Species Composition of Phytophagous and Entomophagous Insects and Mites on Soybeans in Krasnodar and Stavropol Territories, Russia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AgroXXI.ru. Soya v Rossii—Dejstvitel’nost’ i Vozmozhnost’ [Soybeans in Russia—Reality and Opportunity]. Available online: https://www.agroxxi.ru/monitoring-selskohozjaistvennyh-tovarov/soja-v-rossii-deistvitelnost-i-vozmozhnost.html (accessed on 13 June 2024).
- Ismailov, V.Y.; Pushnya, M.V.; Komantsev, A.A.; Zamotailov, A.S. Development of methods for controlling the number of click beetles on soybeans using synthetic sex pheromones. Proc. Kuban State Agrar. Univ. 2022, 95, 87–93. [Google Scholar] [CrossRef]
- Fedorova, S.N. Vrednaya entomofauna soevogo agrotsenoza v Orlovskoi oblasti [Destructive entomological fauna of soybean agrocenosis in the Oryol region]. Zernobobovye Krupyanye Kult. 2013, 4, 58–63. [Google Scholar]
- Lee, S.T.; Li, C.; Davis, J.A. Predator-Pest Dynamics of Arthropods Residing in Louisiana Soybean Agroecosystems. Insects 2022, 13, 154. [Google Scholar] [CrossRef]
- Kanobe, C.; McCarville, M.T.; O’Neal, M.E.; Tylka, G.L.; MacIntosh, G.C. Soybean Aphid Infestation Induces Changes in Fatty Acid Metabolism in Soybean. PLoS ONE 2015, 10, e0145660. [Google Scholar] [CrossRef]
- Bhusal, S.J.; Koch, R.L.; Lorenz, A.J. Variation in Soybean Aphid (Hemiptera: Aphididae) Biotypes within Fields. J. Econ. Entomol. 2021, 114, 1336–1344. [Google Scholar] [CrossRef]
- Lu, W.; Wang, M.; Xu, Z.; Shen, G.; Wei, P.; Li, M.; Reid, W.; He, L. Adaptation of acaricide stress facilitates Tetranychus urticae expanding against Tetranychus cinnabarinus in China. Ecol. Evol. 2017, 7, 1233–1249. [Google Scholar] [CrossRef]
- Herron, G.A.; Langfield, K.L.; Chen, Y.; Wilson, L.J. Development of abamectin resistance in Tetranychus urticae in Australian cotton and the establishment of discriminating doses for T. lambi. Exp. Appl. Acarol. 2021, 83, 325–341. [Google Scholar] [CrossRef]
- Maksimovich, Y.V. Obosnovanie meropriyatii po zashchite soi ot obyknovennogo pautinnogo kleshcha (Tetranychus urticae Koch.). Zaŝita Rastenij 2017, 41, 255–262. [Google Scholar]
