Soil Aggregate Stability Under Freeze–Thaw Cycles in Mollisols as Evidenced by 15N Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Preparation of the Straw Used in the Experiment
2.3. Soil Column Filling
2.4. Soil Pre-Cultivation, Freeze–Thaw Treatment, and Soil Sampling
2.5. Determination Indicators and Methods
2.5.1. Soil TN, NO3−-N, and NH4+-N Contents and Their 15N-Labeled Contents in Bulk Soils
2.5.2. MBN and 15N-Labeled MBN Contents in Bulk Soils
2.5.3. Soil Aggregate Fractionation Methods, and TN and 15N-Labeled TN Contents in Soil Aggregates
2.6. Data Calculation
2.6.1. Soil Aggregate Stability
2.6.2. Relative Contribution Rate, Allocation Amount, and Allocation Rate
2.7. Statistical Analysis
3. Results
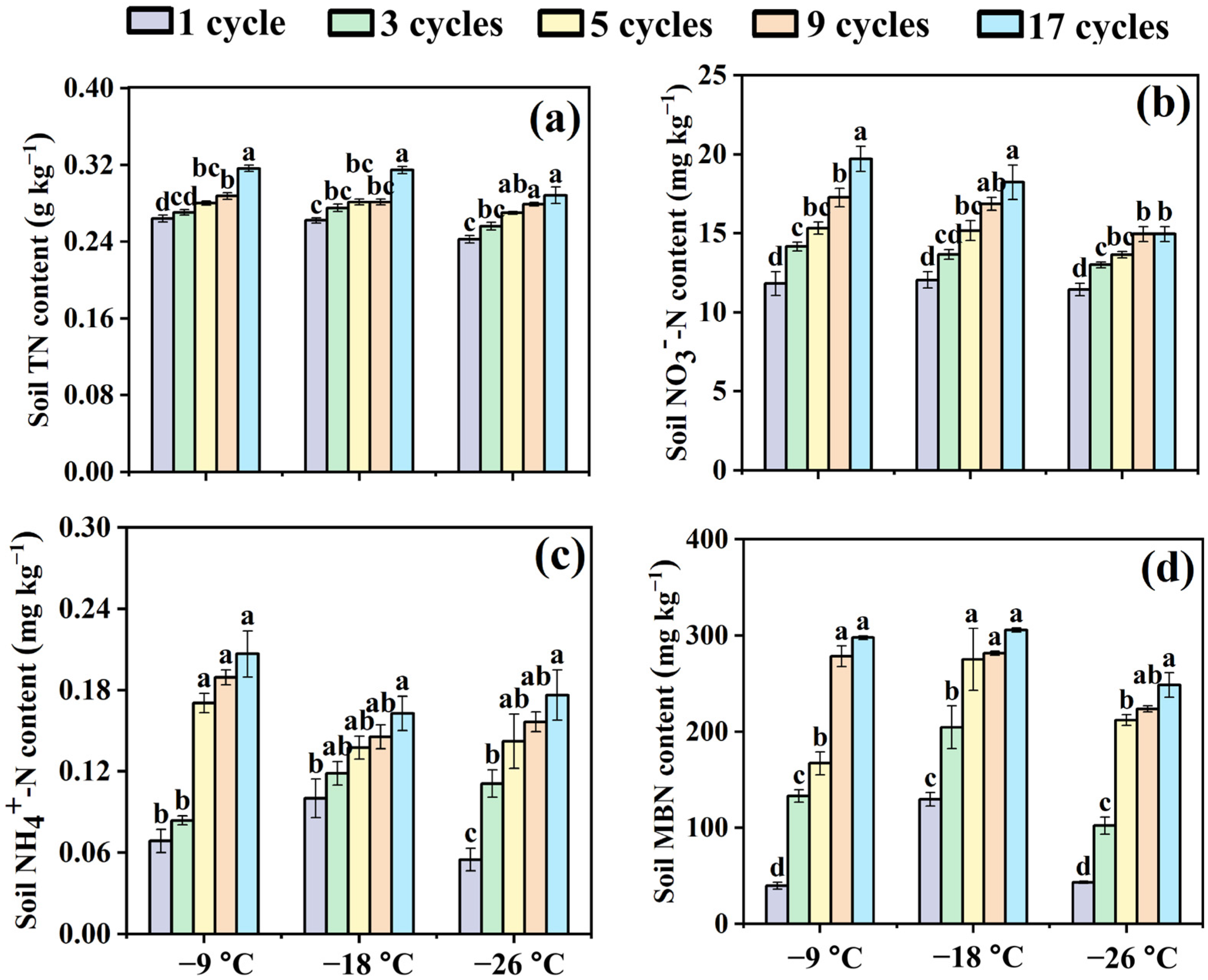
3.1. Effects of Freeze–Thaw Frequency and Temperature on Soil Nitrogen Components in Bulk Soils
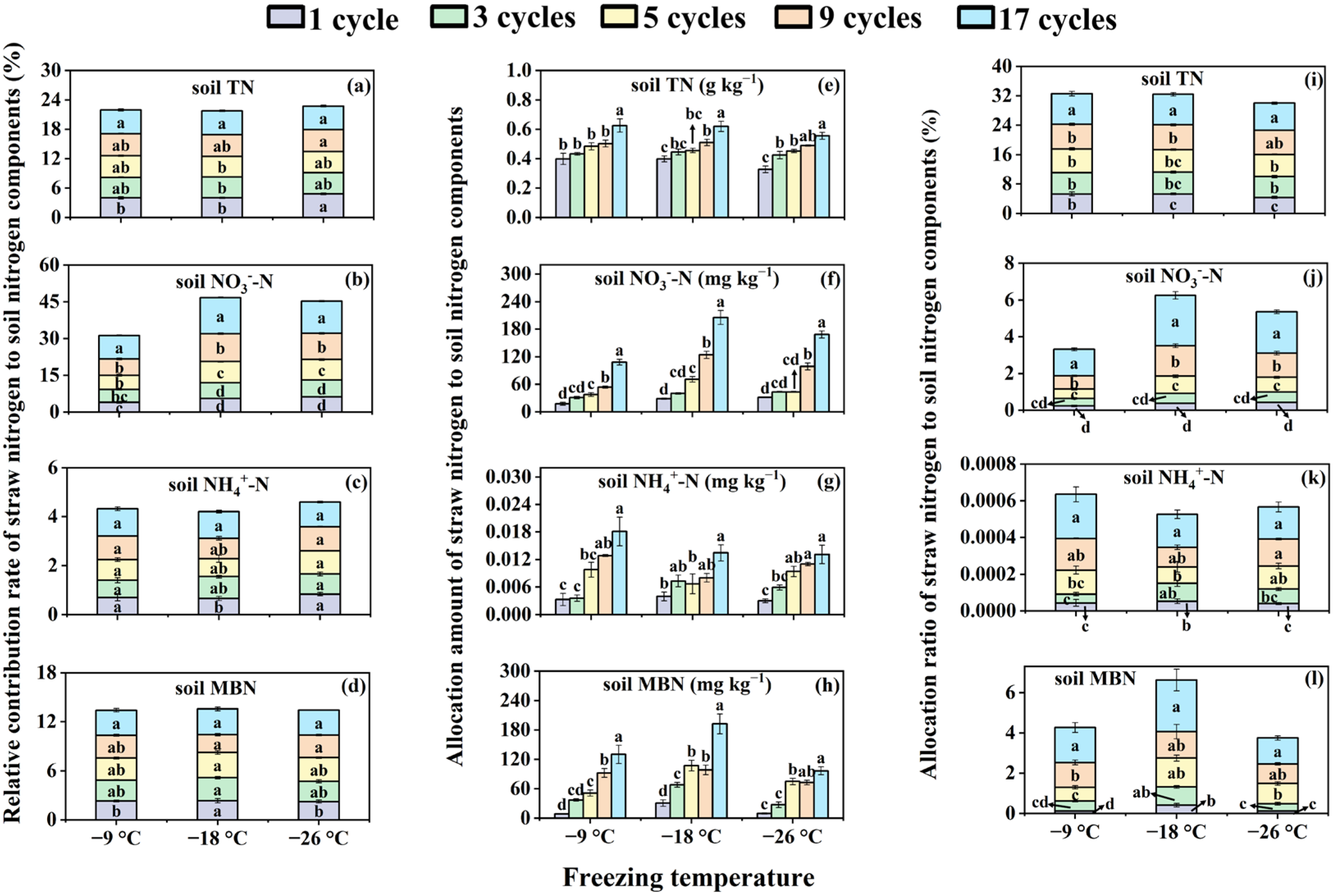
3.2. Effects of Freeze–Thaw Frequency and Temperature on Relative Contribution Rate, Allocation Amount, and Allocation Ratio of Corn Straw Nitrogen in Soil Nitrogen Components for Bulk Soils
3.3. Effects of Freeze–Thaw Frequency and Temperature on Soil Total Nitrogen Content in Soil Aggregates
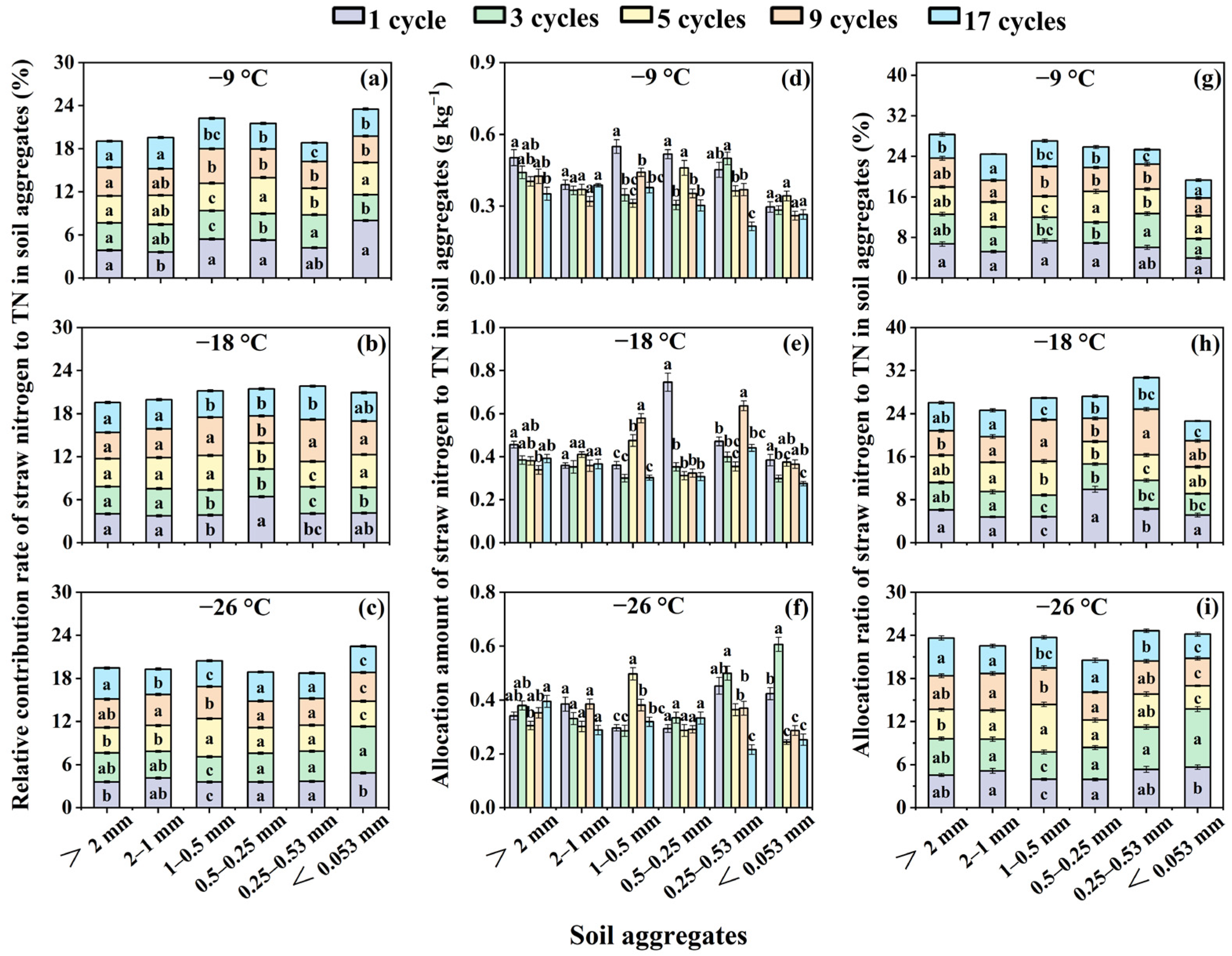
3.4. Effects of Freeze–Thaw Frequency and Temperature on Relative Contribution Rate, Allocation Amount and Allocation Ratio of Corn Straw Nitrogen in Total Nitrogen for Soil Aggregates
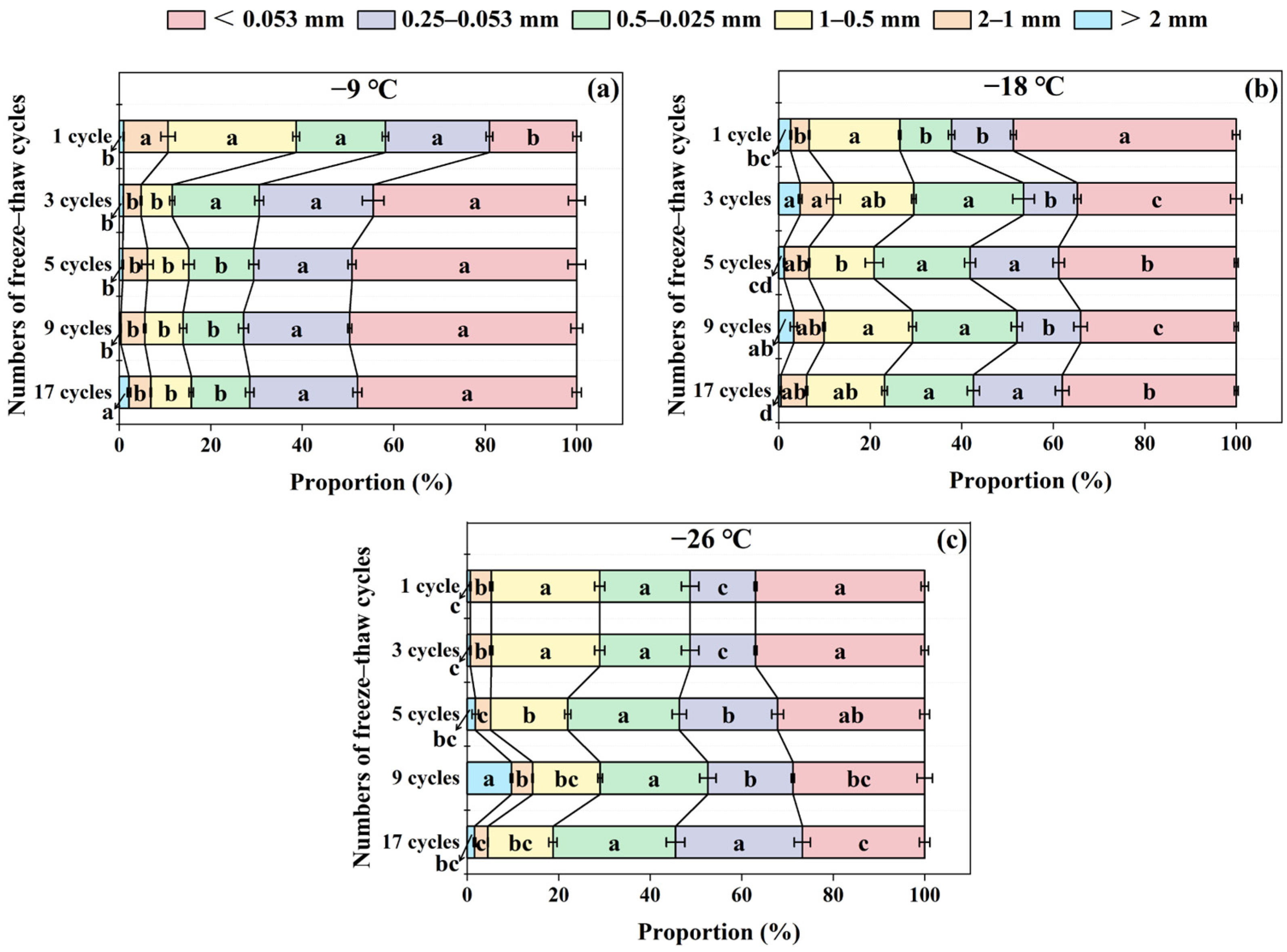
3.5. Effects of Freeze–Thaw Frequency and Temperature on Soil Aggregate Distribution
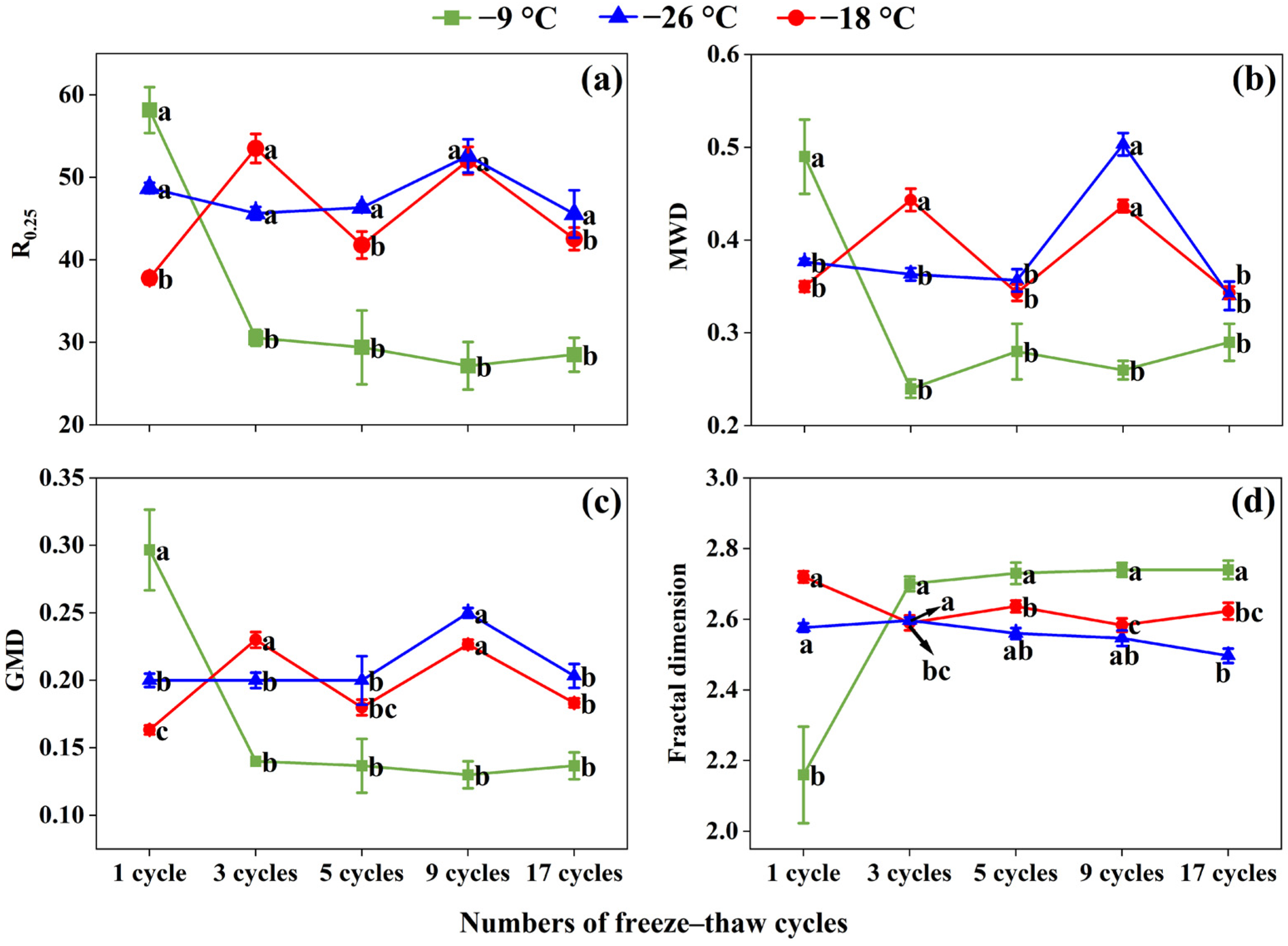
3.6. Effects of Freeze–Thaw Frequency and Temperature on Soil Aggregate Stability
3.7. Relationships of Soil Properties in Bulk Soils and Soil Aggregate Sizes Under Freeze–Thaw Action
4. Discussion
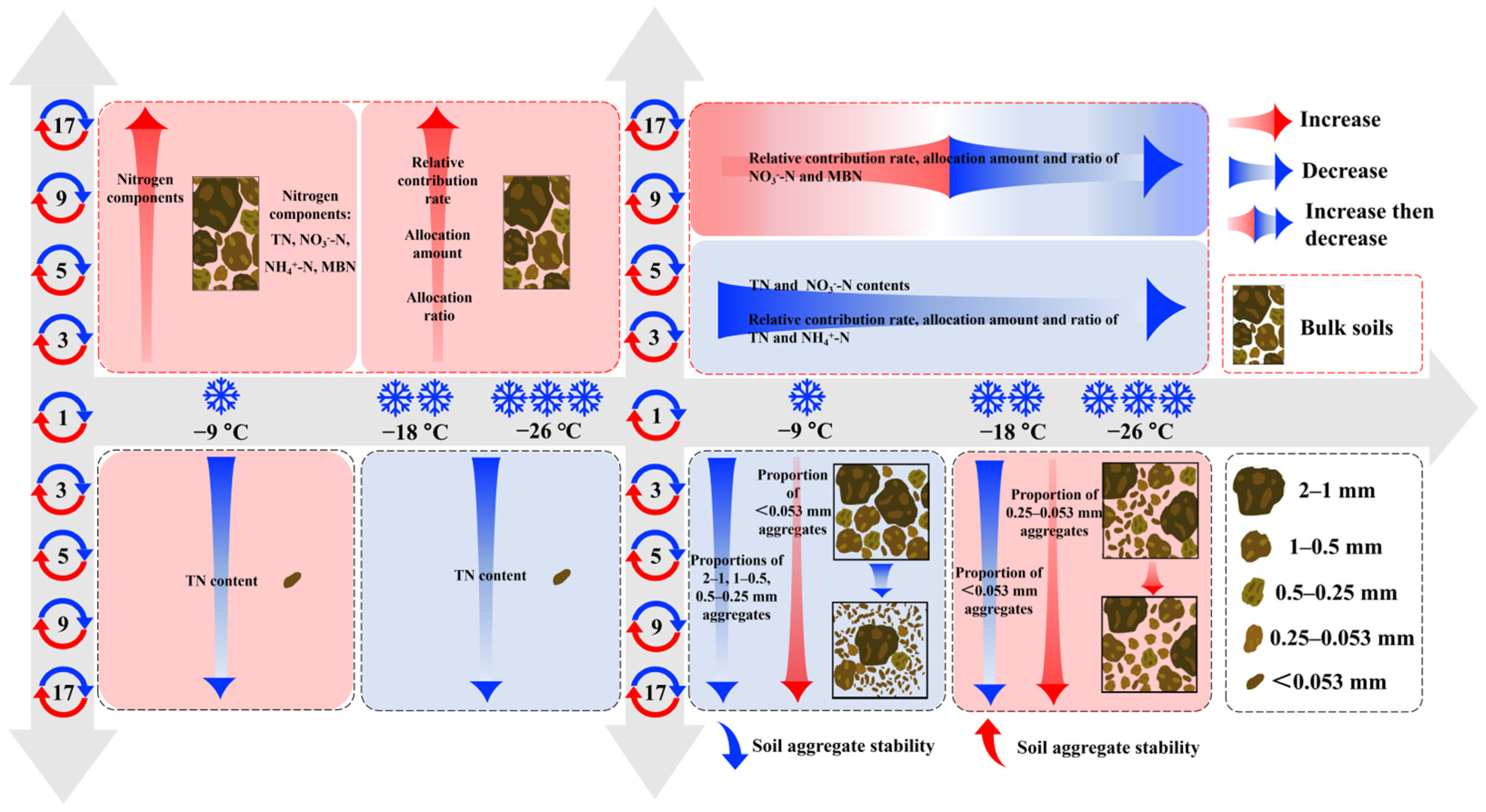
4.1. Dynamics of Nitrogen Components in Bulk Soils Due to Freeze–Thaw Action
4.2. Dynamics of Nitrogen Components in Soil Aggregates Due to Freeze–Thaw Action
4.3. Variations in Soil Aggregate Stability Induced by Freeze–Thaw Action
4.4. The Interactive Impacts of Freeze–Thaw Frequency and Temperature on Soil Nitrogen Dynamics and Aggregate Stability
