Abstract
This study was designed to investigate the relationship between the caloric value and salt tolerance of two varieties of Miscanthus sacchariflorus (Amur silvergrass: M127 and M022). The salt tolerance capacity, photosynthetic characteristics, Na+ and K+ uptake by the roots and aboveground parts, and caloric value of different parts of the aboveground parts were obtained under hydroponic conditions. The results showed that M022 was more tolerant to salt stress than M127 and the former had a higher photosynthetic efficiency as well as a lower aboveground Na+ accumulation, K+ efflux, and larger K+/Na+ ratio. The calorific values of stems, spear leaves, aging leaves, and functional leaves of the two varieties showed a decreasing trend with increasing NaCl concentration. At 270 mM NaCl, the calorific values of the stems, aging leaves, functional leaves, and spear leaves was reduced by 18.10%, 46.73%, 26.11%, and 18.35% for M022 and 41.99%, 39.41%, 34.82%, and 45.09% for M127 compared to the controls, respectively. We observed that the aging leaves of M022 had a faster decline rate in calorific value than those of M127, indicating that the aging leaves of M022 preferentially isolated the harmful Na+ ion, reduced its accumulation in other parts, and increased the K+/Na+ ratio in the corresponding parts, thus inhibiting the decrease in calorific value. Following this result, it can be inferred that M022 inhibited the decline in calorific values during stress by efficiently compartmentalizing the distribution of Na+ and K+. Our results provide a theoretical basis and technical support for the efficient cultivation of salt-tolerant energy plants in saline–alkaline soil.
1. Introduction
Saline–alkaline soils are widely distributed around the world with an estimated total area of 1.10 billion hm2, with China accounting for about 1/10 of the world’s total [1,2]. About 20% of agricultural land is affected by salinity, and this percentage is continuously increasing [3]. Saline-induced stress causes osmotic damage, ion toxicity and the resulting oxidative stress, nutrient imbalance, and decreased photosynthetic capacity, which ultimately leads to slow plant growth and decreased biomass and yield [4,5].
The salt tolerance mechanism in plants follows three main pathways: (1) the osmotic regulation pathway, which mainly relies on the accumulation of small-molecule soluble substances [6]; (2) the ion homeostasis pathway, which relies on the selective uptake of ions by the roots, phloem reflux of ions in stems, compartmentalization of ions from leaves to vesicles, and secretion of excess salt ions from salt glands to the outside of the plant body [7,8]; and (3) the regulatory pathway of the antioxidant system that relies on the scavenging of harmful free radicals by enzymatic and non-enzymatic systems [5,9,10]. Sodium ion (Na+) is a non-essential element for plant growth whereas potassium ion (K+) is one of the essential elements for plant growth. Due to the similarity in the chemical properties of Na+ and K+, Na+ competes for the absorption channel of K+, which may lead to K+ deficiency in plants [11]. To tolerate salt-induced stress, plants limit the transportation of Na+ and effectively isolate it by maintaining a relatively high K+ concentration through selective transportation and by enhancing a balance in the K+/Na+ ratio [11,12]. When M. giganteus was stressed with Na+, K+ enrichment in the leaves reduced the toxic effect of Na+ and improved plant growth [13]. Nevertheless, the effect of salt-induced stress on the caloric value of Miscanthus varieties is yet to be evaluated.
External availability of K+ induces root response to either surpluses or deficiencies. When K+ is deficient, plants initiate a short-term deficiency response that increases the roots’ affinity and uptake of K+. Research suggests that the high-affinity K+-uptake mechanism in plants is mediated by specialized channels, including Arabidopsis HAK5 and AKT1. Generally, these channels are activated by reactive oxygen varieties produced by nicotinamide adenine dinucleotide phosphate oxidases and are accumulated in the elongation zone of root hairs [14,15,16,17,18]. Consequently, under K+-deficient conditions, lateral root growth becomes inhibited. Shankar et al. [19] observed that K+-deficient rice varieties accumulate more Na+ compared to the K+-sufficient varieties. Under excess-ion stress, plants modify conducting tissues to resist the damaging consequences. For example, Sarath et al. [20] observed that the density of the xylem and cell wall thickness of Volkameria inermis were modified under excess Na+ stress. Increased salinity is known to cause damage in the development of the root cap. This damage is often characterized by an alteration in root growth, branching, and shape [21].
Experimental evidence suggests that the content of mineral elements in plants increases under salt stress. For example, in alfalfa (Medicago sativa Linn.), the concentration of Na+ in leaves of different ages showed a shift from spear leaves at the growing point to basal aging leaves under salt stress, while K+ showed the opposite distribution pattern [22]. According to the authors, salt-tolerant alfalfa segregated excessive Na+ in aging leaves and reduced its concentration in the stem by accelerating the senescence of aging leaves, thereby reducing the toxic effect of Na+ on plant growth. However, the effect was the opposite in salt-sensitive varieties where more Na+ was accumulated in the stems.
Miscanthus varieties, including M. sinensis, M. sacchariflorus, and M. floridulus are known to be tolerant to moderate saline-alkali soils [23]. M. sinensis (Amur silvergrass) is a C4 perennial tall grass with high photosynthetic efficiency, high biomass yield, high stress tolerance, low production cost [24], and is a promising and environmentally friendly energy plant [25,26]. Amur silvergrass is widely distributed in flooded wetlands in temperate Asia, and is recognized as an excellent energy crop with high biomass, high cellulose content, good combustion characteristics, and high regeneration capacity [27,28]. Miscanthus varieties have a well-developed root system (to a depth of 3 m) [29], which promotes effective utilization of water and nutrients from deep soil horizons [30]. Miscanthus varieties tolerate less fertile and partly eroded soils [31], and their vigorously branching stems effectively protect soils against erosion [32]. Research on Amur silvergrass has mainly focused on resource surveys and evaluations [33], external trait observations, drought resistance [27,28], and cold tolerance [34], leaving a gap in knowledge about its survival under increasing salinization.
The content of mineral elements directly affects the calorific value of energy plants such as Miscanthus [35,36]. As established above, the K+ content in plants can be significantly altered by Na+ stress. This means that the calorific value of Miscanthus varieties could be negatively impacted by saline-induced stress. Thus, it is important to investigate the effect of salt stress on the combustion characteristics of different parts of Amur silvergrass. The response mechanism to different salt concentration gradients of different varieties with various levels of salt tolerance remains elusive. This study was thus designed to study the growth of two Amur silvergrass varieties under salt-induced stress and to (i) decipher the mechanisms of tolerance to salt stress and its effect on photosynthetic rate and mineral element absorption; (ii) analyze the effects of salt stress on the combustion characteristics of different plant parts; and (iii) explore the relationship between the calorific value of the plant and its salt tolerance.