- Bueno, A.F.; Panizzi, A.R.; Hunt, T.E.; Dourado, P.M.; Pitta, R.M.; Gonçalves, J. Challenges for Adoption of Integrated Pest Management (IPM): The Soybean Example. Neotrop. Entomol. 2021, 50, 5–20. [Google Scholar] [CrossRef]
- Goebel, K.M.; Davros, N.M.; Andersen, D.E.; Rice, P.J. Tallgrass prairie wildlife exposure to spray drift from commonly used soybean insecticides in Midwestern USA. Sci. Total Environ. 2022, 818, 151745. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Ferreira, C.P.; Ramalho, F.S.; Godoy, W.A.C.; Pachú, J.K.S.; Omoto, C.; Neto, D.O.A.; Padovez, F.E.O.; Silva, L.B. Modeling the Resistance Evolution to Insecticides Driven by Lepidopteran Species Competition in Cotton, Soybean, and Corn Crops. Biology 2022, 11, 1354. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, E.A.; Warpechowski, L.F.; Braga, L.E.; Morin, M.; Tenório, C.; Boff, J.S.; Bernardi, O.; Farias, J.R. Intra- and Interspecific Variation in the Susceptibility to Insecticides of Stink Bugs (Hemiptera: Pentatomidae) That Attack Soybean and Maize in Southern Brazil. J. Econ. Entomol. 2022, 115, 631–636. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Franco, E.; Walsh, D.B.; Lavine, M.; Lavine, L.; Zhu, F. Phenotypic and Genotypic Plasticity of Acaricide Resistance in Populations of Tetranychus urticae (Acari: Tetranychidae) on Peppermint and Silage Corn in the Pacific Northwest. J. Econ. Entomol. 2018, 111, 2831–2843. [Google Scholar] [CrossRef]
- İnak, E. Geographical distribution and origin of acetylcholinesterase mutations conferring acaricide resistance in Tetranychus urticae populations from Turkey. Exp. Appl. Acarol. 2022, 86, 49–59. [Google Scholar] [CrossRef]
- Hanson, A.A.; Menger-Anderson, J.; Silverstein, C.; Potter, B.D.; MacRae, I.V.; Hodgson, E.W.; Koch, R.L. Evidence for Soybean Aphid (Hemiptera: Aphididae) Resistance to Pyrethroid Insecticides in the Upper Midwestern United States. J. Econ. Entomol. 2017, 110, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.N.; Pritchard, S.; Tyndall, J.C.; Hodgson, E.W.; O’Neal, M.E. Evaluating Soybean Aphid-Resistant Varieties in Different Environments to Estimate Financial Outcomes. J. Econ. Entomol. 