4.5. Implications and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rooney, E.C.; Bailey, V.; Patel, K.F.; Dragila, M.; Battu, A.K.; Buchko, A.C.; Gallo, A.C.; Hatten, J.; Possinger, A.R.; Qafoku, O.; et al. Soil pore network response to freeze-thaw cycles in permafrost aggregates. Geoderma 2022, 411, 115674. [Google Scholar] [CrossRef]
- de Bruijn, A.M.G.; Butterbach-Bahl, K.; Blagodatsky, S.; Grote, R. Model evaluation of different mechanisms driving freeze-thaw N2O emissions. Agric. Ecosyst. Environ. 2009, 133, 196–207. [Google Scholar] [CrossRef]
- Ji, X.M.; Liu, M.H.; Yang, J.L.; Feng, F.J. Meta-analysis of the impact of freeze-thaw cycles on soil microbial diversity and C and N dynamics. Soil Biol. Biochem. 2022, 168, 108608. [Google Scholar] [CrossRef]
- Henry, H.A.L. Soil freeze-thaw cycle experiments: Trends, methodological weaknesses and suggested improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Wagner-Riddle, C.; Congreves, K.A.; Abalos, D.; Berg, A.A.; Brown, S.E.; Ambadan, J.T.; Gao, X.P.; Tenuta, M. Globally important nitrous oxide emissions from croplands induced by freeze-thaw cycles. Nat. Geosci. 2017, 10, 279–283. [Google Scholar] [CrossRef]
- Wang, X.; Zi, H.B.; Wang, J.B.; Guo, X.W.; Zhang, Z.H.; Yan, T.; Wang, Q.; He, J.S. Grazing-induced changes in soil microclimate and aboveground biomass modulate freeze-thaw processes in a Tibetan alpine meadow. Agric. Ecosyst. Environ. 2023, 357, 108659. [Google Scholar] [CrossRef]
- Chen, Z.M.; Wang, H.Y.; Liu, X.W.; Zhao, X.L.; Lu, D.J.; Zhou, J.M.; Li, C.Z. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice-wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Hu, N.J.; Yang, M.F.; Zhan, X.H.; Zhang, Z.W. Effects of different tillage and straw return on soil organic carbon in a rice-wheat rotation system. PLoS ONE 2014, 9, e88900. [Google Scholar] [CrossRef]
- Liang, B.; Yang, X.Y.; He, X.H.; Zhou, J.B. Effects of 17-year fertilization on soil microbial biomass C and N and soluble organic C and N in loessial soil during maize growth. Biol. Fertil. Soils 2011, 47, 121–128. [Google Scholar] [CrossRef]
- Pabst, H.; Kuhnel, A.; Kuzyakov, Y. Effect of land-use and elevation on microbial biomass and water extractable carbon in soils of Mt. Kilimanjaro ecosystems. Appl. Soil Ecol. 2013, 67, 10–19. [Google Scholar] [CrossRef]
- Mi, W.H.; Sun, Y.; Zhao, C.; Wu, L.H. Soil organic carbon and its labile fractions in paddy soil as influenced by water regimes and straw management. Agric. Water Manag. 2019, 224, 105752. [Google Scholar] [CrossRef]
