Effects of Elevated CO2 on Maize Physiological and Biochemical Processes
Abstract
1. Introduction
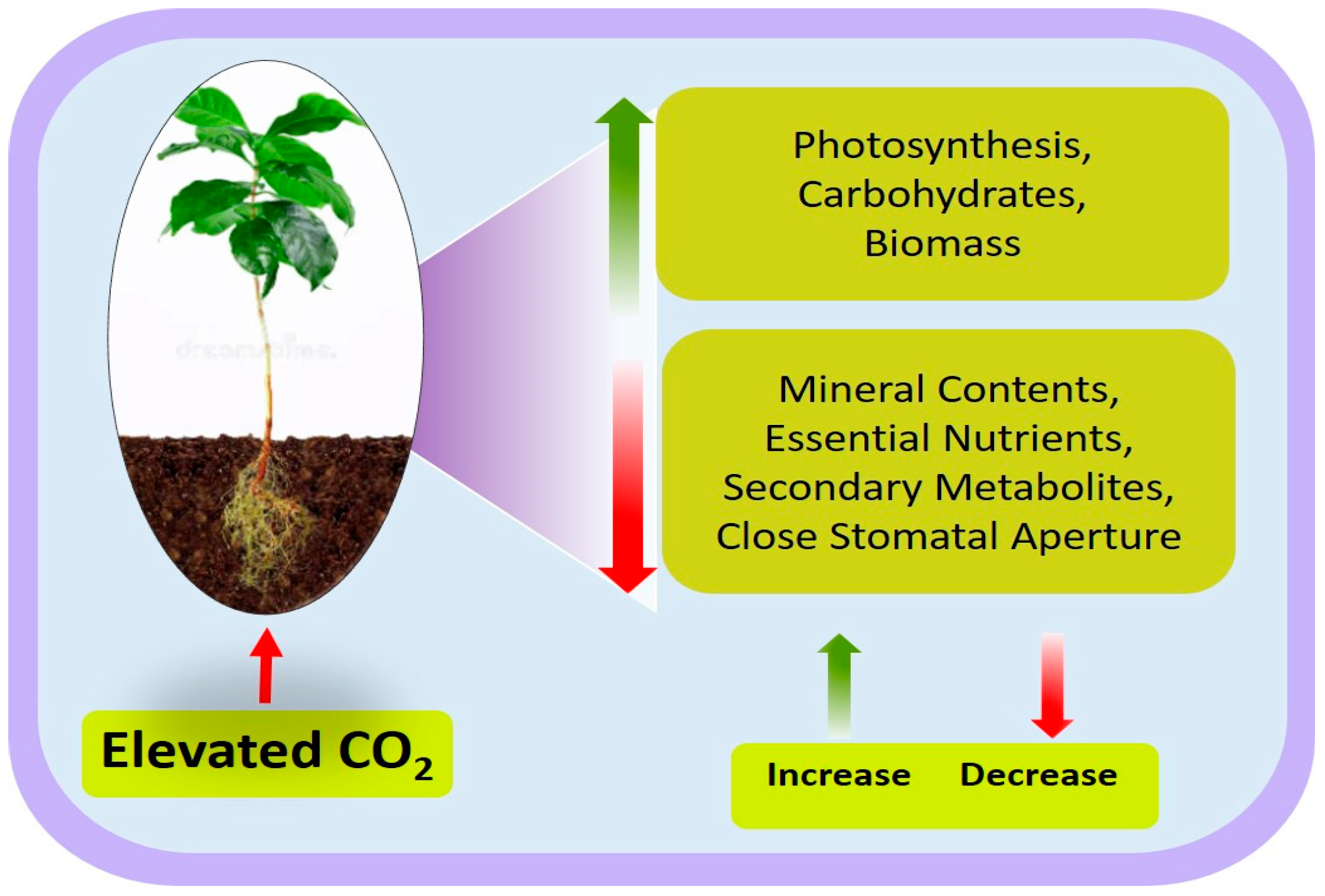
2. Effects of Elevated CO2 on Maize Growth and Development
3. Positive and Negative Impacts of Elevated CO2 on Plant Nutrition
4. Genetic Responses of Maize to Elevated CO2
5. Impact on Water Use Efficiency and Drought Resistance
6. Folate Role in Maize Growth and Development Under Stress Condition
6.1. Importance and Significance
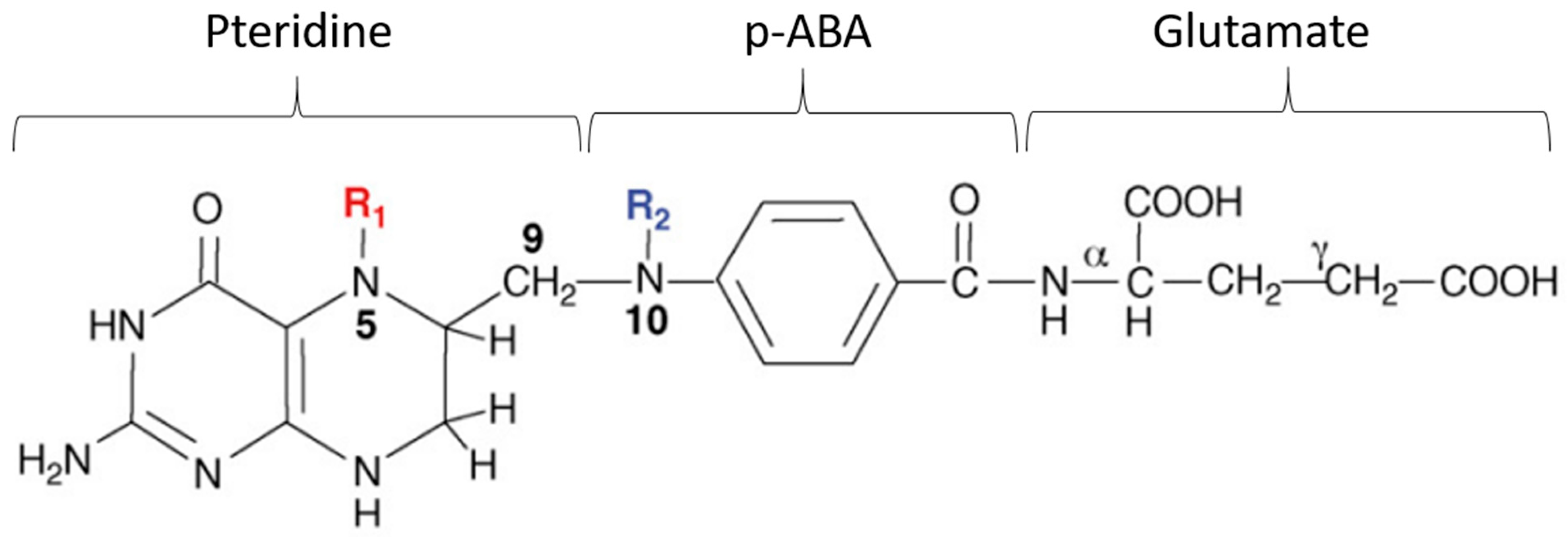
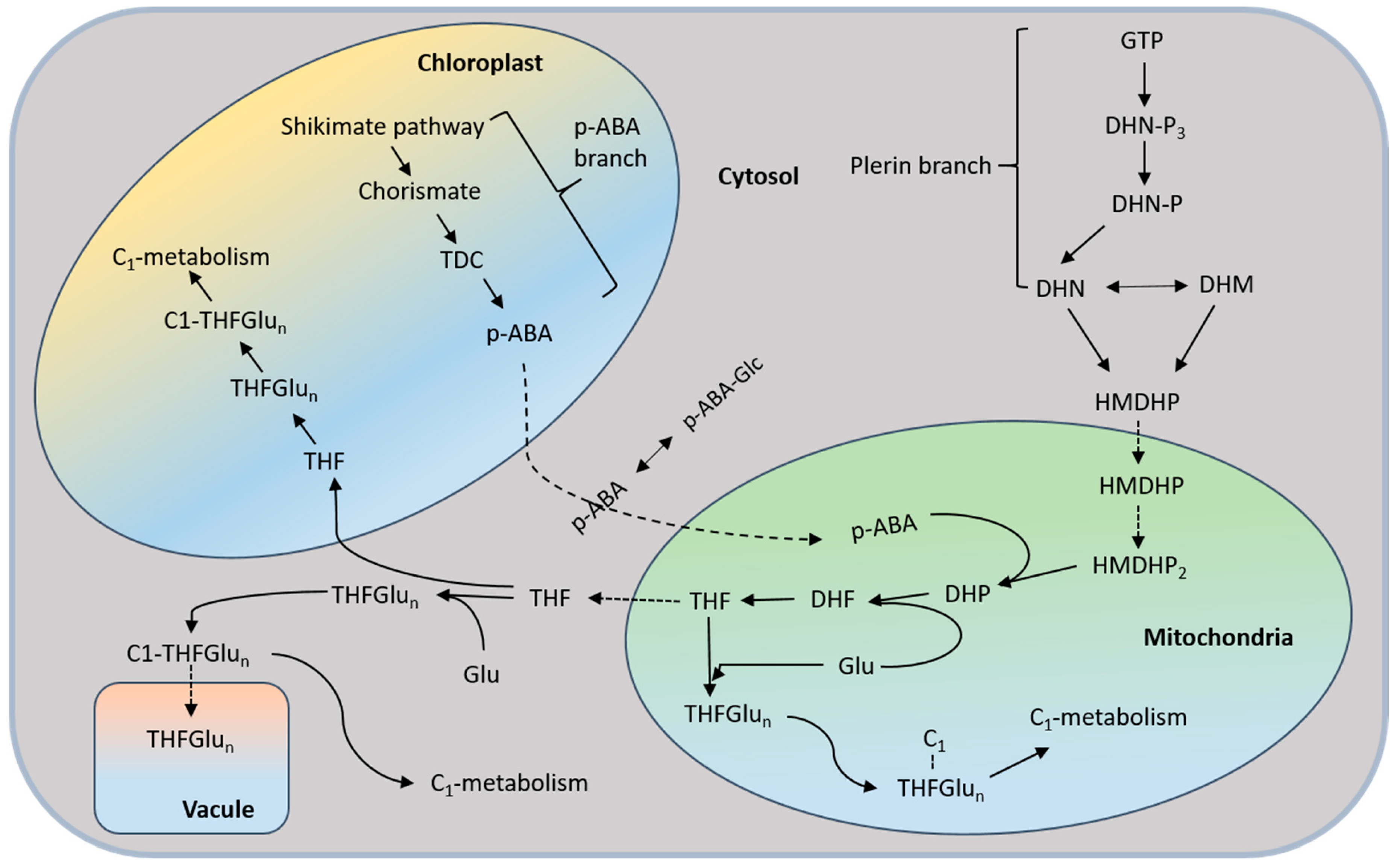
6.2. Folate Biosynthesis in Plants
6.3. Effects of Elevated CO2 on Folates
6.4. Folate Stability and Degradation Under Environmental Stresses
6.5. Biofortification Strategies for Folate Enhancement in Crops
7. Lignin’s Role in Maize Growth and Development Under Stress Condition
7.1. Importance and Significance of Lignin
7.2. Lignin Biosynthesis in Grasses

7.3. Effect of Elevated CO2 on Lignin
7.4. Lignin’s Role in Carbon Sequestration Under Elevated CO2
7.5. Impacts of Lignin Modifications on Biofuel Production
8. Future Directions and Prospects
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasanna, B.; Pixley, K.; Warburton, M.L.; Xie, C.-X. Molecular marker-assisted breeding options for maize improvement in Asia. Mol. Breed. 2010, 26, 339–356. [Google Scholar] [CrossRef]
- Vasal, S. The quality protein maize story. Food Nutr. Bull. 2000, 21, 445–450. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Meng, H.; Jiang, L.; Xu, B.; Guo, W.; Li, J.; Zhu, X.; Qi, X.; Duan, L.; Meng, X.; Fan, Y. Arabidopsis plastidial folylpolyglutamate synthetase is required for seed reserve accumulation and seedling establishment in darkness. PLoS ONE 2014, 9, e101905. [Google Scholar] [CrossRef]
- Tariq, M.; Iqbal, H. Maize in Pakistan–an overview. Agric. Nat. Resour. 2010, 44, 757–763. [Google Scholar]
- Hall, M.C.; Stiling, P.; Moon, D.C.; Drake, B.G.; Hunter, M.D. Effects of elevated CO2 on foliar quality and herbivore damage in a scrub oak ecosystem. J. Chem. Ecol. 2005, 31, 267–286. [Google Scholar] [CrossRef]
- Worku, A.; Teketay, D.; Lemenih, M.; Fetene, M. Diversity, regeneration status, and population structures of gum and resin producing woody species in Borana, Southern Ethiopia. For. Trees Livelihoods 2012, 21, 85–96. [Google Scholar] [CrossRef]
- Mina, U.; Kumar, R.; Gogoi, R.; Bhatia, A.; Harit, R.; Singh, D.; Kumar, A.; Kumar, A. Effect of elevated temperature and carbon dioxide on maize genotypes health index. Ecol. Indic. 2019, 105, 292–302. [Google Scholar] [CrossRef]
- Florides, G.A.; Christodoulides, P. Global warming and carbon dioxide through sciences. Environ. Int. 2009, 35, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- West, T.O.; Marland, G. A synthesis of carbon sequestration, carbon emissions, and net carbon flux in agriculture: Comparing tillage practices in the United States. Agric. Ecosyst. Environ. 2002, 91, 217–232. [Google Scholar] [CrossRef]
- Hansen, J.E.; Sato, M.; Lacis, A.; Ruedy, R.; Tegen, I.; Matthews, E. Climate forcings in the industrial era. Proc. Natl. Acad. Sci. USA 1998, 95, 12753–12758. [Google Scholar] [CrossRef]
- Nunes, L.J. The rising threat of atmospheric CO2: A review on the causes, impacts, and mitigation strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Sonnemann, G.; Grygalashvyly, M. Effective CO2 lifetime and future CO2 levels based on fit function. In Annales Geophysicae; Copernicus Publications: Göttingen, Germany, 2013; pp. 1591–1596. [Google Scholar]
