Rubus idaeus RiACS1 Gene Is Involved in Ethylene Synthesis and Accelerates Fruit Ripening in Solanum lycopersicum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Cloning of RiACS1 Gene
2.3. RiACS1 Bioinformatics Analysis
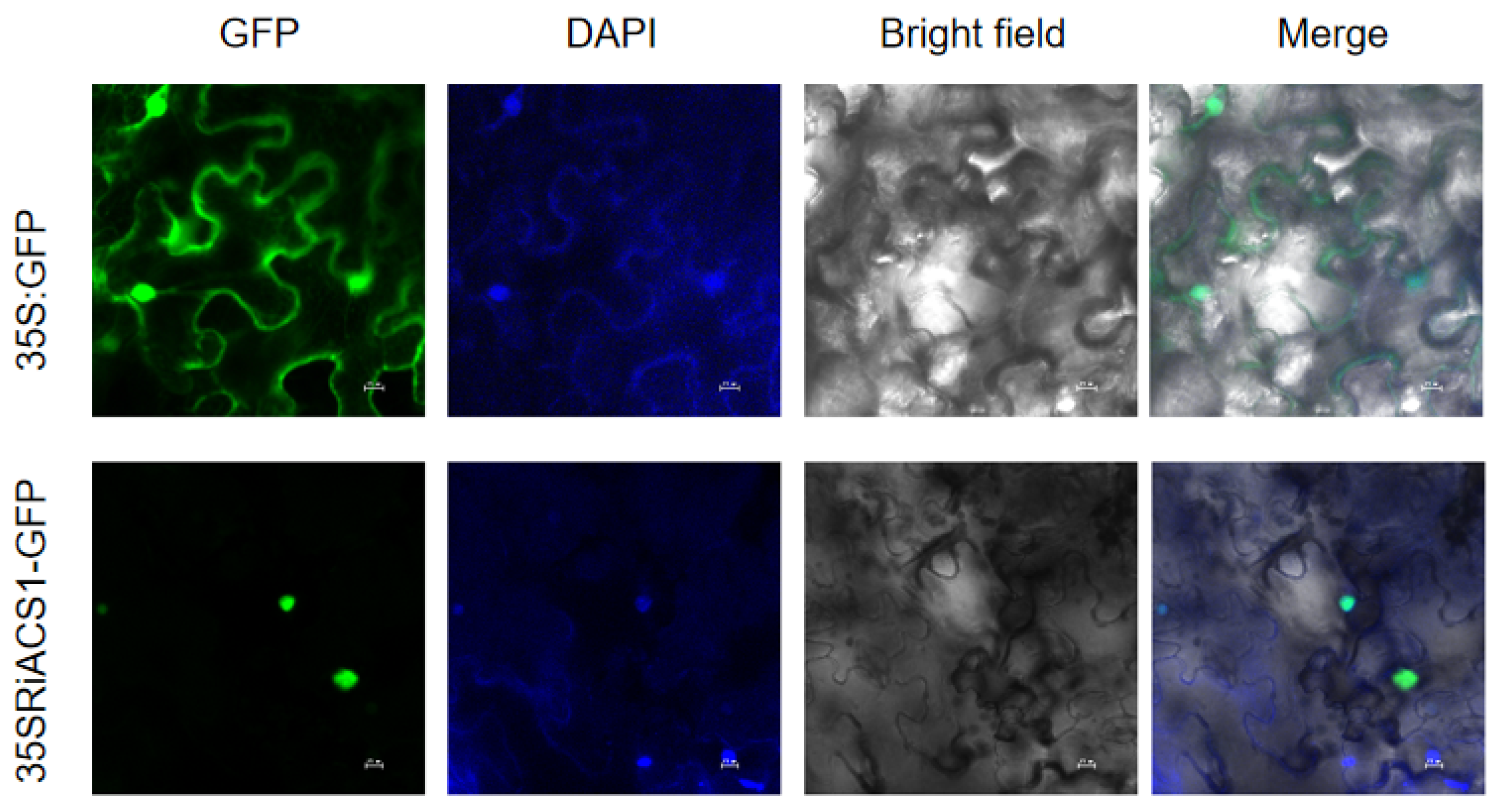
2.4. Subcellular Localization of RiACS1 Protein
2.5. Quantitative Real-Time PCR (qPCR) Analysis of RiACS1
2.6. Genetic Transformation of Tomatoes
2.7. Determination of Physiological Indicators Related to Transgenic Tomatoes
2.8. Expression Analysis of Ethylene Synthesis-Related Genes in Tomato Fruits with Transgenic Tomatoes
2.9. Statistical Analysis
3. Results
3.1. Property and Sequence Analysis of RiACS1 Protein
3.2. Subcellular Localization of RiACS1 Protein
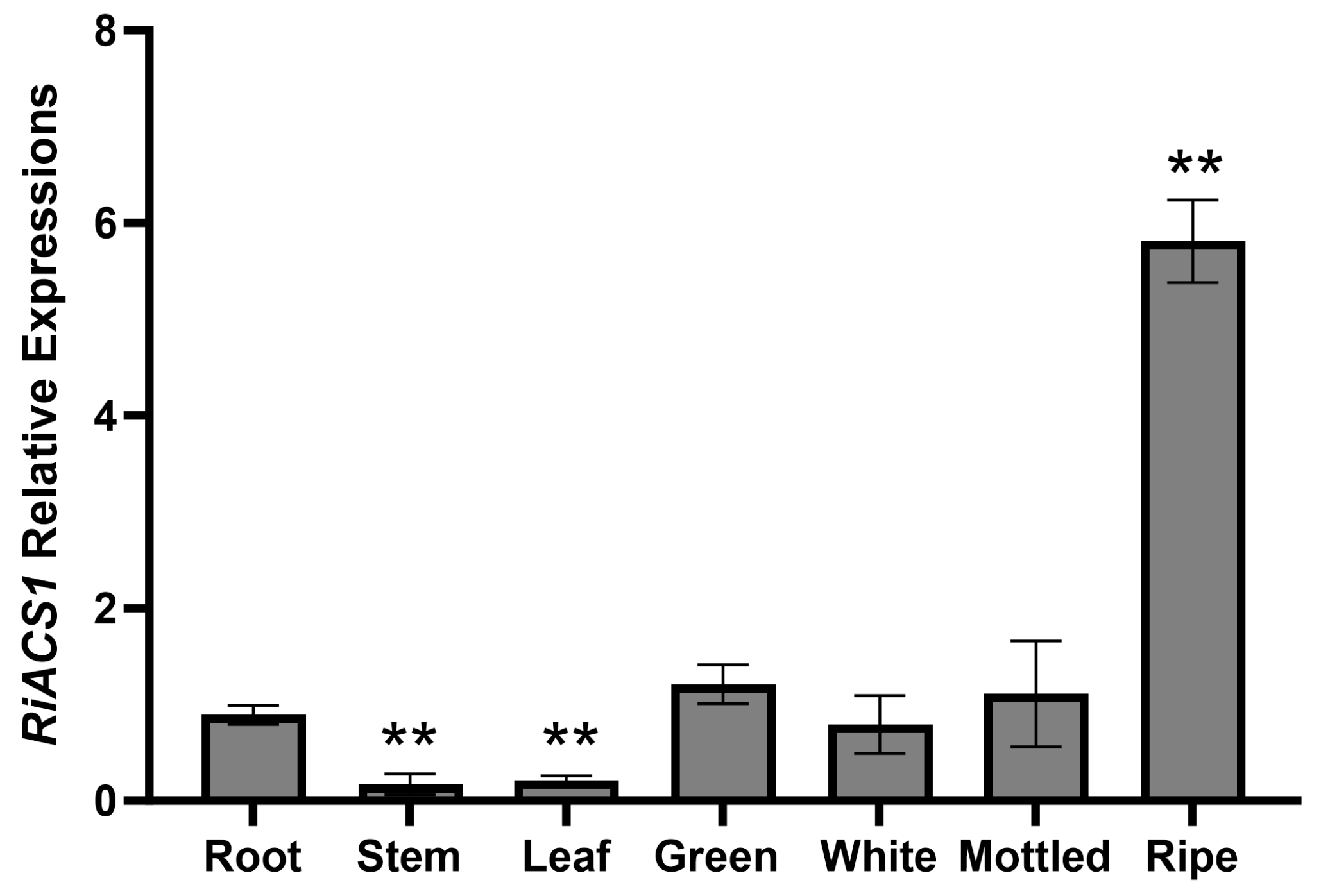
3.3. Expression Analysis of RiACS1 Gene in Different Tissues and Fruit Ripening Stages of Raspberry
3.4. Phenotypic Observation and Identification of Transgenic Tomato Plants
3.5. Overexpression of RiACS1 Promotes Tomato Fruit Ripening
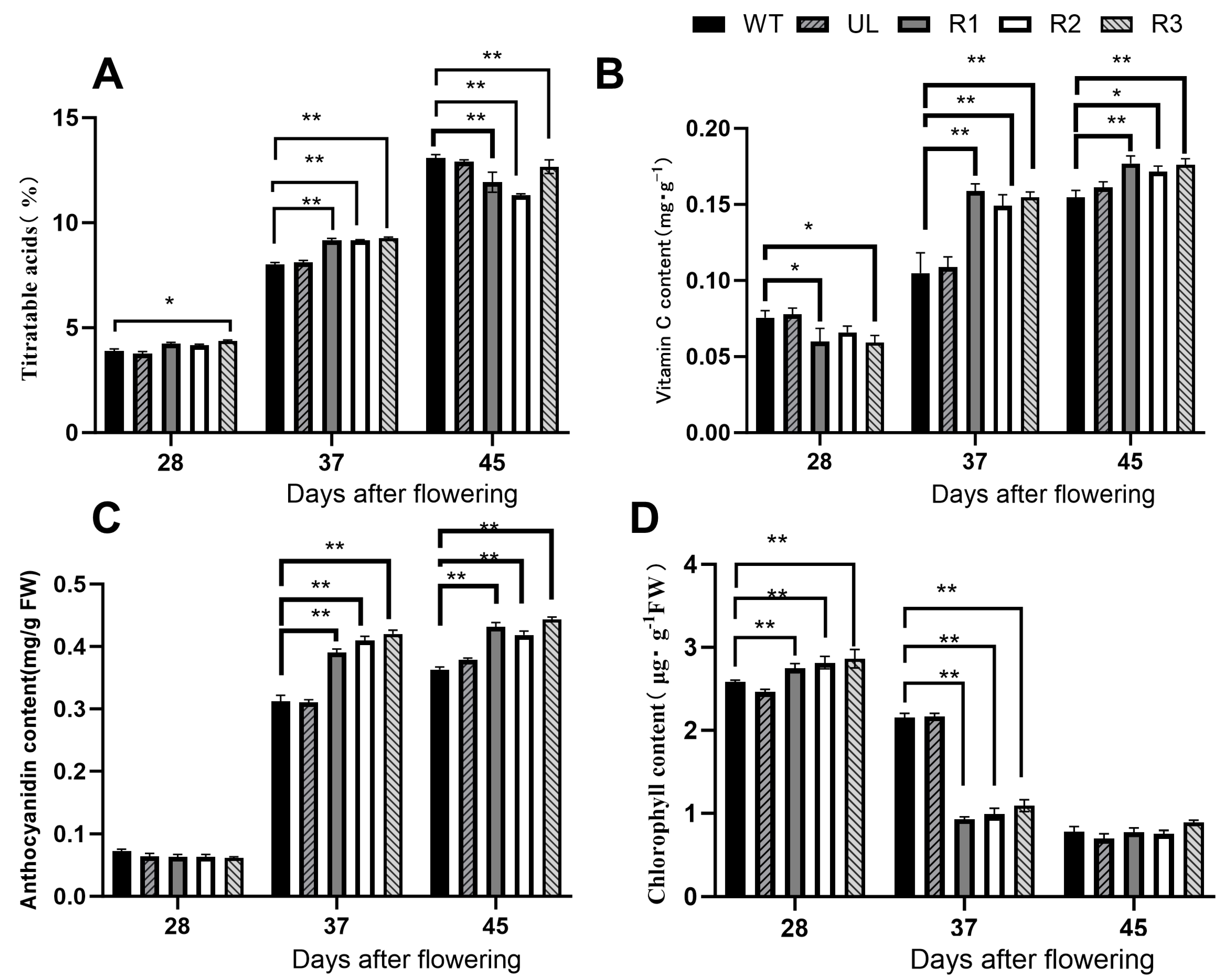
3.6. Material Changes in Tomato Fruits at Different Ripening Stages
3.7. Analysis of Gene Expression Related to Ethylene Synthesis
4. Discussion
4.1. RiACS1 Gene Sequence and Subcellular Localization Analysis
4.2. Relationship Between RiACS1 Gene and Ripening and Softening of Raspberry Fruit
4.3. Overexpression of RiACS1 Gene Promoted Early Flowering and Fruit Softening in Tomato
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kafkaletou, M.; Fasseas, C.; Tsantili, E. Increased firmness and modified cell wall composition by ethylene were reversed by the ethylene inhibitor 1-methylcyclopropene (1-MCP) in the non-climacteric olives harvested at dark green stage—Possible implementation of ethylene for olive quality. J. Plant Physiol. 2019, 238, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Duan, Z.; Li, J.; Jiang, Y.; Cheng, S.; Xue, T.; Zhao, F.; Sheng, W.; Duan, Y. Ultra-low concentration of chlorine dioxide regulates stress-caused premature leaf senescence in tobacco by modulating auxin, ethylene, and chlorophyll biosynthesis. Plant Physiol. Biochem. 2022, 186, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, N.E.; Gatica-Meléndez, C.; Figueroa, C.R. Ethylene application at the immature stage of Fragaria chiloensis fruit represses the anthocyanin biosynthesis with a concomitant accumulation of lignin. Food Chem. 2021, 358, 129913. [Google Scholar] [CrossRef]
