Strategic Selenium Application Methods and Timing Enhance Grain Yield, Minimize Cadmium Bioaccumulation, and Optimize Selenium Fortification in Triticum aestivum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Determination of Cd Subcellular Distribution and Chemical Forms in Leaves
2.3. Determination of Cd Transporter Gene Expression Levels
2.4. Determination of Phenotypic and Yield-Related Traits
2.5. Determination of Cd Content, Se Content, and Cd Translocation Factor
2.6. Statistical Analysis
3. Results
3.1. Agronomic Traits
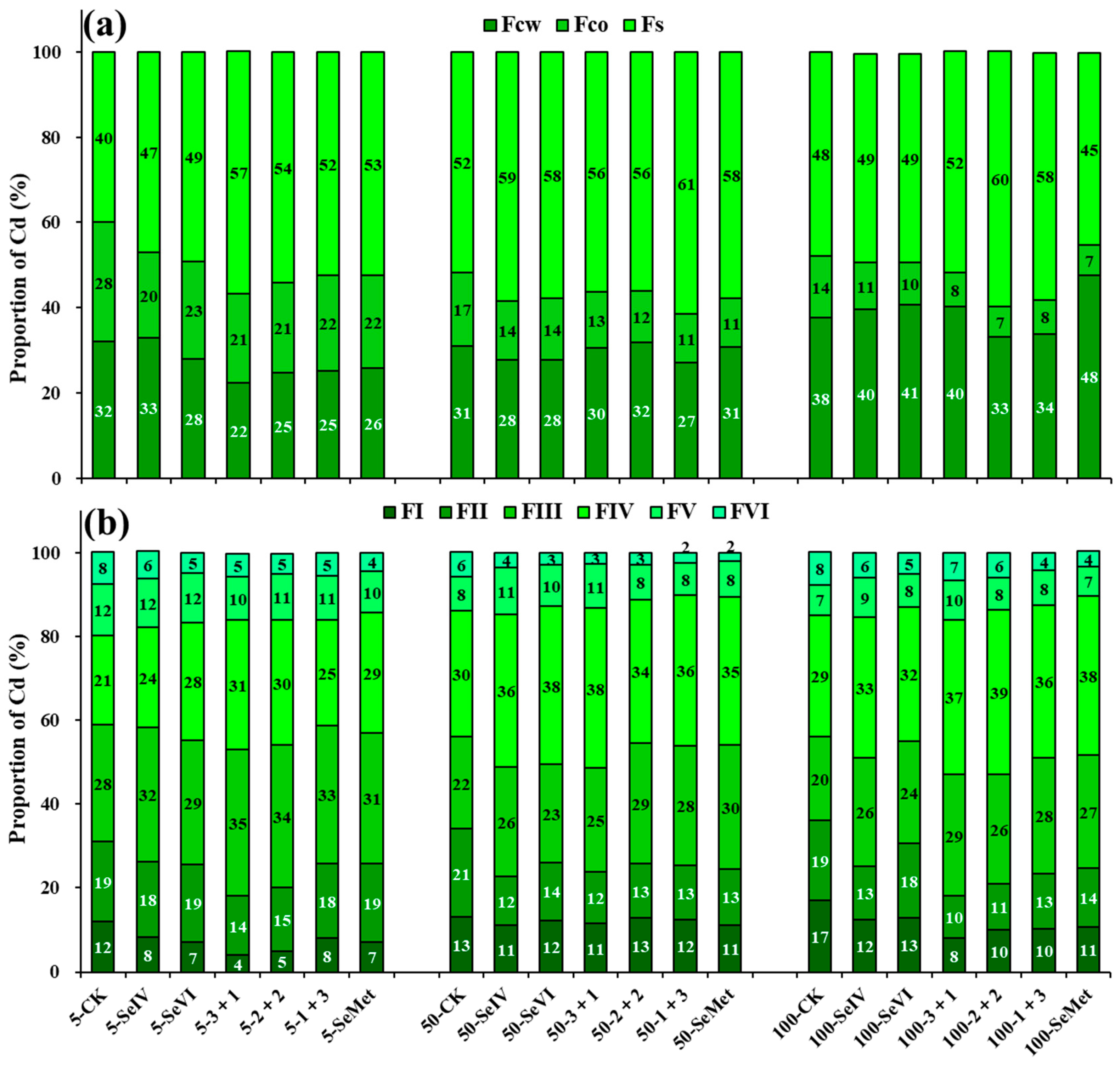
3.2. Se Treatment at Different Stages Increased the Proportion of Cd in Insoluble and Bound Forms Under Cd Stress
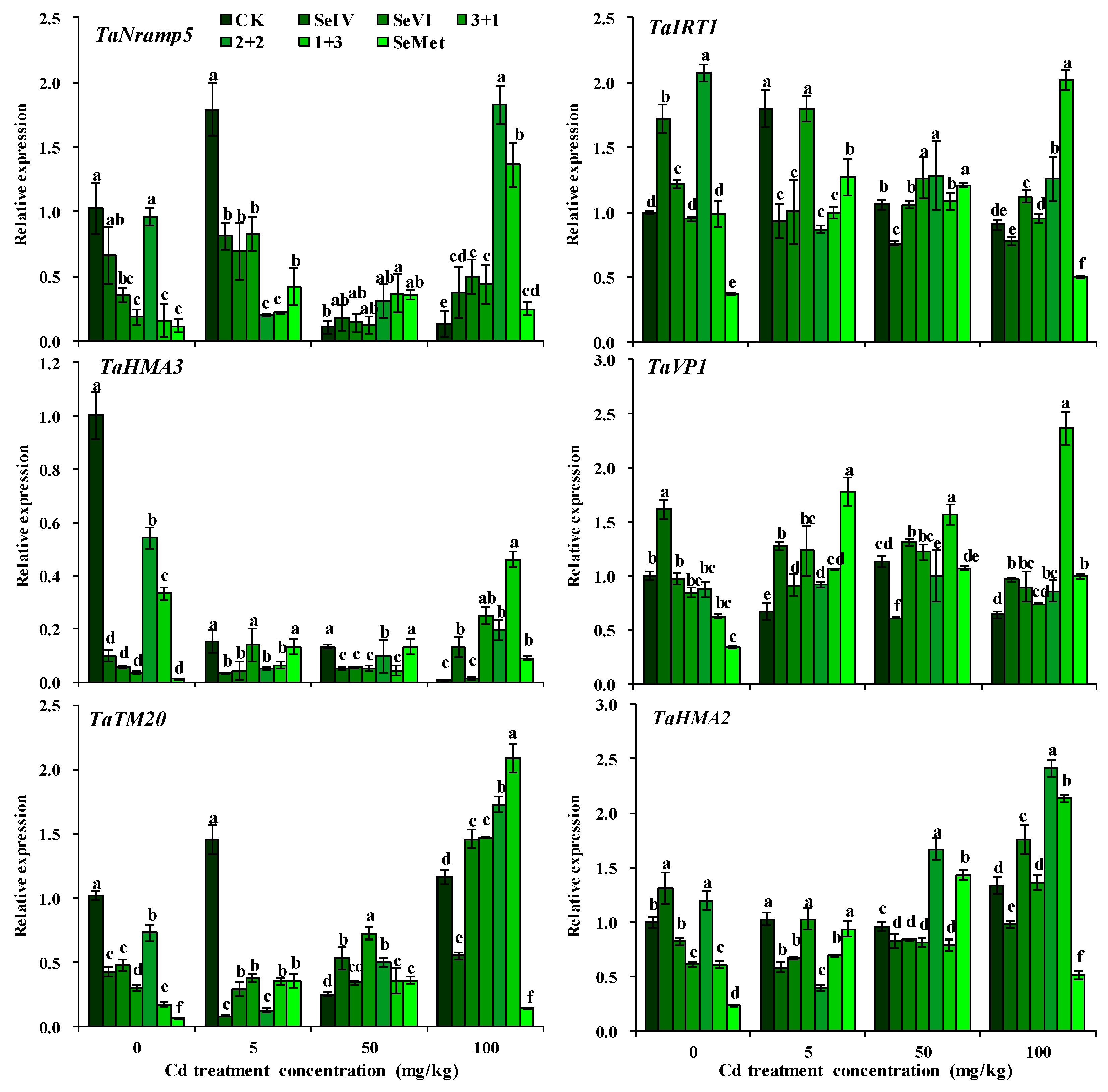
3.3. Expression Level of Cd Transporter Genes
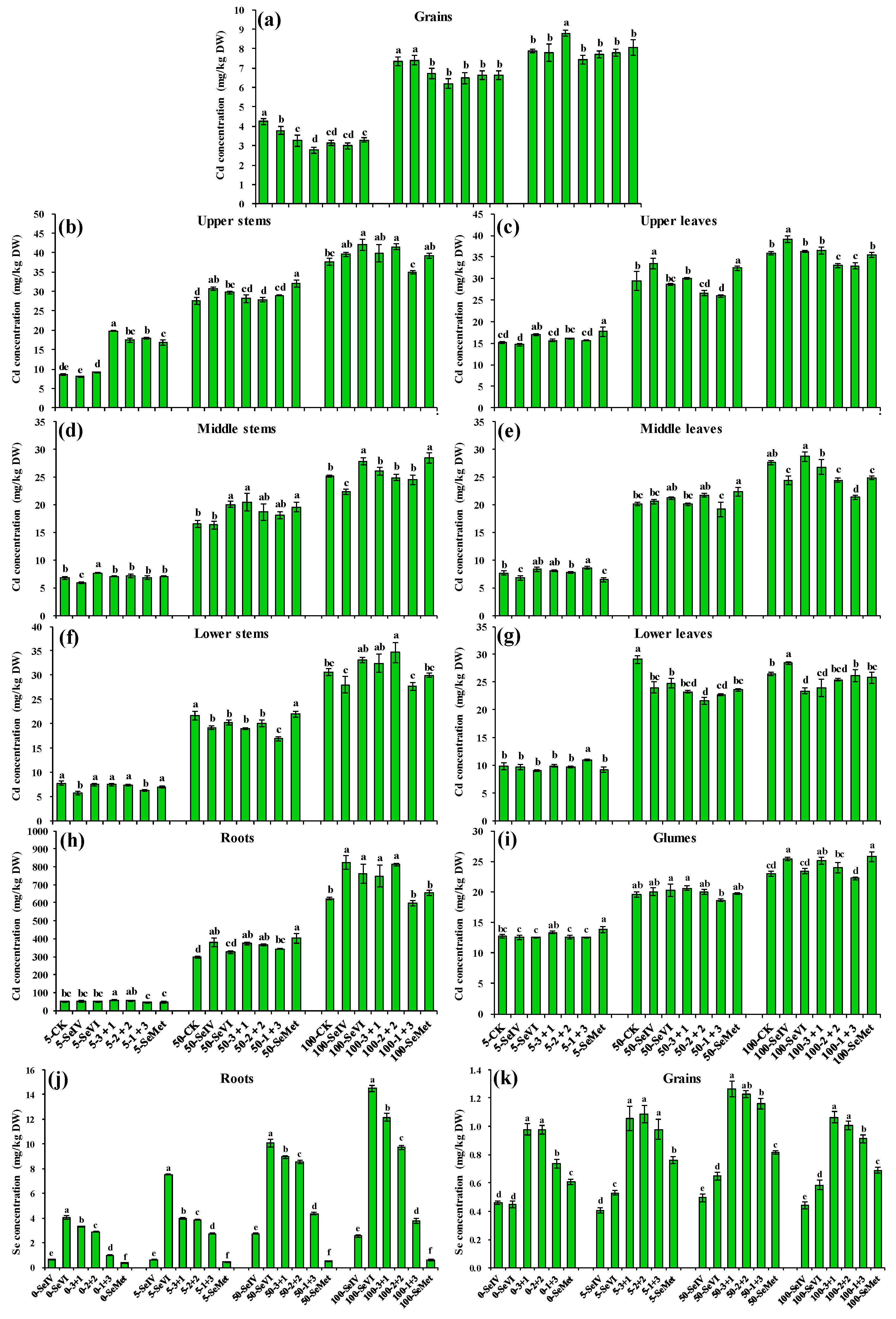
3.4. 3 + 1 Treatment Effectively Reduced Cd Accumulation and Increased Se Content in the Grains
3.5. Se Treatment Reduced the Translocation of Se from Nutrient Organs to the Grains
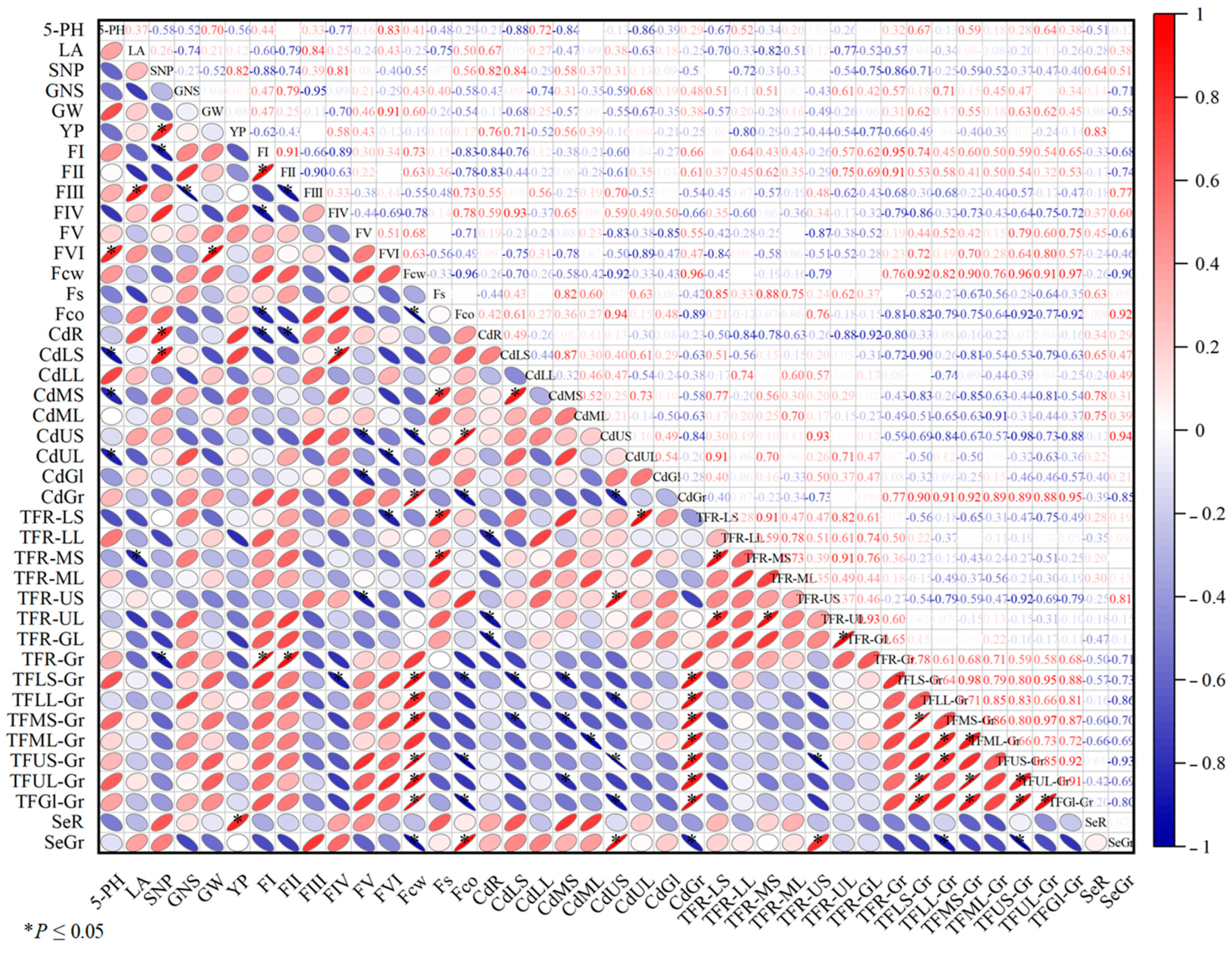
3.6. Trends of Correlation Among Different Indices
4. Discussion
4.1. Se Treatment Relieves the Inhibitory Effect of Cd Stress on Wheat Yield Traits
4.2. Se Treatment Alleviates Cd Toxicity by Reducing the Cd Proportion in the Organelles and Active Forms
4.3. Combined Application of SeVI and SeMet Enhances Cd Retention in Nutrient Organs and Reduces Cd Accumulation in the Grains
4.4. Combined SeVI and SeMet Application Had the Best Effect on Grain Se Biofortification in Wheat
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Cui, X.; Mao, P.; Sun, S.; Huang, R.; Fan, Y.; Li, Y.; Li, Y.; Zhuang, P.; Li, Z. Phytoremediation of cadmium contaminated soils by Amaranthus Hypochondriacus L.: The effects of soil properties highlighting cation exchange capacity. Chemosphere 2021, 283, 131067. [Google Scholar] [CrossRef] [PubMed]
- Barman, F.; Kundu, R. Foliar application of selenium affecting pollen viability, grain chalkiness, and transporter genes in cadmium accumulating rice cultivar: A pot study. Chemosphere 2023, 313, 137538. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Darwish, W.S.; Chiba, H.; Elhelaly, A.E.; Hui, S.-P. Estimation of cadmium content in Egyptian foodstuffs: Health risk assessment, biological responses of human HepG2 cells to food-relevant concentrations of cadmium, and protection trials using rosmarinic and ascorbic acids. Environ. Sci. Pollut. Res. Int. 2019, 26, 15443–15457. [Google Scholar] [CrossRef]