2020, 113, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Valmorbida, I.; Muraro, D.S.; Hodgson, E.W.; O’Neal, M.E. Soybean aphid (Hemiptera: Aphididae) response to lambda-cyhalothrin varies with its virulence status to aphid-resistant soybean. Pest. Manag. Sci. 2020, 76, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.F.; Tavares, C.S.; Araújo, E.O.; Picanço, M.C.; Oliveira, E.E.; Pereira, E.J.G. Plant Resistance in Some Modern Soybean Varieties May Favor Population Growth and Modify the Stylet Penetration of Bemisia tabaci (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2021, 114, 970–978. [Google Scholar] [CrossRef]
- Klai, K.; Chénais, B.; Zidi, M.; Djebbi, S.; Caruso, A.; Denis, F.; Confais, J.; Badawi, M.; Casse, N.; Mezghani Khemakhem, M. Screening of Helicoverpa armigera Mobilome Revealed Transposable Element Insertions in Insecticide Resistance Genes. Insects 2020, 11, 879. [Google Scholar] [CrossRef]
- da Silva, F.R.; Trujillo, D.; Bernardi, O.; Verle Rodrigues, J.C.; Bailey, W.D.; Gilligan, T.M.; Carrillo, D. Comparative Toxicity of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) to Selected Insecticides. Insects 2020, 11, 431. [Google Scholar] [CrossRef]
- Wu, C.; Ding, C.; Chen, S.; Wu, X.; Zhang, L.; Song, Y.; Li, W.; Zeng, R. Exposure of Helicoverpa armigera Larvae to Plant Volatile Organic Compounds Induces Cytochrome P450 Monooxygenases and Enhances Larval Tolerance to the Insecticide Methomyl. Insects 2021, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Zaostrovnyh, V.I.; Dubovickaya, L.K. Vrednye Organizmy soi i Sistema Fitosanitarnoj Optimizacii eyo Posevov [Harmful Organisms of Soybeans and the System of Phytosanitary Optimization of Its Crops]; Izdatel’stvo Maksachuk N. L.: Novosibirsk, Russia, 2003. [Google Scholar]
- Hunt, L.; Bonetto, C.; Marrochi, N.; Scalise, A.; Fanelli, S.; Liess, M.; Lydy, M.J.; Chiu, M.C.; Resh, V.H. Species at Risk (SPEAR) index indicates effects of insecticides on stream invertebrate communities in soy production regions of the Argentine Pampas. Sci. Total Environ. 2017, 580, 699–709. [Google Scholar] [CrossRef]