- Misra, V.; Shrivastava, A.; Mall, A.; Solomon, S.; Singh, A.K.; Ansari, M.I. Can sugarcane cope with increasing atmospheric CO2 concentration? Aust. J. Crop Sci. 2019, 13, 780–784. [Google Scholar] [CrossRef]
- Butterfield, R.E.; Morison, J.I. Modelling the impact of climatic warming on winter cereal development. Agric. For. Meteorol. 1992, 62, 241–261. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO₂ concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Change Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Prins, A.; Mukubi, J.M.; Pellny, T.K.; Verrier, P.J.; Beyene, G.; Lopes, M.S.; Emami, K.; Treumann, A.; Lelarge-Trouverie, C.; Noctor, G. Acclimation to high CO2 in maize is related to water status and dependent on leaf rank. Plant Cell Environ. 2011, 34, 314–331. [Google Scholar] [CrossRef]
- Walker, B.; Steffen, W. An overview of the implications of global change for natural and managed terrestrial ecosystems. Conserv. Ecol. 1997, 1, 17. [Google Scholar] [CrossRef]
- De Souza, A.P.; Gaspar, M.; Da Silva, E.A.; Ulian, E.C.; Waclawovsky, A.J.; Nishiyama, M.Y., Jr.; Dos Santos, R.V.; Teixeira, M.M.; Souza, G.M.; Buckeridge, M.S. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ. 2008, 31, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Vu, J.C.; Allen, L.H., Jr.; Gesch, R.W. Up-regulation of photosynthesis and sucrose metabolism enzymes in young expanding leaves of sugarcane under elevated growth CO2. Plant Sci. 2006, 171, 123–131. [Google Scholar] [CrossRef]
- Prasad, P.V.; Vu, J.C.; Boote, K.J.; Allen, L.H. Enhancement in leaf photosynthesis and upregulation of Rubisco in the C4 sorghum plant at elevated growth carbon dioxide and temperature occur at early stages of leaf ontogeny. Funct. Plant Biol. 2009, 36, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.; Sims, J.; Martins, V.F.; Swerbilow, J.; Romero, M.; Alborn, H.T. Effects of elevated [CO2] on maize defence against mycotoxigenic F usarium verticillioides. Plant Cell Environ. 2014, 37, 2691–2706. [Google Scholar] [CrossRef]
- Silva, R.G.d.; Alves, R.d.C.; Zingaretti, S.M. Increased [CO2] causes changes in physiological and genetic responses in C4 crops: A brief review. Plants 2020, 9, 1567. [Google Scholar] [CrossRef]
- Iglesias, A.A.; Andreo, C.S. Kinetic and structural properties of NADP-malic enzyme from sugarcane leaves. Plant Physiol. 1990, 92, 66–72. [Google Scholar] [CrossRef]
- Maitra, S.; Singh, V. Invited review on ’maize in the 21st century’ Emerging trends of maize biorefineries in the 21st century: Scientific and technological advancements in biofuel and bio-sustainable market. J. Cereal Sci. 2021, 101, 103272. [Google Scholar] [CrossRef]
- Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLoS ONE 2019, 14, e0217148. [Google Scholar] [CrossRef]
- Willeit, M.; Ganopolski, A.; Calov, R.; Brovkin, V. Mid-Pleistocene transition in glacial cycles explained by declining CO2 and regolith removal. Sci. Adv. 2019, 5, eaav7337. [Google Scholar] [CrossRef]
- Tausz-Posch, S.; Tausz, M.; Bourgault, M. Elevated [CO2] effects on crops: Advances in understanding acclimation, nitrogen dynamics and interactions with drought and other organisms. Plant Biol. 2020, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Schjoerring, J.K. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of current hypotheses. Agric. Ecosyst. Environ. 2013, 178, 57–63. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef]