- Kerketta, A.; Kumar, H.; Powell, M.A.; Sahoo, P.K.; Kapoor, H.S.; Mittal, S. Trace element occurrence in vegetable and cereal crops from parts of Asia: A meta-data analysis of crop-wise differences. Curr. Pollut. Rep. 2023, 9, 1–21. [Google Scholar] [CrossRef]
- Shi, G.; Lu, H.; Liu, H.; Lou, L.; Zhang, P.; Song, G.; Zhou, H.; Ma, H. Sulfate application decreases translocation of arsenic and cadmium within wheat (Triticum aestivum L.) plant. Sci. Total Environ. 2020, 713, 136665. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Zhou, H.; Wang, H.; Gao, Y.; Shao, L.; Ding, Q.; Ma, L. Chalcogens reduce grain Cd accumulation by enhancing Cd root efflux and upper organ retention in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2022, 201, 104975. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Huang, Y.; Liu, B.; Liu, Z.; Huang, Y.; Cheng, L.; Huang, Y.; Zhang, C. Foliar application of the sulfhydryl compound 2,3-dimercaptosuccinic acid inhibits cadmium, lead, and arsenic accumulation in rice grains by promoting heavy metal immobilization in flag leaves. Environ. Pollut. 2021, 285, 117355. [Google Scholar] [CrossRef]
- Liu, X.; Yin, Z.; Sheng, H.; Xiao, H.; Zhang, L.; Zhou, P. Response of microbial community, enzyme activity, and physicochemical property in paddy soil to continuous organic fertilizer and lime amendments. J. Plant Nutr. Fertil. 2024, 30, 63–73. (In Chinese) [Google Scholar]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, H.; Gao, M.; Zhou, J.; Zhou, J. Effects of soil amendments, foliar sprayings of silicon and selenium and their combinations on the reduction of cadmium accumulation in rice. Pedosphere 2022, 32, 649–659. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, X.; Ren, T.; Marowa, P.; Meng, C.; Wang, J.; Yang, H.; Li, C.; Zhang, L.; Xu, Z. Heterologous overexpression of Apocynum venetum flavonoids synthetase genes improves Arabidopsis thaliana salt tolerance by activating the IAA and JA biosynthesis pathways. Front. Plant Sci. 2023, 14, 1123856. [Google Scholar] [CrossRef]