- Di Bartolomeis, M.; Kegley, S.; Mineau, P.; Radford, R.; Klein, K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS ONE 2019, 14, e0220029. [Google Scholar] [CrossRef]
- Moscardi, F. Assessment of the application of baculoviruses for control of Lepidoptera. Annu. Rev. Entomol. 1999, 44, 257–289. [Google Scholar] [CrossRef]
- Pastucha, A. Wplyw zaprawiania nasion chitozanem na adrowinoce I plutomania soi (Glycine max (L.) Merrill) [The effect of chitosan seed treatment on soybean health and yield (Glycine max (L.) Merrill)]. Biul. Inst. Hod. Aklim. Rosl. 2001, 217, 287–295. [Google Scholar]
- Schünemann, R.; Knaak, N.; Fiuza, L.M. Mode of Action and Specificity of Bacillus thuringiensis Toxins in the Control of Caterpillars and Stink Bugs in Soybean Culture. ISRN Microbiol. 2014, 2014, 135675. [Google Scholar] [CrossRef]
- Borges, F.S.P.; Loureiro, E.S.; Jaurretche, J.E.; Pessoa, L.G.A.; Arruda, L.A.; Dias, P.M.; Navarrete, A.A. Performance of phytosanitary products for control of soybean caterpillar. An. Acad. Bras. Cienc. 2021, 93, e20200205. [Google Scholar] [CrossRef]
- Faria, M.; Mascarin, G.M.; Butt, T.; Lopes, R.B. On-farm Production of Microbial Entomopathogens for use in Agriculture: Brazil as a Case Study. Neotrop. Entomol. 2023, 52, 122–133. [Google Scholar] [CrossRef]
- Lartigue, S.; Yalaoui, M.; Belliard, J.; Caravel, C.; Jeandroz, L.; Groussier, G.; Calcagno, V.; Louâpre, P.; Dechaume-Moncharmont, F.X.; Malausa, T.; et al. Consistent variations in personality traits and their potential for genetic improvement in biocontrol agents: Trichogramma evanescens as a case study. Evol. Appl. 2022, 15, 1565–1579. [Google Scholar] [CrossRef]
- Bzowska-Bakalarz, M.; Bulak, P.; Bereś, P.K.; Czarnigowska, A.; Czarnigowski, J.; Karamon, B.; Pniak, M.; Bieganowski, A. Using gyroplane for application of Trichogramma spp. against the European corn borer in maize. Pest. Manag. Sci. 2020, 76, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Atashi, N.; Shishehbor, P.; Seraj, A.A.; Rasekh, A.; Hemmati, S.A.; Riddick, E.W. Effects