Effects of Sewage Sludge Compost on Carbon, Nitrogen, Phosphorus, and Sulfur Ratios and Soil Enzyme Activities in a Long-Term Experiment
Abstract
1. Introduction
2. Materials and Methods
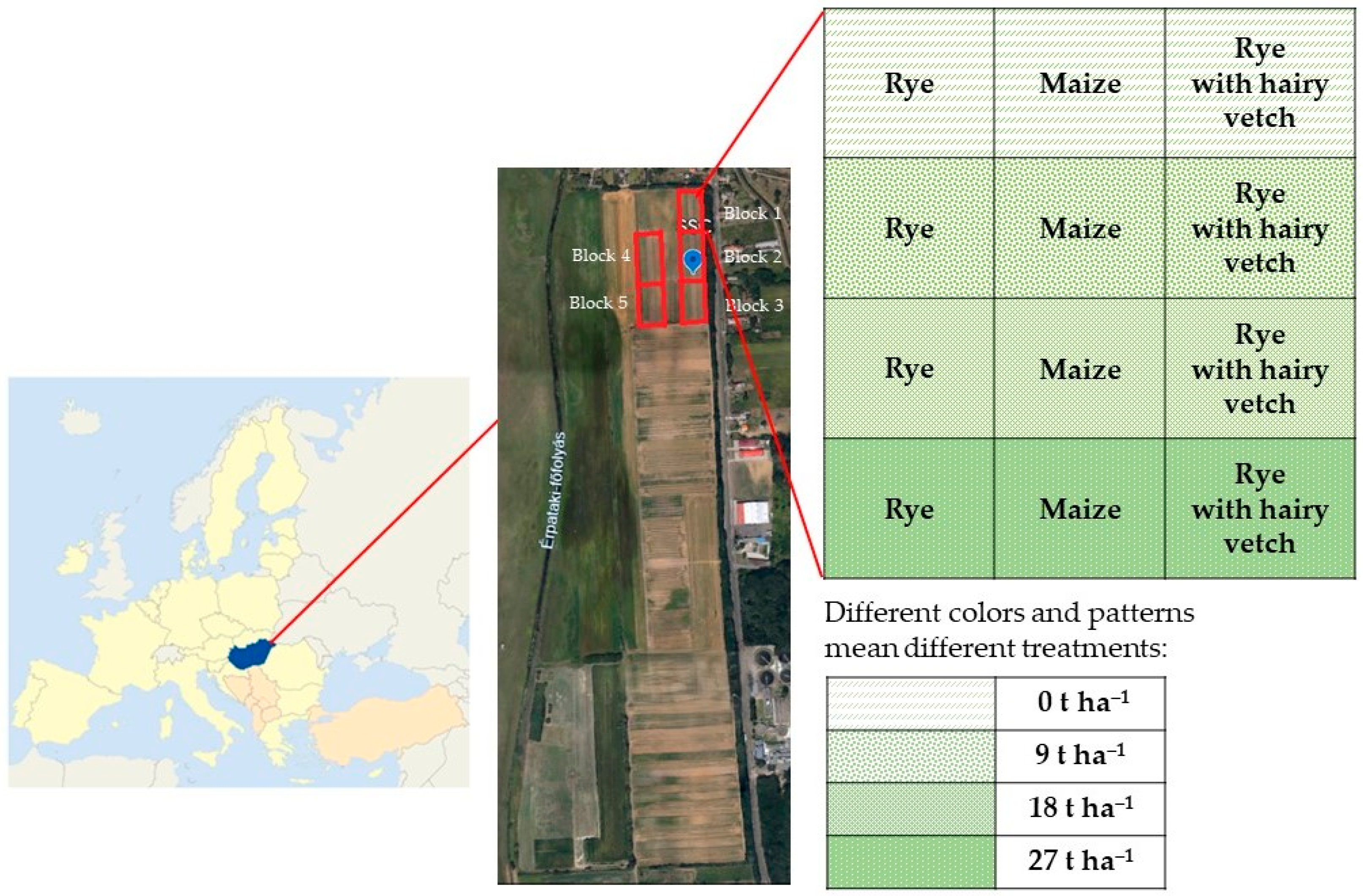
2.1. Experimental Site and Soil Sampling
2.2. Soil Chemical Analysis and Moisture Content Measurement
2.3. Soil Enzyme Activity Measurement
2.4. Statistical Analysis
2.5. Quality Control and Assurance
3. Results
3.1. Chemical Properties of the Soil
3.2. Soil Enzyme Activities
3.3. Evaluation of the Most Effective Soil Chemical Parameters
3.4. Grain Yield of Rye
4. Discussion
4.1. Soil Chemical Properties
4.2. Enzyme Activities
4.3. CNPS Contents and Ratios in Soil
4.4. Yield of Rye
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 4th ed.; Academic Press: London, UK, 2020; pp. 433–526. [Google Scholar]
- Xue, Y.; Kang, H.; Cui, Y.; Lu, S.; Yang, H.; Zhu, J.; Fu, Z.; Yan, C.; Wang, D. Consistent Plant and Microbe Nutrient Limitation Patterns During Natural Vegetation Restoration. Front. Plant Sci. 2022, 13, 885984. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. Recent advances and future research in ecological stoichiometry. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125611. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A.A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J.; Hill, B.H.; Elonen, C.M. Ecoenzymatic Stoichiometry of Stream Sediments with Comparison to Terrestrial Soils. Biogeochemistry 2012, 111, 455–467. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.; Post, W. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Rodrigues, M.; Withers, P.J.A.; Soltangheisi, A.; Vargas, V.; Holzschuh, M.; Pavinato, P.S. Tillage systems and cover crops affecting soil phosphorus bioavailability in Brazilian Cerrado Oxisols. Soil Till. Res. 2021, 205, 104770. [Google Scholar] [CrossRef]
- Peñuelas, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sardans, J. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Glob. Change Biol. 2020, 26, 1962–1985. [Google Scholar] [CrossRef]
- Xu, H.; Wang, M.; You, C.; Tan, B.; Xu, L.; Li, H.; Zhang, L.; Wang, L.; Liu, S.; Hou, G.; et al. Warming Effects on C: N: P Stoichiometry and Nutrient Limitation in Terrestrial Ecosystems. Soil Till. Res. 2024, 235, 105896. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Kirkegaard, J.A.; Richardson, A.E.; Wade, L.J.; Blanchard, C.; Batten, G. Stable soil organic matter: A comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 2011, 163, 197–208. [Google Scholar] [CrossRef]
- Amaleviciute-Volunge, K.; Tripolskaja, L.; Kazlauskaite-Jadzevice, A.; Slepetiene, A.; Baksiene, E. Effect of Long-Term Different Land Uses on Improving Stable Humic Compounds in Arenosol. Agriculture 2024, 14, 250. [Google Scholar] [CrossRef]