- Zhong, W.; Xie, C.; Hu, D.; Pu, S.; Xiong, X.; Ma, J.; Sun, L.; Huang, Z.; Jiang, M.; Li, X. Effect of 24-epibrassinolide on reactive oxygen species and antioxidative defense systems in tall fescue plants under lead stress. Ecotoxicol. Environ. Saf. 2020, 187, 109831. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, J.; Zhou, S.; Wu, X.; Xu, X.; Yang, R.; Yuan, Z. Selenium and toxic metals in human hair of the Dashan Region, China: Concentrations, sources, and antagonism effect. Ecotoxicol. Environ. Saf. 2023, 250, 114479. [Google Scholar] [CrossRef]
- Asgher, M.; Rehaman, A.; Islam, S.N.; Arshad, M.; Khan, N.A. Appraisal of functions and role of selenium in heavy metal stress adaptation in plants. Agriculture 2023, 13, 1083. [Google Scholar] [CrossRef]
- Wang, L.; Wu, K.; Liu, Z.; Li, Z.; Shen, J.; Wu, Z.; Liu, H.; You, L.; Yang, G.; Rensing, C.; et al. Selenite reduced uptake/translocation of cadmium via regulation of assembles and interactions of pectins, hemicelluloses, lignins, callose and Casparian strips in rice roots. J. Hazard. Mater. 2023, 448, 130812. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Kang, Y.; Liu, J.; Wang, Y.; Sun, H.; Ao, T.; Chen, W. Selenium alleviates toxicity in Amaranthus hypochondriacus by modulating the synthesis of thiol compounds and the subcellular distribution of cadmium. Chemosphere 2022, 291 Pt 3, 133108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Selenium Regulated Plant Growth and Cadmium Response in Root of Rape Under Cadmium Stress. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]
- Ding, Y.; Feng, R.; Wang, R.; Guo, J.; Zheng, X. A dual effect of Se on Cd toxicity: Evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 2014, 375, 289–301. [Google Scholar] [CrossRef]
- Qin, X.; Nie, Z.; Liu, H.; Zhao, P.; Qin, S.; Shi, Z. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, S.; Zhuang, J.; Wan, Y.; Wang, Q.; Zhang, J.; Li, H. Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol. Environ. Saf. 2018, 162, 571–580. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Wang, Q.; Qiao, Y.; Li, H. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol. Environ. Saf. 2016, 133, 127–134. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Zheng, J.; Bao, G.; Tao, R.; Wu, C.; Ma, Y.; Ye, W. Effects of basal and foliar selenium fertilizers in reducing cadmium and increasing selenium in selenium enriched, cadmium-polluted rice fields. J. Agro-Environ. Sci. 2024, 43, 974. [Google Scholar]
- Huang, B.; Xin, J.; Dai, H.; Zhou, W. Effects of Interaction between Cadmium (Cd) and Selenium (Se) on Grain Yield and Cd and Se Accumulation in a Hybrid Rice (Oryza sativa) System. J. Agric. Food Chem. 2017, 65, 9537–9546. [Google Scholar] [CrossRef]
- Li, X. Technical solutions for the safe utilization of heavy metal-contaminated farmland in China: A critical review. Land Degrad. Dev. 2019, 30, 1773–1784. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Wan, Y.; Huang, Q.; Li, H. Transcriptome analysis reveals different mechanisms of selenite and selenate regulation of cadmium translocation in Brassica rapa. J. Hazard. Mater. 2023, 452, 131218. [Google Scholar] [CrossRef]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: The relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, P.; Nie, Z.; Shi, H.; Li, C.; Wang, Y.; Qin, S.; Qin, X.; Liu, H. Selenium supply alters the subcellular distribution and chemical forms of cadmium and the expression of transporter genes involved in cadmium uptake and translocation in winter wheat (Triticum aestivum). BMC Plant Biol. 2020, 20, 550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, M.; Tao, Q.; Luo, J.; Li, J.; Guo, X.; Liang, Y.; Yang, X.; Li, T. A comparative study of root cadmium radial transport in seedlings of two wheat (Triticum aestivum L.) genotypes differing in grain cadmium accumulation. Environ. Pollut. 2020, 266 Pt 3, 115235. [Google Scholar] [CrossRef]
- Zhang, G.; Fukami, M.; Sekimoto, H. Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. Field Crops Res. 2002, 77, 93–98. [Google Scholar] [CrossRef]
- Paniz, F.P.; Pedron, T.; Procópio, V.A.; Lange, C.N.; Freire, B.M.; Batista, B.L. Selenium biofortification enhanced grain yield and alleviated the risk of arsenic and cadmium toxicity in rice for human consumption. Toxics 2023, 11, 362. [Google Scholar] [CrossRef]
