Rice Stripe Virus Infection Facilitates the Reproductive Potential of Laodelphax striatellus
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. RNA Interference
2.3. Injection of CP Antibody
2.4. Sample Preparation and RNA Extraction
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Reproduction Assays
2.7. Statistical Analyses
3. Results
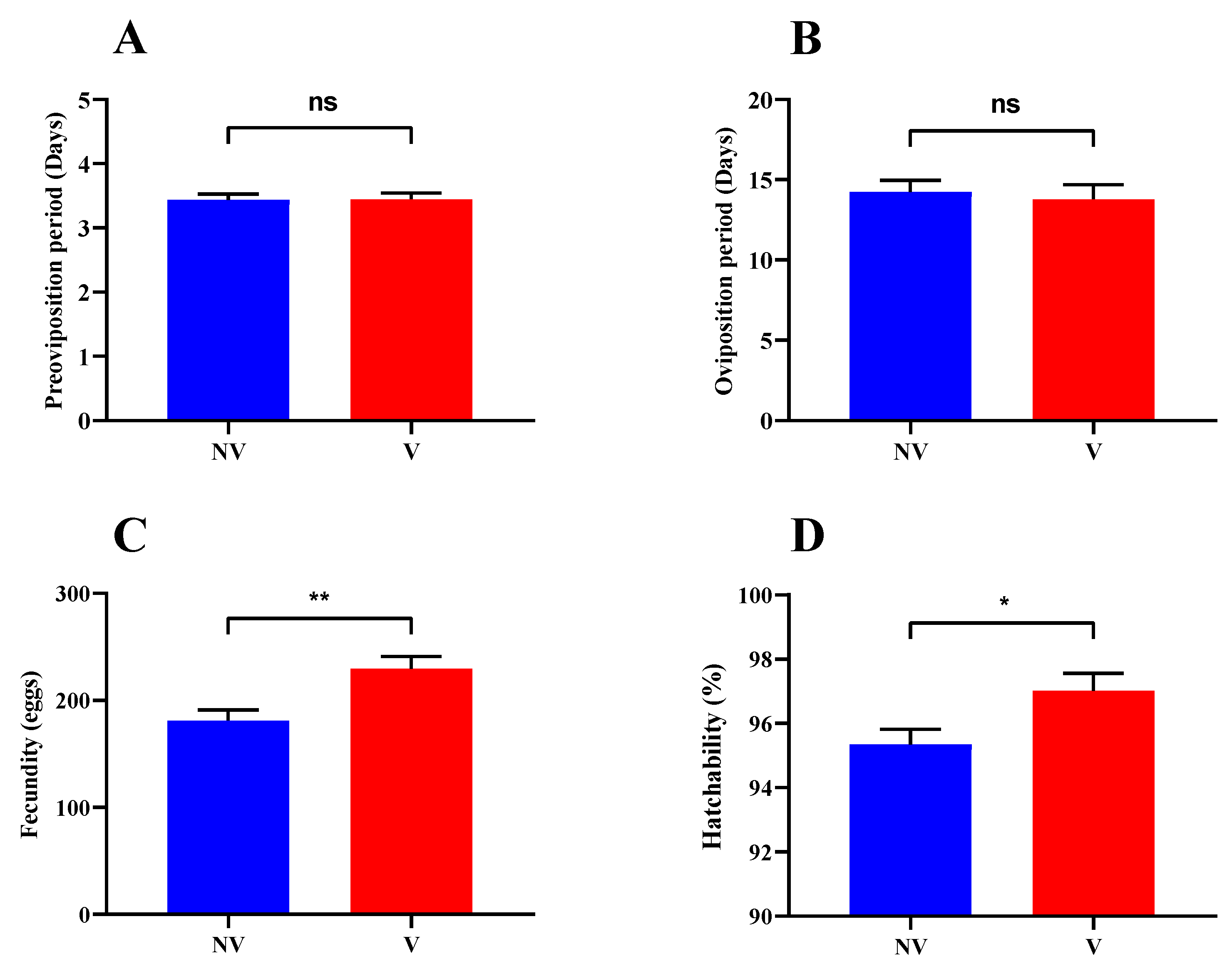
3.1. Effects of RSV Infection on Reproductive Parameters of L. striatellus
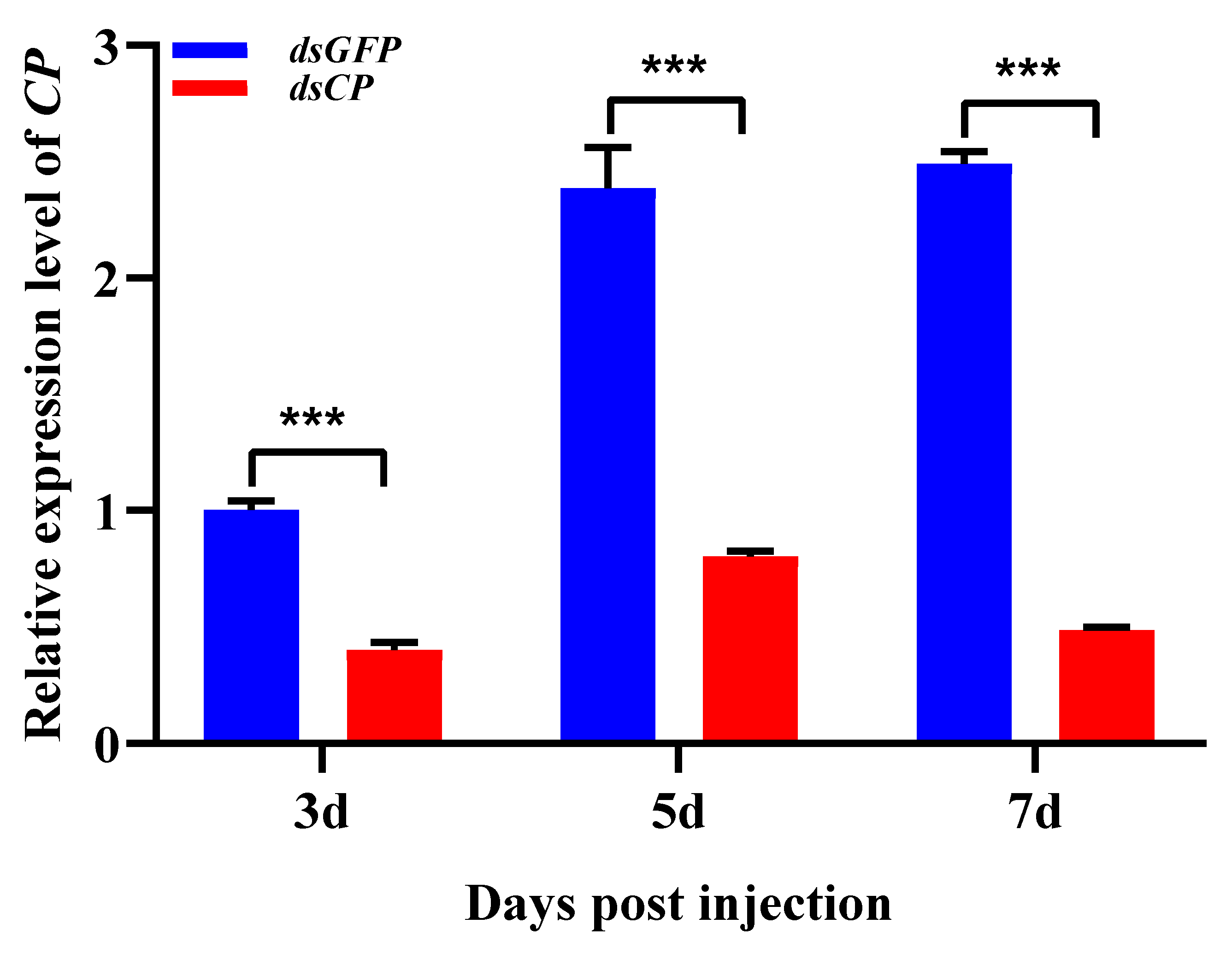
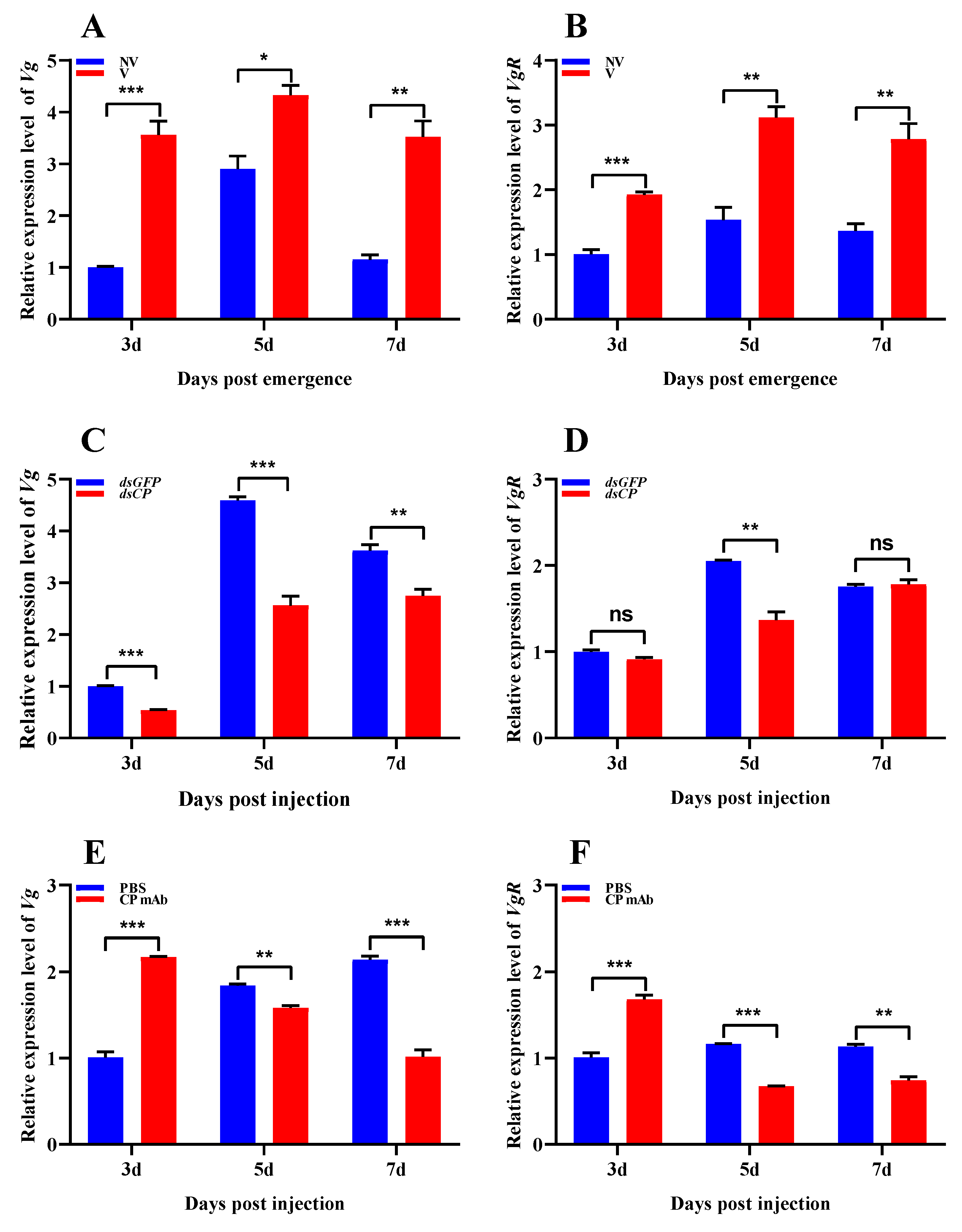
3.2. Effects of CP Silencing on Reproductive Parameters of L. striatellus
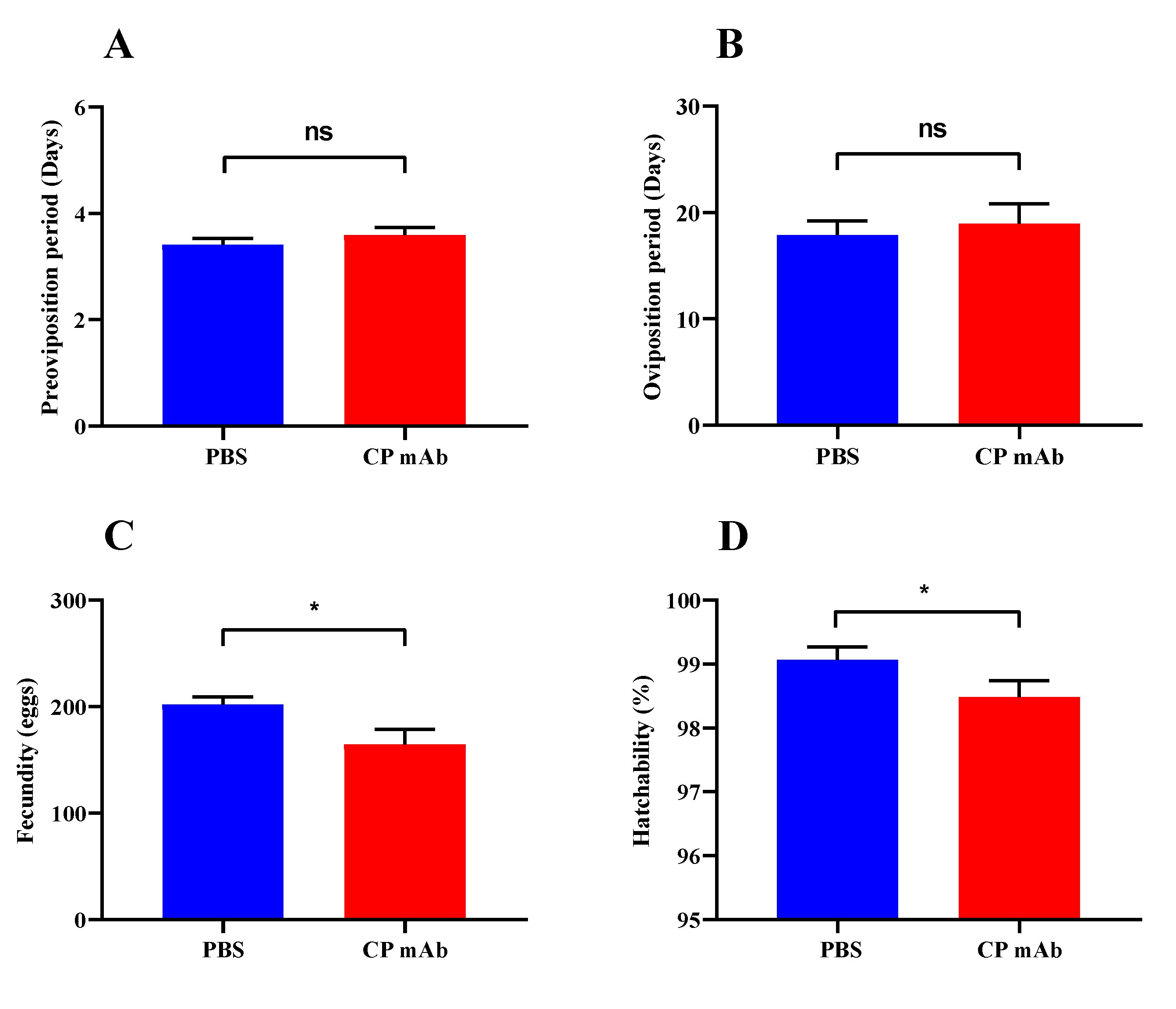
3.3. Effects of CP Antibody Injection on Reproductive Parameters of L. striatellus
3.4. Effects of RSV Infection on Vg/VgR Expressions of L. striatellus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, S.; Krainer, K.M.C. Pandemics of people and plants: Which is the greater threat to food security? Mol. Plant 2020, 13, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef] [PubMed]
- Fereres, A. Insect vectors as drivers of plant virus emergence. Curr. Opin. Virol. 2015, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, F.; Barrès, B.; Bonafos, R.; Desneux, N.; Escobar-Gutiérrez, A.J.; Gachet, E.; Laville, J.; Siegwart, M.; Thiéry, D.; Jactel, H. Producing sugar beets without neonicotinoids: An evaluation of alternatives for the management of viruses-transmitting aphids. Entomol. Gen. 2022, 42, 491–498. [Google Scholar] [CrossRef]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-Borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Evolutionary determinants of host and vector manipulation by plant viruses. Adv. Virus. Res. 2018, 101, 189–250. [Google Scholar] [PubMed]