- Lai, R.; Lagomarsino, A.; Ledda, L.; Roggero, P.P. Variation in soil C and microbial functions across tree canopy projection and open grassland microenvironments. Turk. J. Agric. For. 2014, 38, 62–69. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Hurtarte, L.C.C.; Souza, I.F.; Zinn, Y.L. C: N ratios of bulk soils and particle-size fractions: Global trends and major drivers. Geoderma 2022, 425, 116026. [Google Scholar] [CrossRef]
- Bai, T.; Wang, P.; Qiu, Y.; Zhang, Y.; Hu, S. Nitrogen availability mediates soil carbon cycling response to climate warming: A meta-analysis. Glob. Change Biol. 2023, 29, 2608–2626. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Mao, C.; Qin, S.; Wang, J.; Liu, F.; Blagodatsky, S.; Yang, G.; Zhang, Q.; Zhang, D.; et al. Nitrogen availability regulates topsoil carbon dynamics after permafrost thaw by altering microbial metabolic efficiency. Nat. Commun. 2018, 9, 3951. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Olivier, B.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef]
- Siwik-Ziomek, A.; Lemanowicz, J. The content of carbon, nitrogen, phosphorus and sulphur in soil against the activity of selected hydrolases as affected by crop rotation and fertilization. Zemdirbyste 2014, 101, 367–372. [Google Scholar] [CrossRef]
- Wang, L.; Lu, P.; Feng, S.; Hamel, C.; Sun, D.; Siddique, K.H.M.; Gan, G.Y. Strategies to improve soil health by optimizing the plant-soil-microbe-anthropogenic activity nexus. Agr. Ecosyst. Environ. 2024, 359, 108750. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, J.; Wang, S.; Liang, Y.; Wang, D.; Zhu, Y.; Wang, Y. Soil and microbial C:N:P stoichiometries play vital roles in regulating P transformation in agricultural ecosystems: A review. Pedosphere 2024, 34, 44–51. [Google Scholar] [CrossRef]
- Bennett, J.; Klironomos, J. Mechanisms of plant–soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2019, 222, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Frene, J.P.; Pandey, B.K.; Castrillo, G. Under pressure: Elucidating soil compaction and its effect on soil functions. Plant Soil 2024, 502, 267–278. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and Adaptive Management of Nitrification in Agricultural Soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Zavarzina, A.G.; Danchenko, N.N.; Demin, V.V.; Artemyeva, Z.S.; Kogut, B.M. Humic Substances: Hypotheses and Reality (a Review). Eurasian Soil Sc. 2021, 54, 1826–1854. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J. Differential nutrient limitation of soil microbial biomass and metabolic quotients (qCO2): Is there a biological stoichiometry of soil microbes? PLoS ONE 2013, 8, e57127. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.F.; Naves, E.R.; da Mota, R.P. Soil Quality: Enzymatic Activity of Soil β-Glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–450. [Google Scholar]
