Abstract
Livestock and poultry waste liquid contains a lot of nitrogen, phosphorus, and microorganisms, and direct discharge causes great harm to the environment. Chicken manure was selected as the research object, and nanoparticle nano-Fe2O3, nano-C60, antibiotics enrofloxacin, sulfamethoxazole, and oxytetracycin were selected as additives to carry out medium-temperature sequential batch anaerobic digestion experiment. The experiment lasted for 55 days. The results showed that (1) gas production reached its peak in the first 1–2 days of a single stress experiment, and the cumulative gas production in the first 10 days was as follows: R5 > R4 > R3 > R2 > CK > R1; (2) the concentrations of total volatile fatty acids (TVFAs) in the groups increased rapidly from day 1 to 10, and the concentrations of TVFAs in the nano-Fe2O3 and nano-C60 groups were higher than those in the other four groups. The pH of the system decreased, and the soluble chemical oxygen demand (SCOD) was consistent with the trend of TVFAs, while the pH of the nanoparticle group was lower; (3) changes in the horizontal structure of bacterial community of Firmicutes and Bacteroidetes were dominant bacteria in each group on the first day. On day 5, the relative abundance of actinomycetes and Bacteroidetes increased significantly. This experiment contributes to the study of the effects of adding nanoparticles and antibiotics to anaerobic digestion substrates on gas production characteristics, provides data support, and characterizes the microbial situation during digestion. This paper can help to realize carbon emission reduction in agriculture and rural areas. Based on the above background, a self-designed system for testing the anaerobic digestion potential of methane was used in this study using chicken manure. Based on the single pollutant stresses of nano-Fe2O3, nano-C60, enrofloxacin, sulfamethoxazole, and hygromycin, the effects of different pollutants under independent stresses on methane production and the changes in the performance of the anaerobic digestion system for gas production were investigated. The chemical parameters and microbial diversity in the anaerobic digestion process were analyzed, and the effects of different nanoparticles and antibiotics on the anaerobic system of chicken manure were elucidated. The results of the study can provide data support for the stable operation of biogas projects, which is of great significance in promoting the sustainable development of ecological agriculture.
1. Introduction
With the major decision and deployment of “Carbon peaking and carbon neutrality”, promoting green and low-carbon development is not only an important measure to achieve the goal of “dual carbon” but also a potential. The central document from 2023 points out that accelerating rural carbon emission reduction and environmental governance is an inevitable demand for promoting rural revitalization and the high-quality development of rural agriculture [1]. China’s livestock breeding industry is large in quantity and scale, but the waste liquid of livestock and poultry waste lacks effective treatment. By the end of 2020, the annual output of livestock and poultry waste liquid reached about 4 billion tons, of which about 40% of livestock and poultry waste liquid has not been effectively disposed of and utilized as resources [2]. Livestock and poultry manure is rich in carbon, nitrogen, phosphorus, organic matter, and microorganisms; if not effectively treated or used for resource utilization, it can easily cause soil, water, and air pollution and bring hidden dangers to human health [3]. Achieving carbon neutrality is an important strategic goal in China. Accelerating the treatment of livestock manure and the utilization of agricultural resources in agricultural and rural development is one of the important ways to achieve the goal of carbon neutrality and realize the two goals of environmental protection and resource conservation.
According to the Second National Census of Pollution Sources, the emissions of pollutants amounted to 21.44 million tons in 2017, of which the main source of this pollution was livestock and poultry excreta, which accounted for 46.67% of the total [4]. Among the methods of livestock and poultry manure treatment in our country, the pile treatment of livestock and poultry manure is very common. Nitrogen, phosphorus, heavy metals, pathogenic microorganisms, antibiotics, etc., from feces during the stacking process enter the soil and groundwater through rainfall and the surface, causing environmental problems and threatening human health [5]. At the same time, livestock manure in the accumulation process will release NH3, H2S, and other malodorous gases; these gases will cause air pollution, so the rapid collection and green treatment of livestock manure is the focus of environmental protection. Antibiotics, as the most important drugs for prevention, are widely used in clinical and daily treatment. In the livestock industry, adding antibiotics to feed can promote animal growth and prevent disease. In 2013, the total usage of antibiotics in China was about nine times the total amount used in the United States. The use of antibiotics in China is also a major reason for the growth of animals [6]. Cultures commonly use tetracycline, sulfonamides, quinolones, macrolides, β-lactam. Bacanlı [7] determined the antibiotic residues in 54 animal feces samples from nine cities in Northeast China and found that tetracycline, sulfonamides, and quinolones all had high residues. The maximum concentration of oxytetracycline in chicken feces was 13.39 mg/kg, the maximum concentration of sulfamethoxazole was 7.11 mg/kg, and the maximum detectable concentration of enrofloxacin was 15.43 mg/kg. Capana et al. investigated the environmental pollution caused by antibiotics in livestock feces; the results showed that the maximum detectable concentrations of tetracycline and oxytetracycline in livestock feces reached 143.97 mg/kg and 47.25 mg/kg, respectively, and the maximum detectable concentrations of most typical sulfonamides reached 18.00 mg/kg. The detection range of oxytetracycline in chicken feces was 3.96~23.43 mg/kg. The highest content of enoxamycin in chicken feces was 1420.76 mg/kg [8,9].
The biogas produced by anaerobic digestion technology is a clean energy source that can help alleviate the problem of energy shortage, and anaerobic digestion is also a pollution-free treatment method for livestock and poultry manure and domestic waste [10]. In anaerobic digestion experiments using livestock manure as a substrate, the level of antibiotics tends to have a strong influence on gas production performance. Because of their special structural characteristics, nanoparticles have unusually excellent properties but also have a unique toxic mechanism [11,12]. Nano-scale materials are usually much smaller than the diameter of most cells, which makes it possible for them to enter the cell, and cells containing nanoparticles will continue to cycle metabolism in the organism, causing damage to the function of the organism [13]. Simultaneously, particle size decreases, resulting in enhanced chemical reactivity that facilitates interactions with other chemical substances. Animal manure contains rich bacterial communities, and the number of easy methane-producing bacteria in the microflora can effectively characterize the gas production effect of the system at this time and can also effectively respond to the developmental stage of the digestion, which can provide help in exploring the stability of the digestive system. Therefore, this experiment set the microbial flora detection among the indicators of the reaction digestion system. Exploring the effect of these nanoparticles and antibiotics on the anaerobic digestion system can help to increase methane production and reduce greenhouse gas emissions, which are very helpful for the treatment of livestock and poultry manure.
Nanoparticles and antibiotics are becoming more and more popular in human society, but the introduction of nanoparticles and antibiotics in anaerobic digestion is still less studied. One of the nanoparticles that has received much attention is the element Fe. The metal Fe is often found in common digestate, which can participate in metabolic activities of methanogenic bacteria, and many studies have focused only on the introduction of different forms or valence states of Fe into anaerobic digestion systems to enhance the metabolic activities of methanogenic bacteria. Therefore, the depth and breadth of research on nanoparticles and antibiotics are not high enough in this field of study [14]. In the family of nanoparticles, nano-C60 is a very popular material, and its unique characteristic structure can also promote the development of microbial communities well. Antibiotics are metabolic substances produced by microorganisms such as bacteria and fungi during their development. Enrofloxacin, a common animal-specific drug, along with sulfamethoxazole and oxytetracycline are commonly found in chicken fecal effluent, where sulfamethoxazole is often difficult to degrade in the soil, and oxytetracycline is an antibiotic that is found in high concentrations in chicken feces. The variation of antibiotic resistance genes in chicken manure is also related to environmental factors and bacterial communities [15]. The independent contaminants chosen for the experiment to better suit the characteristics of chicken manure were nano Fe2O3, nano C60, enrofloxacin, sulfamethoxazole and hygromycin. In summary, in existing studies, nanoparticles tend to be centered around iron and antibiotics tend to be centered around sulfamethoxazole and hygromycin. In the results of the study, the gas production of the stress experiment containing nanoparticles will be more than 30% higher than that of the blank group [16]. This shows that the physicochemical properties of nanoparticles are beneficial for anaerobic digestion experiments. Whereas experiments on the effects of anaerobic digestion containing antibiotics are still few and far between, and a single type of antibiotic is used, the experimental results of this study show that the effect of adding antibiotics on gas production characteristics is not always facilitated. After reading a great deal of literature on the experiments, our research efforts were focused on the anaerobic digestion of livestock manure, exploring the effects of different species of nanoparticles and antibiotics individually. Our experiments were able to analyze the facilitation effects of several more common pollutants and provide data to support the facilitation of the anaerobic digestion of chicken manure. It is hoped that the experimental study of the anaerobic digestion of these three antibiotics and two nanoparticles can contribute to the realization of carbon peaking and carbon neutrality, as well as add to the development of the field afterward.
