Abstract
Imbibing watermelon seeds in 1 mM sodium tetraborate (Na2B4O7) for 24 h systemically protected plants against foliar infection by Stagonosporopsis cucurbitacearum in detached leaves and under greenhouse conditions. The treatment resulted in both a reduction in the overall percentage of leaf infection as well as in the size of lesions. Studies of the mechanisms by which Na2B4O7 protected watermelon showed that there was no direct effect on the S. cucurbitacearum mycelium growth in vitro. On the other hand, plants raised from seeds primed with Na2B4O7 showed a higher frequency of fluorescent epidermal cells compared to the plants treated with water. This indicates that a higher number of cells expressed the hypersensitive response after Na2B4O7 priming. In addition, there was an increase in peroxidase activity and an enhanced accumulation of a 45 kDa acidic peroxidase isoform during the early stages of infection in plants treated with Na2B4O7 compared to plants treated with water and this was positively correlated to the reduction of leaf infection caused by the pathogen. These results indicate that Na2B4O7 is able to induce systemic resistance in watermelon against S. cucurbitacearum by activating the hypersensitive reaction at penetration sites, increasing peroxidase activity and altering the peroxidase isozyme profile. Although each individual response may only have had a minor effect, their combined effects had a reducing effect on the disease.
1. Introduction
Gummy stem blight caused by Stagonosporopsis cucurbitacearum (syn. Didymella bryoniae) is a very destructive disease in watermelon and other cucurbit species [1,2,3]. The fungus can attack all plant parts, including leaves, stems, fruit and even roots [1,4]. The disease can cause very serious losses in watermelon in the field as well as after harvest under favourable conditions [3,5,6,7].
Control of the disease is difficult for several reasons. Consequently, the disease develops rapidly at high levels of humidity [5], which is often present in production fields. In addition, no highly resistant cultivars of watermelon are available [8], although extensive searches for new sources of resistance are ongoing [9,10,11,12,13]. Furthermore, the pathogen rapidly develops resistance to fungicides [2,14,15,16], rendering chemical control difficult.
To reduce the pesticide input, induced resistance has received considerable attention as an environmentally friendly strategy [17,18,19]. Induced resistance denotes the phenomenon that a plant, appropriately stimulated, can defend itself against pathogens [18,20]. Thus, treatment of the plants with biotic or abiotic inducers can activate defence responses in the plant against subsequent pathogen attack [21,22]. Research on induced resistance in cucurbit plants has, for example, included studies of cucumber against Gloeosporium orbiculare (syn. Colletotrichum lagenaria) and Podosphaera fuliginea (syn. Sphaerotheca fuliginea) [23,24,25,26,27,28] and of melon against Sclerotinia sclerotiorum and Stagonosporopsis cucurbitacearum [29,30,31]. There are apparently only few reports from watermelon implying that protection against pathogens involves induced resistance [31]. Several chemicals like acibenzolar-S-methyl, methyl jasmonate, K2HPO4 and MnCl2 have been reported to be able to induce resistance in cucumber and melon [24,26,27,28,29].
Various defence responses have been inferred as being important in induced resistance in cucurbit plants. Examples include accumulation of phytoalexins [32,33]. Likewise, the hypersensitive response as well as reinforcement of plant cell walls appear to be positively correlated to the reduction of pathogen penetration and this effect is mediated by papilla formation and accumulation of callose, lignin, phenolic compounds, H2O2 and silicon at the sites of attempted penetration [30,31,33,34,35,36,37]. Accumulation of PR-proteins like peroxidase, chitinase and β-1,3-glucanase has also been reported as an important mechanism [29,31,37,38,39,40]. A specific peroxidase isoform (45 kDa) was found to accumulate during induction of resistance against S. cucurbitacearum in watermelon using rhizobacteria [31] and this isoform was also found to be important when explaining differences in the level of resistance against S. cucurbitacearum between a susceptible and a moderately resistant accession of watermelon [41]. Furthermore, activities of important enzymes in the phenyl propanoid pathway have also been increased, such as phenylalanine ammonia lyase, polyphenol oxidase and peroxidase, which are involved in lignification and accumulation of phytoalexins and phenolics [31,37,38,42,43]. The hypersensitive reaction has also been mentioned as an important mechanism in phosphate-induced resistance in cucumber [27] as well as accumulation of reactive oxygen species in cucumber and watermelon, respectively [27,31].
Boron is an essential plant micronutrient, usually taken up by the roots, although it can also be applied as seed treatments or foliar sprays [44,45] However, in excess, boron causes toxicity to the plants, so an appropriate dosage is critical [44,45]. Borax or sodium tetraborate (Na2B4O7) can be used as a fertiliser [46], but is also known as a pesticide, for example, against insect pests [47]. It has previously been reported to induce local as well as systemic resistance in rice against Pyricularia oryzae in Vietnam in greenhouse as well as in field trials [48,49]. Boric acid has also been reported to induce local and systemic resistance in cucumber against powdery mildew caused by Podosphaera fuliginea [24] and different sources of boron were able to control clubroot in oilseed rape [50]. Recently, safflower seeds were primed with 5–10 ppm boron or seed dressed with boron to reduce the negative effects of seed-borne pathogens and promote germination and seedling growth [51].
The present investigation was undertaken to assess the ability of Na2B4O7 to protect watermelon against S. cucurbitacearum and study the mechanisms involved, with the hypothesis being that the compound induces resistance. We show that priming of watermelon seeds with Na2B4O7 can induce resistance against foliar infection caused by S. cucurbitacearum and the mechanisms of disease protection are associated with a higher frequency of hypersensitive cells, increased peroxidase activity, as well as an altered peroxidase isozyme profile.
2. Materials and Methods
2.1. Plants and Pathogen
Two watermelon accessions (Citrullus spp.) were included in the experiments, i.e., the moderately resistant C. amarus accession PI189225 from USDA, ARS, National Genetic Resources Program (originally collected in Zaire) and the susceptible C. lanatus accession 232-0125/B from India.
S. cucurbitacearum (isolate I16) originated from an infected watermelon leaf collected in Can Tho Province, Vietnam, in 2003. Inoculum was produced on sterilised potato cubes in a 100 mL flask for 5–7 days [41] and conidial inoculum was prepared as earlier described [41]. The susceptible accession 232-0125/B was inoculated with 105 conidia/mL and the moderately resistant accession PI189225 with 106 conidia/mL. A higher inoculum concentration was used on PI189225 because it is less susceptible than 232-0125/B. For in vitro tests, the fungus was grown on potato dextrose agar plates (PDA, Difco, Roskilde, Denmark).
