Polyvinylpolypyrrolidone Immobilized Cu, Cd and Zn in Soils and Reduced Their Uptake by Oilseed Rape
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batch Trials
2.3. Soil Incubation Experiments
2.4. Pot Experiments
2.5. Heavy Metal Determinations and Statistic Analyses
3. Results
3.1. Adsorption Kinetics and Isotherms
3.2. Affinities of Cu, Cd and Zn to Polyvinylpolypyrrolidone
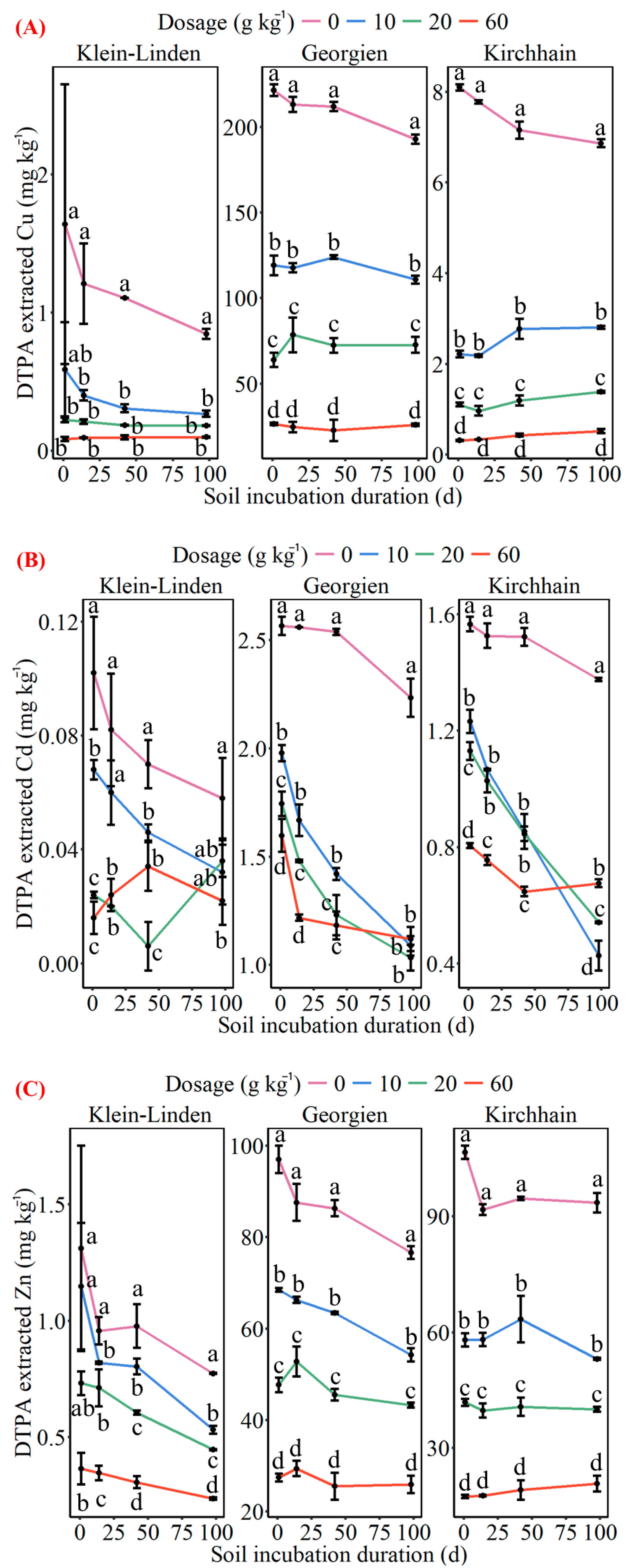
3.3. Effects of Polyvinylpolypyrrolidone on Soil Heavy Metal Immobilization
3.4. Effects of Polyvinylpolypyrrolidone on Plant Uptake of Heavy Metals
4. Discussion
4.1. Adsorption Characteristics of Heavy Metals by Polyvinylpolypyrrolidone
4.2. Immobilization of Heavy Metals in Soils by Polyvinylpolypyrrolidone
4.3. Influences of Polyvinylpolypyrrolidone on Plant Growth and Plant Uptake of Heavy Metals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Y.; Bai, L.; Li, C.; He, Z.; Liu, X. Assessment of heavy metal contamination levels and health risks in environmental media in the northeast region. Cities Soc. 2022, 80, 103796. [Google Scholar] [CrossRef]
- Li, Y.; Ma, G.; Zhou, Q.; Huang, Z. Ranging patterns and foraging patch utilization of Assamese macaques in-habiting limestone forests in southwest Guangxi, China. Glob. Ecol. Conserv. 2020, 21, e00816. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, X.; Zhu, Y.; Zhuo, R. Recent advances in phyto-combined remediation of heavy metal pollution in soil. Biotechnol. Adv. 2024, 72, 108337. [Google Scholar] [CrossRef]
- Ghorbani, A.; Emamverdian, A.; Pehlivan, N.; Zargar, M.; Razavi, S.M.; Chen, M. Nano-enabled agrochemicals: Mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J. Nanobiotechnol. 2024, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Q.; Yuan, Y.; Sun, W. Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chem. 2020, 316, 126213. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Gong, T.; Liang, P. Heavy metal exposure and cardiovascular disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Fu, R.; Li, Q. Removal of inorganic contaminants in soil by electrokinetic remediation technologies: A review. J. Hazard. Mater. 2021, 401, 123345. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Azhar, U.; Ahmad, H.; Shafqat, H.; Babar, M.; Shahzad Munir, H.M.; Sagir, M.; Arif, M.; Hassan, A.; Rachmadona, N.; Rajendran, S.; et al. Remediation techniques for elimination of heavy metal pollutants from soil: A review. Environ. Res. 2022, 214 Pt 4, 113918. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- He, L.; Xu, Y.; Zhang, M.; Gul, S.; Zhang, X.; Zhong, H.; Tang, Y.; Dong, D.; Xu, Y.; Liu, D.; et al. Effect of remediation technologies on soil fertility in heavy metal(loid)-contaminated soils: A critical review. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1417–1435. [Google Scholar] [CrossRef]
- Gao, M.; Tang, F.; Zhao, Y.; Chu, Y.; Yang, Y.; Tian, G.; Wang, Y.; Liu, H. Characteristics of soil pore structure response to electric field strength and their effects on Cr(VI) removal from a historically chromium-contaminated soil. Chem. Eng. J. 2024, 499, 156061. [Google Scholar] [CrossRef]
- Suanon, F.; Tomètin, L.A.S.; Zveushe, O.K.; de Dios, V.R.; Han, Y.; Ifon, B.E.; Atakpa, E.O.; Yete, P.; Sesu, F.; Li, J.; et al. Electrokinetic remediation of chromium-contaminated soils: The potential for advanced materials in three-dimensional EKR approaches. J. Environ. Chem. Eng. 2025, 13, 116774. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Under-standing the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Gondek, K.; Mierzwa-Hersztek, M.; Jarosz, R. Effect of willow biochar and fly ash-derived zeolite in immobilizing heavy metals and promoting enzymatic activity in a contaminated sandy soil. Catena 2023, 232, 107429. [Google Scholar] [CrossRef]
- Yan, Y.; Du, M.; Jing, L.; Zhang, X.; Li, Q.; Yang, J. Green synthesized hydroxyapatite for efficient immobilization of cadmium in weakly alkaline environment. Environ. Res. 2023, 223, 115445. [Google Scholar] [CrossRef] [PubMed]
- Mench, M.; Vangronsveld, J.; Beckx, C.; Ruttens, A. Progress in assisted natural remediation of an arsenic contaminated agricultural soil. Environ. Pollut. 2006, 144, 51–61. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Huang, X.; Jiang, S.; Xiong, J.; Li, J.; Hong, M.; Zhang, J.; Guan, Y.; Feng, X.; et al. Chromium(VI) adsorption and reduction in soils under anoxic conditions: The relative roles of iron (ox-yhr)oxides, iron(II), organic matters, and microbes. Environ. Sci. Technol. 2024, 58, 18391–18403. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Du, W.; Lin, R.; Li, J.; Ai, F.; Yin, Y.; Ji, R.; Wang, X.; Guo, H. In-situ immobilization of cadmium-polluted upland soil: A ten-year field study. Ecotoxicol. Environ. Saf. 2021, 207, 111275. [Google Scholar] [CrossRef]