- Stitt, M.; Krapp, A. The interaction between elevated carbon dioxide and nitrogen nutrition: The physiological and molecular background. Plant Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- Seibert, R.; Andresen, L.C.; Jarosch, K.A.; Moser, G.; Kammann, C.I.; Yuan, N.; Luterbacher, J.; Laughlin, R.J.; Watson, C.J.; Erbs, M.; et al. Plant functional types differ in their long-term nutrient response to eCO2 in an extensive grassland. Ecosystems 2021, 25, 1084–1095. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin III, F.S.; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Loladze, I.; Nolan, J.M.; Ziska, L.H.; Knobbe, A.R. Rising atmospheric CO2 lowers concentrations of plant carotenoids essential to human health: A meta-analysis. Mol. Nutr. Food Res. 2019, 63, 1801047. [Google Scholar] [CrossRef]
- Dippery, J.; Tissue, D.; Thomas, R.; Strain, B. Effects of low and elevated CO2 on C3 and C4 annuals: I. Growth and biomass allocation. Oecologia 1995, 101, 13–20. [Google Scholar] [CrossRef]
- HAMIM, H. Photosynthesis of C3 and C4 species in response to increased CO2 concentration and drought stress. HAYATI J. Biosci. 2005, 12, 131. [Google Scholar] [CrossRef]
- Seneweera, S.P.; Ghannoum, O.; Conroy, J. High vapour pressure deficit and low soil water availability enhance shoot growth responses of a C4 grass (Panicum coloratum cv. Bambatsi) to CO2 enrichment. Funct. Plant Biol. 1998, 25, 287–292. [Google Scholar] [CrossRef]
- Ghannoum, O.; Caemmerer, S.v.; Ziska, L.H.; Conroy, J.P. The growth response of C4 plants to rising atmospheric CO2 partial pressure: A reassessment. Plant Cell Environ. 2000, 23, 931–942. [Google Scholar] [CrossRef]
- Wand, S.J.; Midgley, G.F.; Jones, M.H.; Curtis, P.S. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: A meta-analytic test of current theories and perceptions. Glob. Change Biol. 1999, 5, 723–741. [Google Scholar] [CrossRef]
- Allen, R.J.; Gomez, J.; Horowitz, L.W.; Shevliakova, E. Enhanced future vegetation growth with elevated carbon dioxide concentrations could increase fire activity. Commun. Earth Environ. 2024, 5, 54. [Google Scholar] [CrossRef]
- Mattson, W.J.; Julkunen-Tiitto, R.; Herms, D. CO2 enrichment and carbon partitioning to phenolics: Do plant responses accord better with the protein competition or the growth differentiation balance models? Oikos 2005, 111, 337–347. [Google Scholar] [CrossRef]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P.; et al. Tree mineral nutrition is deteriorating in Europe. Glob. Change Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef]
- Penuelas, J.; Fernández-Martínez, M.; Vallicrosa, H.; Maspons, J.; Zuccarini, P.; Carnicer, J.; Sanders, T.G.; Krüger, I.; Obersteiner, M.; Janssens, I.A.; et al. Increasing atmospheric CO2 concentrations correlate with declining nutritional status of European forests. Commun. Biol. 2020, 3, 125. [Google Scholar] [CrossRef]
- Scheelbeek, P.F.; Bird, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.; Chalabi, Z.; Allen, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef]