of Helicoverpa armigera Egg Age on Development, Reproduction, and Life Table Parameters of Trichogramma euproctidis. Insects 2021, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Borzoui, E.; Naseri, B.; Mohammadzadeh-Bidarani, M. Adaptation of Habrobracon hebetor (Hymenoptera: Braconidae) to Rearing on Ephestia kuehniella (Lepidoptera: Pyralidae) and Helicoverpa armigera (Lepidoptera: Noctuidae). J. Insect Sci. 2016, 16, 12. [Google Scholar] [CrossRef]
- Artohin, K.S. Opredelitel’ Nasekomyh Yuga Rossii [The Determinant of Insects in the South of Russia]; Foundation: Rostov-na-Donu, Russia, 2016; p. 1036. [Google Scholar]
- Misrieva, B.U.; Shamsudinova, M.M. Study of the role of perspective types of entomophages in agrobiocenosis of Dagestan. Veg. Crops Russia 2017, 1, 87–91. [Google Scholar] [CrossRef]
- Reiff, J.M.; Kolb, S.; Entling, M.H.; Herndl, T.; Möth, S.; Walzer, A.; Kropf, M.; Hoffmann, C.; Winter, S. Organic Farming and Cover-Crop Management Reduce Pest Predation in Austrian Vineyards. Insects 2021, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.A.; Smith, J.; Clark, S.; Knupp, K.; Haddad, N.M. Ant Communities and Ecosystem Services in Organic Versus Conventional Agriculture in the U.S. Corn Belt. Environ. Entomol. 2021, 50, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Mupepele, A.C.; Bruelheide, H.; Brühl, C.; Dauber, J.; Fenske, M.; Freibauer, A.; Gerowitt, B.; Krüß, A.; Lakner, S.; Plieninger, T.; et al. Biodiversity in European agricultural landscapes: Transformative societal changes needed. Trends Ecol. Evol. 2021, 36, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batáry, P. Beyond organic farming—Harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Sugonyaev, E.S. Puteshestviya za Nasekomymi. Ex Autopsia [Traveling for Insects. Ex Autopsia]; Russkoe Entomologicheskoe Obshchestvo: St. Petersburg, Russia, 2011. [Google Scholar]
- Agasyeva, I.; Nefedova, M.; Ismailov, V.; Nastasy, A. Entomophages of the Colorado Potato Beetle, Population Dynamics of Perillus bioculatus Fabr. and Its Compatibility with Biological and Chemical Insecticides. Agronomy 2023, 13, 1496. [Google Scholar] [CrossRef]