- De Troyer, I.; Amery, F.; Van Moorleghem, C.; Smolders, E.; Merckx, R. Tracing the source and fate of dissolved organic matter in soil after incorporation of a 13C labelled residue: A batch incubation study. Soil Biol. Biochem. 2011, 43, 513–519. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Hu, N.J.; Zhang, Z.W.; Xu, J.L.; Tao, B.R.; Meng, Y.L. Short-term responses of soil organic carbon and carbon pool management index to different annual straw return rates in a rice–wheat cropping system. Catena 2015, 135, 283–289. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, X.C.; Wen, Y.J.; Cai, H.X.; Song, X.M.; Zhang, Z.P. Effects of freeze-thaw cycles on soil greenhouse gas emissions: A systematic review. Environ. Res. 2024, 248, 118386. [Google Scholar] [CrossRef]
- Gao, D.C.; Zhang, L.; Liu, J.; Peng, B.; Fan, Z.Z.; Dai, W.W.; Jiang, P.; Bai, E. Responses of terrestrial N pools and dynamics to different patterns of freeze-thaw cycle: A meta-analysis. Glob. Change Biol. 2018, 24, 2377–2389. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Fu, Y.; Xu, J.Z.; Li, Y.; Zhao, Y.K.; Wei, S.Y.; Liu, B.J.; Zhang, X.Y.; Lei, H.Z.; Shao, S. Impact of freeze–thaw cycling on the stability and turnover of black soil aggregates. Geoderma 2024, 449, 117004. [Google Scholar] [CrossRef]
- Nie, S.Y.; Jia, X.; Zou, Y.C.; Bian, J.M. Effects of Freeze–Thaw Cycles on Soil N Transformation in Improved Saline Soils from an Irrigated Area in Northeast China. Water 2024, 16, 653. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, C.Z.; Wang, J.; Meng, Q.F.; Yuan, Y.; Ma, X.F.; Liu, X.B.; Zhu, Y.X.; Ding, G.W.; Zhang, J.Z.; et al. Soil aggregates stability and storage of soil organic carbon respond to cropping systems on Black Soils of Northeast China. Sci. Rep. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Yudina, A.; Kuzyakov, Y. Dual nature of soil structure: The unity of aggregates and pores. Geoderma 2023, 434, 116478. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, M.; Liu, X.B.; Zhang, X.Y.; Xiao, L.L.; Liu, J.; Cruse, R.M. Pore connectivity and anisotropy affect carbon mineralization via extracellular enzymes in > 2 mm aggregates under conservation tillage of Mollisols. Soil Tillage Res. 2024, 244, 106253. [Google Scholar] [CrossRef]