- Saban, J.M.; Chapman, M.A.; Taylor, G. FACE facts hold for multiple generations; Evidence from natural CO2 springs. Glob. Change Biol. 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Ainsworth, E.; Davey, P.; Hymus, G.; Osborne, C.; Rogers, A.; Blum, H.; Nösberger, J.; Long, S.P. Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under Free Air CO2Enrichment (FACE). Plant Cell Environ. 2003, 26, 705–714. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Benavente, E.; Giménez, E. Modern Approaches for the Genetic Improvement of Rice, Wheat and Maize for Abiotic Constraints-Related Traits: A Comparative Overview. Agronomy 2021, 11, 376. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, R.; Li, Y.; Liu, X.; Wang, G.; Yin, K.; Jin, J.; Herbert, S.J. Warming and elevated CO2 alter the transcriptomic response of maize (Zea mays L.) at the silking stage. Sci. Rep. 2019, 9, 17948. [Google Scholar] [CrossRef]
- Dermody, O.; Long, S.P.; Mc Connaughay, K.; de Lucia, E.H. How do elevated CO2 and O3 affect the interception and utilization of radiation by a soybean canopy? Glob. Change Biol. 2008, 14, 556–564. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Q.; Zhu, D.; Yu, J. A DREB-like transcription factor from maize (Zea mays), ZmDREB4. 1, plays a negative role in plant growth and development. Front. Plant Sci. 2018, 9, 395. [Google Scholar]
- Allen, L.H., Jr.; Kakani, V.G.; Vu, J.C.; Boote, K.J. Elevated CO2 increases water use efficiency by sustaining photosynthesis of water-limited maize and sorghum. J. Plant Physiol. 2011, 168, 1909–1918. [Google Scholar] [CrossRef]
- Moore, B.; Cheng, S.H.; Sims, D.; Seemann, J. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 1999, 22, 567–582. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Li, R.; Zeng, Y.; Xu, J.; Wang, Q.; Wu, F.; Cao, M.; Lan, H.; Liu, Y.; Lu, Y. Genetic variation for maize root architecture in response to drought stress at the seedling stage. Breed. Sci. 2015, 65, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.M.; Ort, D.R. Diurnal depression in leaf hydraulic conductance at ambient and elevated [CO2] reveals anisohydric water management in field-grown soybean and possible involvement of aquaporins. Environ. Exp. Bot. 2015, 116, 39–46. [Google Scholar] [CrossRef]
- Herrick, J.D. Integrated Responses of Overstory Sweetgum (Liquidambar styracuflua L.) Trees to Elevated Atmospheric Carbon Dioxide: A Field Experiment at the Duke Forest FACE Site; West Virginia University: Morgantown, WV, USA, 2002. [Google Scholar]
- Bekaert, S.; Storozhenko, S.; Mehrshahi, P.; Bennett, M.J.; Lambert, W.; Gregory, J.F.; Schubert, K.; Hugenholtz, J.; van der Straeten, D.; Hanson, A.D. Folate biofortification in food plants. Trends Plant Sci. 2008, 13, 28–35. [Google Scholar] [CrossRef]
- Blancquaert, D.; de Steur, H.; Gellynck, X.; van der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef]
- Díaz de la Garza, R.I.; Gregory, J.F., III; Hanson, A.D. Folate biofortification of tomato fruit. Proc. Natl. Acad. Sci. USA 2007, 104, 4218–4222. [Google Scholar] [CrossRef]
- Nunes, A.C.; Kalkmann, D.C.; Aragao, F.J. Folate biofortification of lettuce by expression of a codon optimized chicken GTP cyclohydrolase I gene. Transgenic Res. 2009, 18, 661–667. [Google Scholar] [CrossRef]
- Blancquaert, D.; Storozhenko, S.; Loizeau, K.; de Steur, H.; de Brouwer, V.; Viaene, J.; Ravanel, S.; Rebeille, F.; Lambert, W.; van der Straeten, D. Folates and folic acid: From fundamental research toward sustainable health. Crit. Rev. Plant Sci. 2010, 29, 14–35. [Google Scholar] [CrossRef]
- Mehrshahi, P.; Gonzalez-Jorge, S.; Akhtar, T.A.; Ward, J.L.; Santoyo-Castelazo, A.; Marcus, S.E.; Lara-Núñez, A.; Ravanel, S.; Hawkins, N.D.; Beale, M.H. Functional analysis of folate polyglutamylation and its essential role in plant metabolism and development. Plant J. 2010, 64, 267–279. [Google Scholar] [CrossRef]