2.2. Cultivation of Plants and Fungi and Plant Inoculation
After priming (see Section 2.5), seeds were sown in square pots (10 × 10 cm) containing the potting medium Pindstrup Substrate (Pindstrup Mosebrug A/S, Ryomgaard, Denmark). Plants were cultivated in a growth chamber as previously described [41].
When the plants had developed the first or second true leaves, they were inoculated with S. cucurbitacearum by atomising a conidial suspension onto the leaf surfaces until run-off. After inoculation, plants were sealed in plastic bags with 100% humidity in darkness. After 48 h, the bags were opened and the plants were placed at normal light regime again.
2.3. Disease Scoring
Disease severity was evaluated at 4 days after inoculation (dai) for accession 232-0125/B and 7 dai for accession PI189225 because symptoms developed slower on the latter. The percentage leaf area covered with symptoms was recorded and symptoms were also assessed by using a 0–5 scale (modified from [52]): where 0 (no symptoms), 1 (very small lesions 0–0.5 cm), 2 (small lesions 0.5–1 cm), 3 (medium lesions 1–2 cm), 4 (large lesions > 2 cm) and 5 (entire leaf infected).
2.4. In Vitro Test of Antifungal Effect of Na2B4O7 on Stagonosporopsis cucurbitacearum
Petri dishes containing PDA with different concentrations of Na2B4O7 (sodium tetraborate-10 hydrate, Merck, Søborg, Denmark) were prepared. The concentrations were 0.25, 0.5, 1, 2, 5 and 10 mM). An agar plug (5 mm in diameter) from a 7-day-old actively growing S. cucurbitacearum culture was placed in the centre of a plate. Plates were incubated in the growth chamber described in Section 2.2. Growth was assessed by measuring the colony diameter of S. cucurbitacearum (mm) at 2, 3, 4 and 5 days after inoculation for each treatment. The antifungal activity of Na2B4O7 was scored by comparing the growth of S. cucurbitacearum mycelium at the different concentrations of Na2B4O7 with the control (water) at each time point. Each treatment comprised four plates.
2.5. Test of Na2B4O7 for Systemic Protection against Foliar Infection
Accession 232-0125/B was used for this experiment. Seeds were primed in different concentrations of Na2B4O7 (0.5, 1 and 2 mM) for 24 h at room temperature. Seeds primed in distilled water served as control. Immediately after treatments, seeds were sown as described in Section 2.2. When the plants had developed the second true leaf (18–20 days after sowing), they were inoculated with S. cucurbitacearum at a concentration of 105 conidia/mL. Inoculation, incubation and disease scoring were as described in Section 2.2 and Section 2.3. There were nine plants for each treatment.
2.6. Ability of 1 mM Na2B4O7 to Protect Detached Watermelon Leaves
Seeds of both watermelon accessions were primed with 1 mM Na2B4O7 or water (control) and sown as described in Section 2.2. When the seedlings were 18 days old, the first and the second true leaf were detached and placed in Petri dishes on two layers of sterile filter paper saturated with sterile distilled water. Subsequently, the centre of leach leaf was inoculated with a 6-mm mycelial plug of S. cucurbitacearum from an actively growing 7-day-old PDA culture. Plates were incubated in the growth chamber described in Section 2.2. The diameters of lesions on leaves were measured at 2, 3, 4 and 5 days after inoculation (dai). The experiment comprised 20 leaves for each accession and treatment.
2.7. Ability of Na2B4O7 to Protect Watermelon Systemically under Greenhouse Conditions
Seeds of both watermelon accessions were primed with 1 mM Na2B4O7 or water (control). When the first true leaf was expanded (ca. 15 days after sowing), plants were inoculated with S. cucurbitacearum by atomising a spore suspension onto the surface of the first true leaf until run-off. Incubation conditions and disease scoring were as described in Section 2.2. There were 28 plants for each treatment for accession PI189225 and 15 plants for accession 232-0125/B.
2.8. Infection Biology of S. cucurbitacearum in Watermelon Plants Treated with Na2B4O7
The experiments were performed with both accessions PI189225 and 232-0125/B. Seeds were primed with 1 mM Na2B4O7 or water (control). Plants were grown and infected with the pathogen as described above. The inoculated first leaves were harvested at 17, 24, 40 and 48 h after inoculation (hai) and cleared using a 3:1 (v/v) mixture of absolute ethanol:glacial acetic acid as described earlier [41]. For observation by light microscopy, leaves were stained with 0.1% (w/v) Evans Blue in lactoglycerol. Twenty-five randomly selected conidia were examined on each of four leaves (a total of 100 conidia per accession, treatment and time point). For each conidium, it was recorded whether it germinated and whether it caused penetration. Furthermore, the frequency of fluorescent epidermal cells (hypersensitive response) at penetration sites was recorded. These cells were detected by epifluorescence microscopy (excitation filter 400–440 nm, dicroitic mirror DM 455, barrier filter > 475).
2.9. Peroxidase Activity and Native PAGE for Detection of Peroxidase Isozymes
Only accession 232-0125/B was studied. Watermelon seeds were primed with 1 mM Na2B4O7 or water (control) and plant growth and inoculation took place as before. The experiment included four treatments: plants, either primed with Na2B4O7 or water were inoculated with pathogen or left uninoculated. First-developed true leaves were harvested at 0, 12, 24, 36, 48, 72 and 96 hai, immediately frozen in liquid nitrogen and stored at −80 °C. Leaf samples were ground in liquid nitrogen and protein extracted in 0.1 M potassium phosphate buffer (pH 7.0). Protein concentration was determined using the Bradford protein assay [53] with bovine serum albumin (Sigma, Søborg, Denmark) as standard.
Peroxidase activity and acidic peroxidase isoforms were determined as described previously [41].
