Evaluating Copper-Doped Biochar Composites for Improving Wheat Nutrition and Growth in Oxisols
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation and Characterization
2.2. Synthesis of Biochar and Copper-Doped Biochar
2.3. Characterization of Biochar and Composite Properties
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
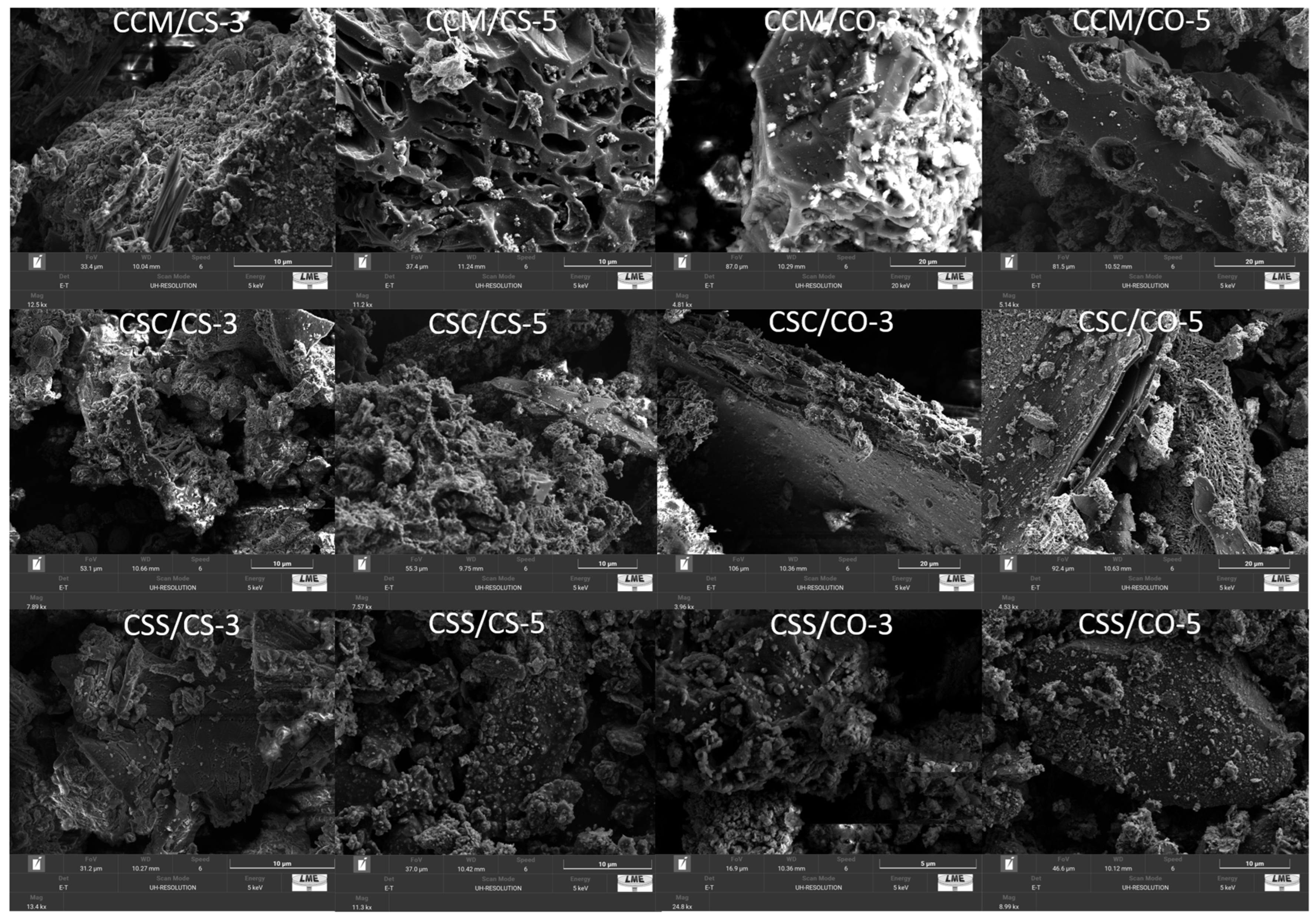
2.5. Scanning Electron Microscopy (SEM)
2.6. Copper Release Kinetics
2.7. Agronomic Performance t of Cu Composites
2.8. Statistical Analysis
3. Results
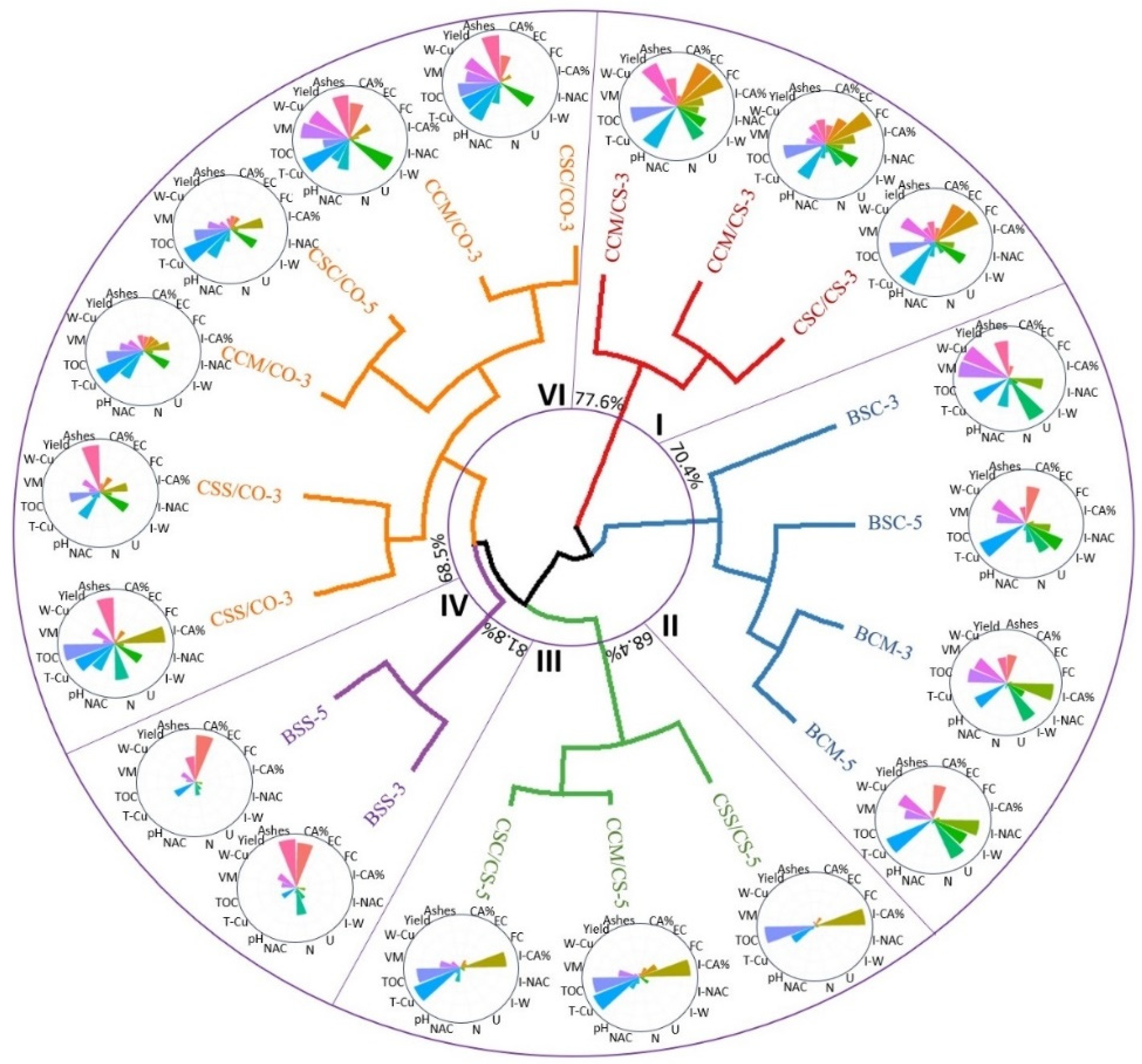
3.1. Chemical Properties of the Composites
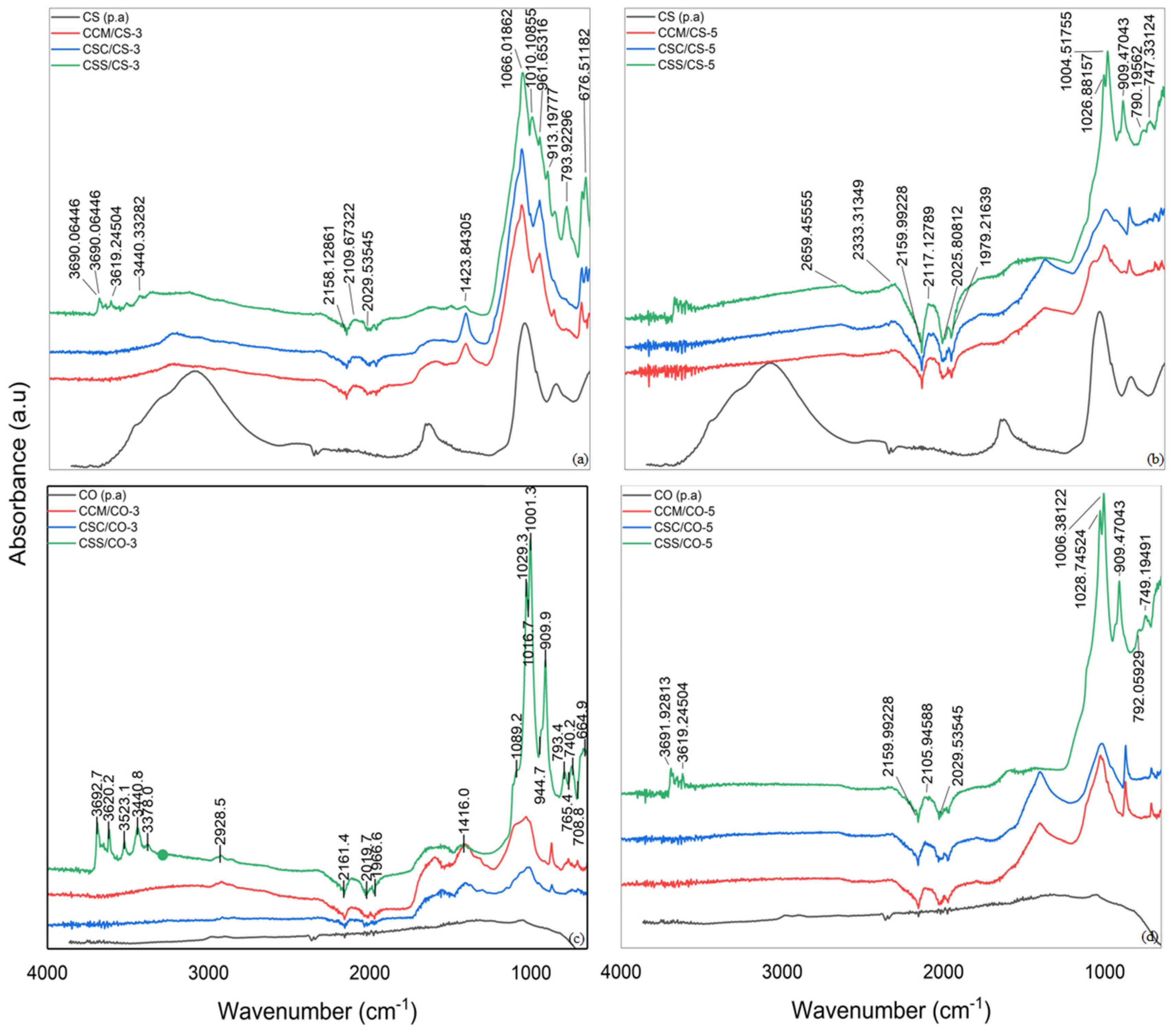
3.2. Infrared Spectroscopy
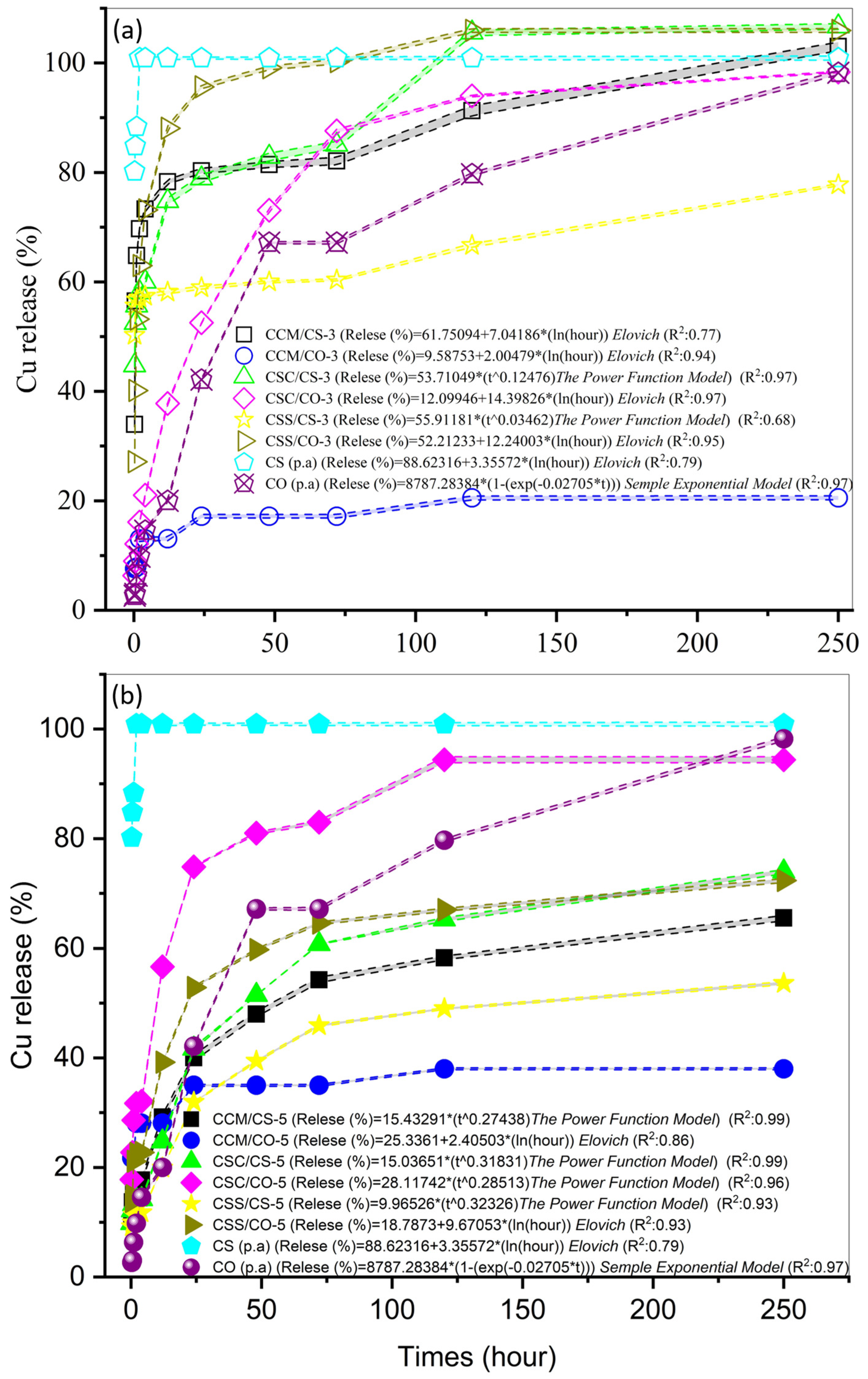
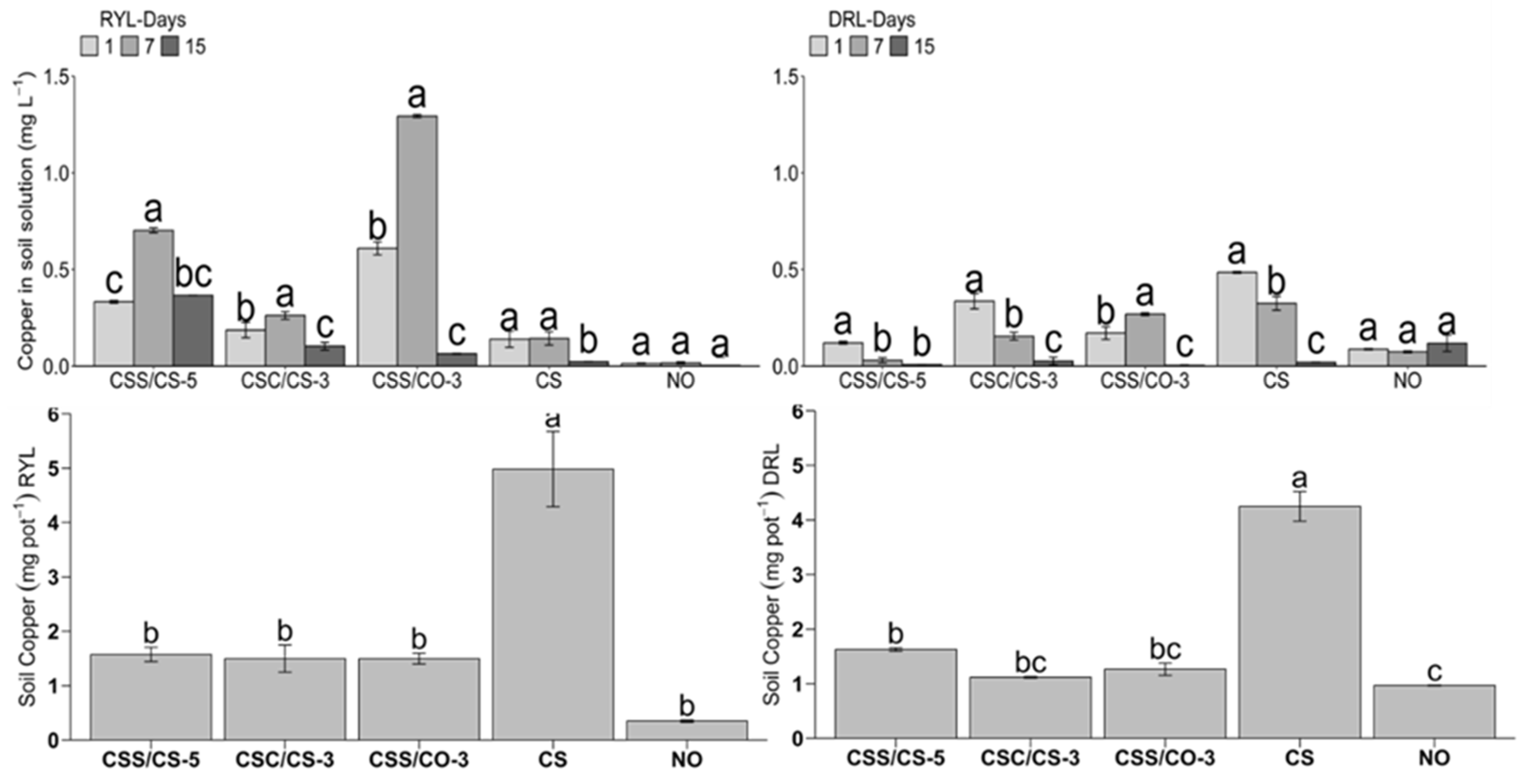
3.3. Kinetics of Cu Release
3.4. Scanning Electron Microscopy
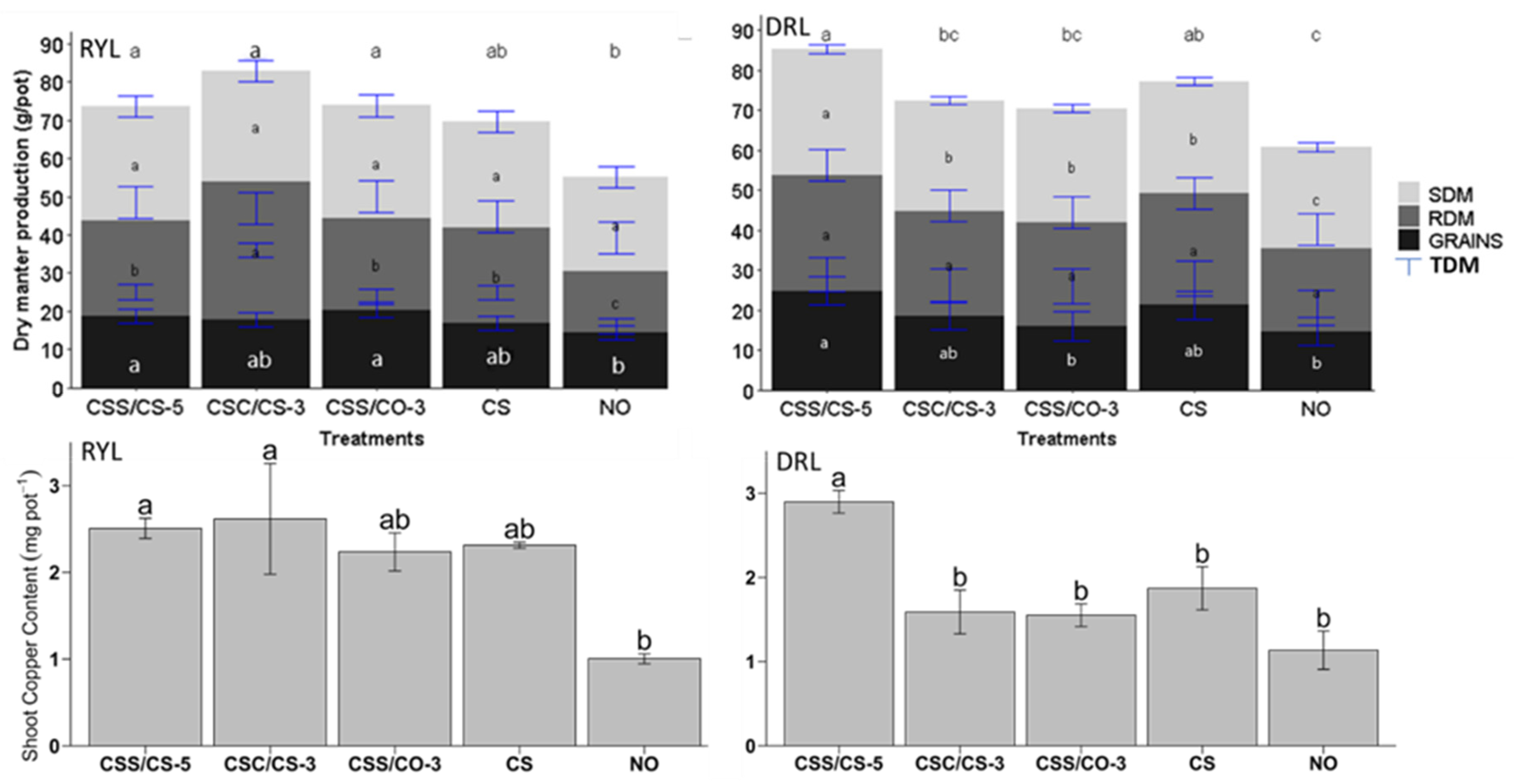
3.5. Agronomic Impact of Composites on Wheat Growth
4. Discussion
4.1. Chemical Properties of the Composites
4.2. Infrared Spectroscopy
4.3. Kinetics of Cu Release
4.4. Scanning Electron Microscopy
4.5. Agronomic Effectiveness of Composites on Wheat Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Al | B | Ca | Cu | Fe | Mg | Mn | Na | P | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| --------------------------------------------- (mg kg−1) ---------------------------------------------------- | ||||||||||
| FCM | 2669 | 299 | 102,606 | 178 | 2533 | 6466 | 462 | 2929 | 21,060 | 551 |
| FSC | 1844 | 222 | 96,764 | 98 | 1137 | 8433 | 92 | 1774 | 18,473 | 109 |
| FSS | 6867 | 47 | 9471 | 348 | 11,106 | 2489 | 200 | 922 | 3843 | 623 |
| BCM-3 | 2780 | 243 | 108,732 | 166 | 2170 | 9516 | 597 | IN | 30,035 | 787 |
| BSC-3 | 2268 | 162 | 86,603 | 147 | 3437 | 8513 | 114 | IN | 23,074 | 1683 |