The use of nanoparticles and antibiotics in large-scale biogas projects promotes microbial activity in the anaerobic digestion process and improves the efficiency of methane production. It also helps to improve the conversion efficiency of biomass energy, thus increasing the economic benefits of energy recovery. Meanwhile, the addition of nanoparticles may reduce the production of hydrogen sulfide in the anaerobic digestion system, mitigating the inhibitory effect of hydrogen sulfide on the system and reducing the risk of ecological contamination. Finally, nanoparticles may reduce the cost of wastewater treatment and sludge treatment by improving the efficiency of anaerobic digestion, especially if there is a need to improve treatment efficiency and reduce sludge production.
Based on the above background, in this study, a methane-producing anaerobic digestion device was designed using chicken manure to investigate the effects of nano-Fe2O3, nano-C60, enrofloxacin, sulfamethoxazole, and oxytetracycline on methane production under the stress of a single pollutant. Data support was provided to investigate the effects of nanoparticles and antibiotics on anaerobic digestion and the stable operation of the biogas plant.
2. Materials and Methods
2.1. Method of Making and Using Experimental Equipment
The physical drawing of the experimental device can be seen from on the left side of Figure 1a,b, and the simplified design drawing is on the right side. The experimental device is composed of two 1 L capacity reaction bottles and a 1 L capacity beaker, which are used as the experimental reaction vessel, the drainage method water storage vessel, and the measuring beaker. Among them, rubber tubes are used to seal the connection, and glass glue is applied to the interface to ensure the air tightness of the experimental device.
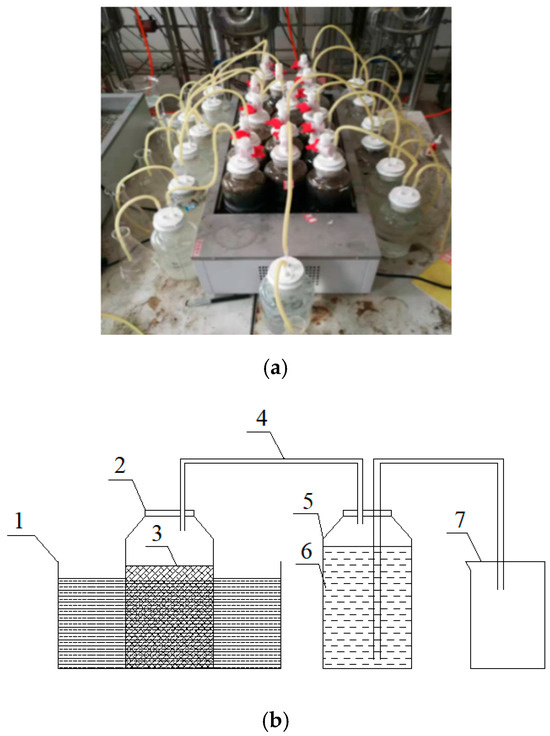
Figure 1.
(a) Physical diagram of the equipment; (b) simplified diagram of the test principle: (1) thermostatic water bath; (2) anaerobic fermentation reaction glass vials; (3) mixture of activated sludge and animal manure; (4) yellow rubber duct biogas; (5) biogas collecting cylinder; (6) distilled water for experiment; (7) beaker with scale.
2.2. Experimental Materials and Reagents
2.2.1. Experimental Material
The chicken manure came from local broiler farms and was tested in advance to confirm that it was antibiotic-free. At the beginning of the experiment, the chicken manure needed to be pre-treated, and the pre-treatment method was to select and remove impurities such as feather eggshells and then store them in the refrigerator at 4 °C.
The microorganisms involved in anaerobic digestion came from the residual sludge, which was obtained from the sewage treatment plant. The residual sludge was acclimated and utilized before the experiment. The method of acclimating sludge was to keep it warm in a 37 °C constant temperature water bath, during which a small amount of material was added for acclimation to maintain the activity of microorganisms. The raw materials (chicken manure, inoculation sludge) are detailed in Table 1. TS is the total solids content, and VS is the volatile solids content.

Table 1.
Water content and elemental properties of chicken manure feedstock and inoculated sludge.
2.2.2. Experimental Reagent
The chemical formula for enrofloxacin (98%, CAS: 93106-60-6) is C19H22FN3O3, the relative molecular mass is 359.39, and purity ≥ 98%. Sulfamethoxazole (98%, CAS: 723-46-6), whose chemical formula is C10H11N3O3S, has a relative molecular mass of 253.28. Oxytetracycin (97%, CAS: 6153-64-6), whose chemical formula C22H24N2O9·2H2O, has a relative molecular mass of 496.46 and was stored in the refrigerator at 4 °C before use.
Preparation of nano-Fe2O3 suspension: nano-Fe2O3 (90 nm) was purchased from Shanghai Maclin Biochemical Technology Co., Ltd. (Shanghai, China), with a purity of 99.8%.
Preparation of nano-C60 suspension: Fullerene C60 was purchased from Shanghai McLean Biochemical Technology Co., Ltd. with a purity of 99.9%. The antibiotics were purchased from Shanghai Aladdin Company.
2.3. Experimental Scheme
In this anaerobic digestion experiment, six experimental groups were set up, and for each experimental group, 120 g of chicken manure and 300 g of activated sludge were pre-placed for fermentation. The maximum residual concentrations of enrofloxacin (ENR), sulfamethoxazole (SMX), and oxytetracycin (OTC) in livestock waste liquid were 15.43, 18.00, and 47.25 mg/kg·TS, respectively, which were approximately 16, 20, and 50 mg/kg·TS. In the R4 and R5 experiments, nano-Fe2O3 of 300 mg/kg·TS and nano-C60 of 100 mg/kg·TS were selected to be added. Table 2 records the six proportioning schemes designed in the experiment. After adding the material and thoroughly mixing, we purged the bottle with nitrogen for at least 2 min to ensure that the bottle was in an anaerobic environment, and then we sealed it. All experimental reaction bottles were placed in a constant temperature water bath of 37 ± 0.5 °C and stored away from light. Each group was set up with 4 parallel experiments; 1of the experimental groups was used only to replace the missing material sampled by the other experimental groups, and the final result was taken as the average of 3 groups. During the experiment, the solution was manually stirred twice a day for 1 min each time.

Table 2.
Ingredient settings for each group.
2.4. Monitoring Method
2.4.1. Physical and Chemical Property Analysis
The test indexes of this study included volatile fatty acids (VFAs) and pH values, and the test objects were daily digestive fluid supernatants. During the test, an appropriate amount of digestive fluid was taken as a sample, centrifuged with a high-speed centrifuge at a speed of 10,000 r/min for 10 min, and filtered with a filter membrane with a pore size of 0.45 μm.
The specific detection methods are as follows:
- VFAs were determined by gas chromatography. The digestive supernatant sample was filtered with a 0.22 μm filter membrane, and a 1 mL sample was mixed with 100 μL formic acid and acidified to pH < 2.Gas production was measured at a fixed time every day. Test method: Connect one side of the reaction bottle to a wide-mouth bottle filled with water, and determine the daily gas volume by displacement.The composition of biogas was determined by gas chromatography. Column: TDX-01, 2 m × 3 m stainless-steel-filled column.