2.10. Experimental Design and Statistical Analysis of Data
Data analysis took place as previously described [41]. Data from the microscopy study on infection biology and frequency of HR cells represent discrete variables and were analysed by logistic regression, assuming a binomial distribution. Data on disease severity, disease scale scorings, colony diameters and enzyme activities represent continuous variables and were analysed by analysis of variance, assuming a normal distribution. Variances were stabilised by appropriate transformations when necessary. All experiments were performed at least three times, except for the in vitro experiment testing the ability of Na2B4O7 to control the pathogen (only performed once). Representative data from individual experiments are presented. Data were analysed by PC-SAS (release 9.4; SAS Institute, Cary, NC, USA) and hypotheses rejected at p < 0.05.
3. Results
3.1. In Vitro Tests
Test of antifungal activity of different concentrations of Na2B4O7 on S. cucurbitacearum growth were conducted on agar plates (Table 1). Concentrations of 0.25 and 0.5 mM showed a significant growth promoting effect whereas a concentration of 1 mM had no significant effect on mycelium growth of S. cucurbitacearum. A concentration of 2 mM inhibited the growth of S. cucurbitacearum at 2 days after inoculation (dai), but not at later time points. Higher concentrations of 5 and 10 mM resulted in clearly significant reductions in colony diameter (Table 1).

Table 1.
Antifungal activity of Na2B4O7 evaluated by comparison of the diameter of fungal colony growth (mm) at different concentrations of Na2B4O7 in agar plates at 2, 3, 4 and 5 days after inoculation.
3.2. Systemic Protection of Watermelon after Seed Priming with Na2B4O7
Three concentrations (0.5, 1 and 2 mM) giving slight promotion, no, or slight inhibition of fungal growth, respectively, were chosen for testing of the ability to protect watermelon accession 232-0125/B systemically against S. cucurbitacearum. Table 2 shows that priming seeds for 24 h in the three tested concentrations of Na2B4O7 reduced S. cucurbitacearum infection on the first and the second true leaves, 1 mM Na2B4O7 giving the largest protection.

Table 2.
Systemic protection of watermelon accession 232-0125/B induced by Na2B4O7 against foliar infection of Stagonosporopsis cucurbitacearum at 4 days after inoculation under greenhouse conditions.
3.3. Ability of 1 mM Na2B4O7 to Protect Detached Watermelon Leaves
The ability of 1 mM Na2B4O7 to induce systemic protection in detached leaves is shown in Table 3. Leaves were detached from plants raised from seeds primed with either water (control) or Na2B4O7. In accession PI189225 (Table 3, Figure 1A), lesion size was significantly smaller in the first true leaves at both 3 and 4 dai after treatment with 1 mM Na2B4O7 compared to treatment with water, whereas there was no significant difference for the second true leaves. In accession 232-0125/B (Table 3, Figure 1B), lesions on the second true leaves were significantly smaller for the Na2B4O7 treatment than for the water treatment at 3 dai whereas there were no differences for the first true leaf at either time point or for the second true leaf for 2 dai.

Table 3.
Detached leaf assay for disease reduction in accessions PI189225 and 232-0125/B raised from seeds primed with 1 mM Na2B4O7 or treated with water (control) at different time points after inoculation.

Figure 1.
Ability of 1 mM Na2B4O7 to induce systemic protection in detached leaves. Leaves were detached from plants raised from seeds primed with either water (control) or Na2B4O7. (A) Lesion development on first developed true leaves of accession PI189225 at 4 days after inoculation and (B) on the second developed true leaf of accession 232-0125/B at 3 days after inoculation. Top rows in (A,B) show leaves from plants raised from seeds primed with water and lower rows in (A,B) show leaves from plants raised from seeds primed with 1 mM Na2B4O7.
3.4. Ability of 1 mM Na2B4O7 to Protect Watermelon under Greenhouse Conditions
Testing the effect of 1 mM Na2B4O7 on systemic protection of the two accessions PI189225 and 232-0125/B against S. cucurbitacearum under greenhouse conditions is shown in Table 4 and Figure 2. In accession PI189225, the percentage leaf coverage with symptoms after treatment with 1 mM Na2B4O7 was not significantly different from treatment with water. However, by assessing the size of lesions according to a scale with six levels (as described in Section Materials and Methods), it was observed that lesion size on plants raised from seeds primed with Na2B4O7 was significantly smaller than on leaves raised from seeds primed with water (Table 4, Figure 2A,B). For accession 232-0125/B, plants raised from seeds primed with 1 mM Na2B4O7 showed significantly less disease, measured as both percentage of leaf coverage with symptoms and lesion size, than plants raised from seeds primed with water (Table 4, Figure 2C).

Table 4.
Disease severity and percentage of protection on the first true leaves of accessions PI189225 and 232-0125/B raised from seeds primed with 1 mM Na2B4O7 or with water (control).
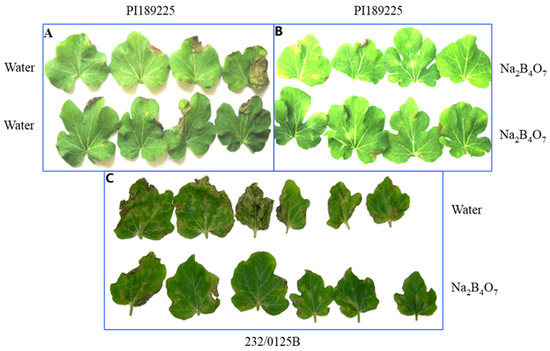
Figure 2.
Leaf symptoms caused by S. cucurbitacearum on watermelon plants raised from seeds primed with 1 mM Na2B4O7 or water (control) under greenhouse conditions. (A,B) accession PI189225 at 7 days after inoculation and (C) accession 232/0125B at 4 days after inoculation. (A) and top row of (C): leaves from plants raised from seeds primed with water. (B) and lower row of (C): leaves from plants raised from seeds primed with 1 mM Na2B4O7.
3.5. Infection Biology of S. cucurbitacearum in Watermelon Plants Treated with Na2B4O7
Studies on the infection biology of S. cucurbitacearum in the first true leaf of plants raised from seeds primed with 1 mM Na2B4O7 or water were performed in both accessions (Table 5 and Table 6). There was no significant difference in spore germination between treatments at any time points (Table 5 and Table 6), whereas there was a significantly lower penetration after Na2B4O7 treatment compared to the water treatment in accession PI189225 at 40 hai (Table 5) and in accession 232-0125/B at 17 hai (Table 6).

Table 5.