- Francini, A.; Ferrante, A. Chapter 14—Ethylene in abiotic stress tolerance in crops. In The Plant Hormone Ethylene; Khan, N.A., Ferrante, A., Munné-Bosch, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 211–220. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Trivellini, A.; Chhillar, H.; Chopra, P.; Ferrante, A.; Khan, N.A.; Ismail, A.M. The significance and functions of ethylene in flooding stress tolerance in plants. Environ. Exp. Bot. 2020, 179, 104188. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, X.; Bürger, M.; Chory, J.; Wang, X. The role of ethylene in plant temperature stress response. Trends Plant Sci. 2023, 28, 808–824. [Google Scholar] [CrossRef]
- Singh, N.; Gaddam, S.R.; Singh, D.; Trivedi, P.K. Regulation of arsenic stress response by ethylene biosynthesis and signaling in Arabidopsis thaliana. Environ. Exp. Bot. 2021, 185, 104408. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Arnold, H.; Dobson, G.; Foito, A.; Austin, C.; Sungurtas, J.; Allwood, J.W.; Stewart, D.; McDougall, G.J. Assessing available phytochemicals from commercial blackcurrant and raspberry pomaces. J. Berry Res. 2022, 12, 415–431. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Chen, H.; Qi, Q.; Ding, Q.; Xue, J.; Ding, J.; Jiang, X.; Hou, X.; Li, Y. Identification and expression analysis of strigolactone biosynthetic and signaling genes reveal strigolactones are involved in fruit development of the woodland strawberry (Fragaria vesca). BMC Plant Biol. 2019, 19, 73. [Google Scholar] [CrossRef]
- Álvarez, F.; Moya, M.; Rivera-Mora, C.; Zúñiga, P.E.; Jara-Cornejo, K.; Muñoz, P.; Ayala-Raso, A.; Munné-Bosch, S.; Figueroa, C.R.; Figueroa, N.E.; et al. Abscisic Acid Synthesis and Signaling during the Ripening of Raspberry (Rubus idaeus ‘Heritage’) Fruit. Plants 2023, 12, 1882. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, C.; Ji, H.; Sun, M.; Liu, T.; Wang, J.; Cao, H.; Zhu, Q. Ethylene biosynthesis and signal transduction during ripening and softening in non-climacteric fruits: An overview. Front. Plant Sci. 2024, 15, 1368692. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yadav, V.; Yan, X.; Cheng, D.; Wei, C.; Zhang, X. Systematic genome-wide analysis of the ethylene-responsive ACS gene family: Contributions to sex form differentiation and development in melon and watermelon. Gene 2021, 805, 145910. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Chen, Y.C.; Kieber, J.J.; Yoon, G.M. Regulation of the turnover of ACC synthases by phytohormones and heterodimerization in Arabidopsis. Plant J. 2017, 91, 491–504. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, Q.; Yin, C.C.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene Biosynthesis, Signaling, and Crosstalk with Other Hormones in Rice. Small Methods 2020, 4, 1900278. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Wang, H.; Mou, Z. Synthesis of pyrrolidinyl-ethylene fluorenyl rare-earth metal complexes and catalysis for 2-vinylpyridine polymerization. J. Rare Earths 2023, 41, 381–387. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.Y.; Yoon, G.M. The regulation of ACC synthase protein turnover: A rapid route for modulating plant development and stress responses. Curr. Opin. Plant Biol. 2021, 63, 102046. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Xu, C.; Hao, B.; Sun, G.; Mei, Y.; Sun, L.; Sun, Y.; Wang, Y.; Zhang, Y.; Zhang, W.; Zhang, M.; et al. Dual activities of ACC synthase: Novel clues regarding the molecular evolution of ACS genes. Sci. Adv. 2021, 7, eabg8752. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, G.; Hou, L.; Wang, W.; Hao, J.; Liu, X. Vitis vinifera VvWRKY13 is an ethylene biosynthesis-related transcription factor. Plant Cell Rep. 2015, 34, 1593–1603. [Google Scholar] [CrossRef]
- Cao, H.; Chen, J.; Yue, M.; Xu, C.; Jian, W.; Liu, Y.; Song, B.; Gao, Y.; Cheng, Y.; Li, Z. Tomato transcriptional repressor MYB70 directly regulates ethylene-dependent fruit ripening. Plant J. 2020, 104, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ge, M.; Yu, A.; Song, W.; Fang, J.; Leng, X. Effects of ethylene on berry ripening and anthocyanin accumulation of ‘Fujiminori’ grape in protected cultivation. J. Sci. Food Agric. 2022, 102, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, K.; BANU, S. Characterizing ethylene pathway genes during the development, ripening, and postharvest response in Citrus reticulata Blanco fruit pulp. Turk. J. Bot. 2019, 43, 173–184. [Google Scholar] [CrossRef]
- Choi, I.; Ahn, C.S.; Lee, D.H.; Baek, S.A.; Jung, J.W.; Kim, J.K.; Lee, H.S.; Pai, H.S. Silencing of the Target of Rapamycin Complex Genes Stimulates Tomato Fruit Ripening. Mol. Cells 2022, 45, 660–672. [Google Scholar] [CrossRef]