- Zhu, X.A.; Liu, W.J.; Yuan, X.; Chen, C.F.; Zhu, K.; Zhang, W.J.; Yang, B. Aggregate stability and size distribution regulate rainsplash erosion: Evidence from a humid tropical soil under different land-use regimes. Geoderma 2022, 420, 115880. [Google Scholar] [CrossRef]
- Almajmaie, A.; Hardie, M.; Acuna, T.; Birch, C. Evaluation of methods for determining soil aggregate stability. Soil Tillage Res. 2017, 167, 39–45. [Google Scholar] [CrossRef]
- Rieke, E.L.; Bagnall, D.K.; Morgan, C.L.S.; Flynn, K.D.; Howe, J.A.; Greub, K.L.H. Evaluation of aggregate stability methods for soil health. Geoderma 2022, 428, 116156. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, M.; Han, X.Z.; Lu, X.C.; Chen, X.; Feng, H.L.; Wu, Z.M.; Liu, C.Z.; Yan, J.; Zou, W.X. Evaluation of the soil aggregate stability under long term manure and chemical fertilizer applications: Insights from organic carbon and humic acid structure in aggregates. Agric. Ecosyst. Environ. 2024, 376, 109217. [Google Scholar] [CrossRef]
- Klöffel, T.; Larsbo, M.; Jarvis, N.; Barron, J. Freeze-thaw effects on pore space and hydraulic properties of compacted soil and potential consequences with climate change. Soil Tillage Res. 2024, 239, 106041. [Google Scholar] [CrossRef]
- Li, X.B.; Chen, X.Y.; Gao, Y.C.; Yang, J.H.; Ding, W.T.; Zvomuya, F.; Azad, N.; Li, J.B.; He, H.L. Freezing induced soil water redistribution: A review and global meta-analysis. J. Hydrol. 2025, 651, 132594. [Google Scholar] [CrossRef]
- Lehrsch, G.A.; Sojka, R.E.; Carter, D.L.; Jolley, P.M. Freezing effects on aggregate stability affected by texture, mineralogy, and organic matter. Soil Sci. Soc. Am. J. 1991, 55, 1401–1406. [Google Scholar] [CrossRef]
- Oztas, T.; Fayetorbay, F. Effect of freezing and thawing processes on soil aggregate stability. Catena 2003, 52, 1–8. [Google Scholar] [CrossRef]
- Edwards, L.M. The effects of soil freeze-thaw on soil aggregate breakdown and concomitant sediment flow in Prince Edward Island: A review. Can. J. Soil Sci. 2013, 93, 459–472. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.Y.; Vinci, G.; Sun, B.B.; Drosos, M.; Li, L.Q.; Piccolo, A.; Pan, G.X. Aggregate fractions shaped molecular composition change of soil organic matter in a rice paddy under elevated CO2 and air warming. Soil Biol. Biochem. 2021, 159, 108289. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhang, L.H.; Mu, Y.H.; Ma, W.; Kong, X.B.; Yang, C.S. Dynamic characteristics of soil pore structure and water-heat variations during freeze-thaw process. Eng. Geol. 2024, 343, 107785. [Google Scholar] [CrossRef]
