1284. [Google Scholar] [CrossRef]
- Grzelakowska, A.; Cieślewicz, J.; Łudzińska, M. The dynamics of vitamin C content in fresh and processed cucumber (Cucumissativus L.). Chem. Didact. Ecol. Metrol. 2013, 18, 97–102. [Google Scholar] [CrossRef]
- Gorzelany, J.; Migut, D.; Matłok, N. Analiza właściwości mechanicznych świeżych owoców wybranych odmian ogórków gruntowych i poddanych procesowi kiszenia. Inż. Przetw. Spoż. 2015, 3/4, 16–21. [Google Scholar]
- Ha, S.H.; Kim, J.B.; Park, J.S.; Lee, S.W.; Cho, K.J. A Comparison of the Carotenoid Accumulation in Capsicum Varieties That Show Different Ripening Colours: Deletion of the Capsanthin-Capsorubin Synthase Gene Is Not a Prerequisite for the Formation of a Yellow Pepper. J. Exp. Bot. 2007, 58, 3135–3144. [Google Scholar] [CrossRef]
- Li, Q.H.; Yang, S.P.; Yu, Y.N.; Khan, A.; Feng, P.L.; Ali, M.; Shao, D.K.; Wang, Y.Y.; Zhang, R.X.; Gai, W.X. Comprehensive transcriptome-based characterization of stages of Capsicum. Sci. Hortic. 2021, 288, 110311. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Witrowa-Rajchert, D.; Rybak, K.; Rolof, J.; Pobiega, K.; Woźniak, Ł.; Gramza-Michałowska, A. The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers. Molecules 2022, 27, 8637. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolic Compounds, Ascorbic Acid, Carotenoids and Antioxidant Properties of Green, Red and Yellow Bell Peppers. J. Food Agricult. Environ. 2003, 1, 22–27. [Google Scholar]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of Chemical Profile and Antioxidant Activity of Twenty Cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A Comparison between Fresh and Processed Peppers. LWT-Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Pérez-López, A.J.; López-Nicolas, J.M.; Núñez-Delicado, E.; Del Amor, F.M.; Carbonell-Barrachina, A.A. Effects of Agricultural Practices on Color, Carotenoids Composition, and Minerals Contents of Sweet Peppers, Cv. Almuden. J. Agric. Food Chem. 2007, 55, 8158–8164. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, R.; Reboredo, F.; Guerra, M.; Ressurreição, S.; Alvarenga, N. Elemental Composition and Some Nutritional Parameters of Sweet Pepper from Organic and Conventional Agriculture. Plants 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I. Tabele Składu i Wartości Odżywczej Żywności; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2020; ISBN 978-83-200-6258-8. [Google Scholar]
- USDA. 2022. Available online: https://fdc.nal.usda.gov/food-details/2258588/nutrients (accessed on 16 July 2025).
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Fermented Vegetables and Legumes vs. Lifestyle Diseases: Microbiota and More. Life 2023, 13, 1044. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M. The Na/K Pump, Cl Ion, and Osmotic Stabilization of Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6257–6262. [Google Scholar] [CrossRef] [PubMed]
- Bonatsou, S.; Iliopoulos, V.; Mallouchos, A.; Gogou, E.; Oikonomopoulou, V.; Krokida, M.; Taoukis, P.; Panagou, E.Z. Effect of Osmotic Dehydration of Olives as Pre-Fermentation Treatment and Partial Substitution of Sodium Chloride by Monosodium Glutamate in the Fermentation Profile of Kalamata Natural Black Olives. Food Microbiol. 2017, 63, 72–83. [Google Scholar] [CrossRef]
- Mi, T.; Wang, D.; Yao, S.; Yang, H.; Che, Y.; Wu, C. Effects of Salt Concentration on the Quality and Microbial Diversity of Spontaneously Fermented Radish Paocai. Food Res. Inter. 2022, 160, 111622. [Google Scholar] [CrossRef]
- Mani, A. Food Preservation by Fermentation and Fermented Food Products. Inter. J. Acad. Res. Develop. 2018, 1, 51–57. [Google Scholar]
- WHO. 2012. Available online: https://apps.who.int/gb/ncds/pdf/a_ncd_2-en.pdf (accessed on 16 July 2025).