- Yuan, H.; Yue, P.; Bu, H.; Han, D.; Wang, A. Genome-wide analysis of ACO and ACS genes in pear (Pyrus ussuriensis). Vitr. Cell. Dev. Biol.-Plant 2020, 56, 193–199. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, A. Recent Advances in Phytohormone Regulation of Apple-Fruit Ripening. Plants 2021, 10, 2061. [Google Scholar] [CrossRef]
- Jia, M.z.; Liu, L.y.; Geng, C.; Jiang, J. Activation of 1-Aminocyclopropane-1-Carboxylic Acid Synthases Sets Stomatal Density and Clustered Ratio on Leaf Epidermis of Arabidopsis in Response to Drought. Front. Plant Sci. 2021, 12, 758785. [Google Scholar] [CrossRef]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Yang, L. Engineering Non-transgenic Gynoecious Cucumber Using an Improved Transformation Protocol and Optimized CRISPR/Cas9 System. Mol. Plant 2017, 10, 1575–1578. [Google Scholar] [CrossRef]
- Chu, L.L.; Yan, Z.; Sheng, X.X.; Liu, H.Q.; Wang, Q.Y.; Zeng, R.F.; Hu, C.G.; Zhang, J.Z. Citrus ACC synthase CiACS4 regulates plant height by inhibiting gibberellin biosynthesis. Plant Physiol. 2023, 192, 1947–1968. [Google Scholar] [CrossRef]
- Fuentes, L.; Monsalve, L.; Morales-Quintana, L.; Valdenegro, M.; Martínez, J.P.; Defilippi, B.G.; González-Agüero, M. Differential expression of ethylene biosynthesis genes in drupelets and receptacle of raspberry (Rubus idaeus). J. Plant Physiol. 2015, 179, 100–105. [Google Scholar] [CrossRef]
- Burdon, J.N.; Sexton, R. Fruit abscission and ethylene production of red raspberry cultivars. Sci. Hortic. 1990, 43, 95–102. [Google Scholar] [CrossRef]
- Iannetta, P.; Van den Berg, J.; Wheatley, R.; McMillan, G.; McNicol, R.; Davies, H. A causal role for ethene in raspberry fruit ripening. Acta Hortic. 1999, 505, 393–399. [Google Scholar] [CrossRef]
- Yang, G.; Chen, Y.; Yu, H.; Zhang, H.; Han, D.; Guo, X.; Yan, E.; Quan, H.; Li, T. Raspberry (Rubus idaeus L.) NCED1 gene enhances high salinity and cold tolerance in Arabidopsis. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 811–819. [Google Scholar] [CrossRef]
- Han, D.; Xu, T.; Han, J.; Liu, W.; Wang, Y.; Li, X.; Sun, X.; Wang, X.; Li, T.; Yang, G. Overexpression of MxWRKY53 increased iron and high salinity stress tolerance in Arabidopsis thaliana. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 266–278. [Google Scholar] [CrossRef]