- Sandhu, S.; Sekaran, U.; Ozlu, E.; Hoilett, N.O.; Kumar, S. Short-Term Impacts of Biochar and Manure Application on Soil Labile Carbon Fractions, Enzyme Activity, and Microbial Community Structure. Biochar 2019, 1, 271–282. [Google Scholar] [CrossRef]
- Stefanoni Rubio, P.J.; Godoy, M.S.; Della Mónica, I.F.; Pettinari, M.J.; Godeas, A.M.; Scervino, J.M. Carbon and nitrogen sources influence tricalcium phosphate solubilization and extracellular phosphatase activity by Talaromyces flavus. Curr. Microbiol. 2016, 72, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, R.; Nielsen, S.; Joseph, S.D.; Huang, D.; Thomas, T. A Combination of Biochar–Mineral Complexes and Compost Improves Soil Bacterial Processes, Soil Quality, and Plant Properties. Front. Microbiol. 2016, 7, 372. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.F.; Dos-Santos, A.L.; Meyer-Fernandes, J.R. Inorganic phosphate as an important regulator of phosphatases. Enzyme Res. 2011, 103980. [Google Scholar] [CrossRef]
- Bajouco, R.; Fraga, I.; Pinheiro, J.; Coutinho, J. Acid Phosphomonoesterase and β-Glucosidase Activities in Volcanic Soils Under Permanent Fertilized Pastures: Distribution Profile and Microbial Effort Toward P Acquisition. Soil Sci. Plant Nutr. 2020, 66, 734–744. [Google Scholar] [CrossRef]
- Gou, X.; Ren, Y.; Qin, X.; Wei, X.; Wang, J. Global patterns of soil phosphatase responses to nitrogen and phosphorus fertilization. Pedosphere 2024, 34, 200–210. [Google Scholar] [CrossRef]
- Gou, X.M.; Cai, Y.; Wang, C.Q.; Li, B.; Zhang, R.P.; Zhang, Y.; Tang, X.Y.; Chen, Q.; Shen, J.; Deng, J.R.; et al. Effects of different long-term cropping systems on phoD-harboring bacterial community in red soils. J. Soils Sediments 2021, 21, 376–387. [Google Scholar] [CrossRef]
- Ibrahim, M.; Iqbal, M.; Tang, Y.-T.; Khan, S.; Guan, D.-X.; Li, G. Phosphorus Mobilization in Plant–Soil Environments and Inspired Strategies for Managing Phosphorus: A Review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Fujita, K.; Kunito, T.; Moro, H.; Toda, H.; Otsuka, S.; Nagaoka, K. Microbial resource allocation for phosphatase synthesis reflects the availability of inorganic phosphorus across various soils. Biogeochemistry 2017, 136, 325–339. Available online: https://www.jstor.org/stable/48720871 (accessed on 6 January 2025). [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Kosobucki, P. Study of Compost Based on Sewage Sludge and Different Structural Materials. Biomass 2024, 4, 273–285. [Google Scholar] [CrossRef]
- Myszura-Dymek, M. Properties of Organic Matter in Composts Based on Sewage Sludge. J. Ecol. Eng. 2024, 25, 70–81. [Google Scholar] [CrossRef]