| BSS-3 | 7399 | 94 | 10,293 | 423 | 5565 | 2347 | 230 | IN | 4745 | 812 |
| BCM-5 | 4719 | 196 | 105,307 | 169 | 3657 | 13,158 | 882 | IN | 46,275 | 968 |
| BSC-5 | 3648 | 164 | 125,101 | 188 | 4749 | 17,627 | 156 | IN | 44,359 | 2238 |
| BSS-5 | 10,537 | 95 | 12,647 | 481 | 11,439 | 2738 | 301 | IN | 6129 | 922 |
| CCM/CS-3 | 6334 | 129.5 | 101,517 | 232,979 | 3271 | <LQ | 1857 | 7527 | 27,145 | 567 |
| CCM/CO-3 | 4879 | 10.0 | 108,679 | 111,412 | 2712 | <LQ | 1843 | 84,240 | 29,341 | 233 |
| CCM/CS-5 | 7342 | 459.0 | 157,750 | 282,412 | 4312 | <LQ | 2143 | 11,461 | 32,208 | 412 |
| CCM/CO-5 | 7169 | 18.4 | 142,800 | 237,358 | 4366 | <LQ | 2185 | 77,565 | 33,387 | 338 |
| CSC/CS-3 | 2275 | 189.3 | 112,591 | 302,437 | 1601 | 12,482 | 52 | 20,552 | 44,250 | 4105 |
| CSC/CO-3 | 2567 | 434.8 | 83,212 | 241,562 | 1547 | 9455 | 62 | 145,502 | 36,883 | 1238 |
| CSC/CS-5 | 3356 | 958.9 | 237,362 | 281,037 | 1839 | 19,350 | 127 | 24,298 | 68,037 | 3865 |
| CSC/CO-5 | 3446 | 491.4 | 292,200 | 233,291 | 1629 | 22,464 | 154 | 129,040 | 66,508 | 1640 |
| CSS/CS-3 | 77,711 | 134.3 | 2465 | 309,250 | 41,466 | 3622 | 126 | 10,527 | 11,182 | 1366 |
| CSS/CO-3 | 260,387 | 475.6 | 2411 | 203,825 | 135,316 | 4983 | 210 | 96,636 | 12,786 | 1151 |
| CSS/CS-5 | 35,608 | 78.2 | <LQ | 337,858 | 28,450 | <LQ | 1240 | 677 | <LQ | 111 |
| CSS/CS-5 | 35,104 | 147.8 | <LQ | 214,850 | 10,251 | <LQ | 1137 | 77,757 | <LQ | 163 |
Appendix B
References
- Vermaat, J.G.; Van Der Bie, G.J. On the Occurrence of Copper in Tropical Soils. Plant Soil 1950, 2, 257–282. [Google Scholar] [CrossRef]
- Hortin, J.M.; Anderson, A.J.; Britt, D.W.; Jacobson, A.R.; McLean, J.E. Copper Oxide Nanoparticle Dissolution at Alkaline PH Is Controlled by Dissolved Organic Matter: Influence of Soil-Derived Organic Matter, Wheat, Bacteria, and Nanoparticle Coating. Environ. Sci. Nano 2020, 7, 2618–2631. [Google Scholar] [CrossRef]