- Hesler, L.S. Inventory and assessment of foliar natural enemies of the soybean aphid (Hemiptera: Aphididae) in South Dakota. Environ. Entomol. 2014, 43, 577–588. [Google Scholar] [CrossRef]
- Vition, P.G. Predatory natural entomophages of soybean pests. In Proceedings of the Biological Plant Protection Is the Basis for Stabilizing Agroecosystems: Materials of the International Research and Practice Conference, Krasnodar, Russia, 11–13 September 2018; Volume 10, pp. 86–90. [Google Scholar]
- Almdal, C.D.; Costamagna, A.C. Annual Crops Contribute More Predators than Perennial Habitats during an Aphid Outbreak. Insects 2023, 14, 624. [Google Scholar] [CrossRef]
- Moonga, M.N.; Kamminga, K.; Davis, J.A. Status of Stink Bug (Hemiptera: Pentatomidae) Egg Parasitoids in Soybeans in Louisiana. Environ. Entomol. 2018, 47, 1459–1464. [Google Scholar] [CrossRef]
- Pereira, F.P.; Reigada, C.; Diniz, A.J.F.; Parra, J.R.P. Potential of Two Trichogrammatidae species for Helicoverpa armigera control. Neotrop. Entomol. 2019, 48, 966–973. [Google Scholar] [CrossRef]
- Fidelis, E.G.; Querino, R.B.; Adaime, R. The Amazon and Its Biodiversity: A Source of Unexplored Potential Natural Enemies for Biological Control (Predators and Parasitoids). Neotrop. Entomol. 2023, 52, 152–171. [Google Scholar] [CrossRef]
- Herlihy, M.V.; Talamas, E.J.; Weber, D.C. Attack and Success of Native and Exotic Parasitoids on Eggs of Halyomorpha halys in Three Maryland Habitats. PLoS ONE 2016, 11, e0150275. [Google Scholar] [CrossRef]
- Pezzini, D.T.; Nystrom Santacruz, E.C.; Koch, R.L. Predation and Parasitism of Halyomorpha halys (Hemiptera: Pentatomidae) Eggs in Minnesota. Environ. Entomol. 2018, 47, 812–821. [Google Scholar] [CrossRef]
- Wang, Y.J.; Nakazawa, T.; Ho, C.K. Warming impact on herbivore population composition affects top-down control by predators. Sci. Rep. 2017, 7, 941. [Google Scholar] [CrossRef]
- Greene, A.D.; Reay-Jones, F.P.F.; Kirk, K.R.; Peoples, B.K.; Greene, J.K. Associating Site Characteristics with Distributions of Pestiferous and Predaceous Arthropods in Soybean. Environ. Entomol. 2021, 50, 477–488. [Google Scholar] [CrossRef]