Infection biology of Stagonosporopsis cucurbitacearum in leaves of watermelon accession PI189225 raised from seeds primed with 1 mM Na2B4O7 or water (control).

Table 6.
Infection biology of Stagonosporopsis cucurbitacearum in leaves of watermelon accession 232-0125/B raised from seeds primed with 1 mM Na2B4O7 or water (control).
Single and multiple HR occurred at penetration sites (Table 5 and Table 6, Figure 3). In accession PI189225, both single and multiple HR were seen at 40 and 48 hai (Table 5), but only single HR was significantly higher at 48 hai after Na2B4O7 treatment compared to water treatment. In accession 232-0125/B, both single and multiple HR were detected already from 24 hai in plants primed with Na2B4O7, but only from 40 hai in plants primed with water (Table 6). However, there was no significant difference between treatments at any time point (Table 6).
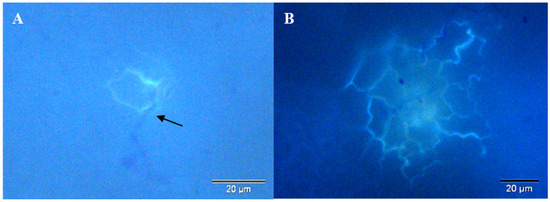
Figure 3.
(A) single and (B) multiple fluorescent epidermal cells at penetration site of S. cucurbitacearum in leaves of accession 232-0125/B at 24 and 48 hai, respectively. Arrow: hypha causing penetration.
3.6. Peroxidase Activity
Total peroxidase activity was investigated in leaves of the susceptible accession 232-0125/B primed with Na2B4O7 or water, with and without inoculation with S. cucurbitacearum (Figure 4). Peroxidase activity after treatment with Na2B4O7 or water, but without inoculation with the pathogen, remained low throughout the experiment. Inoculation with pathogen resulted in a significant increase in peroxidase activity at 12 hai in the Na2B4O7-treated plants, which were not seen for any other treatment. At 24 hai, there was no significant difference between treatments, but peroxidase activity in water treated plants inoculated with the pathogen, started to increase rapidly from 36 hai and became significantly higher than in plants treated with Na2B4O7 at 48 and 96 hai. The increase of peroxidase activity at the late time points correlated with symptom development.
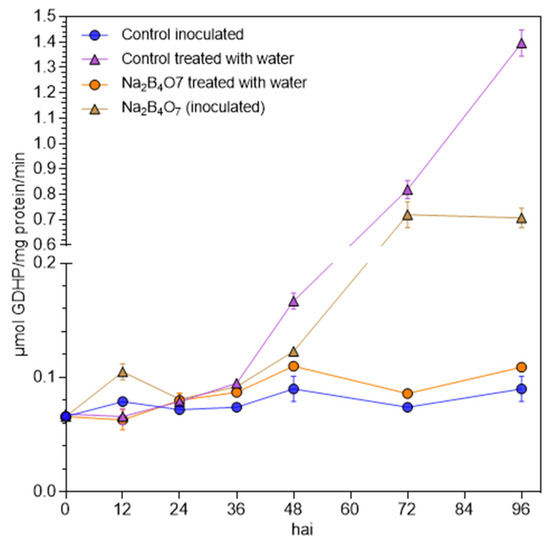
Figure 4.
Total peroxidase activity (µmol guaiacol dehydrogenated product (GDHP)/mg protein/second) in watermelon leaves of accession 232-0125/B raised from seeds primed with 1 mM Na2B4O7 or water (control), followed by inoculation with S. cucurbitacearum or application of water. Error bars represent standard error of the mean.
3.7. Native PAGE for Detection of Peroxidase Isozymes
Basic native-PAGE for detection of acidic isoforms of peroxidase (Figure 5) showed an increased accumulation of an approximately 45 kDa isoform following the progression of infection with a particularly large accumulation from 36 hai after treatment with Na2B4O7.
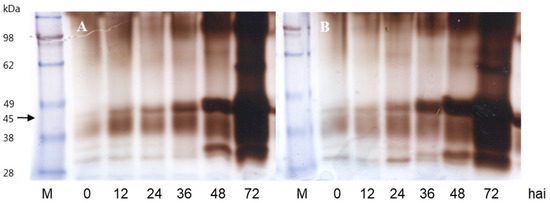
Figure 5.
Basic 10% native PAGE of acidic peroxidase isozymes in leaves of the susceptible watermelon accession 232-0125/B raised from seeds primed with (A) water (control) or (B) 1 mM Na2B4O7 at different time points after inoculation with S. cucurbitacearum.
4. Discussion
Priming of watermelon seeds with Na2B4O7 was able to provide significant levels of protection against S. cucurbitacearum in the first leaves of both living plants and in detached leaves. Priming with 1 mM Na2B4O7 for 24 h gave the highest level of protection under greenhouse conditions, whereas it was not as pronounced in detached leaves. Previously, boron in different chemical formulations, including Na2B4O7, was shown to protect plants against various diseases [24,31,48,49,51]. Na2B4O7 is often considered a biopesticide, chiefly against insect pests [54,55]. However, Na2B4O7 has apparently not been used to protect watermelon and other species in Cucurbitaceae against diseases before.
In vitro tests showed that 1 mM Na2B4O7 had no visible effect on S. cucurbitacearum growth on agar plates. An inhibiting effect on S. cucurbitacearum was seen only in concentrations higher than 2 mM and therefore 1 mM Na2B4O7 was chosen for further studies. Since 1 mM Na2B4O7 applied to seeds could protect watermelon plants efficiently, without a direct effect on the pathogen, the results could indicate that Na2B4O7 induces systemic resistance against foliar infection by S. cucurbitacearum. Similarly, Na2B4O7 was found to protect rice systemically from blast at a concentration of 1 mM and this concentration did not inhibit P. oryzae mycelium growth in in vitro tests [49]. Interestingly, boron and Na2B4O7 have been found to inhibit other pathogens like Monilinia laxa strongly in vitro [56]. Concentration of the compounds plays a pivotal role, but there might also be differences in the sensitivity of different pathogens to the compounds.