- Jez, E.; Pellegrini, E.; Contin, M. Copper Bioavailability and Leaching in Conventional and Organic Viticulture under Environmental Stress. Appl. Sci. 2023, 13, 2595. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, J.; Li, Q.; Song, H.; Xia, D. Cu-Doped Magnetic Loofah Biochar for Tetracycline Degradation via Peroxymonosulfate Activation. New J. Chem. 2022, 46, 17223–17234. [Google Scholar] [CrossRef]
- Carneiro, J.S.d.S.; Leite, D.A. da C.; Castro, G.M. de; Franca, J.R.; Botelho, L.; Soares, J.R.; Oliveira, J.E. de; Melo, L.C.A. Biochar-Graphene Oxide Composite Is Efficient to Adsorb and Deliver Copper and Zinc in Tropical Soil. J. Clean. Prod. 2022, 360, 132170. [Google Scholar] [CrossRef]
- Cho, D.-W.; Kim, S.; Tsang, Y.F.; Song, H. Preparation of Nitrogen-Doped Cu-Biochar and Its Application into Catalytic Reduction of p-Nitrophenol. Environ. Geochem. Health 2019, 41, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Altıkat, A.; Alma, M.H.; Altıkat, A.; Bilgili, M.E.; Altıkat, S. A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability 2024, 16, 937. [Google Scholar] [CrossRef]
- Otoni, J.P.; Matoso, S.C.G.; Pérez, X.L.O.; da Silva, V.B. Potential for Agronomic and Environmental Use of Biochars Derived from Different Organic Waste. J. Clean. Prod. 2024, 449, 141826. [Google Scholar] [CrossRef]
- Hu, Z.; Wei, L. Review on Characterization of Biochar Derived from Biomass Pyrolysis via Reactive Molecular Dynamics Simulations. J. Compos. Sci. 2023, 7, 354. [Google Scholar] [CrossRef]
- Wijitkosum, S.; Jiwnok, P. Elemental Composition of Biochar Obtained from Agricultural Waste for Soil Amendment and Carbon Sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef]