- Xu, W.S.; Li, K.S.; Chen, L.X.; Kong, W.H.; Liu, C.X. The impacts of freeze-thaw cycles on saturated hydraulic conductivity and microstructure of saline-alkali soils. Sci. Rep. 2021, 11, 18655. [Google Scholar] [CrossRef]
- Zhang, J.; He, P.; Wei, D.; Jin, L.; Zhang, L.; Li, L.; Zhao, S.; Xu, X.; Zhou, W.; Qiu, S. Changes in N pools in the maize–soil system after urea or straw application to a typical intensive agricultural soil: A 15N Tracer Study. Agronomy 2021, 11, 1134. [Google Scholar] [CrossRef]
- Zhao, X.H.; Quan, Z.; Gurmesa, G.A.; Huang, B.; Yu, H.M.; Zhu, F.F.; Xun, Z.F.; Liu, C.; Liu, D.; Yang, X.S.; et al. Effects of nitrification inhibitor and maize straw application on N2O and N2 emissions from two agricultural soils: A 15N tracer study. Soil Ecol. Lett. 2025, 7, 240276. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv. Agron. 2005, 88, 35–66. [Google Scholar] [CrossRef]
- Majumder, B.; Kuzyakov, Y. Effect of fertilization on decomposition of 14C labelled plant residues and their incorporation into soil aggregates. Soil Tillage Res. 2010, 109, 94–102. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.; United States Department of Agriculture Natural Resources Conservation Service; U.S. Government Printing Office: Washington, DC, USA, 2010.
- Lu, X.C.; Han, X.Z.; Chen, X.; Yan, Y.; You, M.Y.; Kwaw-Mensah, D.; Hao, X.X.; Zou, W.X. Changes of soil phosphorus fractions in parent material of a Mollisol at the early pedogenic stage in Northeast China. Appl. Ecol. Environ. Res. 2020, 18, 2935–2948. [Google Scholar] [CrossRef]
- Zhou, M.; Xiao, Y.; Xiao, L.; Li, Y.; Zhang, X.; Cruse, R.M.; Liu, X. Increased soil aggregate stability by altering contents and chemical composition of organic carbon fractions via seven years of manure addition in Mollisols. Agriculture 2023, 13, 88. [Google Scholar] [CrossRef]
- NY/T 1121.3-2006; Soil Testing—Part 3: Determination of Soil Mechanical Composition. Ministry of Agriculture of the People’s Republic of China, Standards Press of China: Beijing, China, 2006.