2. Materials and Methods
2.1. Plant Cultures and NaCl Treatment
The rhizomes of the Amur silvergrasses (Miscanthus sacchariflorus) were collected from a high-salinity region (0.6% soil Na+ content) in Dongying, China (37°43′ N and 119°01′ E), and this study was conducted from the end of April to the end of September (18 weeks). The experiment was conducted in a glass-covered greenhouse at the Institute of Botany, Jiangsu Province and Chinese Academy of Sciences, Nanjing, China (32°02′ N, 118°28′ E). The plants were maintained in a glass-covered greenhouse, with average maximum/minimum temperatures of 30.4 °C/22.6 °C and a photoperiod of 11.5 to 14.3 h. The maximum photosynthetically active radiation on a horizontal plane just above the canopy ranged from 1200 to 1800 μmol m−2 s−1, provided by sunlight. Two Amur silvergrass (M127 and M022) varieties were used in this study, and the cultivation procedure was according to Marcum et al. [37]. Three rhizomes with developing shoots were planted in plastic containers filled with coarse and acid-washed silica sand (the containers measured 5 cm in diameter and 10 cm in height). The bottom of the container was removed and replaced with a nylon screen to hold sand and allow roots to grow through. Each container was then suspended on foam boards supported by wire at the bottom and placed on a large 45-L turnover box (66.5 cm × 45.5 cm × 17 cm). The turnover box was filled with Hoagland nutrient solution containing the following nutrients: 1.25 Ca(NO3)2, 1.25 KNO3, 0.5 MgSO4, and 0.025 NH4H2PO4 (in mM) and 46 H3BO3, 0.3 CuSO4·5H2O, 0.1 Na2MoO4·2H2O, 9.2 MnCl2·4H2O, 0.8 ZnSO4·7H2O, and 286 FeSO4·7H2O-EDTA (in µM). The pH of the nutrient solution was 6.0 ± 0.2. The nutrient solution was submerged at the bottom of the containers and replaced every 7 days.
When the plant grew to 20–30 cm in height, with each plant having 6–7 leaves, the materials with relatively uniform growth were selected to start the salt treatment. The experiment was designed with four levels of NaCl concentrations (0, 90, 180, and 270 mM), and the two Amur silvergrass (M127 and M022) varieties were exposed to this salt concentration for three weeks. The control treatment was a nutrient solution without added NaCl. Each treatment was replicated in 4 plastic containers and randomly distributed. To avoid salinity shock, the salinity level was increased by 45 mM NaCl daily during a 6 days period. When the salt concentration reached the desired concentration, it was maintained for three weeks, during which air was continuously admitted. At the end of the NaCl treatment, the harvested plants were washed three times using deionized water and separated into five parts, i.e., stems, aging leaves, functional leaves, spear leaves, and roots, to be used for the follow-up experiments.
2.2. Determination of Plant Growth Parameters and Leaf Photosynthetic Efficiency
The dry weights of five parts of the Amur silvergrass were determined by oven-drying fresh samples at 60 °C until a constant weight was obtained, and the dry weight was recorded. Plant relative growth rate (RGR), relative spear leaf mass, relative functional leaf mass, relative aging leaf mass, relative stem mass, and relative root mass were calculated from the following equation: (NaCl treatment)/(without NaCl treatment) × 100%.
Before the end of the experiment, the top third of functional leaves with good growth were selected to measure the photosynthetic efficiency at 9:00–12:00 a.m. The Li-Cor 6400XT portable photosynthesis meter (Li-Cor, Lincoln, NB, USA) was used to determine the net photosynthetic rate (Pn, in µmol CO2 m−2 s−1), stomatal conductance (Cond, in mol H2O m−2 s−1), intercellular CO2 concentration (Ci, in µmol CO2 mol−1), and transpiration rate (Tr, in mol H2O m−2 s−1). The CO2 was set to 400 μmol mol−1, and the light intensity was set to 1000 µmol m−2 s−1.
2.3. X-ray Analysis of Root Cross-Section
Through X-ray analysis, the Na+ and K+ contents in a root cross-section were evaluated as described by Xie et al. [38], with some modifications. After exposure to the control or 180 mM NaCl stress for 10 days, the roots were collected and washed in distilled water three times. Two root segments in the root hair zone of each cultivar or treatment were cut with a razor blade, wrapped with filter paper, and immediately cooled by liquid nitrogen. The fractured root segments were freeze-dried and platinized with an ion sputter. The distribution of Na+ and K+ in the samples was analyzed by a model S-3000N SEM equipped with an energy-dispersive X-ray spectrometer (Horiba Inc., Kyoto, Japan). The relative Na+ or K+ contents were calculated and expressed as the percentage of the atomic number for the Na+ or K+ element in a given site based on the total atomic number for all the elements that could be detected.
2.4. Measurement of Na+ and K+ Concentrations in the Plants
Each part of the Amur silvergrass dry sample was crushed with a multi-bead shocker (Yasui Kikai, Kobe, Japan), and then, about 0.02 g powder was placed in a test tube containing 15 mL of distilled water and sealed [39]. After extracting the sample with boiled water for 1 h and filtrating it, the extracted liquid was used to analyze K+ and Na+ concentrations by flame photometry (Model FP6410, Shanghai Xinyi Instruments Inc., Shanghai, China). All ion concentrations were determined by a flame spectrophotometer and calculated on a dry-tissue-weight basis.
The values of the selective transport capacity (ST) from root to shoot for K+ over Na+ were estimated following the equation of Wang et al. [40]: ST = (Na+/K+ in root)/(Na+/K+ in each part). The bigger the ST value, the stronger the root controls Na+ and promotes K+ transport to the shoot, indicating a stronger selective transport capacity of the root.
2.5. Measurement of the Calorific Value
The calorific value of the samples was determined using an XRY-1A Digital Display Oxygen Bomb Calorimeter (Shanghai Changji Geological Instrument Co., Ltd., Shanghai, China) [41]. About 1.0 g of each pelleted sample was put into an adiabatic bomb instrument and burned to ash. The samples for the determination of the calorific value were sieved using a 60-mesh sieve. Powder samples were compacted into pellets for measurement to reduce errors caused by incomplete combustion resulting from dry and loose samples blown away during the sudden release of volatiles.