- Saifullah; Javed, H.; Naeem, A.; Rengel, Z.; Dahlawi, S. Timing of foliar Zn application plays a vital role in minimizing Cd accumulation in wheat. Environ. Sci. Pollut. Res. Int. 2016, 23, 16432–16439. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Wu, S.; Rao, S.; Li, L.; Cheng, S.; Cheng, H. Morphological and physiological indicators and transcriptome analyses reveal the mechanism of selenium multilevel mitigation of cadmium damage in Brassica juncea. Plants 2023, 12, 1583. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Luo, F.; Zhu, D.; Sun, H.; Zou, R.; Duan, W.; Liu, J.; Yan, Y. Wheat Selenium-binding protein TaSBP-A enhances cadmium tolerance by decreasing free Cd(2+) and alleviating the oxidative damage and photosynthesis impairment. Front. Plant Sci. 2023, 14, 1103241. [Google Scholar]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Bukhari, M.A. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem. 2015, 175, 350–357. [Google Scholar] [CrossRef]
- Zhou, X.; Lai, F.; Zhang, C.; Gao, A.; Xu, W. Pathways of selenium to grain relative to form of selenium and variety of rice (Oryza sativa L.). Acta Pedol. Sin. 2017, 54, 1251–1258. (In Chinese) [Google Scholar]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.E.; Wang, Z.H.; Li, F.C.; Li, K.Y.; Yang, N.; Wang, S.; Wang, H.; He, G.; Dai, J. Selenium content of wheat grain and its regulation in different wheat production regions of China. Sci. Agric. Sin. 2016, 49, 1715–1728. [Google Scholar]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at different stages. Field Crops Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. Int. 2020, 27, 717–728. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, L.; Huang, Q.; Li, H.; Wan, Y. Uptake and translocation mechanisms of different forms of organic selenium in rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 970480. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, N.; Hou, Z.; Lou, D.; Ye, J. Effects of different application methods of selenite and selenate on selenium distribution within wheat. J. Plant Nutr. 2018, 41, 2729–2740. [Google Scholar] [CrossRef]
- Ducsay, L.; Ložek, O.; Marček, M.; Varényiová, M.; Hozlár, P.; Lošák, T. Possibility of selenium biofortification of winter wheat grain. Plant Soil Environ. 2016, 62, 379–383. [Google Scholar] [CrossRef]