- Burt, R. (Ed.) Soil Survey Laboratory Methods Manual, 4th ed.; USDA-NRCS: Washington, DC, USA, 2004. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; Agricultural Press: Beijing, China, 2008. [Google Scholar]
- Jimenez, R.R.; Ladha, J.K. Automated elemental analysis: A rapid and reliable but expensive measurement of total carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1993, 24, 1897–1924. [Google Scholar] [CrossRef]
- Salazar, O.; Diaz, R.; Nario, A.; Videla, X.; Alonso-Ayuso, M.; Quemada, M. N fertilizer efficiency determined by the 15N dilution technique in maize followed or not by a cover crop in Mediterranean Chile. Agriculture 2021, 11, 721. [Google Scholar] [CrossRef]
- Linam, F.; Limmer, M.A.; Tappero, R.; Seyfferth, A.L. Rice husk and charred husk amendments increase porewater and plant Si but water management determines grain As and Cd concentration. Plant Soil 2022, 477, 135–152. [Google Scholar] [CrossRef]
- Su, M.M.; Tian, H.W.; Guo, Z.C.; Luo, G.J.; Gong, X.; Li, X.E.; Yan, H.Y.; Shen, L.C.; Yang, S.W.; He, T.B.; et al. Utilization of SiO2-NP-modified biochar from invasive plants to mitigate heavy metal stress in Allium hookeri. Environ. Technol. Innov. 2025, 37, 104041. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. Carbon and N analysis of soils by automated combustion techniques. Commun. Soil Sci. Plant Anal. 1991, 22, 843–850. [Google Scholar] [CrossRef]
- Rhymes, J.M.; Cordero, I.; Chomel, M.; Lavallee, J.M.; Straathof, A.L.; Ashworth, D.; Langridge, H.; Semchenko, M.; de Vries, F.T.; Johnson, D.; et al. Are researchers following best storage practices for measuring soil biochemical properties? Soil 2021, 7, 95–106. [Google Scholar] [CrossRef]
- Zhang, S.S.; Fang, Y.T.; Xi, D. Adaptation of micro-diffusion method for the analysis of 15N natural abundance of ammonium in samples with small volume. Rapid Commun. Mass Spectrom. 2015, 29, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Büks, F. Technical note: The recovery rate of free particulate organic matter from soil samples is strongly affected by the method of density fractionation. Biogeosciences 2023, 20, 1529–1535. [Google Scholar] [CrossRef]
- Bavel, C.H.M.V. Mean weight-diameter of soil aggregates as a statistical index of aggregation. Soil Sci. Soc. Am. J. 1950, 14, 20–23. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distributions. In Methods of Soil Analysis, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 837–871. [Google Scholar]
- Yang, P.L.; Luo, Y.P.; Shi, Y.C. Characterized soil fractal characteristics by weight distribution of particle size. Chin. Sci. Bull. 1993, 38, 1896–1899, (In Chinese with English Abstract). [Google Scholar]
- Chen, Z.M.; Wang, Q.; Wang, H.Y.; Bao, L.; Zhou, J.M. Crop yields and soil organic carbon fractions as influenced by straw incorporation in a rice-wheat cropping system in southeastern China. Nutr. Cycl. Agroecosyst. 2018, 112, 61–73. [Google Scholar] [CrossRef]
- Edwards, K.A.; McCulloch, J.; Kershaw, G.P.; Jefferies, R.L. Soil microbial and nutrient dynamics in a wet arctic sedge meadow in late winter and early spring. Soil Biol. Biochem. 2006, 38, 2843–2851. [Google Scholar] [CrossRef]
- Ping, C.L.; Michaelson, G.J.; Kimble, J.M. Carbon storage along a Latitudinal transect in Alaska. Nutr. Cycl. Agroecosyst. 1997, 49, 235–242. [Google Scholar] [CrossRef]
- Koponen, H.T.; Martikainen, P.J. Soil water content and freezing temperature affect freeze–thaw related N2O production in organic soil. Nutr. Cycl. Agroecosyst. 2004, 69, 213–219. [Google Scholar] [CrossRef]
- Koponen, H.T.; Jaakkola, T.; Keinänen-Toivola, M.M.; Kaipainen, S.; Tuomainen, J.; Servomaa, K.; Martikainen, P.J. Microbial communities, biomass, and activities in soils as affected by freeze thaw cycles. Soil Biol. Biochem. 2006, 38, 1861–1871. [Google Scholar] [CrossRef]
- Sahoo, M. Winter soil temperature and its effect on soil nitrate Status: A Support Vector Regression-based approach on the projected impacts. Catena 2022, 211, 105958. [Google Scholar] [CrossRef]
- Schimel, J.P.; Clein, J.S. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol. Biochem. 1996, 28, 1061–1066. [Google Scholar] [CrossRef]
- Krogstad, K.; Gharasoo, M.; Jensen, G.; Hug, L.A.; Rudolph, D.; Van Cappellen, P.; Rezanezhad, F. N leaching from agricultural soils under imposed freeze-thaw cycles: A column study with and without fertilizer amendment. Front. Environ. Sci. 2022, 10, 915329. [Google Scholar] [CrossRef]
- Gao, D.C.; Bai, E.; Yang, Y.; Zong, S.W.; Hagedorn, F. A global meta-analysis on freeze-thaw effects on soil carbon and phosphorus cycling. Soil Biol. Biochem. 2021, 159, 108283. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, Z.M.; He, P.; Zhang, Y.F.; Li, L.J. Changes in soil microbial community and carbon use efficiency in freeze-thaw period restored after growth season under warming and straw return. Appl. Soil Ecol. 2025, 205, 105779. [Google Scholar] [CrossRef]
- Berns-Herrboldt, E.C.; O’Meara, T.A.; Herndon, E.M.; Sulman, B.N.; Gu, B.H.; Klingeman, D.M.; Lowe, K.A.; Graham, D.E. Dynamic soil columns simulate Arctic redox biogeochemistry and carbon release during changes in water saturation. Sci. Rep. 2025, 15, 3093. [Google Scholar] [CrossRef]
- Wei, C.C.; Li, Y.L.; Yang, P.L.; Nadeem, A.A.; Luo, W.B.; Wang, Y.; Chi, Y.B. Variable soil carbon and N mineralization dynamics under freeze-thaw and constant temperature with brackish water irrigation. Irrig. Sci. 2025, 43, 549–561. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Li, P.; Xu, G.C.; Wang, X.K.; Li, Z.B.; Cheng, S.D.; Huang, M.S. Effects of dynamic factors of erosion on soil N and phosphorus loss under freeze-thaw conditions. Geoderma 2021, 390, 114972. [Google Scholar] [CrossRef]
- Tang, S.R.; Yuan, P.; Tawaraya, K.; Tokida, T.; Fukuoka, M.; Yoshimoto, M.; Sakai, H.; Hasegawa, T.; Xu, X.K.; Cheng, W.G. Winter nocturnal warming affects the freeze-thaw frequency, soil aggregate distribution, and the contents and decomposability of C and N in paddy fields. Sci. Total Environ. 2022, 802, 149870. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.S.; Sun, W.C. Multi-phase-field microporomechanics model for simulating ice-lens growth in frozen soil. Int. J. Numer. Anal. Methods Geomech. 2022, 46, 2307–2336. [Google Scholar] [CrossRef]