- Liu, Q.; Zang, G.-L.; Zhao, Q. Removal of Copper Ions by Functionalized Biochar Based on a Multicomponent Ugi Reaction. RSC Adv. 2021, 11, 25880–25891. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, D.; Yan, Y.; Yang, C.; Fang, B.; Li, X.; Shao, Y.; Wang, H.; Yue, J.; Wang, Y.; et al. Short-Term Application of Chicken Manure under Different Nitrogen Rates Alters Structure and Co-Occurrence Pattern but Not Diversity of Soil Microbial Community in Wheat Field. Front. Microbiol. 2022, 13, 975571. [Google Scholar] [CrossRef] [PubMed]
- Heckler, G.S.; da Costa, R.C.; Fransozo, A.; Rosso, S.; Munehisa Shimizu, R. Long-Term Patterns of Spatial and Temporal Distribution in the Seabob Shrimp Xiphopenaeus Kroyeri (Decapoda: Penaeidae) Population in Southeastern Brazil. J. Crustac. Biol. 2014, 34, 326–333. [Google Scholar] [CrossRef]
- Wydro, U.; Jankowska, M.; Wołejko, E.; Kondzior, P.; Łozowicka, B.; Kaczyński, P.; Rodziewicz, J.; Janczukowicz, W.; Pietryczuk, A.; Cudowski, A.; et al. Changes in Soil Biological Properties after Sewage Sludge and Pesticide Application in Wheat Cultivation. Appl. Sci. 2022, 12, 11452. [Google Scholar] [CrossRef]
- Xu, L.; Xing, X.; Peng, J.; Ji, M. Effects of In Situ Remediation on Copper Distribution and Soil Aggregate Adsorption–Desorption Characteristics in Smelter-Impacted Soil. Front. Environ. Sci. 2022, 10, 816361. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Aleosfoor, A.; Rahimpour, M.R. Highly Dispersed Copper on Biochar Carbon as a Promising Catalyst for Reverse Water Gas Shift Reaction. J. Energy Inst. 2024, 115, 101680. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2017; pp. 23–38. ISBN 9781486305094. [Google Scholar]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM: West Conshohocken, PA, USA, 2013.