- The pH meter was calibrated using a special calibration solution to determine the pH value.
2.4.2. Determination of Enrofloxacin Antibiotic
Five grams of samples were placed in a centrifuge tube, to which 20 mL of 0.1 mol/L EDTA-Mellvaine buffer solution was added. The mixture was vortexed and homogenized for one minute, subjected to ultrasonic treatment for ten minutes, and then centrifuged at 4 °C at 9000 r/min for five minutes. The supernatant was subsequently collected for further analysis.
The HLB column (200 mg, 6 mL) was used for solid phase extraction. The HLB column was activated with 6 mL methanol and ultra-pure water, respectively. Then, all the sample supernatant was passed through the HLB column at a rate of about 1 mL/min, washed with 6 mL ultra-pure water, the eluent was discarded, and the supernatant was pumped for drying for 10 min [17]. Then, 6 mL methanol was eluted at 1.0~3.0 mL/min, and the eluent was gathered. The residue was blown dry with nitrogen at 50 °C, dissolved with 1 mL 0.1% formic acid water, filtered by 0.22 μm organic filter membrane, and then placed in LC-MS for detection.
The chromatography was performed on a 1.8 μm Plus C18 column with a size of 2.1 × 50 mm and a temperature of 40 °C. The sample size was 1.0 μL. Mobile phase A comprised 0.1% formic acid in water, while phase B consisted of acetonitrile. Gradient elution was employed, utilizing electrospray ionization in positive mode (ES+) with a capillary voltage set at 4 kV, and the detection method utilized multiple reaction monitoring (MRM) [18].
2.4.3. Determination of Antibiotic Sulfamethoxazole
Accurately weigh 5 g of sample, add 20 ml of acetonitrile, shake the slurry evenly, rotate, and ultrasonic for 10 min. Add 5 g of anhydrous sodium sulfate, swirl it (without caking), and centrifuge it for 10 min under 10,000 r/min centrifuge. Transfer the liquid supernatant to the bottle, add 7 mL isopropyl alcohol, apply ultrasonic for 1 min, steam it at 45 °C to about 5 mL, and repeat the above operations. The chicken core bottle was washed 3 times with 15 mL 0.1mol/L HCl and transferred to a 50 mL centrifuge tube, then washed with 5 mL n-hexane and transferred to the centrifuge tube, vortexed for 2 min, centrifuged for 5 min at 10,000 r/min, and discarded and purified by the n-hexane layer.
The MCX column (60 mg, 3 mL), methanol column (3 mL), and 0.1 mol/L HCl activation column (3 mL) were used for solid phase extraction, and 8 ml of samples was accurately determined. It was washed with 3 mL 0.1 mol/L hydrochloric acid and 3 ml 50% methanol water, and then it was drained and eluted with 5 mL 5% ammonia water methanol. After nitrogen blowing at 50 °C, the samples were dissolved in a mixture of 0.2% formic acid in water and methanol (1:1). The solution was then filtered through a 0.22 μm or larger organic filter membrane before being analyzed using a bottling machine.
2.4.4. Determination of Antibiotic Oxytetracycline
Weigh 2 g sample into 50 mL centrifuge tube, add 0.1 mol/L EDTA-Mellvaine buffer 10 mL, homogenize it for 1 min, provide ultrasound in a cold bath for 10 min, and centrifuge it at 4 °C at 10,000 r/min for 5 min. Repeat the above steps, combine the supernatant twice, add 15 mL water to saturate the n-hexane vortex, centrifuge it at 4 °C at 10,000 r/min for 5 min, and then set it aside.
Solid phase extraction was determined on an HLB column (3 mL, 60 mg) with a bottling machine.
2.4.5. Microorganism16S rRNA Sequencing
The anaerobic digestive fluid was filtered through a 0.22 μm membrane, and the membrane was collected and stored at −20 °C for testing. The high-throughput sequencing of 16S rRNA was completed by Shanghai Meiji Biomedical Technology Co., Ltd. (Shanghai, China). The samples were extracted using the FastDNA® Spin Kit for Soil extraction kit according to the instructions for DNA extraction. The bacterial 16S rRNA gene V3-V4 region was amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), while the archaeal 16S rRNA gene V4-V5 region was amplified using primers 524F10extF (5′-TGYCAGCCGCCGCGGTAA-3′) and Arch958RmodR (5′-YCCGGCGTTGAVTCCAATT-3′).
2.4.6. Data Analysis
The gas and liquid parameters were measured three times for each experimental reaction device. SPSS 18.0 version of single-factor analysis of variance and multiple comparisons were used to conduct correlation analysis of the data, and the Least Significant Difference method was used to conduct a mean comparison to evaluate the data level. Finally, Origin 8.0 image-making software was used to visualize the data and generate graphics.
3. Results
3.1. Impacts of Nanoparticles and Antibiotics on the Anaerobic Digestion of Livestock and Poultry Waste Liquid Gas Production
3.1.1. Impacts of Nanoparticles and Antibiotics on the Anaerobic Gas Production Characteristics of Livestock Waste Liquid
Figure 2 shows the data of biogas content per day for the anaerobic digestion of two nanoparticles and three antibiotics, and Figure 3 shows the data of the cumulative biogas content in the entire system for the anaerobic digestion of two nanoparticles and three antibiotics. During the 55 days of anaerobic fermentation, basically, two peaks of gas production occurred in all six experimental groups, with the first one clustered in the first 10 days and the second one clustered in the 30th–40th day. R1–R3 without adding nanoparticles reached its peak on days 1, 2, and 2, with peak values of 244 ± 13, 264 ± 23, and 390 ± 16 mL, respectively. CK in the blank group showed a time lag in the first peak gas production, reaching 249 ± 22 mL on the 5th day, while the R4 group in the nanoparticle addition group reached the peak gas production on the 1st day, reaching 345 ± 11 mL, and for the R5 group, reaching its maximum output on the second day, it was 423 ± 11 mL. The cumulative gas production at this stage is 2968 (R5) > 2490 (R4) > 1445 (R3) > 1350 (R2) > 1216 (CK) > 1203 (R1) mL; thus, nanoparticles like nano-Fe2O3 and nano-C60 can be obtained, which can promote the biogas production from anaerobic digestion 10 days before the anaerobic digestion reaction. The cumulative biogas production for groups R4 and R5 increased by 81.09% and 115.8%, respectively, while the gas production levels of the other three antibiotic groups were comparable to that of the control (CK) group. On the 11th day, gas production in each group gradually ceased, resulting in a pH value decline to 5.3 and the subsequent inhibition of methanogenic bacterial activity. Following a week of observation, no self-recovery trend was observed within the system; thus, from the 21st to the 23rd day, NaOH was continuously added to adjust the pH to be above 6.5. Gas production in all groups began to recover progressively after the 25th day. The R1 group supplemented with 16 mg ENR/kg·TS achieved peak gas production once again at 310 mL on the 33rd day, while R2 supplemented with 20 mg SMX/kg·TS reached its peak gas production of 185 mL on the 40th day. Additionally, an addition of 50 mg OTC/kg·TS resulted in peak gas production within a span of just 33 days, yielding a daily output of approximately 410 mL. The addition of nanoparticle groups R4 and R5 reached peak gas production for the second time on the 33rd and 37th days, which were 148 ± 12 and 132 ± 4 mL, respectively. The maximum daily gas production at the second peak was lower than that of the other four groups. The total cumulative gas productions for each group were as follows: 3712 (CK), 3968 (R1), 4459 (R2), 4344 (R3), 4498 (R4), and 4639 (R5) mL, respectively. Three kinds of antibiotics in the experimental concentration can promote anaerobic digestion, and SMX and OTC have more obvious stimulating effects. Batch experiments demonstrated that the concentrations of oxytetracycline at both 5 mg/L and 10 mg/L could enhance methane production from chicken manure by approximately 3.38% and 7.11%, respectively; furthermore, the inhibition threshold was determined to be around 24.32 mg/L [19].