- Samaranayake, K.G.L.I.; Costamagna, A.C. Adjacent habitat type affects the movement of predators suppressing soybean aphids. PLoS ONE 2019, 14, e0218522. [Google Scholar] [CrossRef]
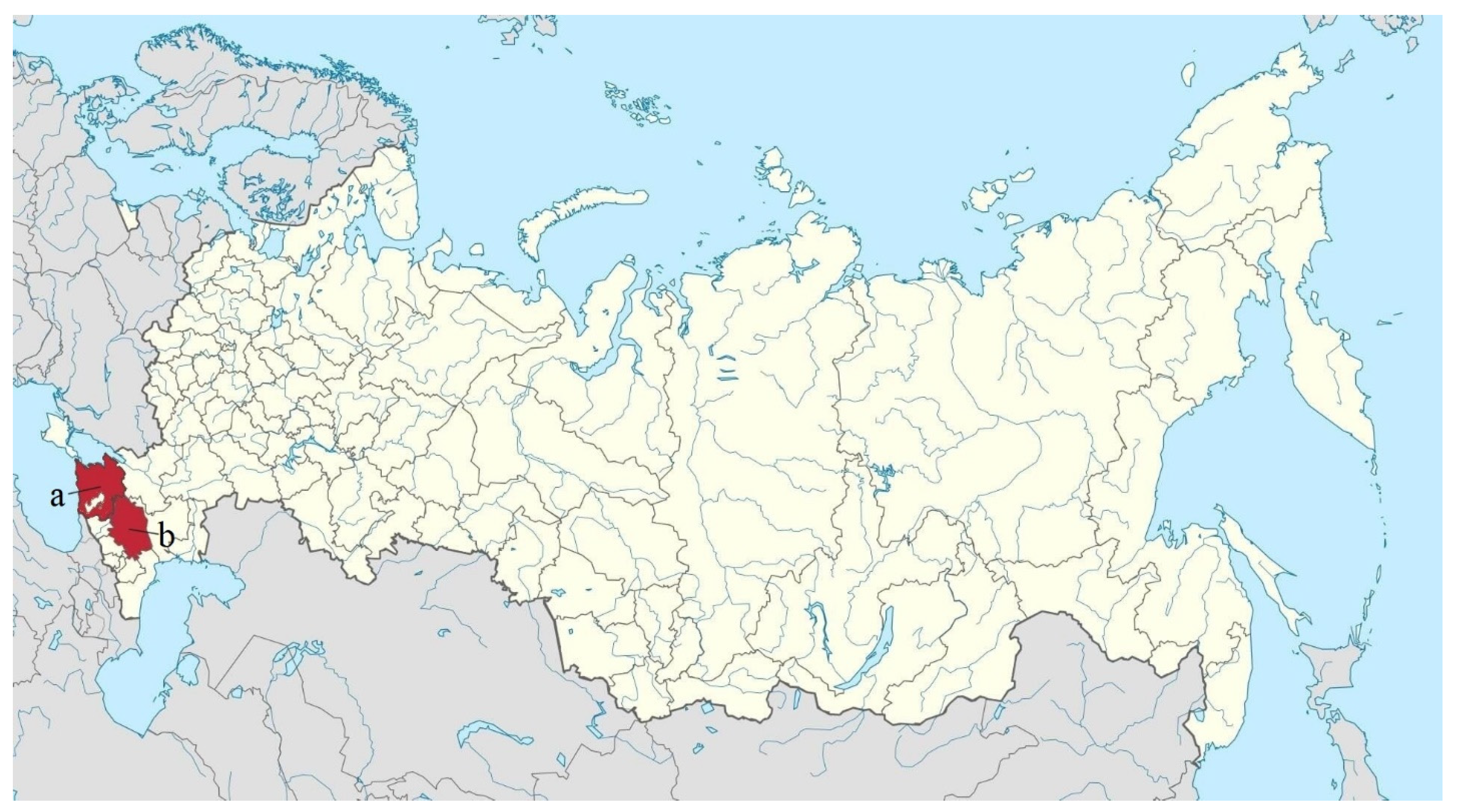


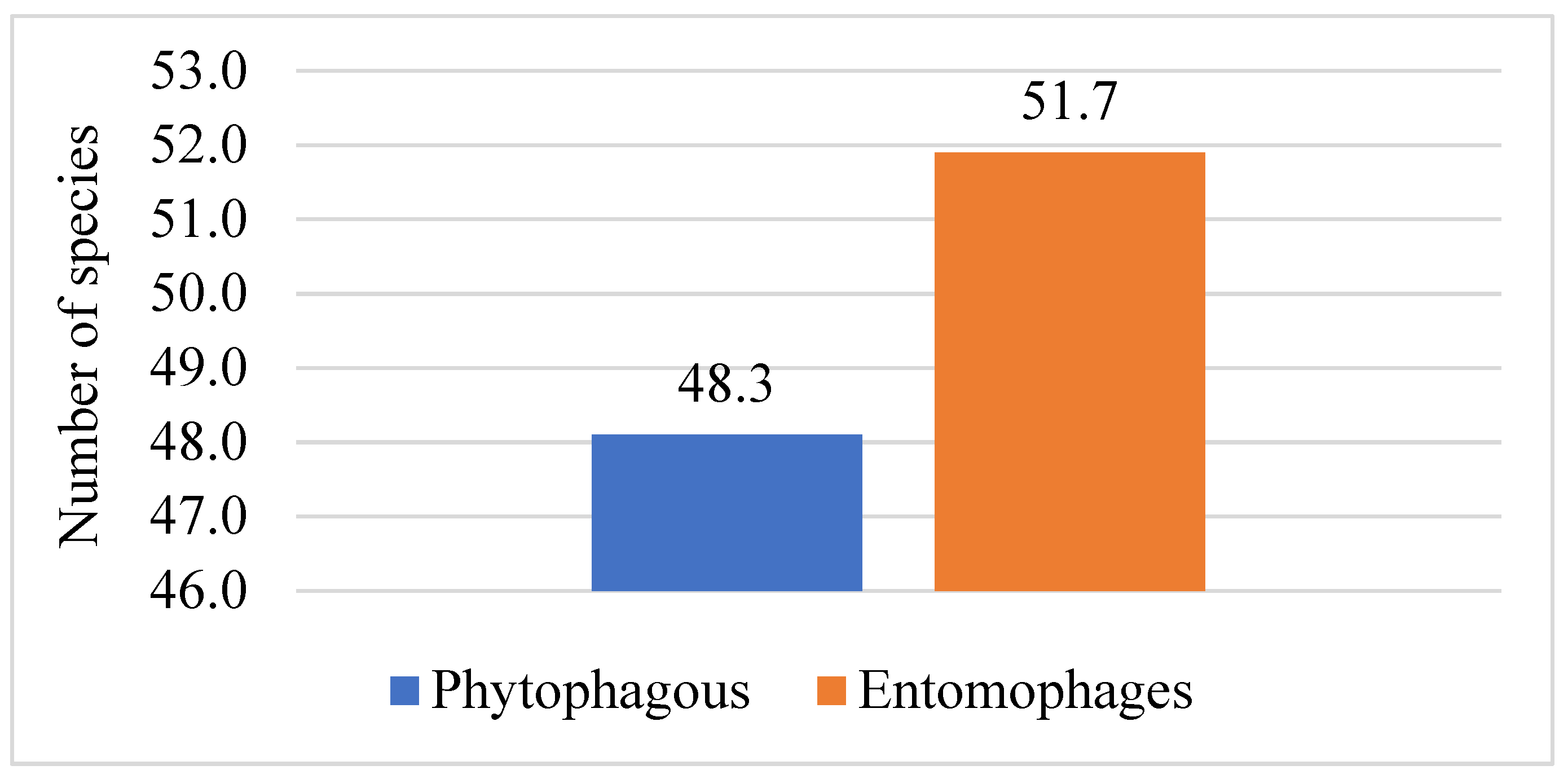
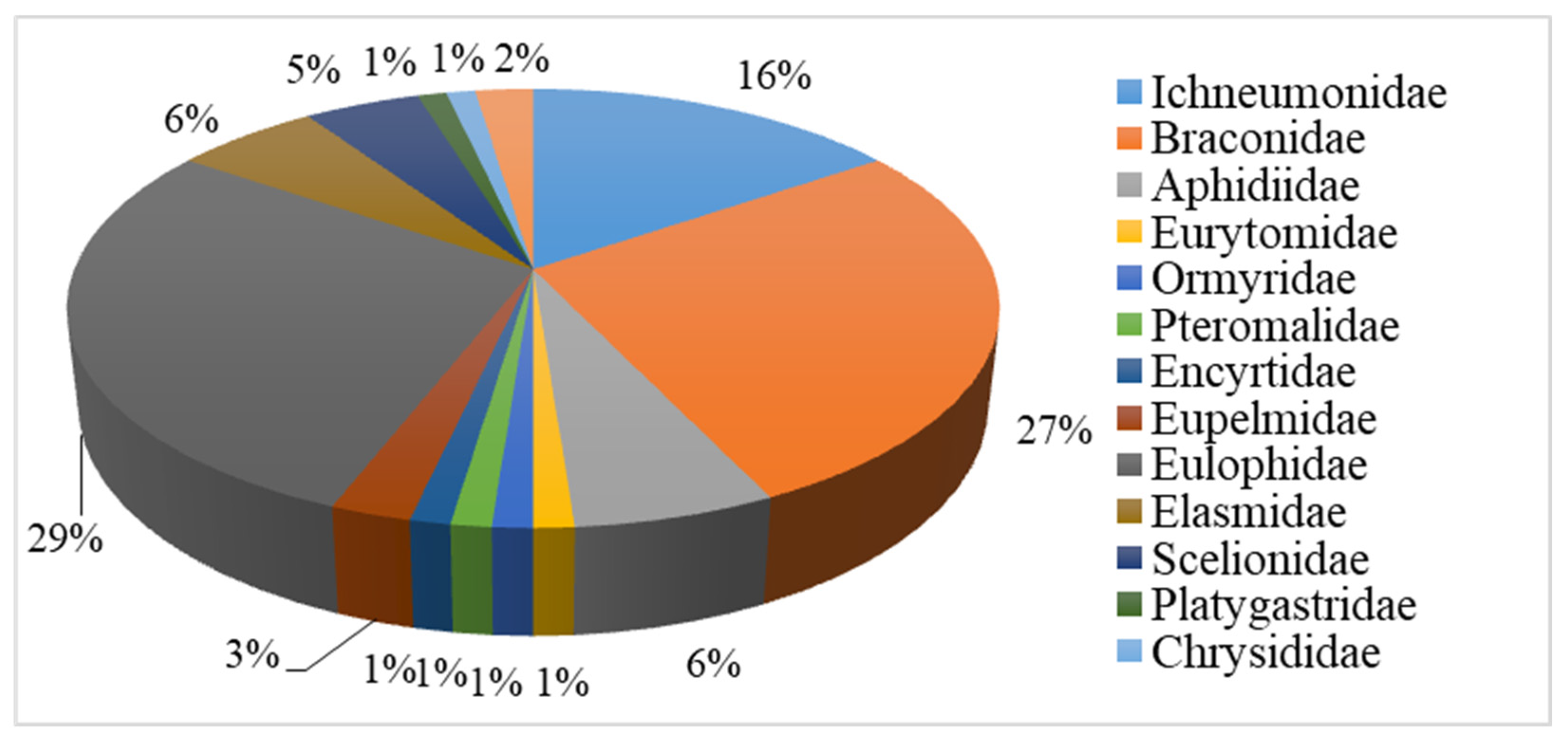
| Order | Number of Families | Number of Species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | % | Total | % | Phytophages | % | Entomophages | % | of These | ||||
| Parasites | % | Predators | % | |||||||||
| Orthoptera | 3 | 5.7 | 12 | 5.7 | 12 | 5.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Homoptera | 2 | 3.9 | 2 | 1.0 | 2 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Thysanoptera | 2 | 3.9 | 4 | 1.9 | 3 | 1.4 | 1 | 0.5 | 0 | 0.0 | 1 | 0.5 |
| Hemiptera | 11 | 21.2 | 40 | 19.0 | 34 | 16.2 | 6 | 2.9 | 0 | 0.0 | 6 | 2.9 |
| Coleoptera | 7 | 13.5 | 27 | 12.9 | 20 | 9.5 | 7 | 3.3 | 0 | 0.0 | 7 | 3.3 |
| Neuroptera | 1 | 1.9 | 6 | 2.9 | 0 | 0.0 | 6 | 2.9 | 0 | 0.0 | 6 | 2.9 |
| Lepidoptera | 10 | 19.2 | 28 | 13.3 | 28 | 13.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hymenoptera | 13 | 25.0 | 85 | 40.5 | 1 | 0.5 | 84 | 40.0 | 84 | 40.0 | 0 | 0.0 |
| Diptera | 3 | 5.7 | 6 | 2.9 | 1 | 0.5 | 5 | 2.4 | 3 | 1.4 | 2 | 1.0 |
| Total | 52 | 100 | 210 | 100 | 101 | 48.1 | 109 | 51.9 | 87 | 41.4 | 22 | 10.5 |
| Family | Species |
|---|---|
| Ichneumonidae | Hyposoter didumator Wesmael |
| Exetastes fornicator Fabricius | |
| Exetastes nigripes Gravenhorst | |
| Banchus falcatorius Fabricius | |
| Banchus volutatorius Linnaeus | |
| Netelia testacea Gravenhorst | |
| Itoplectis melanocephala Gravenhorst | |
| Barylypa pallida Gravenhorst | |
| Barylypa amabilis Tosquinet | |
| Braconidae | Apanteles plutellae Kurdjumov |
| Apanteles vanessae Reinhard | |
| Apanteles kazak Tobias | |
| Apanteles arcticus Tobias | |
| Microgaster vidua Ruthe | |
| Chelonus oculator Fabricius | |
| Chelonus annulipes Wesmael | |
| Macrocentrus collaris Spinola | |
| Rogas rossicus Spinola | |
| Rogas dimidiatus Spinola | |
| Bracon hebetor Say | |