- Farsang, A.; Babcsányi, I.; Ladányi, Z.; Perei, K.; Bodor, A.; Csányi, T.K.; Barta, K. Evaluating the effects of sewage sludge compost applications on the microbial activity, the nutrient and heavy metal content of a Chernozem soil in a field survey. Arab. J. Geosci. 2020, 13, 982. [Google Scholar] [CrossRef]
- Myszura-Dymek, M.; Zukowska, G. The Influence of Sewage Sludge Composts on the Enzymatic Activity of Reclaimed Post-Mining Soil. Sustainability 2023, 15, 4749. [Google Scholar] [CrossRef]
- Sciubba, L.; Cavani, L.; Marzadori, C.; Ciavatta, C. Effect of biosolids from municipal sewage sludge composted with rice husk on soil functionality. Biol. Fertil. Soils 2013, 49, 597–608. [Google Scholar] [CrossRef]
- Curci, M.; Lavecchia, A.; Cucci, G.; Lacolla, G.; De Corato, U.; Crecchio, C. Short-Term Effects of Sewage Sludge Compost Amendment on Semiarid Soil. Soil Syst. 2020, 4, 48. [Google Scholar] [CrossRef]
- Liu, X.; Guo, K.; Huang, L.; Ji, Z.; Jiang, H.; Li, H.; Zhang, J. Responses of absolute and specific enzyme activity to consecutive application of composted sewage sludge in a Fluventic Ustochrept. PLoS ONE 2017, 12, e0177796. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Buzás, I. Manual of Soil and Agrochemical Analysis. 2. Physico-Chemical and Chemical Analytical Methods for Soils; Mezőgazdasági Kiadó: Budapest, Hungary, 1988. (In Hungarian) [Google Scholar]
- Hungarian Standard. (MSZ) 20135 Determination of available nutrient content of soils. In Item 4.2.1: Preparing the Ammonium Lactate Extractant. Item 5.4.2: Determination of Orto-Phosphate Concentration; Hungarian Standard Association: Budapest, Hungary, 1999. (In Hungarian) [Google Scholar]
- Mulvaney, R.L. Nitrogen-inorganic forms. In Methods of Soil Analysis, Part 3, Chemical Methods, 1st ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America Inc., American Society of Agronomy, Inc.: Madison, WI, USA, 1996; SSSA Book Series No. 5; pp. 1123–1184. [Google Scholar]
- Wuenscher, R.; Unterfrauner, H.; Peticzka, R.; Zehetner, F. A comparison of 14 soil phosphorus extraction methods applied to 50 agricultural soils from Central Europe. Plant Soil Environ. 2015, 61, 86–96. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Agronomy Monograph 9; ASA and SSSA: Madison, WI, USA, 1982; pp. 591–592. [Google Scholar]
- Jimenez, R.R.; Ladha, J.K. Automated elemental analysis: A rapid and reliable but expensive measurement of total carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plan. 1993, 24, 1897–1924. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1976, 9, 167–172. [Google Scholar] [CrossRef]
- Hartemink, A.E.; Barrow, N.J. Soil pH—Nutrient relationships: The diagram. Plant Soil 2023, 486, 209–215. [Google Scholar] [CrossRef]