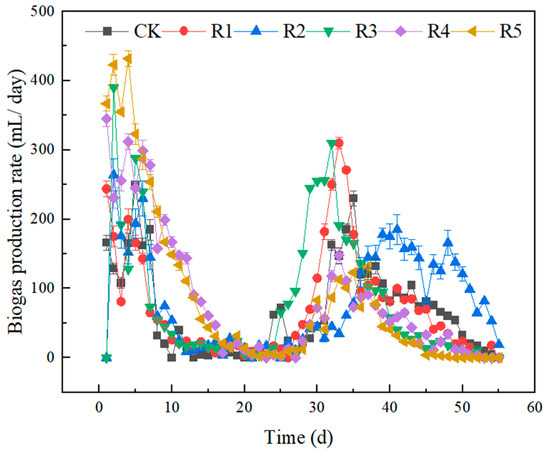
Figure 2.
Variations in daily gas production rates during the anaerobic digestion of livestock waste liquid influenced by nanoparticles and antibiotics.
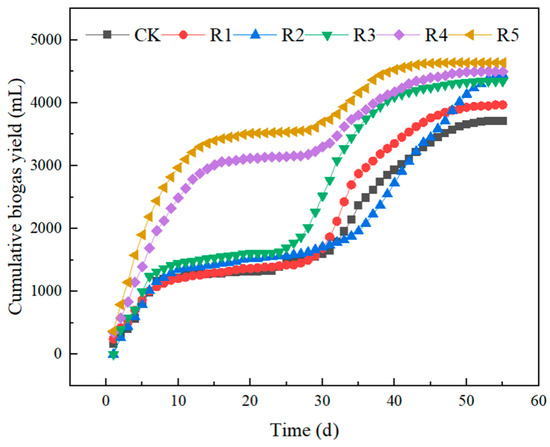
Figure 3.
Variations in accumulated biogas production during the anaerobic digestion of livestock waste liquid influenced by nanoparticles and antibiotics.
Research has found that 500 mg/kg norfloxacin could inhibit the methane production rate, but no inhibition occurred at 10 mg/kg concentration, and the overall effect of biogas production was better than that of control group [20]. Antibiotics also have a positive effect on anaerobic digestive reactions. Research also found that tunicamycin ≤ 20 mg/L temporarily inhibited early gas production, and the bacteria were more active in the later stages, and with the increase in tunicamycin concentration, the concentration of methane also increased by 0.54~9.58%, respectively [21]. According to research, cephalexin at a concentration of 0–2000 mg/L had no obvious implication on the anaerobic digestion gas production of surplus sludge over a 157-day experiment. The inhibitory effect of antibiotics was observed only for the first 25 days, after which the 600 mg/L and 1000 mg/L groups recovered and were stimulated. This resulted in a total gas production increase of 30.3% and 63.8%, respectively [22]. Two scholars have found that extracellular polymeric substances (EPSs) in activated sludge can delay the diffusion of antibiotics in the cell through adsorption, and antibiotics stimulate the secretion of EPS, which plays a protective role in the growth and reproduction of microorganisms. Furthermore, through the OTC series of intermittent anaerobic digestion experiments with a cycle of 180 h, research shows that 5 mg/L of oxyethoxycycline has little negative effect on the system during anaerobic digestion. On the contrary, 50 mg/L ethoxycycline has the effect of inhibiting methane production, and methane production is reduced by 23.75% [23].
The addition of nano-Fe2O3 and nano-C60 can increase the experimental gas production, and the rate of biogas production increased significantly in the first 10 days of the experiment. The total cumulative gas production during the experimental period was also higher than that of other experimental groups. The unique structural traits of nano-Fe2O3 and nano-C60 nanoparticles endow them with exceptional properties that distinguish them from other conventional materials. The ultra-small size of these nanoparticles gives rise to surface effects. Consequently, these nanoparticles exhibit high chemical reactivity and can readily interact with other chemical substances. Therefore, as a medium to promote microbial hydrolysis substrates to produce gas effects, the dissolution of metal ions from nanoparticles such as nano-Fe2O3 and nano-C60 is considered to be one of the most common and major ways to affect sludge anaerobic digestion. Fe3+ is a vital trace element for the growth and metabolic activities of anaerobic microorganisms, providing beneficial effects when present in moderation. Research indicates that at low concentrations of nano-Fe2O3, the released Fe3+ can form chelates with negatively charged residues found in extracellular polymeric substances, thereby mitigating ionic toxicity [24].
3.1.2. Analysis of Changes in pH, TVFAs, and SCOD Concentrations of Livestock Waste Liquid During Anaerobic Digestion by Nanoparticles and Antibiotics
Figure 4, Figure 5, and Figure 6, respectively, show the changes of pH, TVFAs, and SCOD concentrations in the experimental group. The SCOD value can reflect the degradation of organic matter in wastewater treatment, and the higher value indicates the more serious pollution of organic matter. Volatile fatty acids (VFAs) are important intermediate products of the anaerobic digestion process, methanogens mainly utilize VFAs to form methane, and a higher content of VFAs indicates that the work of methanogens is inhibited. VFAs are produced by hydrolytic-acid-producing bacteria during anaerobic digestion, and the VFAs are dynamically changed in the anaerobic digestive system. From the first day to the tenth day of the experiment, the TVFA content of each group accumulated rapidly, and the total concentration showed an increasing trend. Notably, the concentrations of volatile fatty acids were significantly higher in groups R4 and R5 compared to the other four groups. This clearly indicates that the incorporation of nano-Fe2O3 and nano-C60 enhances the hydrolysis process of anaerobic digestion. Its ultrastructure served as a medium to promote decomposition by the hydrolysis and acidification of microorganisms, resulting in higher concentrations of TVFA in the first 10 days than in the other groups. At this stage, with the decomposition of organic acids, the pH of the system decreased, and the trend of SCOD was consistent with that of VFAs. Subsequently, the TVFA concentration in each group continued to increase and reached a peak value of 10,269.25 (CK), 10,454.16 (R1), 9975.94 (R2), 10,568.93 (R3), 10,345 (R4) and 10,987 (R5) mg/L on the 19th day. The SCODs of the same stage were 13,980 ± 57.1 (CK), 13,610 ± 16.1 (R1), 12,770 ± 34.1 (R2), 15,480 ± 66.1 (R3), 14,685 ± 38.1 (R4) and 15,393 ± 66.9 (R5) mg/L, respectively. On the 19th day, the pH of each group dropped to 5.30~5.37. At this time, gas production stopped, indicating that acid inhibition had destroyed the balance of material conversion, In the acidification stage of anaerobic digestion, the acidifying bacteria had a certain range of acid tolerance; when the pH is too low, it will reduce methane production and hydrogen consumption, which in turn affects the anaerobic digestion process. In the first 10 days of the experiment, the pH of each group gradually decreased, and gas production stopped after the observation for a week that the pH fell to about 5. At this time, the overly acidified system had signs of collapse and no longer produced methane, and after adjusting the pH, the alkalized environment was conducive to the work of the acidifying bacteria. The pH of nano-Fe2O3 and nano-C60 groups is lower, by 5.08 ± 0.3 and 5.01 ± 0.1, respectively, which is consistent with the TVFAs research. In the early stage of the experiment, the VFAs had been accumulating and the SCOD values were also at a high level, and both of them had a decreasing trend after the artificial pH adjustment around the 20th day, at which time the gas production of each reaction group began to increase, indicating that the efficiency of the methanogens began to recover. The gas production of each group also reached the maximum value around the 30th day. From day 34 to 37, the TVFAs of the experimental group increased rapidly again, which reflected that the hydrolysis rate of the difficult-to-break-down substances in the digestive solution was higher than the utilization rate of methanogens. On the 37th day, the density of hydrolyzed-acid-producing bacteria gradually decreased with the decrease in the substrate, and then the concentration of TVFAs also gradually decreased. On the 25th to 34th day, the concentration of TVFAs in R3 was always at a low level, but the biogas yield did not match the concentrations of TVFAs and SCOD, the low content of VFAs in the digestive system was not caused by the utilization of methanogens, and enrofloxacin may have had a certain negative effect on the hydrolysis of acid-producing bacteria. Studies have shown that antibiotic inhibition reduces methane production without causing acetic acid accumulation, and its mechanism is similar to the non-competitive inhibition of enzymes; that is, antibiotic-binding enzyme–substrate complexes inactivate the enzyme site, resulting in the obstruction of acetic acid methanogenesis.