- Masek, O.; Buss, W.; Sohi, S. Standard Biochar Materials. Environ. Sci. Technol. 2018, 52, 9543–9544. [Google Scholar] [CrossRef]
- Granulométrica, B.A. Manual de Métodos Analíticos Oficiais para Fertilizantes Minerais, Orgânicos, Organominerais e Corretivos. 2014. Available online: http://sistemasweb.agricultura.gov.br/arquivosislegis/anexos/tm/INM28_2007_MAPA.pdf (accessed on 14 August 2023).
- Liberda, D.; Pięta, E.; Pogoda, K.; Piergies, N.; Roman, M.; Koziol, P.; Wrobel, T.P.; Paluszkiewicz, C.; Kwiatek, W.M. The Impact of Preprocessing Methods for a Successful Prostate Cell Lines Discrimination Using Partial Least Squares Regression and Discriminant Analysis Based on Fourier Transform Infrared Imaging. Cells 2021, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Harfield, D.; Hillen, B.; Stenzel, F.; Homung, A. Long-Termaging of Biochar. In Proceedings of the Biochar: Production, Characterization and Applications, Alba, Italy, 20–25 August 2017. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo 3a Edição Revista e Ampliada. In Man. Métodos Análise Solo—Capítulo 8—Densidade Partículas; Embrapa: Brasília, Brazil, 2017; Volume 574. [Google Scholar]
- Barrow, N.J.; Hartemink, A.E. The Effects of PH on Nutrient Availability Depend on Both Soils and Plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper Uptake, Essentiality, Toxicity, Detoxification and Risk Assessment in Soil-Plant Environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- de Novais, R.F.; Neves, J.C.L.; de Barros, N.F. Ensaio Em Ambiente Controlado. In Métodos de Pesquisa em Fertilidade de Solo; Embrapa-SEA: Brasília, Brazil, 1991; p. 392. [Google Scholar]
- do Carmo, D.L.; Silva, C.A.; de Lima, J.M.; Pinheiro, G.L. Electrical Conductivity and Chemical Composition of Soil Solution: Comparison of Solution Samplers in Tropical Soils. Rev. Bras. Ciência Do Solo 2016, 40, e0140795. [Google Scholar] [CrossRef]
- da Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Literasi Media Publishing: Kota Malang, Indonesia, 2009; Volume 2, ISBN 9786021018187. [Google Scholar]
- Factoextra R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 14 August 2023).
- Agricolae Pacote Agricolae—CRAN—Embalagem Agrícola. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 14 August 2023).
- Extract Extract and Visualize the Results of Multivariate Data Analyses, R Package Factoextra Version 1.0.7. 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 5 January 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Suzuki, R.; Hidetoshi, S. Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling; R Package; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wei, T.; Wei, A.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, T. Package “corrplot”: Visualization of a Correlation Matrix; (R package); R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://cran.r-project.org/web/packages/corrplot/index.html (accessed on 5 January 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; Mcgowan, D.L.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.P.; Delignette-Muller, M.L. A Toolbox for Nonlinear Regression in R: The Package Nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef]
- Akaike, H. A Bayesian Extension of the Minimum AIC Procedure of Autoregressive Model Fitting. Biometrika 1979, 66, 237–242. [Google Scholar] [CrossRef]
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; do Rosário Guimarães, I.; Guilherme, L.R.G. Combining Biochar and Sewage Sludge for Immobilization of Heavy Metals in Mining Soils. Ecotoxicol. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional Heterogeneity of Different Biochar: Effect of Pyrolysis Temperature and Feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, R.; Misztal, E.; Hrabak, J.; Banks, S.W.; Sajdak, M. Various Biomass Pyrolysis Conditions Influence the Porosity and Pore Size Distribution of Biochar. Energy 2023, 263, 126128. [Google Scholar] [CrossRef]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using Sewage Sludge with High Ash Content for Biochar Production and Cu(II) Sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo, Y.T. The Impact of Pyrolysis Temperature on Biochar Properties and Its Effects on Soil Hydrological Properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Walkiewicz, A.; Neugschwandtner, R.W.; Kopecký, M.; Zamanian, K.; Chen, W.-H.; Bucur, D. Feasibility of Biochar Derived from Sewage Sludge to Promote Sustainable Agriculture and Mitigate GHG Emissions—A Review. Int. J. Environ. Res. Public Health 2022, 19, 12983. [Google Scholar] [CrossRef]
- Schellekens, J.; Silva, C.A.; Buurman, P.; Rittl, T.F.; Domingues, R.R.; Justi, M.; Vidal-Torrado, P.; Trugilho, P.F. Molecular Characterization of Biochar from Five Brazilian Agricultural Residues Obtained at Different Charring Temperatures. J. Anal. Appl. Pyrolysis 2018, 130, 106–117. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Wang, Y.; Yu, J.; Li, N.; Wang, S. Cu/CuO-Decorated Peanut-Shell-Derived Biochar for the Efficient Degradation of Tetracycline via Peroxymonosulfate Activation. Catalysts 2023, 13, 1246. [Google Scholar] [CrossRef]
- Wang, C.; Dai, H.; Liang, L.; Li, N.; Cui, X.; Yan, B.; Chen, G. Enhanced Mechanism of Copper Doping in Magnetic Biochar for Peroxymonosulfate Activation and Sulfamethoxazole Degradation. J. Hazard. Mater. 2023, 458, 132002. [Google Scholar] [CrossRef] [PubMed]
- Chaves Fernandes, B.C.; Ferreira Mendes, K.; Dias Júnior, A.F.; da Silva Caldeira, V.P.; da Silva Teófilo, T.M.; Severo Silva, T.; Mendonça, V.; de Freitas Souza, M.; Valadão Silva, D. Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Paiva, I.; de Morais, E.G.; Jindo, K.; Silva, C.A. Biochar N Content, Pools and Aromaticity as Affected by Feedstock and Pyrolysis Temperature. Waste Biomass Valorization 2024, 15, 3599–3619. [Google Scholar] [CrossRef]