- Puissant, J.; Jones, B.; Goodall, T.; Mang, D.; Blaud, A.; Gweon, H.S.; Malik, A.; Jones, D.L.; Clark, I.M.; Hirsch, P.R.; et al. The pH optimum of soil exoenzymes adapt to long term changes in soil pH. Soil Biol. Biochem. 2019, 138, 107601. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Guo, D.; Zhao, J.; Yan, L.; Feng, G.; Gao, O.; Yu, H.; Zhao, L. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Yang, T.; Chu, H. Threshold effects of soil pH on microbial co-occurrence structure in acidic and alkaline arable lands. Sci. Total Environ. 2021, 800, 149592. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Chaplot, V.; Smith, P. Cropping leads to loss of soil organic matter: How can we prevent it? Pedosphere 2023, 33, 8–10. [Google Scholar] [CrossRef]
- Oktaba, L.; Odrobińska, D.; Uzarowicz, Ł. The impact of different land uses in urban area on humus quality. J. Soils Sediments 2018, 18, 2823–2832. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Cong, L.; Wang, X.; Shi, S. Effect of fulvic acid on the phosphorus availability in acid soil. J. Soil Sci. Plant Nut. 2013, 13, 526–533. [Google Scholar] [CrossRef]
- Zalba, P.; Amiotti, N.M.; Juan, A.; Galantini, J.A.; Pistola, S. Soil Humic and Fulvic Acids from Different Land-Use Systems Evaluated By E4/E6 Ratios. Commun. Soil Sci. Plant Anal. 2016, 47, 1675–1679. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Remick, K.A.; Helmann, J.D. Chapter One—The elements of life: A biocentric tour of the periodic table. In Advances in Microbial Physiology, 1st ed.; Poole, R.K., Kelly, D.J., Eds.; Academic Press: London, UK, 2023; Volume 82, pp. 1–127. [Google Scholar] [CrossRef]
- Silva, M.B.; Camargos, L.S.; Teixeira Filho, M.C.M.; Souza, L.A.; Coscione, A.R.; Lavres, J.; Abreu-Junior, C.H.; He, Z.; Zhao, F.; Jani, A.D.; et al. Residual effects of composted sewage sludge on nitrogen cycling and plant metabolism in a no-till common bean-palisade grass-soybean rotation. Front Plant Sci. 2023, 14, 1281670. [Google Scholar] [CrossRef] [PubMed]
- Telman, W.; Dietz, K.-J. Thiol redox-regulation for efficient adjustment of sulfur metabolism in acclimation to abiotic stress. J. Exp. Bot. 2019, 70, 4223–4236. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Maruyama-Nakashita, A. Biosynthesis of Sulfur-Containing Small Biomolecules in Plants. Int. J. Mol. Sci. 2020, 21, 3470. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Cox, M.S.; Oglesby, C.; Dhillon, J.S. Revisiting the role of sulfur in crop production: A narrative review. J. Agric. Food Res. 2024, 15, 101013. [Google Scholar] [CrossRef]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Lambers, H.; Condron, L.M.; Cramer, M.D.; Leake, J.R.; Richardson, A.E.; Smith, S.E. Soil microbial biomass and the fate of phosphorus during long-term ecosystem development. Plant Soil 2013, 367, 225–234. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Wood, S.S.; Mesquita, C.P.B. How Microbes Can, and Cannot, Be Used to Assess Soil Health. Soil Biol. Bioch. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Długosz, J.; Piotrowska-Długosz, A.; Kalisz, B. Vertical changes in P-acquiring enzyme activities and microbial biomass in Luvisols—The effect of different types of agricultural land use and soil-forming processes. Geoderma 2023, 432, 116406. [Google Scholar] [CrossRef]