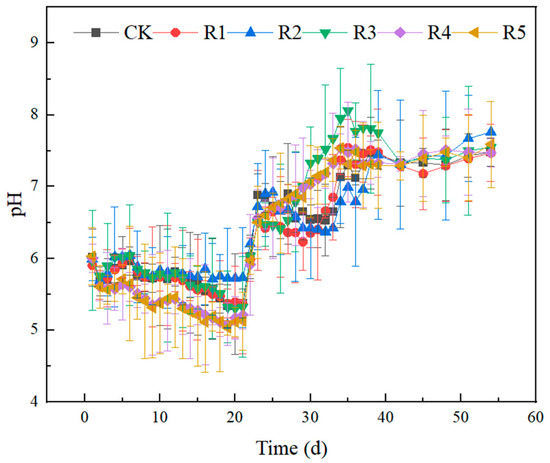
Figure 4.
Change in pH concentration in the anaerobic process of livestock waste liquid treated by nanoparticles and antibiotics.
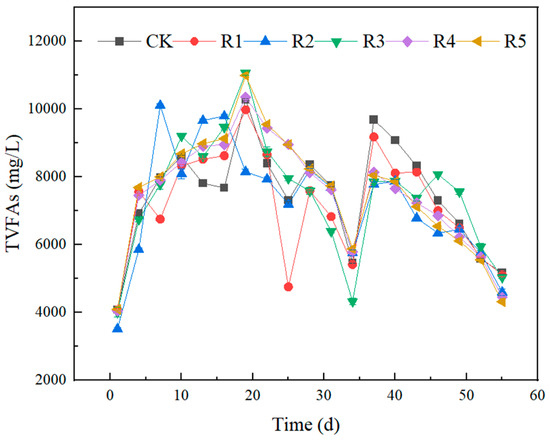
Figure 5.
Effects of nanoparticles and antibiotics on TVFAs during anaerobic digestion.
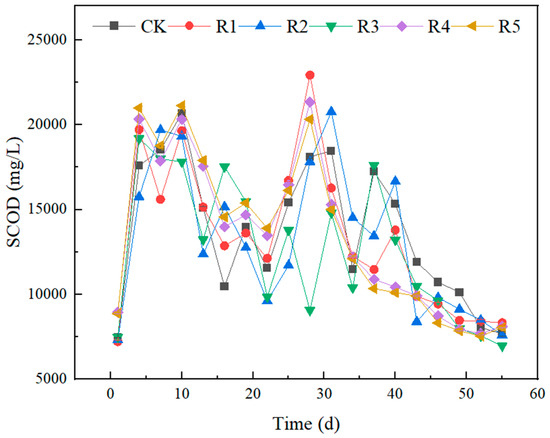
Figure 6.
Variation of SCOD in anaerobic digestion of livestock waste liquid by nanoparticles and antibiotics.
3.2. Effects of Nanoparticles and Antibiotics on Microbial Flora in the Anaerobic Digestion of Livestock Waste Liquid
Figure 7 shows the relative abundance of bacteria in each group during anaerobic digestion as affected by nanoparticles and antibiotics. From the figure, it can be seen that the bacterial community structure of these six groups at each stage of the anaerobic digestion system had similarities at the phylum level. The community of microorganisms during anaerobic digestion is related to the response of the digestive system, and the phylum of Thick-walled Bacteria, Bacteroidetes, and Ascomycetes tend to help convert macromolecular organics into simple organics during the process of hydrolytic acidification. They can metabolize a variety of substrates and can help the hydrolysis of macromolecules and the production of organic acids. Firmicutes can produce a large number of proteases, cellulases, lipases, and other extracellular enzymes. In the degradation of carbohydrates and proteins, the Bacteroides group can convert them into acetic acid and NH3. Proteobacteria possess the capability to reduce sulfate to H2S. Furthermore, Spirochaetota, Synergistota, and Chloroflexi possess the capability to decompose organic acids generated by hydrolytically acidifying bacteria into acetic acid and hydrogen gas (H2). On the initial day of observation, Firmicutes and Bacteroidota emerged as the predominant bacterial groups in each category, with relative abundance levels ranging from 46.56% to 66.18% and 29.32% to 49.52%, respectively. The results of the R4 and R5 bacteria groups with the addition of nanoparticles showed no significant change. On the 5th day of digestion, actinomycetes and Bacteroidetes in digestive fluid increased significantly, which were 6.11–21.75% and 35.20–54.49%, respectively. The number of Bacteroidota in the nanoparticle group was slightly higher than R2. This is mainly due to the presence of nanoparticles as the medium to promote the growth of Bacteroidota. From the 15th to the 50th day, Firmicutes became more competitive with the reaction, while Bacteroidota’s relative abundance decreased continuously, which may have been caused by the reduction in nutrients. However, the number of Bacteroidota in the groups with R4 and R5 nanoparticles added should be higher than that in the other groups. On the 50th day, Firmicutes dominated, and the relative abundance was 83.81–93.44%.
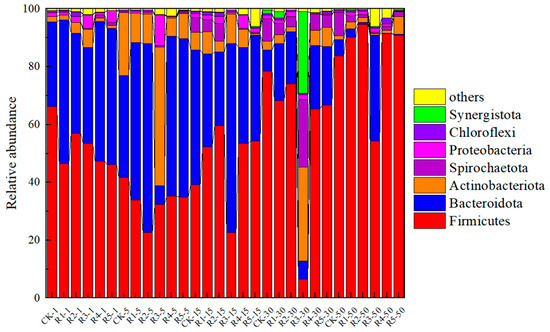
Figure 7.
Effects of addition of nanoparticles and antibiotics on bacterial community structure during digestion.
After adding 20 mg/kg·TS SMX on day 1, the abundance of Firmicutes in the CK group (68.53%) surpassed that in the R2 group (40.74%~56.89%). However, the abundance of Bacteroidetes R2 (34.74–55.37%) > CK (26.16%) in the first five days of the experiment indicated that Bacteroidetes was highly tolerant to SMX. By day 15, the phenomenon of acidification became increasingly pronounced within the system, with a higher relative abundance of Firmicutes observed in the R2 group. During the final 10 days of the system, the relative abundance of fungi across all groups ranged from 85.06% to 94.54%, indicating an absolute dominance of fungi within the digestive system at this stage. During the experiment, SMX had no obvious effect on other phyla-level flora, and the succession was mainly with nutrient consumption. On the 5th day of the reaction, the main Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria accounted for 48% of the total level of Actinobacteria in the R3 experimental group supplemented with 50 mg/kg·TS OTC, and other groups accounted for 8.5–32%. The increasing proportion of Actinobacteriota may be an important reason for promoting gas production. This is because actinomycetes can decompose many organic materials, including cellulose, lignin, and other complex compounds. Actinomycetes have a positive role in the biological treatment of sewage and organic solid waste, and some species of actinomycetes can also produce a variety of enzyme preparations to improve the efficiency of anaerobic digestion. The proportion of Proteobacteria increased from 12% to 38% with the increase in oxytetracycline concentration. In the process of the experiment, the lack of Proteobacteria made it easy to cause the accumulation of VFAs, indicating that it has a strong tolerance to oxytetracycin and that the material transformation in the system is stable under high concentrations of oxytetracycin. It has also been reported that the presence of toxic and hazardous substances tends to promote the growth of Aspergillus in environmental systems.