- Guo, C.Y.; Zhang, L.M.; Li, S.G.; Chen, Y.X. Freeze-thaw events change soil greenhouse gas fluxes through modifying soil carbon and N cycling processes in a Temperate forest in Northeastern China. Forests 2024, 15, 2082. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Sen, S.; Risbud, S.H.; Bartl, M.H. Thermodynamic and Kinetic transitions of liquids in nanoconfinement. Acc. Chem. Res. 2020, 53, 2869–2878. [Google Scholar] [CrossRef]
- Larsen, K.S.; Jonasson, S.; Michelsen, A. Repeated freeze-thaw cycles and their effects on biological processes in two arctic ecosystem types. Appl. Soil Ecol. 2002, 21, 187–195. [Google Scholar] [CrossRef]
- Lipson, D.A.; Schmidt, S.K.; Monson, R.K. Links between microbial population dynamics and N availability in an alpine ecosystem. Ecology 1999, 80, 1623–1631. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Zhang, S.; Song, D.L.; Wu, H.; Wang, L.X.; Wang, X.B. Distribution characteristics of microbial residues within aggregates of fluvo-aquic soil under biochar application. Agronomy 2023, 13, 392. [Google Scholar] [CrossRef]
- Dong, C.J.; Gu, Y.Z.; Jia, Y.L.; Wei, P.J.; Jin, J.W.; Deng, Y.F.; Yang, P.Z.; Chen, S.Y. Effects of freeze-thaw cycles on the size distribution and stability of soil aggregate in the permafrost regions of the Qinghai-Tibetan Plateau. Environ. Res. Commun. 2023, 12, 129501. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, Q.; Ma, W.D.; Niu, B.C.; Liu, F.G.; Chen, Q. Effects of freezing and thawing on soil shear strength in the western farming pastoral ecotone of northern China. Sci. Rep. 2025, 15, 23150. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, L.X.; Zhang, S.Q.; Tian, L.; Ou, Y.; Yan, B.X.; Cui, H.; Bao, M.W.; Liang, A.Z. Freeze-thaw cycles increase the mobility of phosphorus fractions based on soil aggregate in restored wetlands. Catena 2022, 209, 105846. [Google Scholar] [CrossRef]
- Li, J.F.; Lu, X.H.; Wang, P.; Yu, Y.; Sun, L.; Li, M. Influence of freeze-thaw process on as migration and microorganisms in aggregates of paddy soil. J. Environ. Manag. 2024, 370, 122847. [Google Scholar] [CrossRef]
- Liu, B.; Fan, H.M.; Jiang, Y.; Ma, R.M. Evaluation of soil macro-aggregate characteristics in response to soil macropore characteristics investigated by X-ray computed tomography under freeze-thaw effects. Soil Tillage Res. 2023, 225, 105559. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Sosa, F.A.; Chen, Q.; Zhang, X. Black soil quality after 19 years of continuous conservation tillage. Agronomy 2024, 14, 2859. [Google Scholar] [CrossRef]
- Xiang, B.; Liu, E.; Yang, L. Influences of freezing–thawing actions on mechanical properties of soils and stress and deformation of soil slope in cold regions. Sci. Rep. 2022, 12, 5387. [Google Scholar] [CrossRef]
- Li, D.N.; Li, Y.; Yao, S.H.; Zhou, H.; Huang, S.; Peng, X.L.; Meng, Y.L. Dynamics of N mineralization and N cycling functional genes in response to soil pore size distribution. Eur. J. Soil Biol. 2024, 123, 103692. [Google Scholar] [CrossRef]
- Gu, W.Q.; Wang, Y.N.; Sun, Y.Y.; Liu, Z.F.; Wang, W.J.; Wu, D.; Zhang, Y.X.; Sun, W.; Wang, X.; Feng, Z.B.; et al. Assessing the formation and stability of paddy soil aggregate driven by organic carbon and Fe/Al oxides in rice straw cyclic utilization strategies: Insight from a six-year field trial. Sci. Total Environ. 2024, 951, 175607. [Google Scholar] [CrossRef]
- Sawicka, J.E.; Robador, A.; Hubert, C.; Jorgensen, B.B.; Brüchert, V. Effects of freeze-thaw cycles on anaerobic microbial processes in an Arctic intertidal mud flat. ISME J. 2010, 4, 585–594. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, C.X.; He, X.L.; Wang, H.X.; Wang, Y.; Zhou, F.Y.; Qin, D.; Fan, Z.K. Study on the effects of winter irrigation during seasonal freezing-thawing period on soil microbial ecological properties. Sci. Rep. 2025, 15, 23586. [Google Scholar] [CrossRef] [PubMed]
- Choromanska, T.H. DeLuca. Microbial activity and N mineralization in forest mineral soils following heating: Evaluation of post-fire effects. Soil Biol. Biochem. 2002, 34, 263–271. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.S.; Sun, F.H.; Jiang, Q.H.; Shang, H.L.; Zheng, C.L. Effect of biochar and cyanobacteria crust incorporation on soil wind erosion in arid mining area under freeze-thaw action. Sci. Rep. 2025, 15, 16363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Lin, D.; Wei, P.; Zhou, L.; Zeng, L.; Qian, S.; Zhao, L.; Yang, Y.; Zhu, G. Effects of Fractal Dimension and Soil Erodibility on Soil Quality in an Erodible Region: A Case Study from Karst Mountainous Areas. Forests 2023, 14, 1609. [Google Scholar] [CrossRef]
- Gil, J.; Marushchak, M.E.; Rütting, T.; Baggs, E.M.; Pérez, T.; Novakovskiy, A.; Trubnikova, T.; Kaverin, D.; Martikainen, P.J.; Biasi, C. Sources of nitrous oxide and the fate of mineral nitrogen in subarctic permafrost peat soils. Biogeosciences 2022, 19, 2683–2698. [Google Scholar] [CrossRef]
- Liu, M.; Dannenmann, M.; Lin, S.; Saiz, G.; Yan, G.; Yao, Z.; Pelster, D.E.; Tao, H.; Sippel, S.; Tao, Y.; et al. Ground cover rice production systems increase soil carbon and nitrogen stocks at regional scale. Biogeosciences 2015, 12, 4831–4840. [Google Scholar] [CrossRef]
- Liu, X.B.; Burras, C.L.; Kravchenko, Y.S.; Duran, A.; Huffman, T.; Morras, H.; Studdert, G.; Zhang, X.Y.; Cruse, R.M.; Yuan, X.H. Overview of Mollisols in the world: Distribution, land use and management. Can. J. Soil Sci. 2012, 92, 383–402. [Google Scholar] [CrossRef]
- Zhou, M.; Xiao, Y.; Li, Y.S.; Liu, J.; Sui, Y.Y.; Zhang, X.Y.; Liu, X.B. Simulated erosion of A horizon influences the dissolved organic matter chemodiversity and carbon sequestration of B horizon in Mollisols. Soil Biol. Biochem. 2025, 201, 109648. [Google Scholar] [CrossRef]

| Soil Types | Soil Indicators | Nitrogen Components/Aggregate Sizes | Factors | ||
|---|---|---|---|---|---|