2.6. Data Analysis
Statistical analysis and data processing were carried out using SPSS 20.0 for Microsoft Windows (Chicago, IL, USA). In all the experiments, one-way analysis of variance (ANOVA) was used to ascertain whether the differences between the different parameters were significant at p < 0.05. Bivariate correlation analysis was also performed (p < 0.05, Pearson’s correlation).
3. Results
3.1. Changes in the Growth Parameters of Amur Silvergrass Varieties under Salt Stress
There are three main response categories used to evaluate plant tolerance to salt stress: morphological, growth, and physiological indicators [37,42]. Because of their relative simplicity and low cost, morphological and growth indicators are commonly used to evaluate the salt tolerance of turfgrasses. Thus, in this study, relative plant height and biomass content were used to compare the salt tolerance of different Amur silvergrass varieties. Figure 1 shows a decreasing trend in the relative height of both M022 and M127 as the salt stress level was increased, and the higher the concentration of NaCl, the more obvious the decrease in their relative height. The relative height of M127 and M022 decreased from 66.67% to 12.20% and 98.21% to 39.97%, respectively, as the concentration of NaCl increased from 90 mM to 270 mM. The differential relative heights of M022 and M127 suggest that M022 was more tolerant to salt stress.
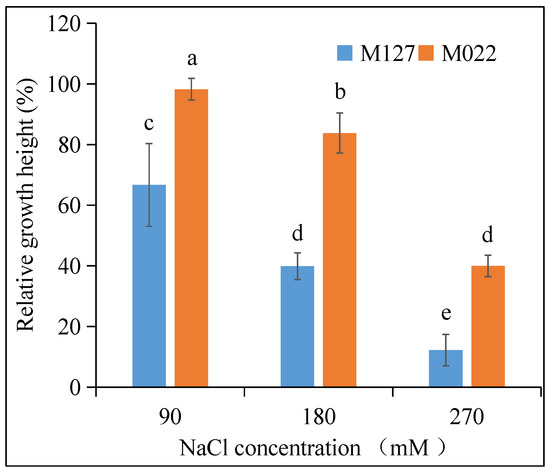
Figure 1.
Changes in the relative height of different Amur silvergrass varieties under salt stress conditions. The lowercase letters indicate significant differences at p < 0.05 between different treatments.
The relative biomass of Amur silvergrass was quantified based on the roots (Figure 2A), stem (Figure 2B), and leaves (Figure 2C–E). The relative root biomass of M127 was highest (108.22%) at 90 mM NaCl and lowest (67.03%) at 270 mM NaCl. For M022, the relative root biomass was 127.18% and 107.06% under similar NaCl stress, respectively (Figure 2A). Also, the plant stem showed similar trends in relative biomass for both varieties (Figure 2B) and ranged between 102.65 and 40.25% and between 108.76 and 83.30% in 90–270 mM NaCl for M127 and M022, respectively. The variation in the relative biomass of aging, functional, and spear leaves followed the same trend as that of the roots and stem. With increasing NaCl concentration (90 to 270 mM), the relative biomass of aging leaves increased from 104.03% to 131.64% for M022, while it decreased from 94.69% to 43.48% for M127 (Figure 2C). For the functional and spear leaves of M022, the relative biomass changed from 106.91% to 73.05% and 108.59% to 89.33% as opposed to 92.82% to 40.18% and 87.99% to 43.09% for M127, respectively (Figure 2C–E). Except for 270 mM NaCl, the relative biomass of different parts of M022 treated with lower NaCl concentrations (90 and 180 mM) was greater than 100% and higher than that of M127, suggesting that the lower salt concentration promoted growth and biomass accumulation in M022.

Figure 2.
Changes in the relative biomass of different Amur silvergrass varieties under salt stress conditions: (A) root relative biomass; (B) stem relative biomass; (C) relative biomass of aging leaves; (D) relative biomass of functional leaves; and (E) relative biomass of spear leaves. The lowercase letters indicate significant differences at p < 0.05 between different treatments.
3.2. Changes in the Photosynthetic Properties of Amur Silvergrass Varieties under Salt Stress
After salt-induced stress, the net photosynthetic rate (Figure 3A), stomatal conductance (Figure 3B), and transpiration rate (Figure 3C) of M022 and M127 were significantly reduced, except for the intercellular CO2 concentration (Figure 3D). The greater the NaCl concentration, the more significant the reduction of net photosynthetic rate, stomatal conductance, and transpiration rate of both plant varieties. However, the reduction recorded for the salt-tolerant M022 was lower than that of the salt-sensitive M127. Compared to the control, the net photosynthetic rate, stomatal conductance, and transpiration rate of M127 treated with 270 mM NaCl decreased by 87.0%, 88.2%, and 84.1%, while the corresponding values of M022 decreased by 64.5%, 76.5%, and 63.4%, respectively. This confirms that M022 was less affected by salt stress and thus maintained a higher photosynthetic efficiency under our study conditions compared to M127.
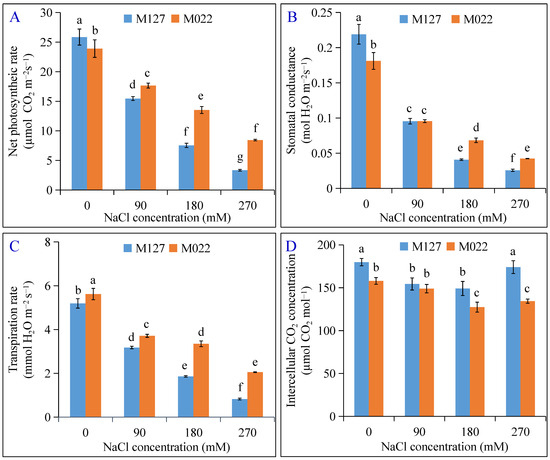
Figure 3.
Effect of salt stress on net photosynthetic rate (A), stomatal conductance (B), transpiration rate (C), and intercellular CO2 concentration (D). The lowercase letters indicate significant differences at p < 0.05 between different treatments.