- Tu, P.; Zhang, G.; Wei, G.; Li, J.; Li, Y.; Deng, L.; Yuan, H. Influence of Pyrolysis Temperature on the Physicochemical Properties of Biochars Obtained from Herbaceous and Woody Plants. Bioresour. Bioprocess. 2022, 9, 131. [Google Scholar] [CrossRef]
- Nepal, J.; Ahmad, W.; Munsif, F.; Khan, A.; Zou, Z. Advances and Prospects of Biochar in Improving Soil Fertility, Biochemical Quality, and Environmental Applications. Front. Environ. Sci. 2023, 11, 1114752. [Google Scholar] [CrossRef]
- Dong, X.; Wang, C.; Li, H.; Wu, M.; Liao, S.; Zhang, D.; Pan, B. The Sorption of Heavy Metals on Thermally Treated Sediments with High Organic Matter Content. Bioresour. Technol. 2014, 160, 123–128. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis Temperature-Dependent Carbon Retention and Stability of Biochar with Participation of Calcium: Implications to Carbon Sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef] [PubMed]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Praus, L.; Száková, J.; Tlustoš, P. The Role of Biochar and Soil Properties in Determining the Available Content of Al, Cu, Zn, Mn, and Cd in Soil. Agronomy 2020, 10, 885. [Google Scholar] [CrossRef]
- Joseph, S.; Graber, E.R.; Chia, C.; Munroe, P.; Donne, S.; Thomas, T.; Nielsen, S.; Marjo, C.; Rutlidge, H.; Pan, G.X.; et al. Shifting Paradigms: Development of High-Efficiency Biochar Fertilizers Based on Nano-Structures and Soluble Components. Carbon Manag. 2013, 4, 323–343. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Liu, H.; Zhao, Y.; Li, L. Effects of Pyrolysis Temperature on Biochar’s Characteristics and Speciation and Environmental Risks of Heavy Metals in Sewage Sludge Biochars. Environ. Technol. Innov. 2022, 26, 102288. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Mohammed, E.A.; Shaban, M.; Abou Kana, M.T.H.; Negm, N.A. Synthesis, Characterization and Catalytic Performances of Activated Carbon-Doped Transition Metals during Biofuel Production from Waste Cooking Oils. J. Mol. Liq. 2020, 306, 112749. [Google Scholar] [CrossRef]
- Li, L.; Niu, W.; Cong, H.; Meng, H.; Niu, Z.; Shen, X.; Cao, L.; Kong, X. Effects of Pyrolysis Temperature on the Release Characteristics of Polycyclic Aromatic Hydrocarbons during Pyrolysis of Corn Stover Pellet. BioResources 2023, 18, 2112–2136. [Google Scholar] [CrossRef]
- Manogaran, M.D.; Shamsuddin, R.; Mohd Yusoff, M.H.; Lay, M.; Siyal, A.A. A Review on Treatment Processes of Chicken Manure. Clean. Circ. Bioeconomy 2022, 2, 100013. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.-V.N.; Arun, J.; Gopinath, K.P. Production, Characterization, Activation and Environmental Applications of Engineered Biochar: A Review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Sieber, V.; Hofer, M.; Brück, W.M.; Garbe, D.; Brück, T.; Lynch, C.A. ChiBio: An Integrated Bio-Refinery for Processing Chitin-Rich Bio-Waste to Specialty Chemicals. In Grand Challenges in Marine Biotechnology. Grand Challenges in Biology and Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 555–578. ISBN 978-3-319-69075-9. [Google Scholar]
- Elsherpiny, M.A. Effect of Organic Amendments and Synthetic Substances on Copper Availability, Absorption, and Wheat Productivity. Egypt. J. Soil Sci. 2023, 63, 429–442. [Google Scholar] [CrossRef]
- Lou, T.; Yin, Y.; Wang, J. Mechanisms of Biochar Promoting Medium Chain Fatty Acids Production from Sewage Sludge: Effect of Biochar Type. J. Clean. Prod. 2024, 451, 141995. [Google Scholar] [CrossRef]
- Xiao, Y.; Xue, Y.; Gao, F.; Mosa, A. Sorption of Heavy Metal Ions onto Crayfish Shell Biochar: Effect of Pyrolysis Temperature, PH and Ionic Strength. J. Taiwan Inst. Chem. Eng. 2017, 80, 114–121. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of Chitin-and Chitosan-Derivatives for the Detoxification of Water and Wastewater—A Short Review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhu, W.; He, L.; Tan, F.; Zhu, N.; Zhou, Q.; He, M.; Hu, G. Calcium-Rich Biochar from Crab Shell: An Unexpected Super Adsorbent for Dye Removal. Bioresour. Technol. 2018, 267, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Wu, S.; Han, J.; Dong, T.; Zhu, C.; Li, X.; Xu, L.; Zhang, Y.; Zhou, M.; Pan, Y. Sulfide-Modified Zero-Valent Iron Activated Periodate for Sulfadiazine Removal: Performance and Dominant Routine of Reactive Species Production. Water Res. 2022, 220, 118676. [Google Scholar] [CrossRef]
- Shuailong, C.; Qintie, L.I.N.; Rongbo, X.; Haoyu, L.U.O.; Hengyi, F.U. Removal of EDTA-Cu in Wastewater by Copper-Based Biochar Activated Sodium Persulfate. Chin. J. Environ. Eng. 2020, 14, 3298–3307. [Google Scholar]
- Karimi, B.; Masson, V.; Guilland, C.; Leroy, E.; Pellegrinelli, S.; Giboulot, E.; Maron, P.-A.; Ranjard, L. Ecotoxicity of Copper Input and Accumulation for Soil Biodiversity in Vineyards. Environ. Chem. Lett. 2021, 19, 2013–2030. [Google Scholar] [CrossRef]
- Meenu; Rani, M.; Shanker, U. Efficient Photodegradation of Hexabromocyclododecane Leached from Polystyrene by Biochar and Sulfur Doped CuO Nanocomposite: Optimization Factors, Kinetics, and Photoactivity. Environ. Pollut. 2024, 340, 122818. [Google Scholar] [CrossRef]
- Alva, A.K.; Graham, J.H.; Anderson, C.A. Soil PH and Copper Effects on Young ‘Hamlin’Orange Trees. Soil Sci. Soc. Am. J. 1995, 59, 481–487. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen Decades of Antimicrobial Copper Compounds Applied in Agriculture. A Review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Xu, P.; Gao, Y.; Cui, Z.; Wu, B.; Yan, B.; Wang, Y.; Zaitongguli, K.; Wen, M.; Wang, H.; Jing, N.; et al. Research Progress on Effects of Biochar on Soil Environment and Crop Nutrient Absorption and Utilization. Sustainability 2023, 15, 4861. [Google Scholar] [CrossRef]
- Brasil Instrução Normativa MAPA No 46 DE 22/11/2016—Federal—LegisWeb. Available online: https://www.legisweb.com.br/legislacao/?id=332894 (accessed on 27 June 2024).