- Yulikasari, A.; Tangahu, B.V.; Nurhayati, E.; Arliyani, I.; Mashudi; Titah, H.S.; Lam, Y.M.; Wang, Y. A comprehensive review of integrated phytoremediation and nanoparticle methods for heavy metal in red mud. Ecotoxicol. Environ. Saf. 2024, 288, 117381. [Google Scholar] [CrossRef] [PubMed]
- Madrid, F.; Díaz-Barrientos, E.; Florido, M.C.; Madrid, L. Inorganic amendments to decrease metal availability in soils of recreational urban areas: Limitations to their efficiency and possible drawbacks. Water Air Soil Pollut. 2008, 192, 117–125. [Google Scholar] [CrossRef]
- Liu, H.; Chen, P.; Wang, H.; Yang, Y.; Wu, Y. Remediation of Cu-, Zn-, and Pb-contaminated soil using different soil washing agents: Removal efficiencies and mechanisms. Water Air Soil Pollut. 2023, 234, 476. [Google Scholar] [CrossRef]
- Hai, N.N.S.; Sanderson, P.; Qi, F.; Du, J.; Nong, N.N.; Bolan, N.; Naidu, R. Effects of chelates (EDTA, EDDS, NTA) on phytoavailability of heavy metals (As, Cd, Cu, Pb, Zn) using ryegrass (Lolium multiflorum Lam.). Environ. Sci. Pollut. Res. 2022, 29, 42102–42116. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Q.; Peng, H.; Zhang, J.; Chen, W.; Zhou, B.; Chen, M. Remediation of heavy metal-contaminated soils with soil Washing: A review. Sustainability 2022, 14, 13058. [Google Scholar] [CrossRef]
- Qu, J.; Li, Z.; Wu, Z.; Bi, F.; Wei, S.; Dong, M.; Hu, Q.; Wang, Y.; Yu, H.; Zhang, Y. Cyclodextrin-functionalized magnetic alginate microspheres for synchronous removal of lead and bisphenol a from contaminated soil. Chem. Eng. J. 2023, 461, 142079. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Z.; Song, M.; Liu, L.; Liu, Z. Soil flushing for remediation of landfill leachate-contaminated soil: A comprehensive evaluation of optimal flushing agents and influencing factors. Waste Manag. 2025, 200, 114771. [Google Scholar] [CrossRef]