- Ximénez-Embún, P.; Alonso, I.; Madrid-Albarrán, Y.; Cámara, C. Establishment of selenium uptake and species distribution in lupine, Indian mustard, and sunflower plants. J. Agric. Food Chem. 2004, 52, 832–838. [Google Scholar] [CrossRef]
- Kikkert, J.; Berkelaar, E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef]
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef]
- Cubadda, F.; Aureli, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Acharya, R.; Reddy, R.A.; Prakash, N.T. Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J. Agric. Food Chem. 2010, 58, 2295–2301. [Google Scholar] [CrossRef]


| Abbreviation | Soil Cd Concentration | Se Spraying Concentration (0.2 mM) | |||
|---|---|---|---|---|---|
| SS | JS | HS | FS | ||
| 0-CK | 0 mg/kg | DW | DW | DW | DW |
| 0-SeIV | SeIV | SeIV | SeIV | SeIV | |
| 0-SeVI | SeVI | SeVI | SeVI | SeVI | |
| 0-3 + 1 | SeVI | SeVI | SeVI | SeMet | |
| 0-2 + 2 | SeVI | SeVI | SeMet | SeMet | |
| 0-1 + 3 | SeVI | SeMet | SeMet | SeMet | |
| 0-SeMet | SeMet | SeMet | SeMet | SeMet | |
| 5-CK | 5 mg/kg | DW | DW | DW | DW |
| 5-SeIV | SeIV | SeIV | SeIV | SeIV | |
| 5-SeVI | SeVI | SeVI | SeVI | SeVI | |
| 5-3 + 1 | SeVI | SeVI | SeVI | SeMet | |
| 5-2 + 2 | SeVI | SeVI | SeMet | SeMet | |
| 5-1 + 3 | SeVI | SeMet | SeMet | SeMet | |
| 5-SeMet | SeMet | SeMet | SeMet | SeMet | |
| 50-CK | 50 mg/kg | DW | DW | DW | DW |
| 50-SeIV | SeIV | SeIV | SeIV | SeIV | |
| 50-SeVI | SeVI | SeVI | SeVI | SeVI | |
| 50-3 + 1 | SeVI | SeVI | SeVI | SeMet | |
| 50-2 + 2 | SeVI | SeVI | SeMet | SeMet | |
| 50-1 + 3 | SeVI | SeMet | SeMet | SeMet | |
| 50-SeMet | SeMet | SeMet | SeMet | SeMet | |
| 100-CK | 100 mg/kg | DW | DW | DW | DW |
| 100-SeIV | SeIV | SeIV | SeIV | SeIV | |
| 100-SeVI | SeVI | SeVI | SeVI | SeVI | |
| 100-3 + 1 | SeVI | SeVI | SeVI | SeMet | |
| 100-2 + 2 | SeVI | SeVI | SeMet | SeMet | |
| 100-1 + 3 | SeVI | SeMet | SeMet | SeMet | |
| 100-SeMet | SeMet | SeMet | SeMet | SeMet | |
| Treatment | PH (cm) | LA (cm2) | SNP | GNS | GW (g) | YP (g) |
|---|---|---|---|---|---|---|
| 0-CK | 67.6 ± 1.5 Aa | 27.4 ± 1.1 Aa | 3.6 ± 0.1 Ac | 38.0 ± 1.7 Aab | 40.6 ± 0.9 Ab | 5.6 ± 0.4 Aa |