In order to verify if induced resistance sensu Kloepper et al. [20] was involved in the protection, the infection biology of the pathogen was studied to determine at which steps it was arrested. As pointed out earlier [41,57], quantitative microscopy of the infection course of the pathogen at consecutive time points provides a unique opportunity for understanding when infection is arrested and link this to defence responses in the host. There was no significant inhibition of spore germination on leaves by 1 mM Na2B4O7, corroborating the in vitro results, whereas the percentage of spores causing penetration was significantly reduced in leaves from Na2B4O7-primed compared to water-primed plants of both the susceptible and the moderately resistant accession.
A comparison of the infection biology of S. cucurbitacearum in Na2B4O7-induced and water-treated plants showed that there was a higher frequency of single fluorescent epidermal cells in leaves of plants raised from seeds primed with Na2B4O7 than in plants raised from seeds primed with water, although the difference was only significant in accession PI189225 at 48 hai. FEC is an indication that the cells are undergoing the hypersensitive reaction (HR) [58,59,60] and HR has previously been reported as a defence response in the interaction between melon and S. cucurbitacearum [30]. HR cells have a major accumulation of reactive oxygen species, phytoalexins and phenolic compounds [61,62,63] and are reported to be an efficient defence response of plants to biotrophic and hemibiotrophic pathogens [27,60,62,63,64,65]. Recently, it was suggested that S. cucurbitacearum might be considered as a hemibiotrophic pathogen, among others, because it was inhibited by H2O2 during penetration and because it sometimes grows in the tissue without causing symptoms [41]. However, as pointed out previously [41], further studies should be carried out to study under which conditions a hemibiotrophic behaviour takes place.
A higher frequency of HR-cells has been related to resistance in various plant pathogen interactions. For example, a higher frequency of HR was found to be correlated to the level of resistance in rice infected by Bipolaris oryzae [59]. Likewise, a high frequency of HR-cells was also found to be associated with induced resistance in cucumber against Colletotrichum lagenaria after pre-treatment with the bacteria Serratia marcescens (isolate 90-166) and Pseudomonas fluorescens (isolate 89B61) [25]. We found that HR occurred at a higher frequency after penetration in plants raised from seeds primed with Na2B4O7 than in the control plants. This indicates that HR is a defence response after penetration and that the enhancement of the response following Na2B4O7 treatment could inhibit the pathogen. A comparative observation was reported earlier for induced resistance in barley against the hemibiotrophic pathogen Pyrenophora teres where inducer treatment enhanced the efficiency of the HR response [66].
Peroxidases are important enzymes in plant defence [67,68]. Thus, they may participate in generation of H2O2, which is important for HR and are involved in many defence responses [65]. Peroxidases also participate in modification of plant cell walls such as lignification, suberisation and in oxidative protein cross-linking in cell walls. Total peroxidase activity was studied in the susceptible accession 232-0125/B and it was found to increase earlier and more rapidly (12 hai) in pathogen-infected plants raised from seeds primed with Na2B4O7 than in pathogen-infected control plants (seeds primed with water). The increase in peroxidase activity at 12 hai correlated with lower penetration frequency in plants treated with Na2B4O7 at 17 hai compared to the control. A similar observation was made in studies of induced resistance of cucumber against Colletotrichum lagenaria by K2HPO4 [26] and studies of induced resistance of watermelon against S. cucurbitacearum using Pseudomonas aeruginosa 231-1 as inducer [31]. Likewise, peroxidases have been implicated in the defence of cucurbit plants against infection by various pathogens, including S. cucurbitacearum [28,30,41,69]. The early increase of peroxidase activity in plants treated with Na2B4O7 may therefore be involved in disease protection. However, at the late stages of infection from 48 to 96 hai, peroxidase activity increased rapidly to a very high level in control plants pre-treated with water compared to plants pre-treated with Na2B4O7 and this correlated with larger disease development in control plants. A similar pattern of peroxidase activity has been found in other host-pathogen interactions such as in cucumber, where pre-treatment with K2HPO4 can induce resistance against Colletotrichum lagenaria [26] and in wheat infected by Zymoseptoria tritici [70]. Also, in watermelon infected by S. cucurbitacearum, a high peroxidase activity was seen in a susceptible compared to a moderately resistant accession at the late stages of infection [41]. This late increase in peroxidase activity in the compatible interaction could be a defence response, which occurred too late to be effective in stopping the pathogen. Furthermore, as pointed out by Nga et al. [41], it could also represent a rapid increase of ascorbate peroxidase activity for scavenging the large amounts of H2O2, which was produced during the later stages of infection.
Native-PAGE for studying the accumulation of specific peroxidase isozymes was performed in the susceptible accession 232-0125/B and it was found that an acidic (45 kDa) isoform accumulated to a higher extent in leaves from plants raised from seeds primed with Na2B4O7 after inoculation with the pathogen than in the control plants raised from seeds primed with water. The same isoform was found to accumulate to a larger extent in previous studies of watermelon infected by S. cucurbitacearum. Thus, increased accumulation during infection was observed in the moderately resistant accession (PI189225) and in the susceptible accession 232-0125/B [41], as well as in watermelon plants protected from infection by the pathogen after protection with the bacterium Pseudomonas aeruginosa 231-1 [31]. As reviewed earlier [41], different peroxidase isoforms have been implicated in resistance of cucurbit plants against different pathogens so further characterisation of peroxidase isoforms may contribute to a better understanding of resistance.
A future practical use of Na2B4O7 in crop production will depend on several issues. Boron is an essential element for plant growth [71,72] and there is increasing evidence that it may also be essential in human nutrition in minute amounts [73]. Boron has also shown promise in health-promoting dietary supplements in animals and humans [74,75]. However, excess boron poses toxicity issues in humans, animals and plants [44,76,77] so the application to agricultural or horticultural systems should be carefully considered. It could be an advantage to use Na2B4O7 to control gummy stem blight in watermelon in future under field conditions. Thus, it is simple to apply by seed priming and only a small amount of chemical is needed for seed priming compared to foliar application, but it is important to make sure that there will be no negative effects on seed germination and vigour, the environment and human health if used at a large scale. Even if Na2B4O7 will not be possible to use in practical crop production, the potential of this compound to induce defence responses may be useful for further studies on the defence mechanisms operating in watermelon against gummy stem blight and other diseases.