| Freeze–Thaw Frequency | Freeze–Thaw Temperature | Freeze–Thaw Frequency × Freeze-Thaw Temperature | |||
| Bulk soils | Soil nitrogen component contents | TN | <0.001 *** | <0.001 *** | 0.401 |
| NO3−-N | <0.001 *** | 0.001 ** | 0.593 | ||
| NH4+-N | <0.001 *** | 0.108 | 0.005 ** | ||
| MBN | <0.001 *** | <0.001 *** | 0.001 ** | ||
| Relative contribution rate | TN | <0.001 *** | 0.753 | 0.859 | |
| NO3−-N | <0.001 *** | <0.001 *** | <0.001 *** | ||
| NH4+-N | <0.001 *** | 0.294 | 0.333 | ||
| MBN | 0.042 * | 0.983 | 0.851 | ||
| Allocation amount | TN | <0.001 *** | 0.034 | 0.807 | |
| NO3−-N | <0.001 *** | <0.001 *** | <0.001 *** | ||
| NH4+-N | <0.001 *** | 0.214 | 0.079 | ||
| MBN | <0.001 *** | 0.001 ** | 0.457 | ||
| Allocation ratio | TN | <0.001 *** | 0.034 * | 0.807 | |
| NO3−-N | <0.001 *** | <0.001 *** | <0.001 *** | ||
| NH4+-N | <0.001 *** | 0.213 | 0.078 | ||
| MBN | <0.001 *** | 0.001 ** | 0.457 | ||
| Soil aggregates | TN contents | >2 mm | <0.001 *** | <0.001 *** | 0.040 * |
| 2–1 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| 1–0.5 mm | 0.062 | <0.001 *** | <0.001 *** | ||
| 0.5–0.25 mm | 0.007 ** | <0.001 *** | 0.006 ** | ||
| 0.25–0.053 mm | <0.001 *** | 0.296 | <0.001 *** | ||
| <0.053 mm | <0.001 *** | <0.001 *** | 0.061 | ||
| Relative contribution rate | >2 mm | 0.151 | 0.563 | 0.034 * | |
| 2–1 mm | 0.273 | 0.431 | 0.001 *** | ||
| 1–0.5 mm | <0.001 *** | 0.004 ** | <0.001 *** | ||
| 0.5–0.25 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| 0.25–0.053 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| <0.053 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| Allocation amount | >2 mm | 0.003 ** | <0.001 *** | 0.003 ** | |
| 2–1 mm | 0.384 | 0.038 | 0.005 ** | ||
| 1–0.5 mm | <0.001 *** | 0.001 ** | <0.001 *** | ||
| 0.5–0.25 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| 0.25–0.053 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| <0.053 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| Allocation ratio | >2 mm | 0.003 ** | <0.001 *** | 0.003 ** | |
| 2–1 mm | 0.384 | 0.038 * | 0.005 ** | ||
| 1–0.5 mm | <0.001 *** | 0.001 ** | <0.001 *** | ||
| 0.5–0.25 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| 0.25–0.053 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| <0.053 mm | <0.001 *** | <0.001 *** | <0.001 *** | ||
| Soil aggregate stability | R0.25 | <0.001 *** | <0.001 *** | <0.001 *** | |
| MWD | <0.001 *** | <0.001 *** | <0.001 *** | ||
| GMD | <0.001 *** | <0.001 *** | <0.001 *** | ||
| D | <0.001 *** | <0.001 *** | <0.001 *** | ||
| Soil Types | Soil Properties | Soil Indicators/Aggregate Sizes | Freezing Temperatures | ||
|---|---|---|---|---|---|
| −9 °C | −18 °C | −26 °C | |||
| Bulk soils | TN | Nitrogen content | 0.521 * | −0.440 | −0.609 * |
| Relative contribution rate | 0.433 | −0.369 | −0.604 * | ||
| Allocation amount | 0.466 | −0.379 | −0.680 ** | ||
| Allocation ratio | 0.466 | −0.379 | −0.680 ** | ||
| NO3−-N | Nitrogen content | 0.723 ** | −0.532 * | −0.726 ** | |
| Relative contribution rate | 0.624 * | −0.418 | −0.711 ** | ||
| Allocation amount | 0.530 * | −0.356 | −0.752 ** | ||
| Allocation ratio | 0.530 * | −0.356 | −0.752 ** | ||
| NH4+-N | Nitrogen content | 0.694 ** | −0.474 | −0.531 * | |
| Relative contribution rate | 0.489 | −0.403 | −0.532 * | ||
| Allocation amount | 0.558 * | −0.388 | −0.595 * | ||
| Allocation ratio | 0.558 * | −0.388 | −0.595 * | ||
| MBN | Nitrogen content | 0.767 ** | −0.589 * | −0.650 ** | |
| Relative contribution rate | 0.593 * | 0.019 | −0.582 * | ||
| Allocation amount | 0.632 * | −0.248 | −0.689 ** | ||
| Allocation ratio | 0.632 * | −0.248 | −0.689 ** | ||
| Soil aggregates | Proportion | >2 mm | 0.061 | 0.007 | 0.007 |
| 2–1 mm | −0.868 ** | 0.597 * | 0.597 * | ||
| 1–0.5 mm | −0.971 ** | 0.098 | 0.098 | ||
| 0.5–0.25 mm | −0.602 * | −0.622 * | −0.622 * | ||
| 0.25–0.053 mm | 0.112 | −0.533 * | −0.533 * | ||
| <0.053 mm | 0.978 ** | 0.861 ** | 0.861 ** | ||
| TN content | >2 mm | −0.817 ** | 0.469 | 0.469 | |
| 2–1 mm | −0.917 ** | 0.599 * | 0.599 * | ||
| 1–0.5 mm | −0.348 | −0.434 | −0.434 | ||
| 0.5–0.25 mm | −0.100 | 0.205 | 0.205 | ||
| 0.25–0.053 mm | −0.631 * | 0.461 | 0.461 | ||
| <0.053 mm | −0.651 ** | 0.435 | 0.435 | ||
| Relative contribution rate | >2 mm | −0.007 | −0.535 * | −0.535 * | |
| 2–1 mm | 0.524 * | 0.247 | 0.247 | ||
| 1–0.5 mm | −0.702 ** | −0.029 | −0.029 | ||
| 0.5–0.25 mm | −0.653 ** | −0.469 | −0.469 | ||
| 0.25–0.053 mm | −0.379 | 0.156 | 0.156 | ||
| <0.053 mm | 0.247 | 0.509 | 0.509 | ||
| Allocation amount | >2 mm | −0.575 * | −0.419 | −0.419 | |
| 2–1 mm | −0.227 | 0.354 | 0.354 | ||
| 1–0.5 mm | −0.734 ** | −0.103 | −0.103 | ||
| 0.5–0.25 mm | −0.698 ** | −0.400 | −0.400 | ||
| 0.25–0.053 mm | −0.429 | 0.360 | 0.360 | ||
| <0.053 mm | −0.131 | 0.541 * | 0.541 * | ||
| Allocation ratio | >2 mm | −0.575 * | −0.420 | −0.420 | |
| 2–1 mm | −0.227 | 0.355 | 0.355 | ||
| 1–0.5 mm | −0.734 ** | −0.103 | −0.103 | ||
| 0.5–0.25 mm | −0.698 ** | −0.400 | −0.400 | ||
| 0.25–0.053 mm | −0.429 | 0.360 | 0.360 | ||
| <0.053 mm | −0.131 | 0.541 * | 0.541 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, M.; Jiao, X.; Ma, L.; Yu, H.; Chen, Y.; Sui, Y. Soil Aggregate Stability Under Freeze–Thaw Cycles in Mollisols as Evidenced by 15N Distribution. Agronomy 2025, 15, 2263. https://doi.org/10.3390/agronomy15102263
Wang Y, Zhou M, Jiao X, Ma L, Yu H, Chen Y, Sui Y. Soil Aggregate Stability Under Freeze–Thaw Cycles in Mollisols as Evidenced by 15N Distribution. Agronomy. 2025; 15(10):2263. https://doi.org/10.3390/agronomy15102263
Chicago/Turabian StyleWang, Yao, Meng Zhou, Xiaoguang Jiao, Liangqian Ma, He Yu, Yimin Chen, and Yueyu Sui. 2025. "Soil Aggregate Stability Under Freeze–Thaw Cycles in Mollisols as Evidenced by 15N Distribution" Agronomy 15, no. 10: 2263. https://doi.org/10.3390/agronomy15102263
APA StyleWang, Y., Zhou, M., Jiao, X., Ma, L., Yu, H., Chen, Y., & Sui, Y. (2025). Soil Aggregate Stability Under Freeze–Thaw Cycles in Mollisols as Evidenced by 15N Distribution. Agronomy, 15(10), 2263. https://doi.org/10.3390/agronomy15102263