| 0-SeIV | 65.6 ± 1.7 Aa | 23.3 ± 2.9 Bc | 4.4 ± 0.3 Aa | 33.3 ± 1.2 Bc | 42.3 ± 0.3 Aa | 6.2 ± 0.5 Aa |
| 0-SeVI | 65.2 ± 2.6 Aa | 28.6 ± 1.2 Aa | 4.1 ± 0.1 Aab | 33.3 ± 1.5 Bc | 41.0 ± 0.5 Ab | 5.6 ± 0.4 Ba |
| 0-3 + 1 | 66.4 ± 0.6 ABa | 27.8 ± 0.9 Aa | 3.8 ± 0.1 Bbc | 36.0 ± 1.0 Bbc | 41.4 ±0.4 Ab | 5.7 ± 0.2 Ba |
| 0-2 + 2 | 68.8 ± 1.7 Aa | 24.3 ± 1.5 Bbc | 3.8 ± 0.2 ABbc | 40.3 ± 0.6 Aa | 39.7 ± 0.7 Ac | 6.1 ± 0.2 Aa |
| 0-1 + 3 | 70.0 ± 2.5 Aa | 27.0 ± 0.5 Aab | 4.1 ± 0.1 Aabc | 40.0 ± 2.6 Aa | 36.2 ± 0.4 Be | 5.9 ± 0.3 Aa |
| 0-SeMet | 68.4 ± 3.6 Aa | 26.9 ± 0.1 Aab | 3.7 ± 0.2 Ac | 38.7 ± 0.6 Bab | 37.2 ± 0.3 Ad | 5.3 ± 0.2 Ba |
| 5-CK | 66.2 ± 2.1 Aa | 27.1 ± 2.9 Aa | 3.2 ± 0.2 Bc | 39.3 ± 1.2 Ab | 37.9 ± 0.3 Bbc | 4.7 ± 0.3 Be |
| 5-SeIV | 69.2 ± 1.5 Aa | 27.0 ± 2.3 ABa | 3.5 ± 0.2 Bc | 42.3 ± 1.5 Aab | 40.8 ± 0.7 Ba | 6.1 ± 0.1 Abcd |
| 5-SeVI | 67.2 ± 2.0 Aa | 25.6 ± 0.3 ABa | 4.1 ± 0.1 Aab | 44.0 ± 1.0 Aa | 38.8 ± 0.3 Cb | 6.9 ± 0.2 Aa |
| 5-3 + 1 | 68.0 ± 1.5 Aa | 27.8 ± 1.4 Aa | 4.2 ± 0.1 Aa | 41.0 ± 1.0 Aab | 38.9 ± 0.4 Bb | 6.7 ± 0.2 Aab |
| 5-2 + 2 | 67.7 ± 1.3 Aa | 26.7 ± 0.6 Aa | 4.4 ± 0.4 Aa | 41.3 ± 1.5 Aab | 36.2 ± 1.9 Bd | 6.5 ± 0.4 Aabc |
| 5-1 + 3 | 69.0 ± 1.0 Aa | 26.3 ± 0.5 Aa | 3.6 ± 0.1 Bc | 41.3 ± 2.1 Aab | 39.2 ± 0.4 Ab | 5.8 ± 0.4 Ad |
| 5-SeMet | 67.4 ± 1.5 Aa | 26.2 ± 0.6 Aa | 3.6 ± 0.2 Abc | 43.7 ± 1.2 Aa | 37.0 ± 0.4 Acd | 5.9 ± 0.3 Acd |
| 50-CK | 65.2 ± 2.5 Aa | 23.9 ± 1.9 Aa | 2.8 ± 0.1 Cd | 36.7 ± 2.5 Aab | 34.7 ± 0.2 Ce | 3.5 ± 0.3 Cb |
| 50-SeIV | 69.6 ± 2.1 Aa | 26.6 ± 1.4 ABa | 3.0 ± 0.2 Ccd | 32.7 ± 2.1 Bb | 36.5 ± 0.4 Cc | 3.6 ± 0.1 Bb |
| 50-SeVI | 67.4 ± 3.6 Aa | 24.3 ± 2.4 Ba | 2.9 ± 0.1 Bd | 39.3 ± 4.2 Aab | 40.0 ± 0.5 Ba | 4.5 ± 0.4 Ca |
| 50-3 + 1 | 65.2 ± 1.2 Ba | 23.3 ± 0.6 Ba | 3.2 ± 0.2 Cbcd | 37.3 ± 3.1 ABab | 37.6 ± 0.3 Cb | 4.4 ± 0.4 Ca |
| 50-2 + 2 | 67.8 ± 0.6 Aa | 24.3 ± 0.5 Ba | 3.7 ± 0.2 Ba | 40.3 ± 0.6 Aa | 33.8 ± 0.2 Bf | 5.0 ± 0.2 Ba |
| 50-1 + 3 | 67.4 ± 1.2 Aa | 23.6 ± 0.9 Ba | 3.5 ± 0.2 Bab | 37.3 ± 0.6 Aab | 33.9 ± 0.4 Cf | 4.4 ± 0.2 Ba |
| 50-SeMet | 68.2 ± 0.6 Aa | 26.2 ± 1.4 Aa | 3.3 ± 0.2 Aabc | 38.3 ± 2.5 Bab | 35.6 ± 0.3 Bd | 4.5 ± 0.3 Ca |
| 100-CK | 65.2 ± 2.5 Aa | 24.8 ± 1.9 Ab | 2.3 ± 0.2 Da | 36.3 ± 0.6 Aa | 30.8 ± 0.5 Dc | 2.6 ± 0.2 Db |