5. Conclusions
Priming of watermelon seeds with 1 mM Na2B4O7 could induce systemic resistance in the plants against S. cucurbitacearum and the effect was more pronounced in the susceptible than in the moderately resistant accession because the latter already had a certain level of protection against the disease. Induced resistance, i.e., activation of plant defence, requires energy to protect the plant and this was illustrated by the fact that the protective effect of Na2B4O7 was not so pronounced in detached leaves as in whole plants. The detached leaves simply could not draw on the energy reserves from an actively growing plant and this shows the limitations in using detached leaves for studies of induced resistance.
The disease protection was associated with a higher frequency of HR cells, earlier and higher peroxidase activity and increased accumulation of an acidic 45 kDa peroxidase isoform. Individually, the responses may only have had slight effects on inhibition of the pathogen, but when combined, a significant effect could be observed. In addition, further defence responses are likely activated in the host, also contributing to the protective effect.
Author Contributions
Conceptualization, N.T.T.N., E.d.N., H.S.S., T.T.T.T., P.V.K. and H.J.L.J.; methodology, N.T.T.N., E.d.N., H.S.S. and H.J.L.J.; formal analysis, N.T.T.N., E.d.N. and H.J.L.J.; investigation, N.T.T.N., E.d.N., H.S.S., T.T.T.T., P.V.K. and H.J.L.J.; data curation, N.T.T.N.; writing—original draft preparation, N.T.T.N., E.d.N., H.S.S., T.T.T.T., P.V.K. and H.J.L.J.; writing—review and editing, N.T.T.N., E.d.N., H.S.S., T.T.T.T. and H.J.L.J.; visualization, N.T.T.N. and H.J.L.J.; supervision, E.d.N., H.S.S., T.T.T.T., P.V.K. and H.J.L.J.; project administration, E.d.N., H.S.S., P.V.K. and H.J.L.J.; funding acquisition, E.d.N., H.S.S., P.V.K. and H.J.L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the DANIDA-ENRECA (Enhancement of Research Capacity) project: “Systemic Acquired Resistance—an eco-friendly strategy for managing diseases in rice and pearl millet” financed by the Danish Ministry of Foreign Affairs.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors wish to thank Todd C. Wehner, North Carolina State University for providing accession PI189225; Sharathchandra R. G., Hindumathi C. K. and Geetha N. P. from Department of Applied Botany, Seed Pathology and Biotechnology, University of Mysore, India, for help with enzyme assays and protein electrophoresis; and Jens Due Jensen for help with in vitro testing of the antifungal activity of Na2B4O7 on the pathogen.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Punithalingam, E.; Holliday, P. Didymella bryoniae. CMI Descriptions of Pathogenic Fungi and Bacteria; No. 332; Commonwealth Mycological Institute: Kew, UK, 1972. [Google Scholar]
- Keinath, A.P.; Zitter, T.A. Resistance to benomyl and thiophanate-methyl in Didymella bryoniae from South Carolina and New York. Plant Dis. 1998, 82, 479–484. [Google Scholar] [CrossRef]
- Seblani, R.; Keinath, A.P.; Munkvold, G. Gummy stem blight: One disease, three pathogens. Mol. Plant Pathol. 2023, 24, 825–837. [Google Scholar] [CrossRef]
- Thinggaard, K. Attack of Didymella bryoniae on roots of cucumber. J. Phytopathol. 1987, 120, 372–375. [Google Scholar] [CrossRef]
- Sherf, A.F.; MacNab, A.A. Vegetable Diseases and Their Control; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Keinath, A.P. Effect of protectant fungicide application schedules on gummy stem blight epidemics and marketable yield of watermelon. Plant Dis. 2000, 84, 254–260. [Google Scholar] [CrossRef]
- Huyen, N.T.Q.; Hoa, T.D.; Huyen, T.T.; Nam, C.G.; Phuong, T.T.X. Situation of watermelon (Citrullus lanatus Thumb.) production in Phu Yen province. Hue Univ. J. Sci. Agric. Rural Dev. 2023, 132, 31–43. [Google Scholar]
- Gusmini, G.; Song, R.; Wehner, T.C. New sources of resistance to gummy stem blight in watermelon. Crop Sci. 2005, 45, 582–588. [Google Scholar] [CrossRef]
- Ren, R.; Xu, J.; Zhang, M.; Liu, G.; Yao, X.; Zhu, L.; Hou, Q. Identification and molecular mapping of a gummy stem blight resistance gene in wild watermelon (Citrullus amarus) germplasm PI 189225. Plant Dis. 2020, 104, 16–24. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, D.-S.; Kim, S.G.; Huh, Y.-C.; Back, C.-G.; Lee, Y.-R.; Siddique, M.I.; Han, K.; Lee, H.-E.; Lee, J. QTL mapping for gummy stem blight resistance in watermelon (Citrullus spp.). Plants 2021, 10, 500. [Google Scholar] [CrossRef]
- Hong, J.-E.; Hossain, M.R.; Jung, H.-J.; Nou, I.-S. QTL associated with gummy stem blight (GSB) resistance in watermelon. BMC Genom. 2022, 23, 632. [Google Scholar] [CrossRef]
- Mahapatra, S.; Rao, E.S.; Hebbar, S.S.; Rao, V.K.; Pitchaimuthu, M.; Sriram, S. Evaluation of rootstocks resistant to gummy stem blight and their effect on the fruit yield and quality traits of grafted watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). J. Hortic. Sci. Biotechnol. 2023, 98, 635–648. [Google Scholar] [CrossRef]
- Gimode, W.; Bao, K.; Fei, Z.; McGregor, C. QTL associated with gummy stem blight resistance in watermelon. Theor. Appl. Genet. 2021, 134, 573–584. [Google Scholar] [CrossRef]
- Kong, Z.; Zhang, Y.; Zhuang, C.; Mao, C.; Zhang, C. Detection and characterization of difenoconazole resistance in Stagonosporopsis citrulli from watermelon and muskmelon in Zhejiang Province of China. Phytopathol. Res. 2023, 5, 1. [Google Scholar] [CrossRef]
- Li, H.-X.; Nuckols, T.A.; Harris, D.; Stevenson, K.L.; Brewer, M.T. Differences in fungicide resistance profiles and multiple resistance to a quinone-outside inhibitor (QoI), two succinate dehydrogenase inhibitors (SDHI), and a demethylation inhibitor (DMI) for two Stagonosporopsis species causing gummy stem blight of cucurbits. Pest Manag. Sci. 2019, 75, 3093–3101. [Google Scholar] [CrossRef]