- Wang, S.F.; Guo, H.J.; Ge, F.; Sun, Y.C. Apoptotic neurodegeneration in whitefly promotes the spread of TYLCV. eLife 2020, 9, e56168. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, M.; Li, J.; Shi, Y.; Gu, Q.S.; Yan, F. Changes in Bemisia tabaci feeding behaviors caused directly and indirectly by cucurbit chlorotic yellows virus. Virol. J. 2019, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Shalileh, S.; Ogada, P.A.; Moualeu, D.P.; Poehling, H.M. Manipulation of Frankliniella occidentalis (Thysanoptera: Thripidae) by Tomato spotted wilt virus, (Tospovirus) via the host plant nutrients to enhance its transmission and spread. Environ. Entomol. 2016, 45, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, W.H.; Kim, H.; Nakada, Y.; Masuta, C. A plant virus satellite RNA directly accelerates wing formation in its insect vector for spread. Nat. Commun. 2021, 12, 7087. [Google Scholar] [CrossRef] [PubMed]
- Moeini, P.; Afsharifar, A.; Homayoonzadeh, M.; Hopkins, R.J. Plant virus infection modifies plant pigment and manipulates the host preference behavior of an insect vector. Entomol. Exp. Appl. 2020, 168, 599–609. [Google Scholar] [CrossRef]
- Gao, D.M.; Qiao, J.H.; Gao, Q.; Zhang, J.W.; Zhang, Y.; Xie, L.; Zhang, Y.; Wang, Y.; Fu, J.Y.; Zhang, H.; et al. A plant cytorhabdovirus modulates locomotor activity of insect vectors to enhance virus transmission. Nat. Commun. 2023, 14, 5754. [Google Scholar] [CrossRef] [PubMed]
- Legarrea, S.; Barman, A.; Marchant, W.; Diffie, S.; Srinivasan, R. Temporal effects of a begomovirus infection and host plant resistance on the preference and development of an insect vector, Bemisia tabaci, and implications for epidemics. PLoS ONE 2015, 10, e0142114. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.B.; Tang, X.; Zhang, X.; Zhang, D.Y.; Li, F.; Yan, F.; Zhang, Y.J.; Zhou, X.G.; Liu, Y. Transmission efficiency, preference and behavior of Bemisia tabaci MEAM1 and MED under the influence of tomato chlorosis virus. Front. Plant Sci. 2018, 8, 2271. [Google Scholar] [CrossRef] [PubMed]