- Stokes, M.E.; Chattopadhyay, A.; Wilkins, O.; Nambara, E.; Campbell, M.M. Interplay between sucrose and folate modulates auxin signaling in Arabidopsis. Plant Physiol. 2013, 162, 1552–1565. [Google Scholar] [CrossRef]
- Wittek, F.; Kanawati, B.; Wenig, M.; Hoffmann, T.; Franz-Oberdorf, K.; Schwab, W.; Schmitt-Kopplin, P.; Vlot, A.C. Folic acid induces salicylic acid-dependent immunity in Arabidopsis and enhances susceptibility to Alternaria brassicicola. Mol. Plant Pathol. 2015, 16, 616–622. [Google Scholar] [CrossRef]
- Baxter, C.J.; Redestig, H.; Schauer, N.; Repsilber, D.; Patil, K.R.; Nielsen, J.; Selbig, J.; Liu, J.; Fernie, A.R.; Sweetlove, L.J. The metabolic response of heterotrophic Arabidopsis cells to oxidative stress. Plant Physiol. 2007, 143, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Neilson, K.A.; Mariani, M.; Haynes, P.A. Quantitative proteomic analysis of cold-responsive proteins in rice. Proteomics 2011, 11, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Gorelova, V.; Bastien, O.; De Clerck, O.; Lespinats, S.; Rébeillé, F.; Van Der Straeten, D. Evolution of folate biosynthesis and metabolism across algae and land plant lineages. Sci. Rep. 2019, 9, 5731. [Google Scholar] [CrossRef] [PubMed]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis regulation of folates and phenols in plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Hanson, A.D.; Gregory, J.F., III. Folate biosynthesis, turnover, and transport in plants. Annu. Rev. Plant Biol. 2011, 62, 105–125. [Google Scholar] [CrossRef]
- Goyer, A.; Illarionova, V.; Roje, S.; Fischer, M.; Bacher, A.; Hanson, A.D. Folate biosynthesis in higher plants. cDNA cloning, heterologous expression, and characterization of dihydroneopterin aldolases. Plant Physiol. 2004, 135, 103–111. [Google Scholar] [CrossRef]
- Quinlivan, E.P.; Roje, S.; Basset, G.; Shachar-Hill, Y.; Gregory, J.F.; Hanson, A.D. The folate precursor p-aminobenzoate is reversibly converted to its glucose ester in the plant cytosol. J. Biol. Chem. 2003, 278, 20731–20737. [Google Scholar] [CrossRef]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef]
- Fangmeier, A.; Grüters, U.; Högy, P.; Vermehren, B.; Jäger, H.-J. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat—II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ. Pollut. 1997, 96, 43–59. [Google Scholar] [CrossRef]
- Seneweera, S.P.; Conroy, J.P. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition. Soil Sci. Plant Nutr. 1997, 43, 1131–1136. [Google Scholar] [CrossRef]
- Lieffering, M.; Kim, H.-Y.; Kobayashi, K.; Okada, M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crops Res. 2004, 88, 279–286. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Myers, S.S. Global health implications of nutrient changes in rice under high atmospheric carbon dioxide. GeoHealth 2019, 3, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Myers-Smith, I.H.; Hik, D.S. Climate warming as a driver of tundra shrubline advance. J. Ecol. 2018, 106, 547–560. [Google Scholar] [CrossRef]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, eaaq1012. [Google Scholar] [CrossRef]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van Der Straeten, D. Folates in plants: Research advances and progress in crop biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef]