- Han, D.; Du, M.; Zhou, Z.; Wang, S.; Li, T.; Han, J.; Xu, T.; Yang, G. Overexpression of a Malus baccata NAC Transcription Factor Gene MbNAC25 Increases Cold and Salinity Tolerance in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 1198. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xin, W.; Li, Y.; Wang, A.; Yang, G. An R2R3-MYB Transcription Factor RoMYB10 Regulates Anthocyanin Biosynthesis in Black Raspberry. Agronomy 2023, 13, 1823. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, X.; Cui, C.; He, Y.; Zhang, D.; Zhang, Y.; Li, L. Transcription factor Constans-like 4 may regulate tomato fruit ripening by interacting with abscisic acid and ethylene signaling. Sci. Hortic. 2025, 339, 113865. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Chen, Y.; Li, J.; Yu, C.; Guo, Z.; Yang, G. Overexpression of the Rubus idaeus Polygalacturonases Gene RiPG2 Accelerates Fruit Softening in Solanum lycopersicum. Agronomy 2024, 14, 160. [Google Scholar] [CrossRef]
- Kumari, A.; Ray, K.; Sadhna, S.; Pandey, A.K.; Sreelakshmi, Y.; Sharma, R. Metabolomic homeostasis shifts after callus formation and shoot regeneration in tomato. PLoS ONE 2017, 12, 1–26. [Google Scholar] [CrossRef]
- López-Casado, G.; Sánchez-Raya, C.; Ric-Varas, P.D.; Paniagua, C.; Blanco-Portales, R.; Muñoz-Blanco, J.; Pose, S.; Matas, A.J.; Mercado, J.A. CRISPR/Cas9 editing of the polygalacturonase FaPG1 gene improves strawberry fruit firmness. Hortic. Res. 2023, 10, uhad011. [Google Scholar] [CrossRef]
- Aprianti, S.; Bintoro, N. The effect of concentrations and exposure durations of ethylene gas on the respiration rate of tomato fruit (Solanum lycopersicum). IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012021. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.C.; Choi, D.; Han, J.H.; Jung, Y.; Lee, S. RNA expression, protein activity, and interactions in the ACC synthase gene family in cucumber (Cucumis sativus L.). Hortic. Environ. Biotechnol. 2018, 59, 81–91. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yan, F.; Gao, H.; Xu, Y.Z.; Guo, Y.Y.; Wang, E.J.; Li, Y.H.; Xie, Z.K. Chlorophyll content, leaf gas exchange and growth of oriental lily as affected by shading. Russ. J. Plant Physiol. 2015, 62, 334–339. [Google Scholar] [CrossRef]