- Thomas, A.; Langston, D.B., Jr.; Stevenson, K.L. Baseline sensitivity and cross-resistance to succinate-dehydrogenase-inhibiting and demethylation-inhibiting fungicides in Didymella bryoniae. Plant Dis. 2012, 96, 979–984. [Google Scholar] [CrossRef]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Reglinski, T.; Havis, N.; Rees, H.J.; de Jong, H. The practical role of induced resistance for crop protection. Phytopathology 2023, 113, 719–731. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Tuzun, S.; Kuć, J.A. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 1992, 2, 349–351. [Google Scholar] [CrossRef]
- van Loon, L.C. Systemic induced resistance. In Mechanisms of Resistance to Plant Diseases; Slusarenko, A.J., Fraser, R.S.S., van Loon, L.C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 521–574. [Google Scholar] [CrossRef]
- De Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.H.; Mauch-Mani, B.; Pastor, V.; Pozo, M.J.; Pieterse, C.M.J.; Ton, J.; et al. The induced resistance lexicon: Do’s and don’ts. Trends Plant Sci. 2021, 26, 685–691. [Google Scholar] [CrossRef]
- Kováts, K.; Binder, A.; Hohl, H.R. Cytology of induced systemic resistance of cucumber to Colletotrichum lagenarium. Planta 1991, 183, 484–490. [Google Scholar] [CrossRef]
- Reuveni, M.; Agapov, V.; Reuveni, R. A foliar spray of micronutrient solutions induces local and systemic protection against powdery mildew (Sphaerotheca fuliginia) in cucumber plants. Eur. J. Plant Pathol. 1997, 103, 581–588. [Google Scholar] [CrossRef]
- Jeun, Y.C.; Park, K.S.; Kim, C.H.; Fowler, W.D.; Kloepper, J.W. Cytological observations of cucumber plants during induced resistance elicited by rhizobacteria. Biol. Control 2004, 29, 34–42. [Google Scholar] [CrossRef]
- Irving, H.R.; Kuć, J.A. Local and systemic induction of peroxidase, chitinase and resistance in cucumber plants by K2HPO4. Physiol. Mol. Plant Pathol. 1990, 37, 355–366. [Google Scholar] [CrossRef]
- Orober, M.; Siegrist, J.; Buchenauer, H. Mechanisms of phosphate-induced disease resistance in cucumber. Eur. J. Plant Pathol. 2002, 108, 345–353. [Google Scholar] [CrossRef]
- Descalzo, R.C.; Rahe, J.E.; Mauza, B. Comparative efficacy of induced resistance for selected diseases of greenhouse cucumber. Can. J. Plant Pathol. 1990, 12, 16–24. [Google Scholar] [CrossRef]
- Buzi, A.; Chilosi, G.; De Sillo, D.; Magro, P. Induction of resistance in melon to Didymella bryoniae and Sclerotinia sclerotiorum by seed treatments with acibenzolar-S-methyl and methyl jasmonate but not with salicylic acid. J. Phytopathol. 2004, 152, 34–42. [Google Scholar] [CrossRef]
- Mahadevaswamy, S.G.; De Britto, S.; Ali, D.; Sasaki, K.; Jogaiah, S.; Amruthesh, K.N. Rhizobial elicitor molecule signaling muskmelon defense against gummy stem blight disease involving innate immune response. Physiol. Mol. Plant Pathol. 2023, 128, 102150. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Giau, N.T.; Long, N.T.; Lübeck, M.; Shetty, N.P.; de Neergaard, E.; Thuy, T.T.T.; Kim, P.V.; Jørgensen, H.J.L. Rhizobacterially induced protection of watermelon against Didymella bryoniae. J. Appl. Microbiol. 2010, 109, 567–582. [Google Scholar] [CrossRef]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Daayf, F.; Jacques, P.; Thonart, P.; Benhamou, N.; Paulitz, T.C.; Bélanger, R.R. Systemic induction of phytoalexins in cucumber in response to treatments with fluorescent pseudomonads. Plant Pathol. 2000, 49, 523–530. [Google Scholar] [CrossRef]
- Schmele, I.; Kauss, H. Enhanced activity of the plasma membrane localized callose synthase in cucumber leaves with induced resistance. Physiol. Mol. Plant Pathol. 1990, 37, 221–228. [Google Scholar] [CrossRef]
- Stumm, D.; Gessler, C. Role of papillae in the induced systemic resistance of cucumbers against Colletotrichum lagenarium. Physiol. Mol. Plant Pathol. 1986, 29, 405–410. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Jogaiah, S.; Satapute, P.; De Britto, S.; Konappa, N.; Udayashankar, A.C. Exogenous priming of chitosan induces upregulation of phytohormones and resistance against cucumber powdery mildew disease is correlated with localized biosynthesis of defense enzymes. Int. J. Biol. Macromol. 2020, 162, 1825–1838. [Google Scholar] [CrossRef]
- Schneider, S.; Ullrich, W.R. Differential induction of resistance and enhanced enzyme activities in cucumber and tobacco caused by treatment with various abiotic and biotic inducers. Physiol. Mol. Plant Pathol. 1994, 45, 291–304. [Google Scholar] [CrossRef]
- Dalisay, R.F.; Kuć, J.A. Persistence of induced resistance and enhanced peroxidase and chitinase activities in cucumber plants. Physiol. Mol. Plant Pathol. 1995, 47, 315–327. [Google Scholar] [CrossRef]
- Roby, D.; Toppan, A.; Esquerré-Tugayé, M.-T. Systemic induction of chitinase activity and resistance in melon plants upon fungal infection or elicitor treatment. Physiol. Mol. Plant Pathol. 1988, 33, 409–417. [Google Scholar] [CrossRef]
- Nga, N.T.T.; de Neergaard, E.; Jørgensen, H.J.L. Infection biology of Stagonosporopsis cucurbitacearum in watermelon and defence responses in the host. Agriculture 2024, 14, 380. [Google Scholar] [CrossRef]
- Chen, C.; Bélanger, R.R.; Benhamou, N.; Paulitz, T.C. Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol. Mol. Plant Pathol. 2000, 56, 13–23. [Google Scholar] [CrossRef]