- Chen, Y.; Du, T.; Zhang, J.; Chen, S.; Fu, J.; Li, H.; Yang, Q. Genes and pathways correlated with heat stress responses and heat tolerance in maize kernels. Front. Plant Sci. 2023, 14, 1228213. [Google Scholar] [CrossRef]
- Osuagwu, G.; Edeoga, H. The influence of water stress (drought) on the mineral and vitamin content of the leaves of Gongronema latifolium (Benth). Int. J. Med. Arom. Plants 2012, 2, 301–309. [Google Scholar]
- Rébeillé, F.; Ravanel, S.; Jabrin, S.; Douce, R.; Storozhenko, S.; Van Der Straeten, D. Folates in plants: Biosynthesis, distribution, and enhancement. Physiol. Plant. 2006, 126, 330–342. [Google Scholar] [CrossRef]
- Kumar, G.; Basak, N.; Priyadarsani, S.; Bagchi, T.B.; Kumar, A.; Pradhan, S.K.; Sanghamitra, P. Alteration in the physico-chemical traits and nutritional quality of rice under anticipated rise in atmospheric CO2 concentration: A review. J. Food Compos. Anal. 2023, 121, 105332. [Google Scholar] [CrossRef]
- Agyenim-Boateng, K.G.; Zhang, S.; Shohag, M.J.I.; Shaibu, A.S.; Li, J.; Li, B.; Sun, J. Folate biofortification in soybean: Challenges and prospects. Agronomy 2023, 13, 241. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Govindaraj, M.; Karthikeyan, A.; Shobhana, V.; Warkentin, T.D. Genomics-integrated breeding for carotenoids and folates in staple cereal grains to reduce malnutrition. Front. Genet. 2020, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S.; Tanumihardjo, S.A. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1809–1834. [Google Scholar] [CrossRef] [PubMed]
- Mahara, F.A.; Nuraida, L.; Lioe, H.N.; Nurjanah, S. The occurrence of folate biosynthesis genes in lactic acid bacteria from different sources. Food Technol. Biotechnol. 2023, 61, 226–237. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Shivay, Y.S.; Nisar, S.; Gaber, A.; Brestic, M.; Barek, V.; et al. Biofortification—A frontier novel approach to enrich micronutrients in field crops to encounter the nutritional security. Molecules 2022, 27, 1340. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K. Impact of micronutrients in mitigation of abiotic stresses in soils and plants—A progressive step toward crop security and nutritional quality. Adv. Agron. 2022, 173, 1–78. [Google Scholar]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Rosler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef]
- Faraji, M.; Fonseca, L.L.; Escamilla-Treviño, L.; Barros-Rios, J.; Engle, N.L.; Yang, Z.K.; Tschaplinski, T.J.; Dixon, R.A.; Voit, E.O. A dynamic model of lignin biosynthesis in Brachypodium distachyon. Biotechnol. Biofuels 2018, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2016, 2, 16050. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Ralph, J.; Hatfield, R.D. Modeling Lignification in Grasses with Monolignol Dehydropolymerisate-Cell Wall Complexes; ACS Publications: Washington, DC, USA, 1998. [Google Scholar]
- Grabber, J.H.; Ralph, J.; Hatfield, R.D. Cross-linking of maize walls by ferulate dimerization and incorporation into lignin. J. Agric. Food Chem. 2000, 48, 6106–6113. [Google Scholar] [CrossRef]
- Lan, W.; Lu, F.; Regner, M.; Zhu, Y.; Rencoret, J.; Ralph, S.A.; Zakai, U.I.; Morreel, K.; Boerjan, W.; Ralph, J. Tricin, a flavonoid monomer in monocot lignification. Plant Physiol. 2015, 167, 1284–1295. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef]