| Bracon simonovi Kokujev | |
| Bracon quadrimaculatus Telenga | |
| Bracon minutator Fabricius | |
| Pteromalidae | Dibrachys cavus Walker |
| Encyrtidae | Copidosoma agrotis Fonscolombe |
| Eulophidae | Eulophus larvarum Linnaeus |
| Eulophus pennicornis Nees | |
| Eulophus tespius Walker | |
| Euplectrus bicolor Swederus | |
| Euplectrus flavipes Fonscolombe | |
| Colpoclipeus florus Walker | |
| Sympiesis viridula Thomson | |
| Sympiesis sericeicornis Nees | |
| Sympiesis xanthostoma Nees | |
| Pediobius pyrgo Walker | |
| Pediobius foliorum Geoffroy | |
| Pediobius cassidae Erdös | |
| Rhicnopelte crassicornis Nees | |
| Trichogrammatidae | Trichogramma evanescens Westwood |
| Trichogramma pintoi Voegelé | |
| Eupelmidae | Eupelmus microzonus Förster |
| Eupelmus urozonus Dalman | |
| Elasmidae | Elasmus unicolor Rondani |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agasyeva, I.S.; Ismailov, V.Y.; Petrishcheva, M.V.; Nastasiy, A.S.; Petrishchev, V.S. Species Composition of Phytophagous and Entomophagous Insects and Mites on Soybeans in Krasnodar and Stavropol Territories, Russia. Agronomy 2024, 14, 1440. https://doi.org/10.3390/agronomy14071440
Agasyeva IS, Ismailov VY, Petrishcheva MV, Nastasiy AS, Petrishchev VS. Species Composition of Phytophagous and Entomophagous Insects and Mites on Soybeans in Krasnodar and Stavropol Territories, Russia. Agronomy. 2024; 14(7):1440. https://doi.org/10.3390/agronomy14071440
Chicago/Turabian StyleAgasyeva, Irina Sergeevna, Vladimir Yakovlevich Ismailov, Maria Vladimirovna Petrishcheva, Anton Sergeevich Nastasiy, and Viktor Sergeevich Petrishchev. 2024. "Species Composition of Phytophagous and Entomophagous Insects and Mites on Soybeans in Krasnodar and Stavropol Territories, Russia" Agronomy 14, no. 7: 1440. https://doi.org/10.3390/agronomy14071440
APA StyleAgasyeva, I. S., Ismailov, V. Y., Petrishcheva, M. V., Nastasiy, A. S., & Petrishchev, V. S. (2024). Species Composition of Phytophagous and Entomophagous Insects and Mites on Soybeans in Krasnodar and Stavropol Territories, Russia. Agronomy, 14(7), 1440. https://doi.org/10.3390/agronomy14071440