- Di Palma, L.; Mecozzi, R. Heavy Metals Mobilization from Harbour Sediments Using EDTA and Citric Acid as Chelating Agents. J. Hazard. Mater. 2007, 147, 768–775. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Zhao, H.; Li, Q. A Comparative Study on Behavior of Heavy Metals in Pyrochar and Hydrochar from Sewage Sludge. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 565–571. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Pan, X.-W.; Tan, J.-Y.; Yang, S.-S.; Wu, J.-T.; Chen, C.; Yuan, Y.; Ren, N.-Q. Sewage Sludge Derived Biochar for Environmental Improvement: Advances, Challenges, and Solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S. Influence of Pyrolysis Temperature on Properties and Environmental Safety of Heavy Metals in Biochars Derived from Municipal Sewage Sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Rúa-Díaz, S.; Forjan, R.; Lago-Vila, M.; Cerqueira, B.; Arco-Lázaro, E.; Marcet, P.; Baragaño, D.; Gallego, J.L.R.; Covelo, E.F. Pyrolysis Temperature Influences the Capacity of Biochar to Immobilize Copper and Arsenic in Mining Soil Remediation. Environ. Sci. Pollut. Res. 2023, 30, 32882–32893. [Google Scholar] [CrossRef]
- Losacco, D.; Campanale, C.; Tumolo, M.; Ancona, V.; Massarelli, C.; Uricchio, V.F. Evaluating the Influence of Nitrogen Fertilizers and Biochar on Brassica oleracea L. var. botrytis by the Use of Fourier Transform Infrared (FTIR) Spectroscopy. Sustainability 2022, 14, 11985. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Chen, H.; Wang, W.; Yang, Y. Comparative Study of Biochar Modified with Different Functional Groups for Efficient Removal of Pb(II) and Ni(II). Int. J. Environ. Res. Public Health 2021, 19, 11163. [Google Scholar] [CrossRef] [PubMed]
- Atchaya, S.; Meena Devi, J. Experimental Investigation on Structural, Optical, Electrical and Magnetic Properties of Copper Oxide Nanoparticles. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2024, 94, 153–160. [Google Scholar] [CrossRef]
- Sukumar, S.; Rudrasenan, A.; Padmanabhan Nambiar, D. Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia Bonducella Seed Extract and Their Applications. ACS Omega 2020, 5, 1040–1051. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Umer, M.; Riaz, S.; Naseem, S. Characterization of Copper Oxide Nanoparticles Fabricated by the Sol–Gel Method. J. Electron. Mater. 2015, 44, 3704–3709. [Google Scholar] [CrossRef]
- Abdullah, R.; Ishak, C.F.; Osman, N.; Halim, N.S.A.; Panhwar, Q.A. Determining the Characteristics and Potential of Plantbased Biochars to Reduce Copper Uptake in Maize. Bragantia 2021, 80, e2221. [Google Scholar] [CrossRef]
- Thapa, S.; Bhandari, A.; Ghimire, R.; Xue, Q.; Kidwaro, F.; Ghatrehsamani, S.; Maharjan, B.; Goodwin, M. Managing Micronutrients for Improving Soil Fertility, Health, and Soybean Yield. Sustainability 2021, 13, 11766. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, F.-Y.; Liu, S.-H.; Ma, C.-Y. Optimized Combination of Zero-Valent Iron and Oxygen-Releasing Biochar as Cathodes of Microbial Fuel Cells to Enhance Copper Migration in Sediment. Bioelectrochemistry 2024, 158, 108699. [Google Scholar] [CrossRef]
- Abbasi, M.; Rizvi, O.S.; Hussain, S.Z.; Jilani, A.; Osra, F.A.; Kajjumba, G.W.; Bhatnagar, A.; Khan, E.; Abbas, T. Nano-Zerovalent Copper Biochar Composite for Treating Selenium Oxyanions in Water: Synthesis, Evaluation, Removal Mechanism, Density Functional Theory, and Molecular Dynamics Simulations. Chem. Eng. J. 2024, 485, 149954. [Google Scholar] [CrossRef]
- Jalali, M.; Imanifard, A.; Jalali, M. Heavy Metals Accumulation in Wheat (Triticum aestivum L.) Roots and Shoots Grown in Calcareous Soils Treated with Non-Spiked and Spiked Sewage Sludge. Environ. Sci. Pollut. Res. 2023, 30, 20862–20873. [Google Scholar] [CrossRef]
- Wu, P.; Guo, Z.; Hua, K.; Wang, D. Long-Term Application of Organic Amendments Changes Heavy Metals Accumulation in Wheat Grains by Affecting Soil Chemical Properties and Wheat Yields. J. Soils Sediments 2023, 23, 2136–2147. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Wen, Y.; Cui, T.; Liu, Y.; Zhang, Y.; Xu, R. Insights into Influence of Aging Processes on Zero-Valent Iron Modified Biochar in Copper(II) Immobilization: From Batch Solution to Pilot-Scale Investigation. Front. Chem. Sci. Eng. 2023, 17, 880–892. [Google Scholar] [CrossRef]
| Acronym | Feedstock | Temperature (°C) | Cu Source |
|---|---|---|---|
| FCM | Chicken Manure | 60 | no |
| FSC | Shrimp Shell | ||
| FSS | Sewage Sludge | ||
| BCM-3 | Chicken Manure | 300 | No |
| BSC-3 | Shrimp Shell | ||
| BSS-3 | Sewage Sludge | ||
| CCM/CS-3 | Chicken Manure | 300 | Copper Sulfate |
| CCM/CO-3 | Chicken Manure | Copper Oxide | |
| CSC/CS-3 | Shrimp Shell | Copper Sulfate | |
| CSC/CO-3 | Shrimp Shell | Copper Oxide | |
| CSS/CS-3 | Sewage Sludge | Copper Sulfate | |
| CSS/CO-3 | Sewage Sludge | Copper Oxide | |
| BCM-5 | Chicken Manure | 550 | No |
| BSC-5 | Shrimp Shell | ||
| BSS-5 | Sewage Sludge | ||
| CCM/CS-5 | Chicken Manure | 550 | Copper Sulfate |
| CCM/CO-5 | Chicken Manure | Copper Oxide | |
| CSC/CS-5 | Shrimp Shell | Copper Sulfate | |