- Martínez, P.M.; Punt, A.M.; Kabel, M.A.; Gruppen, H. Deconstruction of lignin linked p-coumarates, ferulates and xylan by NaOH enhances the enzymatic conversion of glucan. Bioresour. Technol. 2016, 216, 44–51. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D.; Quideau, S.; Helm, R.F.; Grabber, J.H.; Jung, H.-J.G. Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J. Am. Chem. Soc. 1994, 116, 9448–9456. [Google Scholar] [CrossRef]
- Grabber, J.H.; Hatfield, R.D.; Lu, F.; Ralph, J. Coniferyl ferulate incorporation into lignin enhances the alkaline delignification and enzymatic degradation of cell walls. Biomacromolecules 2008, 9, 2510–2516. [Google Scholar] [CrossRef]
- Karlen, S.D.; Zhang, C.; Peck, M.L.; Smith, R.A.; Padmakshan, D.; Helmich, K.E.; Free, H.C.; Lee, S.; Smith, B.G.; Lu, F.; et al. Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci. Adv. 2016, 2, e1600393. [Google Scholar] [CrossRef]
- Christensen, C.S.; Rasmussen, S.K. Low lignin mutants and reduction of lignin content in grasses for increased utilisation of lignocellulose. Agronomy 2019, 9, 256. [Google Scholar] [CrossRef]
- Peñuelas, J.; Estiarte, M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trends Ecol. Evol. 1998, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bidart-Bouzat, M.G.; Imeh-Nathaniel, A. Global change effects on plant chemical defenses against insect herbivores. J. Integr. Plant Biol. 2008, 50, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Coûteaux, M.-M.; Kurz, C.; Bottner, P.; Raschi, A. Influence of increased atmospheric CO2 concentration on quality of plant material and litter decomposition. Tree Physiol. 1999, 19, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Cotrufo, M.F.; Ineson, P.; O’Neill, E.G.; Canadell, J.G. Elevated CO2, litter chemistry, and decomposition: A synthesis. Oecologia 2001, 127, 153–165. [Google Scholar] [CrossRef]
- Porteaus, F.; Hill, J.; Ball, A.; Pinter, P.; Kimball, B.; Wall, G.; Adamsen, F.; Hunsaker, D.; LaMorte, R.; Leavitt, S.; et al. Effect of free air carbon dioxide enrichment (FACE) on the chemical composition and nutritive value of wheat grain and straw. Anim. Feed. Sci. Technol. 2009, 149, 322–332. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Wang, Y.; Huang, J.; Zhu, J.; Yunxia, W.; Dong, G.; Liu, G. Yield formation of CO2-enriched hybrid rice cultivar Shanyou 63 under fully open-air field conditions. Field Crops Res. 2008, 108, 93–100. [Google Scholar] [CrossRef]
- Blaschke, L.; Forstreuter, M.; Sheppard, L.J.; Leith, I.; Murray, M.; Polle, A. Lignification in beech (Fagus sylvatica) grown at elevated CO2 concentrations: Interaction with nutrient availability and leaf maturation. Tree Physiol. 2002, 22, 469–477. [Google Scholar] [CrossRef]
- Khan, P.; Tong, L.; Khan, S.; Zhang, C.; Wang, W. Lignin: A defensive shield halting the environmental stresses-a review. Appl. Ecol. Environ. Res. 2022, 20, 22. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Y.; Wang, J.; Cui, J.; Wang, X.; Li, X.; Li, Y.; Ma, L. Metabolomics analysis reveal the molecular responses of high CO2 concentration improve resistance to Pb stress of Oryza sativa L. seedlings. Ecotoxicol. Environ. Saf. 2023, 251, 114515. [Google Scholar] [CrossRef]
- Cseke, L.J.; Tsai, C.J.; Rogers, A.; Nelsen, M.P.; White, H.L.; Karnosky, D.F.; Podila, G.K. Transcriptomic comparison in the leaves of two aspen genotypes having similar carbon assimilation rates but different partitioning patterns under elevated [CO2]. New Phytol. 2009, 182, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Druart, N.; Rodríguez-Buey, M.; Barron-Gafford, G.; Sjödin, A.; Bhalerao, R.; Hurry, V. Molecular targets of elevated [CO2] in leaves and stems of Populus deltoides: Implications for future tree growth and carbon sequestration. Funct. Plant Biol. 2006, 33, 121–131. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; Van den Ende, W.; Janssens, I.A.; Asard, H. Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: A comparison of fructan and non-fructan accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef] [PubMed]