- Liang, Y.C.; Sun, W.C.; Si, J.; Römheld, V. Effects of foliar- and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005, 54, 678–685. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Rexach, J.; González-Fontes, A. Boron in plants: Deficiency and toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef]
- Saleem, M.; Yusop, M.K.; Ishak, F.; Samsuri, A.W.; Hafeez, B. Boron fertilizers borax and colemanite application on rice and their residual effect on the following crop cycle. Soil Sci. Plant Nutr. 2011, 57, 403–410. [Google Scholar] [CrossRef]
- Bernard, C.E.; Harrass, M.C.; Manning, M.J. Boric acid and inorganic borate pesticides. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 2033–2053. [Google Scholar] [CrossRef]
- Du, P.v.; Bich, T.t.N.; Cuong, N.D.; Kim, P.V. Measurement of localized and systemic resistance of rice plant to Pyricularia grisea by foliar spray of chemical inducers. OmonRice 2001, 9, 87–95. [Google Scholar]
- Du, P.v.; Sau, N.B.; Bich, T.N.; Kim, P.v. Induced resistance of rice plant to blast (Pyricularia grisea) by seed treatment using natri tetraborate (Na2B4O7) under field condition. OmonRice 2001, 9, 96–101. [Google Scholar]
- Deora, A.; Gossen, B.D.; Walley, F.; McDonald, M.R. Boron reduces development of clubroot in canola. Can. J. Plant Pathol. 2011, 33, 475–484. [Google Scholar] [CrossRef]
- Kaya, M.D.; Ergin, N. Boron seed treatments induce germination and seedling growth by reducing seed-borne pathogens in safflower (Carthamus tinctorius L.). J. Plant Prot. Res. 2023, 63, 481–487. [Google Scholar] [CrossRef]
- Tsutsumi, C.Y.; da Silva, N. Screening of melon populations for resistance to Didymella bryoniae in greenhouse and plastic tunnel conditions. Braz. Arch. Biol. Technol. 2004, 47, 171–177. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef]
- Wen, X.; Stoffolano, J.G., Jr.; Greamo, B.; Salemme, V.; Piñero, J.C. Effects of diluted Concord grape juice laced with sodium chloride and selected boron-containing compounds on attraction, consumption, crop muscle contractions, and mortality of adult Drosophila suzukii Matsumura (Diptera: Drosophilidae). Pest Manag. Sci. 2022, 78, 703–710. [Google Scholar] [CrossRef]
- Uysal, A. Control of Monilinia blossom and twig blight (Monilinia laxa) by boron, pyroligneous acid and boscalid. J. Plant Pathol. 2024, 106, 211–223. [Google Scholar] [CrossRef]
- Jensen, B.; Lübeck, P.S.; Jørgensen, H.J.L. Clonostachys rosea reduces spot blotch in barley by inhibiting prepenetration growth and sporulation of Bipolaris sorokiniana without inducing resistance. Pest Manag. Sci. 2016, 72, 2231–2239. [Google Scholar] [CrossRef]
- Shetty, R.; Jensen, B.; Shelton, D.; Jørgensen, K.; Pedas, P.; Jørgensen, H.J.L. Site-specific, silicon-induced structural and molecular defence responses against powdery mildew infection in roses. Pest Manag. Sci. 2021, 77, 4545–4554. [Google Scholar] [CrossRef]
- Thuy, T.T.T.; Lübeck, M.; Smedegaard-Petersen, V.; de Neergaard, E.; Jørgensen, H.J.L. Infection biology of Bipolaris oryzae in rice and defence responses in compatible and less compatible interactions. Agronomy 2023, 13, 231. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Greenberg, J.T. Programmed cell death in plant-pathogen interactions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 525–545. [Google Scholar] [CrossRef]
- Huysmans, M.; Lema, A.S.; Coll, N.S.; Nowack, M.K. Dying two deaths-programmed cell death regulation in development and disease. Curr. Opin. Plant Biol. 2017, 35, 37–44. [Google Scholar] [CrossRef]
- Narusaka, Y.; Narusaka, M.; Park, P.; Kubo, Y.; Hirayama, T.; Seki, M.; Shiraishi, T.; Ishida, J.; Nakashima, M.; Enju, A.; et al. RCH1, a Locus in Arabidopsis that confers resistance to the hemibiotrophic fungal pathogen Colletotrichum higginsianum. Mol. Plant-Microbe Interact. 2004, 17, 749–762. [Google Scholar] [CrossRef]
- Shetty, N.P.; Jørgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Jørgensen, H.J.L.; Lübeck, P.S.; Thordal-Christensen, H.; de Neergaard, E.; Smedegaard-Petersen, V. Mechanisms of induced resistance in barley against Drechslera teres. Phytopathology 1998, 88, 698–707. [Google Scholar] [CrossRef]
- Kawano, T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003, 21, 829–837. [Google Scholar] [CrossRef]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A comprehensive review on function and application of plant peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Smith, J.A.; Hammerschmidt, R. Comparative study of acidic peroxidases associated with induced resistance in cucumber, muskmelon and watermelon. Physiol. Mol. Plant Pathol. 1988, 33, 255–261. [Google Scholar] [CrossRef]
- Shetty, N.P.; Kristensen, B.K.; Newman, M.-A.; Møller, K.; Gregersen, P.L.; Jørgensen, H.J.L. Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol. Mol. Plant Pathol. 2003, 62, 333–346. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. Adv. Agron. 2002, 77, 185–268. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef]
- Alak, G.; Parlak, V.; Yeltekin, A.Ç.; Ucar, A.; Çomaklı, S.; Topal, A.; Atamanalp, M.; Özkaraca, M.; Türkez, H. The protective effect exerted by dietary borax on toxicity metabolism in rainbow trout (Oncorhynchus mykiss) tissues. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 216, 82–92. [Google Scholar] [CrossRef]
- Ince, S.; Kucukkurt, I.; Cigerci, I.H.; Fatih Fidan, A.; Eryavuz, A. The effects of dietary boric acid and borax supplementation on lipid peroxidation, antioxidant activity, and DNA damage in rats. J. Trace Elem. Med. Biol. 2010, 24, 161–164. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef]
- Bolt, H.M.; Başaran, N.; Duydu, Y. Effects of boron compounds on human reproduction. Arch. Toxicol. 2020, 94, 717–724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).