- Margenot, A.J.; Wade, J. Getting the Basics Right on Soil Enzyme Activities: A Comment on Sainju. (2022). Agrosys. Geosci. Environ. 2023, 6, e20405. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Xi, B.; Wei, Z.; Li, X.; Cao, Z. Changes in phosphorus fractions during organic wastes composting from different sources. Bioresource Technol. 2015, 189, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Kuzyakov, Y. Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocation—Coupling soil zymography with 14C imaging. Soil Biol. Biochem. 2013, 67, 106–113. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.W.; Sun, B.; Li, L.; Liu, M.Q.; Zhu, Y.Y.; Guo, S.W.; Ling, N.; Shen, Q.R. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Almási, C.; Orosz, V.; Tóth, T.; Henzsel, I.; Demeter, I.; Mansour, M.M.; Makádi, M. Sewage sludge compost as an alternative source of phosphorus to rye in acidic sandy soil. Acta Agr. Debreceniensis 2024, 1, 11–18. [Google Scholar] [CrossRef]
- Zuccarini, P.; Asensio, D.; Sardans, J.; Ogaya, R.; Peñuelas, J. Changes in soil enzymatic activity in a P-limited Mediterranean shrubland subject to experimental nitrogen deposition. Appl. Soil Ecol. 2021, 168, 104159. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Gu, J.; Yu, L.; Wang, Z. Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in a Larix gmelinii plantation. Ann. For. Sci. 2015, 72, 435–442. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Shen, H.; Zhao, M.; Xu, L.; Xing, A.; Fang, J. Soil extracellular enzyme activity and stoichiometry in China’s forests. Funct. Ecol. 2020, 34, 1461–1471. [Google Scholar] [CrossRef]
- Ladau, J.; Eloe-Fadrosh, E.A. Spatial, Temporal, and Phylogenetic Scales of Microbial Ecology. Trends Microbiol. 2019, 8, 662–669. [Google Scholar] [CrossRef]
- Jobe, T.O.; Zenzen, I.; Karvansara, P.R.; Kopriva, S. Integration of sulfate assimilation with carbon and nitrogen metabolism in transition from C3 to C4 photosynthesis. J. Exp. Bot. 2019, 70, 4211–4221. [Google Scholar] [CrossRef]
- Torres Bazurto, J.; Sanchez, J.D.; Cayón Salinas, D.G. Nutrient accumulation models in the banana (Musa AAA Simmonds cv. Williams) plant under nitrogen doses. Acta Agron. 2017, 66, 391–396. [Google Scholar] [CrossRef]