- Jiu, M.; Zhou, X.P.; Tong, L.; Xu, J.; Yang, X.; Wan, F.H.; Liu, S.S. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2007, 2, e182. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.R.; Hussain, S.; Merchant, A.; Xu, B.Y.; Xie, W.; Wang, S.L.; Zhang, Y.J.; Zhou, X.G.; Wu, Q.J. Tomato spotted wilt orthotospovirus influences the reproduction of its insect vector, western flower thrips, Frankliniella occidentalis, to facilitate transmission. Pest Manag. Sci. 2020, 76, 2406–2414. [Google Scholar] [CrossRef] [PubMed]
- Bragard, C.; Caciagli, P.; Lemaire, O.; Lopez-Moya, J.J.; MacFarlane, S.; Peters, D.; Susi, P.; Torrance, L. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 2013, 51, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.W.; Tsai, J.H. Biology and molecular biology of viruses in the genus Tenuivirus. Annu. Rev. Phytopathol. 1998, 36, 139–163. [Google Scholar] [CrossRef]
- An, S.B.; Choi, J.Y.; Lee, S.H.; Fang, Y.; Kim, J.H.; Park, D.H.; Park, M.G.; Woo, R.M.; Kim, W.J.; Je, Y.H. Silencing of rice stripe virus in Laodelphax striatellus using virus-derived double-stranded RNAs. J. Asia-Pac. Entomol. 2017, 20, 695–698. [Google Scholar] [CrossRef]
- Zhu, J.J.; Eid, F.E.; Tong, L.; Zhao, W.; Wang, W.; Heath, L.S.; Kang, L.; Cui, F. Characterization of protein–protein interactions between rice viruses and vector insects. Insect Sci. 2020, 28, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhu, J.J.; Lu, H.; Zhu, J.M.; Jiang, F.; Wang, W.; Luo, L.; Kang, L.; Cui, F. The nucleocapsid protein of rice stripe virus in cell nuclei of vector insect regulates viral replication. Protein Cell 2021, 13, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Kill, E.; Kill, D. The small brown planthopper (Laodelphax striatellus) as a vector of the rice stripe virus. Arch. Insect Biochem. Physiol. 