- Rahman, S.; Jii, N.; Ni, S.; Harada, Y.; Mashio, A.S.; Begum, Z.A.; Rahman, I.M.M.; Hasegawa, H. Biodegradable chelator-assisted washing and stabilization of arsenic-contaminated excavated soils. Water Air Soil Pollut. 2022, 233, 213. [Google Scholar] [CrossRef]
- Betiha, M.A.; Moustafa, Y.M.; El-Shahat, M.F.; Rafik, E. Polyvinylpyrrolidone-Aminopropyl-SBA-15 schiff Base hybrid for efficient removal of divalent heavy metal cations from wastewater. J. Hazard. Mater. 2020, 397, 122675. [Google Scholar] [CrossRef]
- Hanauer, T.; Jung, S.; Felix-Henningsen, P.; Schnell, S.; Steffens, D. Suitability of inorganic and organic amend-ments for in situ immobilization of Cd, Cu, and Zn in a strongly contaminated Kastanozem of the Mashavera valley, SE Georgia. I. Effect of amendments on metal mobility and microbial activity in soil. J. Plant Nutr. Soil Sci. 2012, 175, 708–720. [Google Scholar] [CrossRef]
- Wang, S.; Tian, C.C. Polyvinylpolypyrrolidone supported brønsted acidic catalyst for esterification. Int. J. Polym. Sci. 2016, 2016, 8104838. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.Y.; Hyun, S.; Kim, J.G. Metal availability in heavy metal-contaminated open burning and open detonation soil: Assessment using soil enzymes, earthworms, and chemical extractions. J. Hazard. Mater. 2009, 170, 382–388. [Google Scholar] [CrossRef]
- Tuezen, M. Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem. J. 2003, 74, 289–297. [Google Scholar] [CrossRef]
- Nabulo, G.; Oryem-Origa, H.; Diamond, M. Assessment of lead, cadmium, and zinc contamination of roadside soils, surface films, and vegetables in Kampala City, Uganda. Environ. Res. 2006, 101, 42–52. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using R; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- Kaschl, A.; Roemheld, V.; Chen, Y. Binding of cadmium, copper, and zinc to humic substances originating from municipal solid waste compost. Isr. J. Chem. 2002, 41, 89–98. [Google Scholar] [CrossRef]
- Yu, S.Q.; Yang, Y.; Kuroda, K.; Pu, J.; Guo, R.; Hou, L.A. Selective removal of Cr(VI) using polyvinylpyrrolidone and polyacrylamide co-modified MoS2 composites by adsorption combined with reduction. Chin. Chem. Lett. 2024, 35, 109130. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Kercheva, M.; Paparkova, T.; Grygorczuk-Płaneta, K.; Siryk, O.; Kukowska, S.; Panek, R. Reclamation of degraded soils: Analysis of selected parameters after organic/inorganic modifications. J. Soils Sediments 2024, 24, 1704–1723. [Google Scholar] [CrossRef]