The structure of bacteria at different genus levels in anaerobic digestion is strongly influenced by the substrate, and the original dominance of some bacterial groups disappears as the digested material is consumed. As shown in Figure 8, Prevotella belongs to the family Bacteroidetes. On day 5 of the experiment, Prevotella was highly tolerant to ENR, so its relative abundance increased. In particular, the relative abundance of Prevotella with added nanoparticles R4 and R5 was 2–3% higher than that of the R2 group. On day 15, the relative abundance of Prevotella in group R2 decreased, and the decline of R4 and R5 was more obvious, which may be due to the rapid degradation of easily degraded nutrients in the substrate within 1–10 days and the better hydrolysis and acidification of R4 and R5 in the early stage, resulting in insufficient nutrients in the substrate. Consequently, the relative abundance of Prevotella decreased. At this juncture, the relative abundance of Treponema in groups R2, R4, and R5 was notably elevated, ranging from 4.22% to 6.12%. Treponema, classified under Spirochaetota, possesses the capability to convert H2 and CO2 into acetic acid; concurrently, the pH levels in R2, R4, and R5 were low, aligning with findings from previous studies. Caproiciproducens (Clostridium rumen) and Ethanoligenenaceae, both belonging to Firmicutes, were more abundant in R4 and R5 than CK, and this phenomenon lasted until day 30. Caproiciproducens has the ability to convert sugars into H2 and supply energy for hydrogen-trophic methanogens. On day 50, the relative abundance of HN-HF0106 in CK was greater than that in R4 and R5, suggesting that residual ENR hindered the activity of this bacterium. In contrast, Ruminiclostridium exhibited robust adaptability in the presence of residual ENR. Ruminiclostridium belongs to Bacteroidota, which can synthesize cellulose-degrading enzymes and degrade lignocellulosic substances. At this time, the organic matter that is easy to decompose is exhausted, and the competitive advantage of the bacteria that take the organic matter that is difficult to decompose, such as cellulose, as the main nutrient source gradually becomes obvious. On the 5th day, the dominant bacteria in R3 were Prevotella, Olsenella, Selenomonas, Megasphaera, and Anaerovibrio. Prevotella was 21% to 30%, Olsenella was 50%, and Selenomonas was 11% to 27% of the total level, significantly lower than CK (37%). Meanwhile, R3 Anaerovibrio was 11% to 29% of the total level, lower than CK (38%). Megasphaera accounted for 17–45% of the total, both higher than CK (15%). On day 5, the community structure of R3 bacteria was significantly different from that of the CK group, indicating that oxytetracycline could affect the community structure.
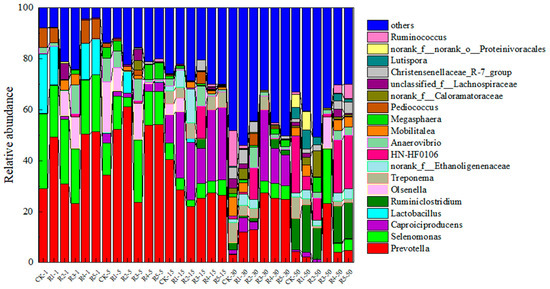
Figure 8.
Changes in horizontal bacterial community structure during the anaerobic digestion of livestock waste liquid induced by nanoparticles and antibiotics.
4. Discussion
Kadam, R. and his companion study pointed out [25] that factors such as pH, ammonia content, and carbon-to-nitrogen ratio in the anaerobic digestion of livestock manure play a vital role in ensuring biogas production, in which microorganisms are involved in showing different tolerances at various stages of digestion and maintaining the pH of the digestate at 6.8 to 7.5, which is optimal for the propagation of microbial populations. Nanoparticles are increasingly used in commercial products for large-scale industrial applications. The addition of 3 g of magnetite and 1 g of natural zeolite to the co-digestion of chicken manure and wheat straw resulted in a significant increase in methane content, both increasing methane production by more than 50%. Microorganisms and enzymes are often used as effective products in digestion pretreatment. These researchers have explored the field of livestock and poultry manure, and their statement that “co-digestion of livestock manure with carbon-rich substrates is a viable option” was a great inspiration for this experiment. In another study, Chubur et al. [26] used citrus plants for anaerobic digestion. Due to the high acidity of the fruits, the pH was at a low level in the early stages of the experiment, which inhibited methane production, and the different types of wastes contained different methane-producing potentials, which emphasized the importance of optimizing the anaerobic digestion process for sustainable waste management. In contrast to the experiments, in terms of pH, a single digestion experiment with livestock manure was used, where the pH of the digestate dropped to around 5.0 for all ten days of the experiment, which was clearly no longer suitable for the digestion process. The experimental group with the addition of nanoparticles increased methane production by more than 50%, responding to the effect of nanoparticles and antibiotics on methane production under a single stress. In future experiments, the effects of nanoparticles and antibiotics will be considered to be explored in combined digestion experiments.
In the early stage of this experiment, the experimental group with two kinds of nanoparticles and three kinds of antibiotics added showed higher peak gas production than the control group; the experimental group with nanoparticles added showed higher production than the other experimental groups, and the cumulative gas production was also significantly increased, indicating that the addition of nanoparticles can promote the early stage of the anaerobic digestion system and effectively strengthen the stability of the anaerobic digestion process. Other studies have looked at the digestion of other nanoparticles and come to similar conclusions. The effect of different concentrations of cobalt nanoparticles (CoNPs) on the anaerobic digestion of sludge was evaluated by Alfredo Córdova Lizama [27]. The results showed that cobalt nanoparticles at doses less than 2 mg/g·VS did not have a significant effect on anaerobic digestion, but the addition of nanoparticles at 3–7 mg/g·VS improved the performance of anaerobic digestion. The analysis of volatile fatty acids, redox potential, and electron transfer system activity showed that the addition of CoNPs stimulated the early stages of anaerobic digestion. The authors also note that CoNPs are effective in enhancing the behavior and stability of anaerobic processes.
In the analysis of cumulative gas production in the first ten days of the experiment, it was found that R5 in the experimental group with nano-C60 increased by about 2.40 times compared with CK in the control group, and R4 in the experimental group with nano-Fe2O3 increased by about 2.04 times compared with CK in the control group. In the cumulative gas production analysis of the entire 55-day experiment period, it was found that the experimental group R5 increased by about 1.25 times compared with the control group CK, and the experimental group R4 increased by about 1.21 times compared with the control group CK. In another similar study, Castro et al. [28] evaluated the effect of adding natural nanoparticles to sludge in order to promote the production of clean energy and showed that the addition of nanoparticles could increase the biogas production by up to two orders of magnitude, but this could be attributed to the variable nature of the nanoparticles applied, and a large number of experiments would be needed to improve the reliability of the results. In addition, the authors observed for magnetite nanoparticles that concentrations of nanoparticles higher than 100 mg/L had a detrimental effect, leading to an overall decrease in biogas production.
Magnetite (Fe3O4) nanoparticle-attached granular activated carbon (GAC) as a biocatalyst was investigated for biogas production enhancement by researchers such as Orrantia et al. [29], who achieved the highest methane productivity response using concentrations of 1.5% and 3% Fe3O4/GAC, respectively. The results showed that 1.5% Fe3O4/GAC was able to promote the consumption of volatile fatty acids and ethanol. Reflecting the close correlation of nanoparticles on microorganisms in anaerobic digestion systems, it is essential to study the effect of micro- and nanomaterial addition on microorganisms in digestive systems.
On this basis, the analysis of microorganisms during digestion was later added to this study, and it was found that the addition of nanoparticles and antibiotics had no significant impact on the development of bacteroides in the early stage, and the abundance of bacteroides decreased in the later stage. However, it was not clear whether it was the cause of the extinction of the digestion level in the later stage itself, and this part needs to be further studied.