2022, 122, e21992. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, S.; Zhou, C.W.; Xin, Q.; Xiang, Q.; Yang, T.Q.; Wu, J.X.; Zhou, X.P.; Zhou, Y.J.; Ding, X.S.; et al. Tenuivirus utilizes its glycoprotein as a helper component to overcome insect midgut barriers for its circulative and propagative transmission. PLoS Pathog. 2019, 15, e1007655. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.S.; Chen, Q.; Mao, Q.Z.; Zhang, X.F.; Wu, W.; Chen, H.Y.; Yu, X.Z.; Wang, Z.Q.; Wei, T.Y. Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 2018, 28, 127–132. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, L.M.; Chen, H.Y.; Jia, D.S.; Li, F.; Wei, T.Y. Nonstructural protein NS4 of Rice stripe virus plays a critical role in viral spread in the body of vector insects. PLoS ONE 2014, 9, e88636. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fu, S.; Tao, X.R.; Zhou, X.P. Rice stripe virus: Exploring molecular weapons in the arsenal of a negative-sense RNA virus. Annu. Rev. Phytopathol. 2021, 59, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Yu, Y.L.; Chen, L.Y.; Li, Q.; Zhang, M.T.; Song, Z.Y.; Chen, X.Y.; Fang, R.X.; Zhang, L.L. Insect tissue-specific vitellogenin facilitates transmission of plant virus. PLoS Pathog. 2018, 14, e1006909. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Yu, Y.L.; Liu, Q.; Liu, D.; Zhang, M.T.; Liang, J.N.; Chen, X.Y.; Zhang, L.L.; Fang, R.X. Rice stripe virus hitchhikes the vector insect vitellogenin ligand-receptor pathway for ovary entry. Philos. Trans. R. Soc. B 2019, 374, 20180312. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Lin, K.J.; Ding, S.M.; Wang, G.R.; Li, F. The vitellogenin receptor has an essential role in vertical transmission of Rice stripe virus during oogenesis in the small brown planthopper. Pest Manag. Sci. 2018, 75, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Du, L.L.; Li, C.Y.; Ma, S.H.; Wang, Z.Y.; Lan, Y.; Lin, F.; Zhou, Y.J.; Wang, Y.Y.; Zhou, T. Rice stripe virus modulates the feeding preference of small brown planthopper from the stems to leaves of rice plants to promote virus infection. Phytopathology 2022, 112, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Zhao, W.; Chen, X.F.; Lu, H.; Xiao, Y.; Li, Q.; Luo, L.; Kang, L.; Cui, F. A plant virus manipulates the long-winged morph of insect vectors. Proc. Natl. Acad. Sci. USA 2024, 121, e2315341121. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.J.; Jiang, S.L.; Wang, W.J.; Li, G.Q.; Tao, X.R.; Pan, W.D.; Sword, G.A.; Chen, F.J. Rice stripe virus counters reduced fecundity in its insect vector by modifying insect physiology, primary endosymbionts and feeding behavior. Sci. Rep. 2015, 5, 12527. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.J.; Xi, W.; Li, X.L.; Zi, J.Y.; Ge, S.S.; Cheng, Z.B.; Zhou, T.; Ji, Y.H.; Deng, J.H.; et al. Rice stripe virus affects the viability of its vector offspring by changing developmental gene expression in embryos. Sci. Rep. 2015, 5, 7883. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.B.; Qin, F.L.; Liu, W.W.; Wang, X.F. Differential proteomics profiling of the ova between healthy and rice stripe virus-infected female insects of Laodelphax striatellus. Sci. Rep. 2016, 6, 27216. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.Z.; Zhang, Y.Y.; Gui, W.; Qian, M.S.; Yu, Y.X.; Xu, G.; Yang, G.Q. Effects of priming rice seeds with decoyinine on fitness traits and virus transmission ability of the small brown planthopper, Laodelphax striatellus. Agronomy 2023, 13, 864. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, Y.X.; Qian, M.S.; Gui, W.; Shah, A.Z.; Xu, G.; Yang, G.Q. Characterization and functional analysis of an α-adrenergic-like octopamine receptor in the small brown planthopper Laodelphax striatellus. Pest. Biochem. Physiol. 2023, 194, 105509. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Zhou, Y.J.; Zhou, X.P. Detection of Rice stripe virus in Laodelphax striatellus by direct dot immunobinding assay. J. Zhejiang Univ. 2005, 31, 37–40. [Google Scholar]
- Bonning, B.C.; Pal, N.; Liu, S.; Wang, Z.; Sivakumar, S.; Dixon, P.M.; King, G.F.; Miller, W.A. Toxin delivery by the coat protein of an aphid-vectored plant virus provides plant resistance to aphids. Nat. Biotechnol. 2014, 32, 102–105. [Google Scholar] [CrossRef]
- Yu, Y.X.; Zhang, Y.Y.; Qian, M.S.; Zhang, Q.X.; Yang, G.Q.; Xu, G. Comparative transcriptomic analysis of head in Laodelphax striatellus upon rice stripe virus infection. Agronomy 2022, 12, 3202. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Ling, B.; Xu, D.L.; Zhang, M.X.; Zhou, G.H. Effects of Southern rice black-streaked dwarf virus on the development and fecundity of its vector, Sogatella furcifera. Virol. J. 2013, 10, 145. [Google Scholar] [CrossRef]
- Xu, H.X.; He, X.C.; Zheng, X.S.; Yang, Y.J.; Tian, J.C.; Lu, Z.X. Southern rice black-streaked dwarf virus (SRBSDV) directly affects the feeding and reproduction behavior of its vector, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Virol. J. 2014, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Guo, J.Y.; Tian, J.C.; Chen, Y.; Ye, G.Y. Effects of rice dwarf virus on ovarian development and fecundity of the green leafhopper, Nephotettix cincticeps (Fabricius). Acta Phytophy. Sin. 2010, 37, 375–376. [Google Scholar]