- Kaplan, H.; Ratering, S.; Felix-Henningsen, P.; Schnell, S. Stability of in situ immobilization of trace metals with different amendments revealed by microbial (13)C-labelled wheat root decomposition and efflux-mediated metal resistance of soil bacteria. Sci. Total Environ. 2019, 659, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Balík, J.; Pavlíková, D.; Tlustoš, P.; Černý, J.; Jakl, M. The fluctuation of copper content in oilseed rape plants (Brassica napus L.) after the application of nitrogen and sulphur fertilizers. Plant Soil Environ. 2007, 53, 143–148. [Google Scholar] [CrossRef]
- Yang, H.F.; Wang, Y.B.; Huang, Y.J. Chemical fractions and phytoavailability of copper to rape grown in the polluted paddy soil. Int. J. Environ. Sci. Technol. 2014, 12, 2929–2938. [Google Scholar] [CrossRef]
- Baryla, A.; Carrier, P.; Franck, F.; Coulomb, C.; Sahut, C.; Havaux, M. Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: Causes and consequences for photosynthesis and growth. Planta 2001, 212, 696–709. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Zeng, X.; Zhang, L.; Zhou, Y.; Anastopoulos, I.; Wang, A.; Zeng, Q.; Xiao, Z. Enhancing cadmium extraction potential of Brassica napus: Effect of rhizosphere interactions. J. Environ. Manag. 2021, 284, 112056. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2017; pp. 88–115. [Google Scholar]
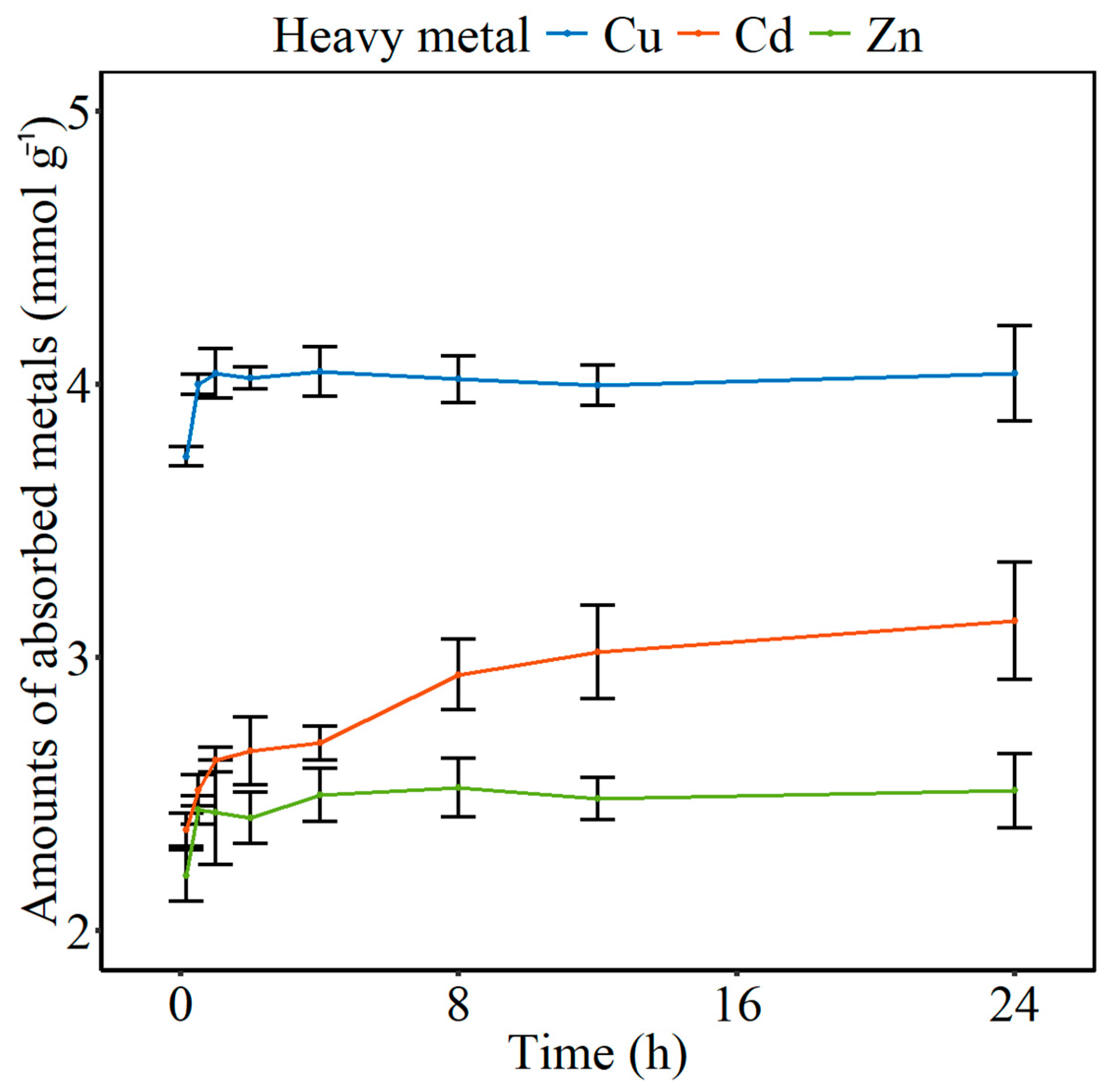


| Models | Heavy Metals | Constants | R2 |
|---|---|---|---|
| Pseudo first order | Cu | qe = 0.256; k1 = 15.81 | 0.963 |
| Cd | qe = 0.315; k1 = 10.93 | 0.232 | |
| Zn | qe = 0.161; k1 = 13.26 | 0.832 | |
| Pseudo second order | Cu | qe = 0.256; k2 = 249.9 | 0.999 |
| Cd | qe = 0.346; k2 = −1.09 | 0.998 | |
| Zn | qe = 0.164; k2 = −2.64 | 0.999 | |
| Elovich | Cu | α = 0.251; β = 0.002 | 0.341 |
| Cd | α = 0.293; β = 0.017 | 0.939 | |
| Zn | α = 0.156; β = 0.003 | 0.631 | |
| Intra-particle diffusion | Cu | C = 0.249; kp = 0.002 | 0.072 |
| Cd | C = 0.269; kp = 0.018 | 0.931 | |
| Zn | C = 0.153; kp = 0.003 | 0.351 | |
| Langmuir | Cu | qmax = 0.327; kL = 0.079; RL = 0.166 | 0.995 |
| Cd | qmax = 0.330; kL = 0.094; RL = 0.086 | 0.995 | |