- Li, J.; Xie, T.; Zhu, H.; Zhou, J.; Li, C.; Xiong, W.; Xu, L.; Wu, Y.; He, Z.; Li, X. Alkaline phosphatase activity mediates soil organic phosphorus mineralization in a subalpine forest ecosystem. Geoderma 2021, 404, 115376. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Araujo, M.A.; Lal, R.; Zinn, Y.L. What C: N ratios in soil particle-size fractions really say: N is preferentially sorbed by clays over organic C. Catena 2023, 230, 107230. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef]
- Park, Y.; Solhtalab, M.; Thongsomboon, W.; Aristilde, L. Strategies of organic phosphorus recycling by soil bacteria: Acquisition, metabolism, and regulation. Environ. Microbiol. Rep. 2022, 14, 3–24. [Google Scholar] [CrossRef]
- Kim, M.; Peng, J.; Sung, K. Causality of climate and soil factors affecting whole crop rye (Secale cereale L.) yield as part of natural ecosystem structure via longitudinal structural equation model in the Republic of Korea. Grassl. Sci. 2020, 66, 110–115. [Google Scholar] [CrossRef]


| 0 t ha−1 SSC | 9 t ha−1 SSC | 18 t ha−1 SSC | 27 t ha−1 SSC | |
|---|---|---|---|---|
| 0 t ha−1 SSC | – | 0.0084 | 0.0086 | 0.0082 |
| 9 t ha−1 SSC | 0.0084 | – | 0.0072 | 0.0075 |
| 18 t ha−1 SSC | 0.0086 | 0.0072 | – | 0.0626 |
| 27 t ha−1 SSC | 0.0082 | 0.0075 | 0.0626 | – |
| Parameter | 0 t ha−1 SSC | 9 t ha−1 SSC | 18 t ha−1 SSC | 27 t ha−1 SSC |
|---|---|---|---|---|
| pH | 4.38 ± 0.47a | 5.37 ± 0.60b | 5.94 ± 0.58b | 6.16 ± 0.32b |
| SOM (%) | 0.62 ± 0.01a | 0.71 ± 0.09ab | 0.75 ± 0.04ab | 0.85 ± 0.11b |
| E4/E6 | 6.67 ± 0.78a | 7.30 ± 0.53a | 7.50 ± 0.26a | 7.66 ± 0.26a |
| NO3-NO2-N (mg kg−1) | 14.53 ± 1.59a | 15.05 ± 1.65a | 19.94 ± 6.44a | 17.20 ± 4.96a |
| AL-P2O5 (mg kg−1) | 106.36 ± 49.32a | 259.75 ± 7.89a | 459.75 ± 29.51b | 696.40 ± 143.71c |
| Total C (mmol kg−1) | 388.224 ± 18.223a | 429.050 ± 7.839ab | 471.443 ± 21.545b | 558.204 ± 53.069c |
| Total N (mmol kg−1) | 39.823 ± 1.008a | 41.914 ± 3.323ab | 47.790 ± 0.450bc | 52.784 ± 5.299c |
| Total P (mmol kg−1) | 8.546 ± 0.783a | 12.858 ± 1.063a | 18.356 ± 2.277b | 21.044 ± 4.273b |
| Total S (mmol kg−1) | 3.000 ± 0.191a | 3.225 ± 0.081ab | 3.653 ± 0.270bc | 4.102 ± 0.383c |
| Moisture (% m/m) | 8.55 ± 0.11a | 8.78 ± 1.06a | 7.94 ± 0.76a | 9.43 ± 0.82a |
| Parameter | 0 t ha−1 SSC | 9 t ha−1 SSC | 18 t ha−1 SSC | 27 t ha−1 SSC |
|---|---|---|---|---|
| C:N | 9.968 ± 0.327a | 10.156 ± 0.318a | 10.068 ± 0.131a | 10.455 ± 0.154a |
| C:P | 47.375 ± 2.238c | 31.783 ± 1.220b | 28.720 ± 1.714ab | 24.375 ± 2.86a |
| C:S | 129.502 ± 5.183a | 127.366 ± 11.006a | 127.456 ± 10.193a | 136.372 ± 11.078a |
| N:P | 4.805 ± 0.294c | 3.177 ± 0.061b | 2.883 ± 0.196b | 2.335 ± 0.288a |
| N:S | 12.982 ± 0.734a | 12.370 ± 0.197a | 12.383 ± 0.443a | 12.876 ± 0.822a |
| P:S | 2.755 ± 0.101a | 3.842 ± 0.249b | 4.752 ± 0.793bc | 5.280 ± 0.165c |
| Parameter | 0 t ha−1 SSC | 9 t ha−1 SSC | 18 t ha−1 SSC | 27 t ha−1 SSC |
|---|---|---|---|---|
| Glucosidase (µg PNP g−1 hr−1) | 39.715 ± 14.911a | 66.375 ± 7.251b | 83.041 ± 6.779bc | 93.926 ± 92.817c |