3.3. Changes in the Na+ and K+ Uptake Properties of Amur Silvergrass Varieties under Salt Stress
Salt stress significantly promoted the accumulation of Na+ and reduced the concentration of K+ in the roots and aboveground plant parts, and the corresponding change in Na+ and K+ concentrations increased with an increase in salt concentration (Table 1). There were differences in the accumulation of Na+ and K+ in different parts of both varieties under salt stress. The Na+ accumulation rate and K+ transfer rate in the roots of M022 were lower than those of M127, whereas the K+/Na+ ratio between the roots of M022 and M127 showed the opposite results with increasing salt stress. In the aboveground parts (aging leaves, functional leaves, and spear leaves), the accumulation rate of Na+, K+ transfer capacity, and K+/Na+ ratio of M022 and M127 showed opposite trends to those of the roots. For example, under 270 mM NaCl treatment, Na+ concentration in the roots of M022 and M127 increased by 347.8 and 206.0 times, and K+ concentration decreased by 67.6% and 46.1%, while the corresponding K+/Na+ ratios were 0.41 and 0.82, respectively. Under the same concentration of NaCl, the Na+ concentration in the stems, aging leaves, functional leaves, and spear leaves of M022 increased by 208.2, 201.1, 253.0, and 417.4 times, the K+ concentration decreased by 9.1%, 47.4%, 17.3%, and 19.4%, and the K+/Na+ ratios were 1.14, 0.62, 1.54, and 1.63, respectively. In addition, the Na+ concentration in the corresponding organs of M127 increased by 302.2, 258.0, 351.9, and 470.1 times, the K+ concentration decreased by 55.8%, 55.2%, 59.2%, and 44.1%, and the K+/Na+ ratios were 0.52, 0.54, 0.48, and 0.77, respectively.

Table 1.
Effect of salt stress on the accumulation rate of Na+, K+ transfer capacity, K+/Na+ ratio of 2 Amur silvergrass roots, stems, spear leaves, aging leaves, and functional leaves.
3.4. Changes in the Calorific Properties of Amur Silvergrass Varieties under Salt Stress and the Relationship with Na+ and K+
The calorific values of different parts of both varieties of Amur silvergrass can be ranked as follows: spear leaves > stems > functional leaves > aging leaves (Figure 4). With the increase in NaCl concentration, the calorific values showed a decreasing trend, and the rate of decrease was different for both plant varieties. The calorific values of all parts (stems, spear leaves, aging leaves, and functional leaves) of the salt-sensitive M127 decreased significantly at lower NaCl concentrations (90 mM), and the decrease was more significant at higher NaCl concentrations. However, the changing trend was only significant for M022 at NaCl concentrations > 180 mM, except for the aging and functional leaves that showed significant decreases in calorific values from NaCl concentrations of 90 mM. This result indicates that the stems and spear leaves of M022 maintained a relatively stable calorific value at NaCl concentrations ≤ 180 mM.
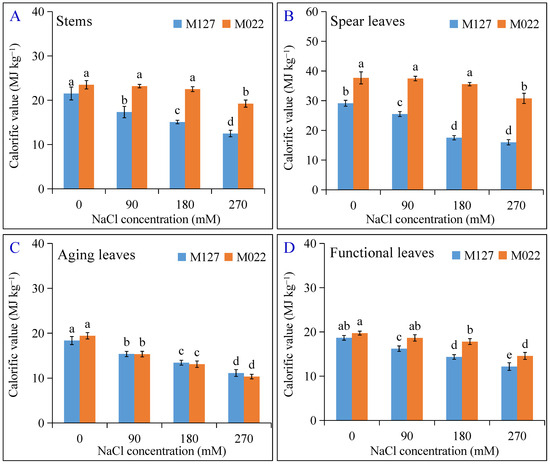
Figure 4.
Effect of different salt concentrations on the calorific values of 2 Amur silvergrass stems (A), spear leaves (B), aging leaves (C), and functional leaves (D). The lowercase letters indicate significant differences at p < 0.05 between different treatments.
For the different plant parts, the decreasing trend of the calorific value ranged between 19.45 and 41.99%, 16.29 and 39.41%, 13.12 and 34.82%, and 12.42 and 45.09% in the stem, aging leaves, functional leaves, and spear leaves of M127 as the salt concentration increased from 90–270 mM compared to the control (0 mM), respectively. Compared to the control, the calorific value of the stem of M022 decreased non-significantly by 1.30% and 4.23% at NaCl concentrations of 90 and 180 mM, respectively, but significantly decreased by 18.10% at 270 mM. Also, at 90 mM NaCl, the calorific values of the aging, functional, and spear leaves decreased by 21.02%, 5.53%, and 0.61% as opposed to 46.73%, 26.11%, and 18.35% at 270 mM NaCl, respectively. The calorific value of M022 was less affected by salt stress compared to M127 except for that of the aging leaves. For example, the calorific value of the aging leaves of M127 decreased by 16.29%, 26.90%, and 39.41% as opposed to 21.02%, 32.58%, and 46.73% for M022 compared to the corresponding controls, respectively.
The relationship between the calorific values of Amur silvergrass varieties and the concentrations of Na+ and K+ and the ratio of K+/Na+ were computed to evaluate what parameter had the greatest effect and to determine on which plant varieties the effect was most pronounced (Figure 5). Our result shows that there was a significant positive linear relationship between the plant contents of K+ and Na+ or the K+/Na+ ratio with the varieties’ calorific value, and the relationship was more significant for M127. It was estimated that the concentrations of K+ and Na+ and the K+/Na+ ratio could explain 76.67%, 52.15%, and 48.73% (p < 0.01) of the changes observed in the calorific value of M127, while they explained 33.19%, 26.38%, and 13.67% of the changes observed for M022 (p < 0.05), respectively.
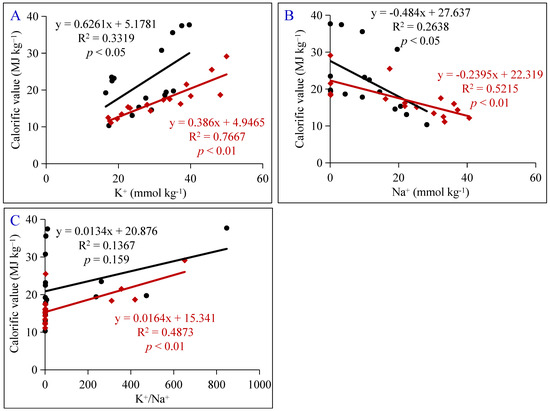
Figure 5.
Relationships of the calorific values in different parts of Amur silvergrass varieties with their K+ (A), Na+ (B), and K+/Na+ ratio (C). The red line is M127, and the black line is M022.