- Albuquerque, T.G.; Santos, J.; Silva, M.A.; Oliveira, M.; Beatriz, P.P.; Costa, H.S. An Update on Processed Foods: Relationship between Salt, Saturated and Trans Fatty Acids Contents. Food Chem. 2018, 267, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, T.H.; Min, B.; Hwang, S.J. Sodium (Na+) Concentration Effects on Metabolic Pathway and Estimation of ATP Use in Dark Fermentation Hydrogen Production through Stoichiometric Analysis. J. Environ. Manag. 2012, 108, 22–26. [Google Scholar] [CrossRef]
- Soltan, M.; Elsamadony, M.; Mostafa, A.; Awad, H.; Tawfik, A. Nutrients Balance for Hydrogen Potential Upgrading from Fruit and Vegetable Peels via Fermentation Process. J. Environ. Manag. 2019, 242, 384–393. [Google Scholar] [CrossRef]
- Srikanth, S.; Mohan, S.V. Regulatory Function of Divalent Cations in Controlling the Acidogenic Biohydrogen Production Process. RSC Adv. 2012, 2, 6576–6589. [Google Scholar] [CrossRef]
- Soltan-Dallal, M.; Zamaniahari, S.; Davoodabadi, A.; Hosseini, M.; Rajabi, Z. Identification and Characterization of Probiotic Lactic Acid Bacteria Isolated from Traditional Persian Pickled Vegetables. GMS Hyg. Infect. Control 2017, 12, Doc15. [Google Scholar] [CrossRef]
- Ifesan, B.O.T.; Egbewole, O.O.; Ifesan, B.T. Effect of Fermentation on Nutritional Composition of Selected Commonly Consumed Green Leafy Vegetables in Nigeria. Int. J. Appl. Sci. Biotechnol. 2014, 2, 291–297. [Google Scholar] [CrossRef]
- Chawla, P.; Bhandari, L.; Sadh, P.K.; Kaushik, R. Impact of Solid-State Fermentation (Aspergillus oryzae) on Functional Properties and Mineral Bioavailability of Black-Eyed Pea (Vigna unguiculata) Seed Flour. Cereal Chem. 2017, 94, 437–442. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Ilich, J.Z. Utilizing Dietary Micronutrient Ratios in Nutritional Research May Be More Informative than Focusing on Single Nutrients. Nutrients 2018, 10, 107. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Ilich, J.Z. Utilizing Dietary Nutrient Ratios in Nutritional Research: Expanding the Concept of Nutrient Ratios to Macronutrients. Nutrients 2019, 11, 282. [Google Scholar] [CrossRef]
- Kemi, V.E.; Kärkkäinen, M.U.M.; Rita, H.J.; Laaksonen, M.M.L.; Outila, T.A.; Lamberg-Allardt, C.J.E. Low Calcium: Phosphorus Ratio in Habitual Diets Affects Serum Parathyroid Hormone Concentration and Calcium Metabolism in Healthy Women with Adequate Calcium Intake. Br. J. Nutr. 2010, 103, 561–568. [Google Scholar] [CrossRef]
- Ogunlade, I.; Alebiosu, A.; Osasona, A. Proximate, Mineral Composition, Antioxidant Activity, and Total Phenolic Content of Some Pepper Varieties (Capsicum species). Int. J. Bio. Chem. Sci. 2013, 6, 2221–2227. [Google Scholar] [CrossRef]
- O’Dell, B.L. Mineral Interactions Relevant to Nutrient Requirements. J. Nutr. 1989, 119, 1832–1838. [Google Scholar] [CrossRef]
- Biernacka, B.; Dziki, D.; Kozłowska, J.; Kowalska, I.; Soluch, A. Dehydrated at Different Conditions and Powdered Leek as a Concentrate of Biologically Active Substances: Antioxidant Activity and Phenolic Compound Profile. Materials 2021, 14, 6127. [Google Scholar] [CrossRef]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic Crossroads of Iron and Copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; pp. 224–257. Available online: https://www.ncbi.nlm.nih.gov/books/nbk222310/ (accessed on 10 July 2025).
- Minerals|Linus Pauling Institute|Oregon State University. Available online: https://lpi.oregonstate.edu/mic/minerals (accessed on 15 July 2025).
- Salehizadeh, H.; Shojaosadati, S.A. Removal of Metal Ions from Aqueous Solution by Polysaccharide Produced from Bacillus Firmus. Water Res. 2003, 37, 4231–4235. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.S.; Goyal, D. Microbial and Plant Derived Biomass for Removal of Heavy Metals from Wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Ahuja, P.; Khan, S.; Saxena, R.K.; Mohapatra, H. Microbial Biosorbents: Meeting Challenges of Heavy Metal Pollution in Aqueous Solutions. Curr. Sci. 2000, 78, 967–973. [Google Scholar]
- Commission Regulation. 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 16 July 2025).