| 100-SeIV | 55.6 ± 1.7 Bb | 29.6 ± 0.8 Aa | 2.7 ± 0.2 Ca | 33.3 ± 1.5 Bb | 33.8 ± 0.2 Da | 3.0 ± 0.1 Bab |
| 100-SeVI | 63.2 ± 1.5 Aa | 19.7 ± 2.7 Cc | 2.5 ± 0.2 Ca | 39.0 ± 1.0 Aa | 34.2 ± 0.4 Da | 3.3 ± 0.3 Da |
| 100-3 + 1 | 64.4 ± 1.2 Ba | 19.1 ± 3.0 Cc | 2.4 ± 0.3 Da | 39.7 ± 1.2 ABa | 34.1 ± 0.4 Da | 3.3 ± 0.3 Da |
| 100-2 + 2 | 62.6 ± 2.1 Ba | 19.1 ± 0.9 Cc | 2.4 ± 0.3 Ca | 37.3 ± 1.5 Ba | 34.3 ± 0.3 Ba | 3.1 ± 0.3 Cab |
| 100-1 + 3 | 56.8 ± 2.1 Bb | 17.9 ± 2.4 Cc | 2.6 ± 0.1 Ca | 39.0 ± 1.0 Aa | 31.7 ± 0.3 Db | 3.3 ± 0.1 Ca |
| 100-SeMet | 54.8 ± 1.0 Bb | 20.5 ± 1.9 Bc | 2.4 ± 0.1 Ba | 36.7 ± 1.5 Ba | 31.7 ± 0.3 Cb | 2.8 ± 0.0 Dab |
| Source of variation | ||||||
| Cd | 72.9 ** | 57.5 ** | 304.1 ** | 36.7 ** | 662.4 ** | 1097.5 ** |
| Se | 1.2 | 6.8 ** | 13.5 ** | 9.2 ** | 88.0 ** | 34.4 ** |
| Cd×Se | 6.6 ** | 9.0 ** | 8.2 ** | 4.3 ** | 22.0 ** | 19.3 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Liu, J.; Cheng, T.; Wang, H.; Zhou, Y.; Gong, Z.; Hu, T. Strategic Selenium Application Methods and Timing Enhance Grain Yield, Minimize Cadmium Bioaccumulation, and Optimize Selenium Fortification in Triticum aestivum L. Agronomy 2025, 15, 199. https://doi.org/10.3390/agronomy15010199
Zhang D, Liu J, Cheng T, Wang H, Zhou Y, Gong Z, Hu T. Strategic Selenium Application Methods and Timing Enhance Grain Yield, Minimize Cadmium Bioaccumulation, and Optimize Selenium Fortification in Triticum aestivum L. Agronomy. 2025; 15(1):199. https://doi.org/10.3390/agronomy15010199
Chicago/Turabian StyleZhang, Dazhong, Jiajia Liu, Tingting Cheng, Hongyi Wang, Yongzhen Zhou, Zhengwu Gong, and Tiezhu Hu. 2025. "Strategic Selenium Application Methods and Timing Enhance Grain Yield, Minimize Cadmium Bioaccumulation, and Optimize Selenium Fortification in Triticum aestivum L." Agronomy 15, no. 1: 199. https://doi.org/10.3390/agronomy15010199
APA StyleZhang, D., Liu, J., Cheng, T., Wang, H., Zhou, Y., Gong, Z., & Hu, T. (2025). Strategic Selenium Application Methods and Timing Enhance Grain Yield, Minimize Cadmium Bioaccumulation, and Optimize Selenium Fortification in Triticum aestivum L. Agronomy, 15(1), 199. https://doi.org/10.3390/agronomy15010199