3.5. Distribution of Na+ and K+ in Amur Silvergrass Varieties under Salt Stress
A spectrometer combined with an SEM was used to perform the X-ray microanalysis of Na+ and K+ distribution in root transections from the pith to the epidermis (Figure 6). In the control, there were low relative Na+ and high relative K+ contents in the roots of the varieties. The relative content of Na+ in the stele was lower than that of other tissues in both Amur silvergrass varieties; however, 180 mM NaCl stress increased the concentration of Na+, while that of K+ decreased, and this is consistent with the observation in Table 1 and the results of Shankar et al. [19], who observed that salt stress decreased the content of K+ in K+-deficient rice varieties. Also, the relative content of Na+ in the exodermis and middle cortex was higher than in the stele of M022; however, the opposite was true for M127 (Table 2). Additionally, the high relative content of K+ was found in the cortex under the control condition; however, it decreased in both Amur silvergrass varieties and was higher in the stele and middle cortex than the exodermis of M022 under 180 mM NaCl stress. Nevertheless, K+ was lower in the stele of M127 than in the cortex.
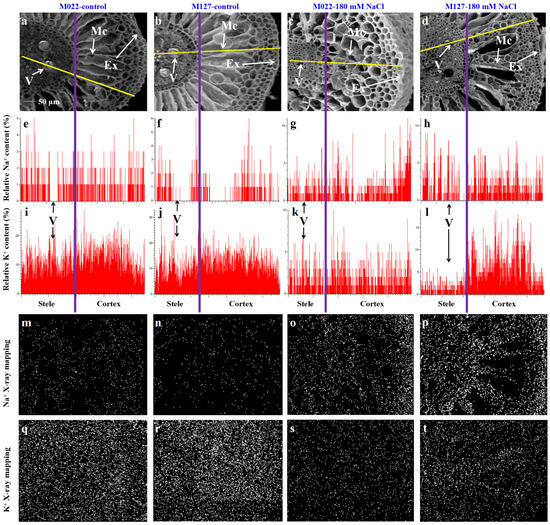
Figure 6.
Compositional map of the elements in the transection of the root hair zone of M127 and M022 with/without 180 mM NaCl. Scanning micrograph of the analyzed root transection (a–d); distribution of Na+ (e–h) and K+ (i–l) in the root transection; and EDS map of the relative distribution of Na+ (m–p) and K+ (q–t) in the root transection. V: vessel; Mc: middle cortex; and Ex: exodermis. The purple line is the boundary between the stele and the cortex and the yellow line is the location of the line-scanning of an X-ray.

Table 2.
The weight percentage of exodermis, middle cortex, and stele in the root transection of the salt-sensitive M127 and salt-tolerant M022 under 180 mM NaCl stress.
Our result also revealed that the relative content of K+ in the vessels of M022 was higher than that of other tissues around the vessels when the plant was stressed, while it was lower in M127. Using X-ray microanalysis, it was observed that the content of Na+ in M022 was higher in the root exodermis and middle cortex than in the stele under salinity stress, but the opposite was true for M127 (Figure 6), suggesting that M022 had a greater ability to inhibit the entry of Na+ into the stele through the cortex. Furthermore, M022 accumulated less Na+ and more K+ in the vessels compared to M127, indicating that M022 can reduce the transfer of Na+ to the aboveground parts while maintaining a higher K+ in the aboveground parts. This is consistent with the ST result (Table 1).
4. Discussion
Miscanthus varieties are an ideal type of herbaceous energy plants with C4 metabolic pathways and perennial growth characteristics that result in a significant redistribution of nutrient elements in their bodies [43]. The mineral element content in the biomass of energy plants has a great effect on their combustion quality and the efficiency of conversion and utilization of biomass energy [44,45,46]. We observed that the calorific values of different parts of the Amur silvergrass varieties decreased with the increase in NaCl concentration (Figure 4) and were related to the increased accumulation of mineral elements in the plant tissues (Table 1). This observation is consistent with other studies that reported increased contents of mineral elements and decreased calorific potentials of giant reed, an energy plant, during salt stress [47].
The calorific value of the salt-tolerant M022 was significantly higher than that of the salt-sensitive M127 (Figure 4). This was related to the significantly lower Na+ content and higher K+/Na+ ratio of the former compared to the latter. Compared to M127, M022 inhibited the decline in calorific values under salt stress by reducing Na+ accumulation and increasing the K+/Na+ ratio through self-regulation, suggesting that the calorific value of plants is closely related to their salt tolerance. In addition, the Na+ concentration in the aging leaves of the salt-tolerant M022 was significantly higher than that in stems, spear leaves, and functional leaves, while the accumulation of Na+ in the salt-sensitive M127 did not show significant positional differences. This suggests that the salt-tolerant variety, i.e., M022, isolated Na+ in the aging leaves and reduced its content in other plant parts by accelerating the senescence of the aging leaves, thus alleviating the toxic effect of Na+ on its growth. These differential results were corroborated by the much more significant correlation between the calorific value of M127 and the concentrations of Na+ and K+ and the ratio of K+/Na+ than for M022 (Figure 5). Similar results have been reported for Medicago sativa [22], Helianthus annuus [48], Diplachne fusca [49], and Saccharum officinarum [50].
Photosynthesis is the basis of plant growth and energy production, and its response to the surrounding environment can reflect the adaptability of plants to salt stress [5,51]. Our results reveal that salt stress negatively impacted the net photosynthetic rate, stomatal conductance, and transpiration rate (Figure 5). Of the two plant varieties, M022 was more tolerant to salt stress, and its photosynthetic rate, stomatal conductance, and transpiration rate were 1.79, 1.68, and 1.80 times higher, while its intercellular CO2 concentration was 21.76 µmol CO₂ mol−1 lower compared to M127. Research with potato plants subjected to salt stress indicated that the over-expression of AtHKT1 genes minimized osmotic imbalance in the plant and inhibited reductions in net photosynthetic rate, stomatal conductance, and transpiration rate [52]. Following the alteration of plant photosynthesis due to stress, plant growth becomes stunted, and the relative growth rate is reduced (Figure 1); hence, plant biomass accumulation is reduced (Figure 2). Previous research with Spartina pectinata L. and Panicum virgatum L. showed that S. pectinata with stronger salt tolerance accumulated more biomass and produced second-generation tillers and greater root-to-shoot biomass ratio than P. virgatum with weaker salt tolerance [53]. Also, under salt stress conditions, Cynodon dactylon decreased the accumulation of Na+ and stabilized the levels of K+ in the leaves by inhibiting the migration of Na+ into the root vessels, selectively promoting K+ transport to the leaves, enhancing Na+ secretion, and balancing stomatal conductance [54]. Generally, the intensity of these changes depends on how stress-tolerant a plant is, and based on our results, M022 presented a much better response to salt stress, and its stomatal conductance was much higher than that of M127 at NaCl concentrations > 90 mM (Figure 3B).