- Huang, L.P.; Shi, X.B.; Shi, J.Z.; Zhang, Z.; Fang, Y.; Zhang, Z.H.; Pan, Q.Y.; Zheng, L.M.; Gao, Y.; Zhang, D.Y.; et al. Tomato chlorosis virus infection facilitates Bemisia tabaci MED reproduction by elevating vitellogenin expression. Insects 2021, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Moeini, P.; Afsharifar, A.; Izadpanah, K.; Sadeghi, S.E.; Eigenbrode, S.D. Maize Iranian mosaic virus (family Rhabdoviridae) improves biological traits of its vector Laodelphax striatellus. Arch. Virol. 2019, 165, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Q.S.; Xu, Z.T.; Liu, R.Y.; Cui, F. Distinct replication and gene expression strategies of the rice stripe virus in vector insects and host plants. J. Gen. Virol. 2019, 100, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.; Wang, W.; Zhang, L.L.; Cui, F. Evaluation of rice stripe virus transmission efficiency by quantification of viral load in the saliva of insect vector. Pest. Manag. Sci. 2019, 75, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Rotenberg, D. Disruption of insect transmission of plant viruses. Curr. Opin. Insect Sci. 2015, 8, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, X.; Ye, X.; Xu, H.; Zhou, X.; Liu, S.; Wang, X. Expression and functional characterisation of a soluble form of tomato yellow leaf curl virus coat protein. Pest Manag. Sci. 2014, 70, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; He, Y.Z.; Guo, Q.; Guo, T.; Liu, Y.Q.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 6746–6751. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hoffmann, A.A.; Xu, X.Q.; Mo, P.W.; Huang, H.J.; Gong, J.T.; Ju, J.F.; Hong, X.Y. Vertical transmission of wolbachia is associated with host vitellogenin in Laodelphax striatellus. Front. Microbiol. 