In the study of anaerobic digestion, the digestion of plant waste is also the focus of attention. Some studies have studied the addition of magnetite to combined anaerobic digestion, but with the lack of characterization and comparison of the advantages of combined digestion itself, it is difficult to explain the specific promotion degree of the addition of magnetite to the digestive system. Liu et al. [30] have carried out experimental analysis on the addition of magnetite to animal manure and wheat straw and conducted batch experiments under the condition of 35 degrees Celsius and medium temperature. The results show that when the digestible ratio of animal manure and wheat straw is 52%, adding 2.7 g magnetite can result in the best methane yield.
Given that the accumulation of antibiotics in activated sludge (WAS) not only affects the performance of anaerobic digestion but also induces the generation of antibiotic-resistant genes (ARGs), which is a great ecological risk, scholars such as Meng et al. [31] systematically emphasized the fate and role of antibiotics in the anaerobic digestion of WAS, the recent progress in the generation and dissemination of ARGs associated with antibiotics and co-pollutants, and strategies for the reduction in and control of antibiotics and ARGs. Because the breakdown of WAS is a physicochemical process, the potential physicochemical effects of low-dose antibiotics on WAS are negligible. The effect of antibiotics on WAS dissolution is thought to be dose-dependent, with higher doses resulting in more soluble organic matter.
These dose-dependent studies are far from adequate and were not considered in this study. It is hoped that more dose-dependent experimental protocols will be included in the next antibiotic studies. The structure, class, and dosage of antibiotic disorders are hot topics of interest in the field, and water content and pH are also key environmental factors affecting changes in the relative abundance of microorganisms in the digestive system; characterizing the development of microbial communities in the anaerobic digestive system is yet to be further refined. In the next experimental studies, we can focus on the type and dosage of nanoparticles and antibiotics added to the anaerobic digestion system and analyze the microbial changes in more detail and dynamically in the course of the study. Combined anaerobic digestion has been an important protocol to improve the stability of the anaerobic digestion system, and in future experiments, we will consider exploring the effects of nanoparticles and antibiotics added in the combined plant and animal experiments. In addition, considering that enzyme activities are also closely related to anaerobic digestion systems, the next step of the study may be to include the analysis of enzyme activities.
5. Conclusions
- (1)
- The experimental group, to which two nanoparticles and three antibiotics were added, showed two peaks of gas production during 55 days of anaerobiosis, with the first peaks of the test being 244±13 (R1), 264±23 (R2), 390±16 (R3), 345±11 (R4), and 423±11 (R5) ml, respectively. In the control group, the peak gas production occurred on the fifth day, and the gas production was 249 ± 22 mL. The cumulative gas productions of each group in the first 10 days are as follows: 2968 (R5) > 2490 (R4) > 1445 (R3) > 1350 (R2) > 1216 (CK) > 1203 (R1) mL. Cumulative gas production was 3712 (CK), 3968 (R1), 4459 (R2), 4344 (R3), 4498 (R4), and 4639 (R5) mL, respectively.
- (2)
- The concentration of TVFAs increased rapidly in all groups during the first 10 days of the digestive system, and the TVFA concentration in the nano-Fe2O3 and nano-C60 groups was higher than that in the other four groups. The pH of the system decreased, and the SCOD showed the same trend as TVFAs. TVFAs reached a peak on day 19, with a value of 10,269.25 (CK), 10,454.16 (R1), 9975.94 (R2), 10,568.93 (R3), 10,345 (R4), and 10,987 (R5) mg/L; the SCODs of the same stage were 13,980 ± 57.1 (CK), 13,610 ± 16.1 (R1), 12,770 ± 34.1 (R2), 15,480 ± 66.1 (R3), 14,685 ± 38.1 (R4), and 15,393 ± 66.9 (R5) mg/L, respectively. The pH of each group decreased to 5.30~5.37, and the pH of nano-Fe2O3 and nano-C60 groups was lower, which was 5.08 ± 0.3 and 5.01 ± 0.1, respectively.
- (3)
- Changes in the horizontal structure of the anaerobically digested bacterial community of livestock manure were investigated in this experiment: At the outset, Firmicutes and Bacteroidota were the main dominant bacteria in each group, and the relative abundance levels were 46.56~66.18% and 29.32~49.52%, respectively. The addition of nanoparticles and antibiotics showed no significant changes in the results of bacteroidota. On the 5th day, the relative abundance of Actinobacteriota and Bacteroidota increased significantly, ranging from 6.11 to 21.75% and 35.20% to 54.49%, respectively. On day 5 of the reaction, the predominant flora in solution were bacillus-like, fungi, actinomycetes and ascomycetes. In R3 with 50 mg OTC/kg-TS, actinomycetes accounted for 48% of the total flora, and the other flora each accounted for 8.5–32%. The thick-walled phylum is the dominant hydrolytic-acid-producing bacterium in anaerobic digestion, secreting proteases and other hydrolytic enzymes to increase the rate of degradation of macromolecules, and it is responsible for the production of VFA. The thick-walled bacterial phylum has a mutualistic association reaction with hydrogenophilic methanogenic bacteria, which play a key role in increasing methane production in the reactor. The role of the Actinobacteria phylum in anaerobic digestion includes increasing the VFA content of the fermentation broth by degrading proteins, lipids, and complex organic matter.
- (4)
- The impacts of single stress on the level structure of the Bacteroidota community were as follows: Prevotella belonged to Bacteroidota, the relative abundance of Prevotella increased on the 5th day, and the relative abundance of Prevotella with respect to R4 and R5 nanoparticles was higher than that of R2 group by 2%~3%. On day 15, the relative abundance of Prevotella in group R2 decreased, while in groups R4 and R5, the decrease was more significant. Additionally, the relative abundance of Prevotella decreased overall. The relative abundance of Treponema in groups R2, R4, and R5 was higher, ranging from 4.22% to 6.12%. The Bacteroidetes phylum is mainly responsible for the degradation of cellulose and hemicellulose in anaerobic digestion, and it plays a key role in the anaerobic fermentation of nitrogen-rich substrates, which can accelerate the decomposition of organic matter that is not easily degraded in the substrate.
The results of this study contribute to the study of adding nanoparticles and antibiotics to chicken manure anaerobic digestion systems, indicating that the addition of nanoparticles and antibiotics will both promote methane production to some extent. However, whether it promotes it and how well it promotes it are highly dependent on the concentration of the pollutant added. For example, 10 mg/kg of norfloxacin did not inhibit the anaerobic digestion of chicken manure, but 500 mg/kg of norfloxacin showed significant inhibition. Since nanoparticles are highly chemically reactive and easily interact with other chemicals, nano-Fe2O3 and nano-C60 would promote gas production in the pre-experimental period after addition. In this experiment, we investigated the inhibition of methanogenesis under the stress of pollutants alone to provide data support for the study of nanoparticles and antibiotics on the stability of anaerobic digestion, which will help the development of biogas engineering.
Different metal oxide nanoparticles have different fates and long-term effects on anaerobic granular sludge, including their effects on microbial community structure, their effects on gas production characteristics, and their accumulation and fate in the system. Different types of nanoparticles have different effects on the stability and gas production characteristics of anaerobic systems, and further studies are needed to fully assess their long-term effects. The cumulative effects of antibiotics on anaerobically digesting microorganisms include effects on metabolic pathways, gas production characteristics, microbial community structure, and the propagation and enrichment of resistance genes, which may negatively affect the efficacy and stability of anaerobic digestion systems. Therefore, the rational use of antibiotics and the development of effective pretreatment technologies are essential to protect the stability and efficacy of anaerobic digestion systems.
This study lacks the characterization of the type and concentration of nanomaterials; these characterizations can help us to understand the interactions and effects of microbial communities and dynamics; the structures of micro- and nanomaterials need to be further analyzed; and the loss of nanomaterials in the experiments is also a problem. The next study can place the focus on the characterization of the structure of the material and the calculation of the loss.