- Karppinen, K.; Tegelberg, P.; Häggman, H.; Jaakola, L. Abscisic Acid Regulates Anthocyanin Biosynthesis and Gene Expression Associated With Cell Wall Modification in Ripening Bilberry (Vaccinium myrtillus L.) Fruits. Front. Plant Sci. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Tsegay, Z.T. Total titratable acidity and organic acids of wines produced from cactus pear (Opuntia-ficus-indica) fruit and Lantana camara (L. Camara) fruit blended fermentation process employed response surface optimization. Food Sci. Nutr. 2020, 8, 4449–4462. [Google Scholar] [CrossRef]
- Hebail, F. Determination of Vitamin C Concentration in Various Fresh Orange and Lemon Samples from Janzour Region Using Volumetric Titration. AlQalam J. Med Appl. Sci. 2024, 7, 1214–1218. [Google Scholar] [CrossRef]
- Wu, T.; Liu, H.T.; Zhao, G.P.; Song, J.X.; Wang, X.L.; Yang, C.Q.; Zhai, R.; Wang, Z.G.; Ma, F.W.; Xu, L.F. Jasmonate and Ethylene-Regulated Ethylene Response Factor 22 Promotes Lanolin-Induced Anthocyanin Biosynthesis in ‘Zaosu’ Pear (Pyrus bretschneideri Rehd.) Fruit. Biomolecules 2020, 10, 278. [Google Scholar] [CrossRef]
- Liu, S.; Lei, C.; Zhu, Z.; Li, M.; Chen, Z.; He, W.; Liu, B.; Chen, L.; Li, X.; Xie, Y. Genome-Wide Analysis and Identification of 1-Aminocyclopropane-1-Carboxylate Synthase (ACS) Gene Family in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2023, 24, 11158. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Chen, F.; Cheng, Y.; Huang, X.; Zou, B.; Wang, Y.; Xu, W.; Qu, S. Genome-Wide Identification and Characterization of Members of the ACS Gene Family in Cucurbita maxima and Their Transcriptional Responses to the Specific Treatments. Int. J. Mol. Sci. 2022, 23, 8476. [Google Scholar] [CrossRef]
- Zhang, J.G.; Du, W.; Fan, J.; Yang, X.P.; Chen, Q.L.; Liu, Y.; Hu, H.J.; Luo, Z.R. Genome-Wide Identification of the 1-Aminocyclopropane-1-carboxylic Acid Synthase (ACS) Genes and Their Possible Role in Sand Pear (Pyrus pyrifolia) Fruit Ripening. Horticulturae 2021, 7, 401. [Google Scholar] [CrossRef]
- Eun, H.D.; Ali, S.; Jung, H.; Kim, K.; Kim, W.C. Profiling of ACC synthase gene (ACS11) expression in Arabidopsis induced by abiotic stresses. Appl. Biol. Chem. 2019, 62, 42. [Google Scholar] [CrossRef]
- Chen, K.E.; Liu, Y.F.; Liu, Y.C.; Sulistio, M.; Chen, C.C.; Wu, C.T. Nonclimacteric ripening characteristics of ‘Jen-Ju Bar’ guava conferred by a defect in the expression of the system-2 ACC synthase gene PgACS1. Postharvest Biol. Technol. 2023, 195, 112147. [Google Scholar] [CrossRef]