| Zn | qmax = 0.186; kL = 0.156; RL = 0.089 | 0.997 | |
| Freundlich | Cu | n = 2.11; kF = 0.041 | 0.954 |
| Cd | n = 2.63; kF = 0.054 | 0.927 | |
| Zn | n = 2.60; kF = 0.037 | 0.936 |
| Soil | Doses g kg−1 | Dry Weight g pot−1 | Cu | Cd | Zn | Fe | K | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | % | ||||||||
| Georgien | 0 | 0.28 ± 0.08 ab | 43.4 ± 4.4 a | 7.2 ± 0.8 a | 161 ± 26 a | 70 ± 15 a | 5.1 ± 1.0 a | 4.3 ± 1.0 a | 0.66 ± 0.07 a |
| 10 | 0.36 ± 0.04 ab | 32.9 ± 1.5 b | 6.2 ± 0.3 ab | 130 ± 17 ab | 72 ± 3 a | 5.2 ± 0.3 a | 4.0 ± 1.0 a | 0.66 ± 0.03 a | |
| 20 | 0.44 ± 0.07 a | 28.5 ± 1.2 bc | 4.5 ± 2.3 b | 126 ± 15 b | 87 ± 16 a | 5.6 ± 0.4 a | 3.8 ± 0.8 a | 0.64 ± 0.05 a | |
| 60 | 0.45 ± 0.03 a | 23.9 ± 2.0 c | 1.8 ± 0.2 c | 98 ± 4 b | 80 ± 10 a | 4.8 ± 0.3 a | 4.0 ± 1.0 a | 0.68 ± 0.06 a | |
| Kirchhain | 0 | 0.58 ± 0.06 a | 9.9 ± 0.9 a | 6.0 ± 1.1 a | 318 ± 45 a | 79 ± 7 b | 3.3 ± 0.2 a | 3.0 ± 0.6 a | 0.53 ± 0.04 a |
| 10 | 0.52 ± 0.03 a | 7.5 ± 1.4 b | 2.1 ± 0.1 b | 98 ± 3 b | 110 ± 17 a | 3.1 ± 0.1 a | 3.1 ± 0.7 a | 0.48 ± 0.07 a | |
| 20 | 0.57 ± 0.09 a | 7.0 ± 1.2 b | 1.1 ± 0.4 b | 96 ± 25 b | 107 ± 10 a | 3.0 ± 0.2 a | 3.5 ± 0.8 a | 0.52 ± 0.02 a | |
| 60 | 0.53 ± 0.03 a | 5.5 ± 0.5 b | 1.6 ± 0.3 b | 65 ± 5 b | 109 ± 12 a | 3.1 ± 0.3 a | 3.1 ± 0.7 a | 0.47 ± 0.06 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Steffens, D.; Jia, Y.; Wang, H. Polyvinylpolypyrrolidone Immobilized Cu, Cd and Zn in Soils and Reduced Their Uptake by Oilseed Rape. Agronomy 2025, 15, 2258. https://doi.org/10.3390/agronomy15102258
Wang Y, Steffens D, Jia Y, Wang H. Polyvinylpolypyrrolidone Immobilized Cu, Cd and Zn in Soils and Reduced Their Uptake by Oilseed Rape. Agronomy. 2025; 15(10):2258. https://doi.org/10.3390/agronomy15102258
Chicago/Turabian StyleWang, Yiliu, Diedrich Steffens, Yunsheng Jia, and Huoyan Wang. 2025. "Polyvinylpolypyrrolidone Immobilized Cu, Cd and Zn in Soils and Reduced Their Uptake by Oilseed Rape" Agronomy 15, no. 10: 2258. https://doi.org/10.3390/agronomy15102258
APA StyleWang, Y., Steffens, D., Jia, Y., & Wang, H. (2025). Polyvinylpolypyrrolidone Immobilized Cu, Cd and Zn in Soils and Reduced Their Uptake by Oilseed Rape. Agronomy, 15(10), 2258. https://doi.org/10.3390/agronomy15102258