| Acidic phosphatase (µg PNP g−1 hr−1) | 448.301 ± 68.330a | 440.787 ± 195.797a | 323.369 ± 99.263a | 385.936 ± 130.754a |
| Alkaline phosphatase (µg PNP g−1 hr−1) | 60.773 ± 8.079a | 151.404 ± 50.506b | 241.525 ± 59.081c | 289.576 ± 48.266c |
| lnGLUC/lnACP | 0.597 ± 0.031a | 0.700 ± 0.028b | 0.750 ± 0.025b | 0.768 ± 0.010b |
| lnGLUC/lnALP | 0.843 ± 0.021a | 0.847 ± 0.022a | 0.810 ± 0.021a | 0.789 ± 0.009a |
| lnGLUC/ln(ACP + ALP) | 0.579 ± 0.025a | 0.660 ± 0.018b | 0.683 ± 0.002b | 0.698 ± 0.014b |
| GLUC (µg PNP g−1 hr−1) | ACP (µg PNP g−1 hr−1) | ALP (µg PNP g−1 hr−1) | |
|---|---|---|---|
| pH (KCl) | 0.815 ** | −0.666 ** | 0.936 ** |
| SOM (%) | 0.765 ** | ns | 0.502 * |
| NO3-NO2-N (mg/kg) | ns | ns | ns |
| AL-P2O5 (mg/kg) | 0.860 ** | ns | 0.859 ** |
| E4/E6 | 0.751 ** | ns | 0.697 ** |
| Moisture % | ns | 0.714 ** | ns |
| C (mmol/kg) | 0.832 ** | ns | 0.704 ** |
| N (mmol/kg) | 0.788 ** | ns | 0.621 * |
| S (mmol/kg) | 0.830 ** | ns | 0.749 ** |
| P (mmol/kg) | 0.834 ** | ns | 0.785 ** |
| C:N | ns | ns | ns |
| C:S | ns | ns | ns |
| C:P | −0.812 ** | ns | −0.812 ** |
| N:S | ns | ns | ns |
| N:P | −0.811 ** | ns | −0.827 ** |
| P:S | 0.770 ** | ns | 0.836 ** |
| Treatment | Yield (t ha−1) | S.D. |
|---|---|---|
| 0 t ha−1 SSC | 4.35a | 0.26 |
| 9 t ha−1 SSC | 4.68a | 0.36 |
| 18 t ha−1 SSC | 4.60a | 0.35 |
| 27 t ha−1 SSC | 4.90a | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almási, C.; Orosz, V.; Tóth, T.; Mansour, M.M.; Demeter, I.; Henzsel, I.; Bogdányi, Z.; Szegi, T.A.; Makádi, M. Effects of Sewage Sludge Compost on Carbon, Nitrogen, Phosphorus, and Sulfur Ratios and Soil Enzyme Activities in a Long-Term Experiment. Agronomy 2025, 15, 143. https://doi.org/10.3390/agronomy15010143
Almási C, Orosz V, Tóth T, Mansour MM, Demeter I, Henzsel I, Bogdányi Z, Szegi TA, Makádi M. Effects of Sewage Sludge Compost on Carbon, Nitrogen, Phosphorus, and Sulfur Ratios and Soil Enzyme Activities in a Long-Term Experiment. Agronomy. 2025; 15(1):143. https://doi.org/10.3390/agronomy15010143
Chicago/Turabian StyleAlmási, Csilla, Viktória Orosz, Timea Tóth, Mostafa M. Mansour, Ibolya Demeter, István Henzsel, Zsolt Bogdányi, Tamás András Szegi, and Marianna Makádi. 2025. "Effects of Sewage Sludge Compost on Carbon, Nitrogen, Phosphorus, and Sulfur Ratios and Soil Enzyme Activities in a Long-Term Experiment" Agronomy 15, no. 1: 143. https://doi.org/10.3390/agronomy15010143
APA StyleAlmási, C., Orosz, V., Tóth, T., Mansour, M. M., Demeter, I., Henzsel, I., Bogdányi, Z., Szegi, T. A., & Makádi, M. (2025). Effects of Sewage Sludge Compost on Carbon, Nitrogen, Phosphorus, and Sulfur Ratios and Soil Enzyme Activities in a Long-Term Experiment. Agronomy, 15(1), 143. https://doi.org/10.3390/agronomy15010143