- Sun, L.; Nasrullah; Ke, F.; Nie, Z.; Xu, J.; Huang, X.; Sun, J.; Wang, P. Genome-wide identification and transcript analysis during fruit ripening of ACS gene family in sweet orange (Citrus sinensis). Sci. Hortic. 2022, 294, 110786. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, R.K.; Rajam, M.V. Delayed ripening and improved fruit processing quality in tomato by RNAi-mediated silencing of three homologs of 1-aminopropane-1-carboxylate synthase gene. J. Plant Physiol. 2013, 170, 987–995. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Liu, X.F.; Fu, B.L.; Zhang, Q.Y.; Tong, Y.; Wang, J.; Wang, W.Q.; Grierson, D.; Yin, X.R. Methyl Jasmonate Enhances Ethylene Synthesis in Kiwifruit by Inducing NAC Genes That Activate ACS1. J. Agric. Food Chem. 2020, 68, 3267–3276. [Google Scholar] [CrossRef]
- Sharma, K.; Gupta, S.; Sarma, S.; Rai, M.; Sreelakshmi, Y.; Sharma, R. Mutations in tomato 1-aminocyclopropane carboxylic acid synthase2 uncover its role in development beside fruit ripening. Plant J. 2021, 106, 95–112. [Google Scholar] [CrossRef]
- Sheng, J.; Li, X.; Zhang, D. Gibberellins, brassinolide, and ethylene signaling were involved in flower differentiation and development in Nelumbo nucifera. Hortic. Plant J. 2022, 8, 243–250. [Google Scholar] [CrossRef]
- Trusov, Y.; Botella, J.R. Silencing of the ACC synthase gene ACACS2 causes delayed flowering in pineapple [Ananas comosus (L.) Merr.]. J. Exp. Bot. 2006, 57, 3953–3960. [Google Scholar] [CrossRef]
- Chu, L.L.; Zheng, W.X.; Liu, H.Q.; Sheng, X.X.; Wang, Q.Y.; Wang, Y.; Hu, C.G.; Zhang, J.Z. ACC SYNTHASE4 inhibits gibberellin biosynthesis and FLOWERING LOCUS T expression during citrus flowering. Plant Physiol. 2024, 195, 479–501. [Google Scholar] [CrossRef]
- Chen, T.; Qin, G.; Tian, S. Regulatory network of fruit ripening: Current understanding and future challenges. New Phytol. 2020, 228, 1219–1226. [Google Scholar] [CrossRef]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different regulatory mechanisms of plant hormones in the ripening of climacteric and non-climacteric fruits: A review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Zhou, J.; Wu, C.E.; Yang, S.; Liu, Y.; Chai, L.; Xue, Z. The interplay between ABA/ethylene and NAC TFs in tomato fruit ripening: A review. Plant Mol. Biol. 2021, 106, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, E.E.; Huberman, M.; Goren, R. Probing the role of endogenous ethylene in the degreening of citrus fruit with ethylene antagonists. Plant Growth Regul. 1993, 12, 325–329. [Google Scholar] [CrossRef]