- Goverde, M.; Arnone III, J.A.; Erhardt, A. Species-specific reactions to elevated CO2 and nutrient availability in four grass species. Basic Appl. Ecol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Luo, R.; Kuzyakov, Y.; Zhu, B.; Qiang, W.; Zhang, Y.; Pang, X. Phosphorus addition decreases plant lignin but increases microbial necromass contribution to soil organic carbon in a subalpine forest. Glob. Change Biol. 2022, 28, 4194–4210. [Google Scholar] [CrossRef]
- Srivastava, S.; Vishwakarma, R.K.; Arafat, Y.A.; Gupta, S.K.; Khan, B.M. Abiotic stress induces change in Cinnamoyl CoA Reductase (CCR) protein abundance and lignin deposition in developing seedlings of Leucaena leucocephala. Physiol. Mol. Biol. Plants 2015, 21, 197–205. [Google Scholar] [CrossRef]
- Jat, M.L.; Chakraborty, D.; Ladha, J.K.; Parihar, C.M.; Datta, A.; Mandal, B.; Nayak, H.S.; Maity, P.; Rana, D.S.; Chaudhari, S.K.; et al. Carbon sequestration potential, challenges, and strategies towards climate action in smallholder agricultural systems of South Asia. Crop Environ. 2022, 1, 86–101. [Google Scholar] [CrossRef]
- Graham, R.L.; Turner, M.G.; Dale, V.H. How increasing CO2 and climate change affect forests. BioScience 1990, 40, 575–587. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Handa, N.; Sharma, R.; Kaur, H.; Kohli, S.; Kumar, V.; Kaur, P. Lignins and abiotic stress: An overview. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2014; Volume 1, pp. 267–296. [Google Scholar]
- Grabber, J.H. Relationships between cell wall digestibility and lignin content as influenced by lignin type and analysis method. Crop Sci. 2019, 59, 1122–1132. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Dien, B.S.; Miller, D.J.; Hector, R.E.; Dixon, R.A.; Chen, F.; McCaslin, M.; Reisen, P.; Sarath, G.; Cotta, M.A. Enhancing alfalfa conversion efficiencies for sugar recovery and ethanol production by altering lignin composition. Bioresour. Technol. 2011, 102, 6479–6486. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Hisano, H.; Nandakumar, R.; Wang, Z.-Y. Genetic modification of lignin biosynthesis for improved biofuel production. Biofuels Glob. Impact Renew. Energy Prod. Agric. Technol. Adv. 2011, 223–235. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, P.; Aziz, T.; Jan, R.; Kim, K.-M. Effects of Elevated CO2 on Maize Physiological and Biochemical Processes. Agronomy 2025, 15, 202. https://doi.org/10.3390/agronomy15010202
Khan P, Aziz T, Jan R, Kim K-M. Effects of Elevated CO2 on Maize Physiological and Biochemical Processes. Agronomy. 2025; 15(1):202. https://doi.org/10.3390/agronomy15010202
Chicago/Turabian StyleKhan, Pirzada, Tariq Aziz, Rahmatullah Jan, and Kyung-Min Kim. 2025. "Effects of Elevated CO2 on Maize Physiological and Biochemical Processes" Agronomy 15, no. 1: 202. https://doi.org/10.3390/agronomy15010202
APA StyleKhan, P., Aziz, T., Jan, R., & Kim, K.-M. (2025). Effects of Elevated CO2 on Maize Physiological and Biochemical Processes. Agronomy, 15(1), 202. https://doi.org/10.3390/agronomy15010202