Author Contributions
Supervision, Resources, Writing—original draft, X.Z.; Investigation, Methodology, Writing—original draft, H.Z.; Visualization, Writing—review and editing, K.L.; Project administration, Writing—review and editing, R.J.; Conceptualization, Writing—original draft, L.F.; Funding acquisition, Writing—review and editing, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 52206255), the Youth Science and Technology Talent Lift Program of Gansu Province (Project No. GXH20220530-14), the Youth Science Foundation Projects of Lanzhou Jiaotong University (Project Nos. 2020018 and 2020011), and the Gansu Province Youth Science Fund project (No. 22JR11RA148).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qing, S. Research on the Optimal Operation of Smart Agricultural Park Considering the Synergy of Low-Carbon Ecology and Biomass Energy. Master’s Thesis, Xi’an University of Technology, Xi’an, China, 2023. [Google Scholar]
- Song, D.D. Study on Biological Fermentation and Application of Aquaculture Waste Liquid. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2023. [Google Scholar] [CrossRef]
- Malghani, S.; Yoo, G.Y.; Giesemann, A.; Well, R.; Kang, H. Combined application of organic manure with urea does not alter the dominant biochemical pathway producing N2O from urea treated soil. Biol. Fertil. Soils Coop. J. Int. Soc. Soil Sci. 2020, 56, 331–343. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, H. Biogas as a renewable energy source—A review. Energy Sources 2009, 31, 1280–1293. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the River Basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Bacanlı, G.M. The two faces of antibiotics: An overview of the effects of antibiotic residues in foodstuffs. Arch. Toxicol. 2024, 98, 1717–1725. [Google Scholar] [CrossRef]
- Capana, A.; Martins, Q.; Crespi, M.; Ribeiro, C.; Barud, H. Thermal behavior of residues (sludge) originated from Araraquara water and sewage treatment station. J. Therm. Anal. Calorim. 2009, 97, 601–604. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Zhang, F.D.; Liu, X.M.; Wang, Y.J.; Zou, S.W.; He, X.S. Study on the determination and analysis of main harmful components in manure of large-scale breeding livestock and poultry. J. Plant Nutr. Fertil. 2005, 11, 116–123. [Google Scholar] [CrossRef]
- McClure, M.E.; Goldenberg, L.R. Use of antibiotics to reduce preterm birth. Lancet Glob. Health 2019, 7, e18–e19. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Ahn, D. Effects of carbon to nitrogen ratio on the performance and stability of aerobic granular sludge. Environ. Eng. Res. Environ. Eng. Res. 2021, 26, 114–122. [Google Scholar] [CrossRef]
- Feng, L.Y.; Luo, J.Y.; Chen, Y.G. Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 2015, 49, 4781–4782. [Google Scholar] [CrossRef] [PubMed]
- Strubbe, L.; van Dijk, E.J.; Deenekamp, P.J.; van Loosdrecht, M.C.; Volcke, E.I. Oxygen transfer efficiency in an aerobic granular sludge reactor: Dynamics and influencing factors of alpha. Chem. Eng. J. 2023, 452, 139548. [Google Scholar] [CrossRef]
- Qian, F.Y. Research on the Effect of Fe3O4 Nanoparticles on the Process of Anaerobic Digestion for Methane Production. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar]
- Zhang, D.; Peng, S.; Wang, D.Q.; Wang, Y.M.; Lin, X.G. Dynamic Changes in Antibiotic Resistance Genes During Biological Fermentation of Chicken Manure and Pig Manure. Environ. Sci. 2023, 44, 1780–1791. [Google Scholar]
- Xiang, Y. Nano Zero-Valent Iron and Nano Iron Tetraoxide Enhancement of Sludge Anaerobic Digestion for Gas Production and Resistance Gene Reduction. Master’s Thesis, Hunan University, Changsha, China, 2020. [Google Scholar]
- Tie, D.; Lei, F.; Xiao, Z. Microbial community structures and antibiotic biodegradation characteristics during anaerobic digestion of chicken manure containing residual enrofloxacin. J. Environ. Sci. Health Part B 2022, 57, 102–113. [Google Scholar]
- Xiao, Z.; Shange, L.; Ruo, J.; Wen, W.; Ti, D.; Jia, L. Effects of simulated extreme precipitation flooding on the degradation of anaerobic digestion effluent by algal-bacterial symbiosis system. Ecol. Indic. 2023, 154, 110677. [Google Scholar]
- Di Cesare, A.; Eckert, E.M.; D’Urso, S.; Bertoni, R.; Gillan, D.C.; Wattiez, R.; Corno, G. Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res. 2016, 94, 208–214. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.G.; Campanaro, S.; Bassani, I.; Angelidaki, I. Deeper insight into the structure of the anaerobic digestion microbial community; the biogas microbiome database is expanded with 157 new genomes. Bioresour. Technol. 2016, 216, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Contreras, C.; Vidal, G. Methanogenic toxicity evaluation of chlortetracycline hydrochloride. Electron. J. Biotechnol. 2015, 18, 445–450. [Google Scholar] [CrossRef][Green Version]
- Ge, H.; Jensen, D.P.; Batstone, J.D. Temperature phased anaerobic digestion increases apparent hydrolysis rate for waste activated sludge. Water Res. 2010, 45, 1597–1606. [Google Scholar] [CrossRef]
- Sreela-Or, C.; Plangklang, P.; Imai, T.; Reungsang, A. Co-digestion of food waste and sludge for hydrogen production by anaerobic mixed cultures: Statistical key factors optimization. Int. J. Hydrogen Energy 2011, 36, 14227–14237. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of low pH on continuous anaerobic digestion of waste activated sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.; Jo, S.; Lee, J.; Khanthong, K.; Jang, H.; Park, J. A Review on the Anaerobic Co-Digestion of Livestock Manures in the Context of Sustainable Waste Management. Energies 2024, 17, 546. [Google Scholar] [CrossRef]
- Chubur, V.; Hasan, G.; Kára, J.; Hanzlíková, I.; Chernysh, Y.; Sedláček, J.; Wang, J.; Roubík, H. Utilization of citrus, date, and jujube substrates for anaerobic digestion processes. Biofuels Bioprod. Biorefining 2024, 18, 1917–1929. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Pedreguera, A.Z.; Saady, N.M.C.; Ruiz Espinoza, J.E. Improving the anaerobic digestion of sewage sludge by adding cobalt nanoparticles. Environ. Technol. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.C.D.; Resende, E.; Taveira, I.; Enrich-Prast, A.; Abreu, F. Nanotechnology boosts the production of clean energy via nanoparticle addition in anaerobic digestion. Front. Nanotechnol. 2024, 6, 1406344. [Google Scholar] [CrossRef]
- Orrantia, M.; Armenta, M.A.; Alvarez, L.H.; Burboa-Charis, V.A.; Meza-Escalante, E.R.; Olivas, A.; Arroyo, E.; Maytorena, V.M. Enhanced methane production via anaerobic digestion assisted with Fe3O4 nanoparticles supported on microporous granular activated carbon. Fuel 2024, 360, 130517. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Wan, H.; Chen, Y.; Wang, X.; Yang, G.; Ren, G. Anaerobic co-digestion of animal manure and wheat straw for optimized biogas production by the addition of magnetite and zeolite. Energy Convers. Manag. 2015, 97, 132–139. [Google Scholar] [CrossRef]
- Meng, Q.B.; He, Z.W.; Yang, W.; Li, W.T.; Tang, C.C.; Zhou, A.J.; Ren, Y.X.; Liu, W.; Li, Z.; Wang, A. Roles and fates of antibiotics in anaerobic digestion of waste activated sludge: Insights to pro- and re-duction of antibiotic resistance genes. Chem. Eng. J. 2024, 500, 156633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).