Peng et al. [55] reported that the glandular trichome of the salt-tolerant Gossypium hirsutum genotype secreted more salt ions compared to the salt-sensitive genotype. The authors suggested that ion compartmentalization and subsequent excretion of ions from the tissues is an important mechanism used by plants to regulate short-term salt stress. After evaluating 70 different genotypes of Miscanthus seedlings, it was found that salt-tolerant genotypes had lower Na+ concentrations and relatively high K+/Na+ ratios in the shoots, indicating that lowering Na+ accumulation in the leaves was important for salt stress regulation in Miscanthus [56]. Compared to the salt-sensitive M127, the salt-tolerant M022 alleviated salt stress by regulating the K+ and Na+ concentrations in different organs under salt stress (Figure 6). By so doing, more Na+ was accumulated in the roots, while lower Na+ and higher K+ contents were maintained in the aboveground plant parts (Table 1). This reduced the toxic effect of excess Na+ on the aboveground organs and thus alleviated the damage of salt stress. This finding is supported by the fact that the selective transport capacity (ST) of the salt-tolerant M022 was higher than that of the salt-sensitive variety, i.e., M127 (Table 1). Accordingly, the roots of the salt-tolerant M022 showed a stronger control for Na+ and promoted the transport of K+ to the aboveground organs and, hence, led to a better growth response (Figure 1 and Figure 2). Thus, maintaining a higher K+/Na+ ratio in plant tissues is more important than simply maintaining a lower Na+ content [57]. A similar response mechanism has been reported for rice varieties with different sensitivities [19]. Specifically, the authors reported that K+ deprivation activated multiple genes and networks that acted together to sense external K availability and regulate uptake by the roots. These regulatory mechanisms are important for ion distribution and plant adaptation to stress.
The balance of intracellular K+ and Na+ concentrations is an important indicator of plant salt tolerance and a key factor in ensuring normal physiological metabolism under salt stress [54,55]. Our results suggest that salt stress affects the distribution of K+ and Na+ in the tissues of different Amur silvergrass varieties, which in turn affects their salt tolerance. Previous studies have shown that limiting Na+ accumulation and K+ efflux and maintaining a balance of higher K+ and lower Na+ in the cytoplasm of plants are important methods for improving salt tolerance and plant resistance to salt damage [58,59]. Based on the results and discussion above, a higher salt tolerance is essential to efficiently slow down the decline in calorific values. When M022 was stressed with 270 mM NaCl, the calorific values of the stems, aging leaves, functional leaves, and spear leaves were reduced by 18.10%, 46.73%, 26.11%, and 18.35% as opposed to 41.99%, 39.41%, 34.82%, and 45.09% for M127 relative to the controls, respectively. These results indicate that, compared to the salt-sensitive M127, the salt-tolerant M022 showed a better response by slowing down the decline in calorific values during stress by compartmentalizing the distribution of Na+ and K+ (Figure 6). Specifically, we observed that the aging leaves of the salt-tolerant M022 had a faster decline rate in calorific value than the salt-sensitive M127, which implied that the aging leaves increased the accumulation of Na+ and reduced the Na+ concentration in other parts of M022. Under such conditions, the salt-tolerant M022 is recommended to maintain relative stability in the calorific values of other plant parts. Also, more studies are required to ascertain some of the observed results in this study under varied conditions. For example, long-term incubation and field studies are required to evaluate the effects of the salt tolerance of plants on calorific values in the soil. This will provide more details on the specific mechanisms of how the salt tolerance of plants alters calorific values when applied to soils from different regions under different conditions. Nevertheless, the salt-tolerant variety, i.e., M022, is recommended for application in saline–alkaline soils.
5. Conclusions
Amur silvergrass M022 showed more tolerance to salt-induced stress compared to M127, and the salt-tolerant M022 had a higher calorific value. Importantly, the rapid accumulation of Na+ in the aging leaves of M022 was ascribed as the main reason for the higher salt tolerance and higher calorific values in the stems, spear leaves, and functional leaves compared to M127. Our results suggest that M022 accelerated the senescence of aging leaves by increasing the accumulation of Na+ in aging leaves, thus slowing down an increase in Na+ concentration in other plant parts to maintain relative stability in the calorific values of other plant parts. Consequently, the biomass of M022 exhibited the phenomenon of “low promotion and high suppression” under salt stress. This was characterized by higher photosynthetic efficiency, lower Na+ accumulation, and K+ efflux in aboveground organs, which effectively maintained the higher K+/Na+ ratio in the plant body.
Author Contributions
H.L. and L.L.: Conceptualization, Formal analysis, and Writing—original draft; J.C.: Methodology; J.N.N. and D.H.: Conceptualization and Writing—review and editing; J.W. and J.L. (Jianjian Li): Investigation; D.L. and J.L. (Jianxiu Liu): Data curation and Formal analysis; H.G.: Supervision; J.Z.: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, and Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific and Technological Innovation Fund of Carbon Emissions Peak and Neutrality of Jiangsu Provincial Department of Science and Technology (Grant No. BE2022304), the Natural Science Foundation of Jiangsu Province, China (Grant number: BK20230756), the National Natural Science Foundation of China (Grant numbers: 31771870, 31201262, 32102424, and 42307401), and the Jiangsu Institute of Botany Talent Fund (Grant number: JIBTF202302).