| CSC/CO-5 | Shrimp Shell | Copper Oxide | |
| CSS/CS-5 | Sewage Sludge | Copper Sulfate | |
| CSS/CO-5 | Sewage Sludge | Copper Oxide |
| Oxisol | pH | C | Clay | Silt | Sand | N | P | K | Ca | Mg | Fe | Mn | B | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ---------- g kg−1 ---------- | --- mg dm−3 --- | cmolc dm−3 | ------------ mg dm−3 ------------- | ||||||||||||
| RYL | 4.7 | 4.5 | 460 | 85 | 455 | 329 | 6.1 | 217.5 | 0.8 | 0.4 | 35.7 | 3.0 | 0.1 | 0.0 | 0.2 |
| DRL | 4.5 | 19.8 | 770 | 100 | 130 | 2580 | 8.3 | 30.1 | 0.3 | 0.2 | 50.6 | 3.4 | 0.06 | 1.1 | 0.3 |
| Biochar | pH | EC | Yield | VM | Ash | FC | Elemental Composition | C:N Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| (dS m−1) | --------------------------------(%)------------------------------------- | ||||||||
| C | N | C:N | |||||||
| BCM-3 | 9.1 | 6.4 | 69 | 57 | 52 | 9.3 | 35 | 2.1 | 16.6 |
| BCM-5 | 10.7 | 8.2 | 53 | 56 | 60 | 11.1 | 26 | 1.9 | 13.1 |
| BSC-3 | 9.2 | 7.3 | 81 | 72 | 32 | 3.9 | 41 | 6.5 | 6.4 |
| BSC-5 | 10.4 | 2.9 | 60 | 53 | 60 | 12.5 | 25 | 2.4 | 10.7 |
| BSS-3 | 6.2 | 1.3 | 88 | 34 | 71 | 4.8 | 16 | 2.1 | 7.6 |
| BSS-5 | 7.1 | 1.5 | 69 | 28 | 78 | 5.4 | 12 | 1.2 | 10.3 |
| Composite | pH | EC | Yield | VM | Ashes | FC | Elemental Composition | C:N Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| (dS m−1) | --------------------------------------------(%)-------------------------------- | ||||||||
| C | N | C:N | |||||||
| CCM/CS-3 | 4.8 ± 0.1 | 21 ± 0.1 | 65 | 25 ± 0.5 | 36 ± 0.1 | 36 ± 0.5 | 17 ± 0.1 | 3 ± 0.1 | 5 ± 0.1 |
| CCM/CS-5 | 10 ± 0.1 | 9 ± 0.1 | 47 | 14 ± 0.1 | 19 ± 0.9 | 66 ± 0.9 | 19 ± 0.1 | 2.3 ± 0.1 | 8 ± 0.1 |
| CCM/CO-3 | 8 ± 0.1 | 7 ± 0.1 | 71 | 44 ± 0.1 | 45 ± 1.4 | 10 ± 1.4 | 27 ± 0.1 | 4.6 ± 0.1 | 6 ± 0.1 |
| CCM/CO-5 | 11 ± 0.1 | 8 ± 0.1 | 59 | 29 ± 0.5 | 33 ± 1.3 | 37 ± 1.8 | 22 ± 0.1 | 2 ± 0.1 | 11 ± 0.5 |
| CSC/CS-3 | 5 ± 0. 1 | 23 ± 0.1 | 64 | 56 ± 0.1 | 33 ± 1.3 | 9 ± 1.1 | 13 ± 0.1 | 4 ± 0.1 | 3.5 ± 0.1 |
| CSC/CS-5 | 10 ± 0.1 | 3.5 ± 0.1 | 44 | 20 ± 0.8 | 15 ± 0.9 | 64 ± 1.5 | 19.5 ± 0.1 | 4 ± 0.1 | 5.5 ± 0.1 |
| CSC/CO-3 | 9 ± 0.1 | 5 ± 0.1 | 84 | 50 ± 0.4 | 46 ± 0.1 | 1.6 ± 0.5 | 26.5 ± 0.1 | 4.4 ± 0.4 | 6 ± 0.5 |
| CSC/CO-5 | 10 ± 0.1 | 3 ± 0.1 | 54 | 22 ± 0.2 | 32 ± 1.2 | 46 ± 1.3 | 21 ± 0.1 | 3.3 ± 0.1 | 6.3 ± 0.2 |
| CSS/CS-3 | 4 ± 0.1 | 25 ± 0.1 | 72 | 30 ± 0.2 | 29 ± 0.4 | 39 ± 0.6 | 6 ± 0.1 | 1.4 ± 0.2 | 4 ± 0.6 |
| CSS/CS-5 | 7 ± 0.1 | 2 ± 0.1 | 48 | 8 ± 0.04 | 19 ± 0.1 | 73 ± 0.1 | 6 ± 0.1 | 1.2 ± 0.1 | 5.4 ± 0.1 |
| CSS/CO-3 | 5 ± 0.1 | 3 ± 0.1 | 90 | 33 ± 0.1 | 27 ± 1.7 | 40 ± 1.6 | 13.5 ± 0.1 | 2.2 ± 0.1 | 6 ± 0.1 |
| CSS/CO-5 | 8 ± 0.1 | 1 ± 0.1 | 72 | 28 ± 0.5 | 19 ± 1.4 | 46 ± 2.0 | 11 ± 0.1 | 1.4 ± 0.1 | 7.5 ± 0.1 |
| Copper (Cu) | |||||||
|---|---|---|---|---|---|---|---|
| Acronym | W | T | NAC | CA% | W Index | NAC Index | CA% Index |
| ------------------------(g kg−1)----------------------- | -----------------(%)---------------- | ||||||
| CCM/CS-3 | 78 ± 8.2 | 233 ± 3.3 | 147 ± 6.7 | 79 ± 6.2 | 33± 3.5 | 63 ± 2.6 | 34 ± 2.6 |
| CCM/CS-5 | 0.1 ± 0. 1 | 282 ± 1.2 | 57 ± 0.7 | 30 ± 0.1 | 0.1 ± 0.1 | 20 ± 0.2 | 11 ± 0.1 |
| CCM/CO-3 | 0.3 ± 0.1 | 111 ± 0.4 | 73 ± 3.9 | 8 ± 2.0 | 0.3 ± 0.1 | 65± 3.3 | 7 ± 1.8 |
| CCM/CO-5 | 0.1 ± 0. 1 | 237 ± 1.0 | 141 ± 0.6 | 52 ± 1.3 | 0. 1 ± 0.1 | 60 ± 0.5 | 22 ± 0.6 |
| CSC/CS-3 | 66 ± 0.1 | 302 ± 2.8 | 221 ± 0.7 | 135 ± 0.1 | 22 ± 0.2 | 72 ± 0.4 | 45 ± 0.4 |
| CSC/CS-5 | 0.1 ± 0. 1 | 281 ± 0.8 | 29 ± 5.2 | 28 ± 2.3 | 0. 1 ± 0.1 | 10± 1.9 | 10 ± 0.8 |
| CSC/CO-3 | 1.2 ± 0.2 | 241± 0.1 | 168 ± 2.1 | 16 ± 2.0 | 0.5 ± 0.1 | 70 ± 0.9 | 6.5 ± 0.8 |
| CSC/CO-5 | 0.1 ± 0.1 | 233 ± 3.3 | 142 ± 0.1 | 42 ± 10.7 | 0.1 ± 0.1 | 61 ± 0.9 | 18 ± 4.5 |
| CSS/CS-3 | 194 ± 7.2 | 309 ± 1.7 | 213 ± 1.5 | 155 ± 4.5 | 63 ± 2.1 | 69 ± 0.4 | 50 ± 1.6 |
| CSS/CS-5 | 0.2 ± 0.1 | 338 ± 5.6 | 19 ± 1.1 | 31 ± 2.4 | 0.1 ± 0.1 | 6 ± 0.4 | 9 ± 0.6 |
| CSS/CO-3 | 6 ± 0.1 | 204 ± 1.7 | 138 ± 4.2 | 55 ± 5.5 | 3 ± 0.3 | 68 ± 1.5 | 27 ± 2.9 |
| CSS/CO-5 | 0.2 ± 0.1 | 215 ± 0.8 | 89 ± 2.4 | 30 ± 0.2 | 0.1 ± 0.1 | 42 ± 1.0 | 14 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisté, L.; Silva, C.A.; Rabêlo, F.H.S.; Jindo, K.; Melo, L.C.A. Evaluating Copper-Doped Biochar Composites for Improving Wheat Nutrition and Growth in Oxisols. Agronomy 2025, 15, 144. https://doi.org/10.3390/agronomy15010144
Chisté L, Silva CA, Rabêlo FHS, Jindo K, Melo LCA. Evaluating Copper-Doped Biochar Composites for Improving Wheat Nutrition and Growth in Oxisols. Agronomy. 2025; 15(1):144. https://doi.org/10.3390/agronomy15010144
Chicago/Turabian StyleChisté, Loren, Carlos Alberto Silva, Flávio Henrique Silveira Rabêlo, Keiji Jindo, and Leônidas Carrijo Azevedo Melo. 2025. "Evaluating Copper-Doped Biochar Composites for Improving Wheat Nutrition and Growth in Oxisols" Agronomy 15, no. 1: 144. https://doi.org/10.3390/agronomy15010144
APA StyleChisté, L., Silva, C. A., Rabêlo, F. H. S., Jindo, K., & Melo, L. C. A. (2025). Evaluating Copper-Doped Biochar Composites for Improving Wheat Nutrition and Growth in Oxisols. Agronomy, 15(1), 144. https://doi.org/10.3390/agronomy15010144