2018, 9, 02016. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, B.Q.; Chen, Z.B.; Li, C.Q.; Li, X.Y.; Hong, J.S.; Luan, J.B. Vitellogenin facilitates associations between the whitefly and a bacteriocyte symbiont. mBio 2023, 14, e02990-22. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jiang, Y.; Zhang, N.N.; Liu, F.; Yang, G.Q. Triazophos-induced vertical transmission of rice stripe virus is associated with host vitellogenin in the small brown planthopper Laodelphax striatellus. Pest. Manag. Sci. 2020, 76, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Lu, C.C.; Guo, S.D.; Guo, Y.X.; Wei, T.Y.; Chen, Q. Leafhopper salivary vitellogenin mediates virus transmission to plant phloem. Nat. Commun. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, Y.; Xu, M.; Liao, J.; Shao, C.; Fu, L.; Qian, M.; Xu, G.; Yang, G. Rice Stripe Virus Infection Facilitates the Reproductive Potential of Laodelphax striatellus. Agronomy 2024, 14, 714. https://doi.org/10.3390/agronomy14040714
Zhang Y, Yu Y, Xu M, Liao J, Shao C, Fu L, Qian M, Xu G, Yang G. Rice Stripe Virus Infection Facilitates the Reproductive Potential of Laodelphax striatellus. Agronomy. 2024; 14(4):714. https://doi.org/10.3390/agronomy14040714
Chicago/Turabian StyleZhang, Yuanyuan, Youxin Yu, Meiqi Xu, Jingyan Liao, Chenjia Shao, Liran Fu, Mingshi Qian, Gang Xu, and Guoqing Yang. 2024. "Rice Stripe Virus Infection Facilitates the Reproductive Potential of Laodelphax striatellus" Agronomy 14, no. 4: 714. https://doi.org/10.3390/agronomy14040714
APA StyleZhang, Y., Yu, Y., Xu, M., Liao, J., Shao, C., Fu, L., Qian, M., Xu, G., & Yang, G. (2024). Rice Stripe Virus Infection Facilitates the Reproductive Potential of Laodelphax striatellus. Agronomy, 14(4), 714. https://doi.org/10.3390/agronomy14040714