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chernousenko, G.I.; Pankova, E.I.; Kalinina, N.V.; Ubugunova, V.I.; Rukhovich, D.I.; Ubugunov, V.L.; Tsyrempilov, E.G. Salt-affected soils of the Barguzin depression. Eurasian Soil Sci. 2017, 50, 646–663. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Shen, X.J. Progress of Water and Salt Transport in Saline Lands and Hydrus Model: A Review. Acad. J. Sci. Technol. 2023, 6, 33–39. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Kataria, S.; Verma, S.K. Salinity stress responses and adaptive mechanisms in major glycophytic crops: The story so far. Salin. Responses Toler. Plants 2018, 1, 1–39. [Google Scholar]
- Ndiate, N.I.; Saeed, Q.; Haider, F.U.; Liqun, C.; Nkoh, J.N.; Mustafa, A. Co-application of biochar and Arbuscular mycorrhizal fungi improves salinity tolerance, growth and lipid metabolism of maize (Zea mays L.) in an alkaline soil. Plants 2021, 10, 2490. [Google Scholar] [CrossRef]
- Gomes Silveira, J.A.; De Almeida Viégas, R.; Almeida Da Rocha, I.M.; De Oliveira Monteiro Moreira, A.C.; De Azevedo Moreira, R.D.; Abreu Oliveira, J.T. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J. Plant Physiol. 2003, 160, 115–123. [Google Scholar] [CrossRef]
- Lu, C.X.; Zhang, Y.Z.; Mi, P.; Guo, X.Y.; Wen, Y.X.; Han, G.L.; Wang, B.S. Proteomics of Salt Gland–Secreted Sap Indicates a Pivotal Role for Vesicle Transport and Energy Metabolism in Plant Salt Secretion. Int. J. Mol. Sci. 2022, 23, 13885. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Hossain, Z.; Mandal, A.K.A.; Datta, S.K.; Biswas, A.K. Development of NaCl-tolerant line in Chrysanthemum morifolium Ramat. through shoot organogenesis of selected callus line. J. Biotechnol. 2007, 129, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Blumwald, E.; Aharon, G.S.; Apse, M.P. Sodium transport in plant cells. BBA-Biomembranes 2000, 1465, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Gierth, M.; Schroeder, J.I. Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 2002, 247, 43–54. [Google Scholar] [CrossRef]
- Płażek, A.; Dubert, F.; Kościelniak, J.; Tatrzańska, M.; Maciejewski, M.; Gondek, K.; Żurek, G. Tolerance of Miscanthus × giganteus to salinity depends on initial weight of rhizomes as well as high accumulation of potassium and proline in leaves. Ind. Crop. Prod. 2014, 52, 278–285. [Google Scholar] [CrossRef]
- Shin, R.; Schachtman, D.P. Hydrogen peroxide mediates plant rootcell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Shin, R. Nutrient sensing and signaling: NPKS. Annu. Rev. Plant Biol. 2007, 58, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Kim, M.J.; Ruzicka, D.; Shin, R.; Schachtman, D.P. The Arabidopsis AP2/ERF transcription factor RAP2.11 modulates plant response to low-potassium conditions. Mol. Plant 2012, 5, 1042–1057. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.; Shin, R. Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 2014, 56, 231–249. [Google Scholar] [CrossRef]
- Shankar, A.; Singh, A.; Kanwar, P.; Srivastava, A.K.; Pandey, A.; Suprasanna, P.; Kapoor, S.; Pandey, G.K. Gene expression analysis of rice seedling under potassium deprivation reveals major changes in metabolism and signaling components. PLoS ONE 2013, 8, e70321. [Google Scholar] [CrossRef]
- Sarath, N.G.; Shackira, A.M.; Puthur, J.T. Adaptive physio-anatomical modulations and ionomics of Volkameria inermis L. in response to NaCl. Int. J. Phytoremed. 2024, 26, 114–130. [Google Scholar] [CrossRef]
- Baranova, E.N.; Gulevich, A.A. Asymmetry of plant cell divisions under salt stress. Symmetry 2021, 13, 1811. [Google Scholar] [CrossRef]
- Ashraf, M.; O’Leary, J.W. Ion distribution in leaves of varying age in salt-tolerant lines of alfalfa under salt stress. J. Plant Nutr. 1994, 17, 1463–1476. [Google Scholar] [CrossRef]
- Stavridou, E.; Webster, R.J.; Robson, P.R.H. Novel Miscanthus genotypes selected for different drought tolerance phenotypes show enhanced tolerance across combinations of salinity and drought treatments. Ann. Bot. 2019, 124, 653–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xue, S.; Kang, W.W.; Qian, Z.X.; Yi, Z.L. Genetic diversity and population structure of Miscanthus lutarioriparius, an endemic plant of China. PLoS ONE 2019, 14, e0211471. [Google Scholar] [CrossRef] [PubMed]
- Brosse, N.; Dufour, A.; Meng, X.Z.; Sun, Q.N.; Ragauskas, A. Miscanthus: A fast-growing crop for biofuels and chemicals production. Biofuel. Bioprod. Bior. 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Meeting US biofuel goals with less land: The potential of Miscanthus. Global Change Biol. 2008, 14, 2000–2014. [Google Scholar] [CrossRef]
- Atkinson, C. Establishing perennial grass energy crops in the UK: A review of current propagation options for Miscanthus. Biomass Bioenerg. 2009, 33, 752–759. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Lewandowski, I. Screening Miscanthus genotypes in field trials to optimize biomass yield and quality in Southern Germany. Eur. J. Agron. 2002, 16, 97–110. [Google Scholar] [CrossRef]
- Mann, J.J.; Barney, J.N.; Kyser, G.B.; DiTomaso, J.M. Root system dynamics of miscanthus × giganteus and Panicum virgatum in response to rainfed and irrigated conditions in California. Bioenerg. Res. 2013, 6, 678–687. [Google Scholar] [CrossRef]
- Pope, C.; Mays, N. The Role of Theory in Qualitative Research Differences in Ontology and Epistemology. In Qualitative Research in Health Care, 4th ed.; Pope, C., Mays, N., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2020; pp. 15–26. [Google Scholar]
- Yost, M.A.; Randall, B.K.; Kitchen, N.R.; Heaton, E.A.; Myers, R.L. Yield potential and nitrogen requirements of miscanthus × giganteus on eroded soil. Agron. J. 2017, 109, 684–695. [Google Scholar] [CrossRef]
- Szulczewski, W.; Zyromski, A.; Jakubowski, W.; Biniak-Pierog, M. A new method for the estimation of biomass yield of giant miscanthus (Miscanthus giganteus) in the course of vegetation. Renew. Sust. Energy Rev. 2018, 82, 1787–1795. [Google Scholar] [CrossRef]