- Hassan, Q.U.; Sarfraz, R.A. Effect of Different Nutraceuticals on Phytochemical and Mineral Composition as Well as Medicinal Properties of Home Made Mixed Vegetable Pickles. Food Biol. 2018, 7, 24–27. [Google Scholar] [CrossRef]
- Yousefi, M.; Arianfar, A.; Hakimzadeh, V.; Rafe, A. Enhancing the Texture and Sensory Properties of Pickled Cucumbers with Different Brine Solutions. Foods 2025, 14, 336. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Geothermal Water—Uniejów | Tap Water—Skierniewice |
|---|---|---|
| Potassium K+ | 19.0 | 3.24 |
| Phosphorus P+ | 0.01 | 0.02 |
| Magnesium Mg+ | 34.03 | 14.5 |
| Calcium Ca+ | 140.3 | 77.6 |
| Sodium Na+ | 2290.0 | 8.66 |
| Sulfur SO4−2 | 127.6 | 24.6 |
| Boron B+ | 0.75 | 0.11 |
| Copper Cu+ | 0.04 | 0.01 |
| Iron Fe2+/3+ | 1.0 | 0.01 |
| Manganese Mn2+ | 0.002 | 0.03 |
| Zinc Zn2+ | 0.0175 | 0.17 |
| Chlorides Cl− | 3615.9 | 15.4 |
| Content of Hg, As, Cd, Pb | <0.001 | <0.001 |
| Cutivar | Dry Matter [%] | pH | Total Sugars [g·100 g−1] | Ascorbic Acid [mg·kg−1] | Total Polyphenols [mg·kg−1] | Total Carotenoids [mg·kg−1] |
|---|---|---|---|---|---|---|
| ‘Yecla F1’ | 8.34 b | 6.34 b | 5.64 b | 1407 b | 1369 b | 87.3 b |
| ‘Salomon F1’ | 9.59 a | 6.62 a | 5.80 a | 1506 a | 1483 a | 96.5 a |
| Water Used | Dry Matter [%] | pH | Total Sugars [g·100 g−1] | Ascorbic Acid [mg·kg−1] | Total Polyphenols [mg·kg−1] | Total Carotenoids [mg·kg−1] |
|---|---|---|---|---|---|---|
| ‘Yecla F1’ | ||||||
| Geothermal water | 7.84 a | 4.40 a | 1.52 a | 954 a | 996 a | 72.6 a |
| Tap water | 7.77 a | 4.30 a | 1.60 a | 907 b | 955 b | 66.4 b |
| ‘Salomon F1’ | ||||||
| Geothermal water | 7.32 a | 4.45 a | 1.36 a | 745 a | 1191 a | 78.5 a |
| Tap water | 7.21 a | 4.35 a | 1.32 a | 709 b | 1077 b | 71.8 b |
| Mineral Compounds | Poland | USDA [75] |
|---|---|---|
| ([74]) | ||
| Calcium (Ca) | 13 | 7 |
| Iron (Fe) | 0.6 | 0.36 |
| Magnesium (Mg) | 11 | 10,3 |
| Phosphorus (P) | 31 | 25 |
| Potassium (K) | 255 | 197 |
| Sodium (Na) | 3 | <2.5 |
| Zinc (Zn) | 0.26 | 0.19 |
| Copper (Cu) | 0.08 | 0.045 |
| Manganese (Mn) | 0.1 | 0.142 |
| Water Used | Minerals [mg∙100 g−1] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P | K | Mg | Ca | Na | Cu | Fe | Mn | Zn | |
| ‘Yecla F1’ | |||||||||
| Geothermal water | 13.5 a | 115.5 a | 8.20 a | 15.3 a | 607.9 a | 0.005 b | 0.217 b | 0.048 b | 0.066 b |
| Tap water | 13.1 a | 111.1 b | 7.47 b | 14.7 b | 587.6 b | 0.009 a | 0.233 a | 0.062 a | 0.140 a |
| ‘Salomon F1’ | |||||||||
| Geothermal water | 13.9 a | 120.8 a | 8.25 a | 18.7 a | 639.4 b | 0.005 a | 0.223 a | 0.059 a | 0.084 b |
| Tap water | 13.0 a | 106.4 b | 7.15 b | 17.3 b | 654.3 a | 0.003 b | 0.204 b | 0.051 b | 0.107 a |
| Water Used | Minerals [mg∙kg−1] | |||
|---|---|---|---|---|
| NO3 | As | Cd | Pb | |
| ‘Yecla F1’ | ||||
| Geothermal water | 12.3 a | 0.003 a | 0.004 a | 0.002 b |
| Tap water | 8.84 b | 0.003 a | 0.004 a | 0.005 a |
| ‘Salomon F1’ | ||||
| Geothermal water | 5.41 b | 0.003 a | 0.003 b | 0.004 b |
| Tap water | 7.07 a | 0.003 a | 0.004 a | 0.006 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrzodak, A.; Szwejda-Grzybowska, J.; Popińska, W.; Mieszczakowska-Frąc, M. Red Pepper Fermentation with Geothermal Mineral Water: Impact on Nutritional Profile and Quality Characteristics. Agronomy 2025, 15, 2279. https://doi.org/10.3390/agronomy15102279
Wrzodak A, Szwejda-Grzybowska J, Popińska W, Mieszczakowska-Frąc M. Red Pepper Fermentation with Geothermal Mineral Water: Impact on Nutritional Profile and Quality Characteristics. Agronomy. 2025; 15(10):2279. https://doi.org/10.3390/agronomy15102279
Chicago/Turabian StyleWrzodak, Anna, Justyna Szwejda-Grzybowska, Wioletta Popińska, and Monika Mieszczakowska-Frąc. 2025. "Red Pepper Fermentation with Geothermal Mineral Water: Impact on Nutritional Profile and Quality Characteristics" Agronomy 15, no. 10: 2279. https://doi.org/10.3390/agronomy15102279
APA StyleWrzodak, A., Szwejda-Grzybowska, J., Popińska, W., & Mieszczakowska-Frąc, M. (2025). Red Pepper Fermentation with Geothermal Mineral Water: Impact on Nutritional Profile and Quality Characteristics. Agronomy, 15(10), 2279. https://doi.org/10.3390/agronomy15102279