- Liu, M.Y.; Song, C.Z.; Chi, M.; Wang, T.M.; Zuo, L.L.; Li, X.L.; Zhang, Z.W.; Xi, Z.M. The effects of light and ethylene and their interaction on the regulation of proanthocyanidin and anthocyanin synthesis in the skins of Vitis vinifera berries. Plant Growth Regul. 2016, 79, 377–390. [Google Scholar] [CrossRef]
- Li, S.; Zhu, B.; Pirrello, J.; Xu, C.; Zhang, B.; Bouzayen, M.; Chen, K.; Grierson, D. Roles of RIN and ethylene in tomato fruit ripening and ripening-associated traits. New Phytol. 2020, 226, 460–475. [Google Scholar] [CrossRef]
- Li, C.; Lu, X.; Xu, J.; Liu, Y. Regulation of fruit ripening by MADS-box transcription factors. Sci. Hortic. 2023, 314, 111950. [Google Scholar] [CrossRef]
- Nguyen, T.M.V.; Tran, D.T.; Van de Poel, B.; Hertog, M.L.; Nicolai, B. The impact of growing season on the ethylene biosynthesis and signaling pathways of a heat tolerant tomato during off-vine postharvest ripening. Postharvest Biol. Technol. 2024, 207, 112637. [Google Scholar] [CrossRef]
- Rahman, H.; Ramanathan, V.; Nallathambi, J.; Duraialagaraja, S.; Muthurajan, R. Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol. 2016, 16, 35. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Xin, W.; Zhang, H.; Jiang, J.; Ding, K.; Liu, M.; Li, N.; Yang, G. Rubus idaeus RiACS1 Gene Is Involved in Ethylene Synthesis and Accelerates Fruit Ripening in Solanum lycopersicum. Agronomy 2025, 15, 164. https://doi.org/10.3390/agronomy15010164
Li T, Xin W, Zhang H, Jiang J, Ding K, Liu M, Li N, Yang G. Rubus idaeus RiACS1 Gene Is Involved in Ethylene Synthesis and Accelerates Fruit Ripening in Solanum lycopersicum. Agronomy. 2025; 15(1):164. https://doi.org/10.3390/agronomy15010164
Chicago/Turabian StyleLi, Tiemei, Wenjiao Xin, Hang Zhang, Jiarong Jiang, Kunmiao Ding, Mengyu Liu, Nanyan Li, and Guohui Yang. 2025. "Rubus idaeus RiACS1 Gene Is Involved in Ethylene Synthesis and Accelerates Fruit Ripening in Solanum lycopersicum" Agronomy 15, no. 1: 164. https://doi.org/10.3390/agronomy15010164
APA StyleLi, T., Xin, W., Zhang, H., Jiang, J., Ding, K., Liu, M., Li, N., & Yang, G. (2025). Rubus idaeus RiACS1 Gene Is Involved in Ethylene Synthesis and Accelerates Fruit Ripening in Solanum lycopersicum. Agronomy, 15(1), 164. https://doi.org/10.3390/agronomy15010164