- Deuter, M. Breeding approaches to improvement of yield and quality in Miscanthus grown in Europe. In European Miscanthus Improvement Final Report September; Lewandowski, I., Clifton-Brown, J.C., Eds.; Institute of Crop Production and Grassland Research, University of Hohenheim: Stuttgart, Germany, 2000; Volume 2000, pp. 28–52. [Google Scholar]
- Farrell, A.D.; Clifton-Brown, J.C.; Lewandowski, I.; Jones, M.B. Genotypic variation in cold tolerance influences the yield of Miscanthus. Ann. Appl. Biol. 2006, 149, 337–345. [Google Scholar] [CrossRef]
- Miles, T.R.; Miles, T.R., Jr.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Boiler deposits from firing biomass fuels. Biomass Bioenergy 1996, 10, 125–138. [Google Scholar] [CrossRef]
- Obernberger, I.; Biedermann, F.; Widmann, W.; Riedl, R. Concentrations of inorganic elements in biomass fuels and recovery in the different ash fractions. Biomass Bioenergy 1997, 12, 211–224. [Google Scholar] [CrossRef]
- Marcum, K.B.; Anderson, S.J.; Engelke, M.C. Salt gland ion secretion: A salinity tolerance mechanism among five zoysiagrass species. Crop Sci. 1998, 38, 806–810. [Google Scholar] [CrossRef]
- Xie, Y.J.; Ling, T.F.; Han, Y.; Liu, K.L.; Zheng, Q.S.; Huang, L.Q.; Yuan, X.X.; He, Z.Y.; Hu, B.; Fang, L.; et al. Carbon Monoxide Enhances Salt Tolerance by Nitric Oxide-Mediated Maintenance of Ion Homeostasis and Up-Regulation of Antioxidant Defence in Wheat Seedling Roots. Plant Cell Environ. 2008, 31, 1864–1881. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Matoh, T. Characterization of Na+ exclusion mechanisms of salt-tolerant reed plants in comparison with salt-sensitive rice plants. Physiol. Plant. 1991, 83, 170–176. [Google Scholar] [CrossRef]
- Wang, S.M.; Zheng, W.J.; Ren, J.Z.; Zhang, C.L. Selectivity of various types of salt-resistant plants for K+ over Na+. J. Arid Environ. 2002, 52, 457–472. [Google Scholar] [CrossRef]
- Yue, X.; Chen, D.Z.; Luo, J.; Xin, Q.F.; Huang, Z. Upgrading of reed pyrolysis oil by using its biochar-based catalytic esterification and the influence of reed sources. Appl. Energy 2020, 268, 114970. [Google Scholar] [CrossRef]
- Marcum, K.B.; Pessarakli, M.; Kopec, D.M. Relative salinity tolerance of 21 turf-type desert salt grasses compared to bermudagrass. HortScience 2005, 40, 827–829. [Google Scholar] [CrossRef]
- Lewandowski, I.; Heinz, A. Delayed harvest of miscanthus—Influences on biomass quantity and quality and environmental impacts of energy production. Eur. J. Agron. 2003, 19, 45–63. [Google Scholar] [CrossRef]
- Lewandowski, I.; Kicherer, A. Combustion quality of biomass: Practical relevance and experiments to modify the biomass quality of Miscanthus × giganteus. Eur. J. Agron. 1997, 6, 163–177. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Singh, M.P.; Erickson, J.E.; Sollenberger, L.E.; Woodard, K.R.; Vendramini, J.M.B.; Fedenko, J.R. Mineral composition and biomass partitioning of sweet sorghum grown for bioenergy in the southeastern USA. Biomass Bioenergy 2012, 47, 1–8. [Google Scholar] [CrossRef]
- Ge, X.M.; Xu, F.Q.; Vasco-Correa, J.; Li, Y.B. Giant reed: A competitive energy crop in comparison with miscanthus. Renew. Sust. Energy Rev. 2016, 54, 350–362. [Google Scholar] [CrossRef]
- Ashraf, M.; O’Leary, J.W. Distribution of cations in leaves of salt-tolerant and salt-sensitive lines of sunflower under saline conditions. J. Plant Nutr. 1995, 18, 2379–2388. [Google Scholar] [CrossRef]
- Bhatti, A.S.; Steinert, S.; Sarwar, G.; Hilpert, A.; Jeschke, W.D. Ion distribution in relation to leaf age in Leptochloa fusca* (L.) Kunth (Kallar grass) I. K, Na, Ca and Mg. New Phytol. 1993, 123, 539–545. [Google Scholar] [CrossRef]
- Plaut, Z.; Meinzer, F.C.; Federman, E. Leaf development, transpiration and ion uptake and distribution in sugarcane cultivars grown under salinity. Plant Soil 2000, 218, 59–69. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.Q. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 2023, 14, 1217193. [Google Scholar]
- Wang, L.; Liu, Y.H.; Li, D.; Feng, S.J.; Yang, J.W.; Zhang, J.J.; Zhang, J.L.; Wang, D.; Gan, Y.T. Improving salt tolerance in potato through overexpression of AtHKT1 gene. BMC Plant Biol. 2019, 19, 357. [Google Scholar] [CrossRef]
- Kim, S.; Rayburn, A.L.; Voigt, T.; Parrish, A.; Lee, D.K. Salinity effects on germination and plant growth of prairie cordgrass and switchgrass. Bioenerg. Res. 2012, 5, 225–235. [Google Scholar] [CrossRef]
- Chen, J.B.; Zong, J.Q.; Li, D.D.; Chen, Y.; Wang, Y.; Guo, H.L.; Li, J.J.; Li, L.; Guo, A.G.; Liu, J.X. Growth response and ion homeostasis in two bermudagrass (Cynodon dactylon) cultivars differing in salinity tolerance under salinity stress. Soil Sci. Plant Nutr. 2019, 65, 419–429. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.P.; Sun, J.L.; Pan, Z.E.; Gong, W.F.; Lu, Y.L.; Du, X.M. Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci. Rep. 2016, 6, 34548. [Google Scholar]
- Chen, C.L.; Van der Schoot, H.; Dehghan, S.; Alvim Kamei, C.L.; Schwarz, K.U.; Meyer, H.; Visser, R.G.F.; Van der Linden, C.G. Genetic diversity of salt tolerance in Miscanthus. Front. Plant Sci. 2017, 8, 187. [Google Scholar]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Shabala, L.; Pottosin, I.; Zeng, F.R.; Velarde-Buendía, A.M.; Massart, A.; Poschenrieder, C.; Hariadi, Y.; Shabala, S. Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K+-permeable channels to reactive oxygen species: Physiological traits that differentiate salinity tolerance between pea and barley. Plant Cell Environ. 2014, 37, 589–600. [Google Scholar] [CrossRef]
- Wu, H.H.; Shabala, L.; Zhou, M.X.; Su, N.N.; Wu, Q.; Ul-Haq, T.; Zhu, J.J.; Mancuso, S.; Azzarello, E.; Shabala, S. Root vacuolar Na+ sequestration but not exclusion from uptake correlates with barley salt tolerance. Plant J. 2019, 100, 55–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).