Comparison of Twenty Selected Fenugreek Genotypes Grown under Irrigated and Dryland Conditions: Morphology, Yield, Quality Properties and Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Total Alkaloid Content Extraction
2.3. Trigonelline Content Extraction and UHPLC Analysis
2.4. Isolation of Fixed Oil Content (%)
2.5. Determination of Fatty Acids (%)
2.6. Seed Extraction of Fenugreek Seeds
2.7. DPPH Method
2.8. FRAP Method
2.9. Total Phenolic Content
2.10. Total Flavonoid Content
2.11. Statistical Analysis
3. Results
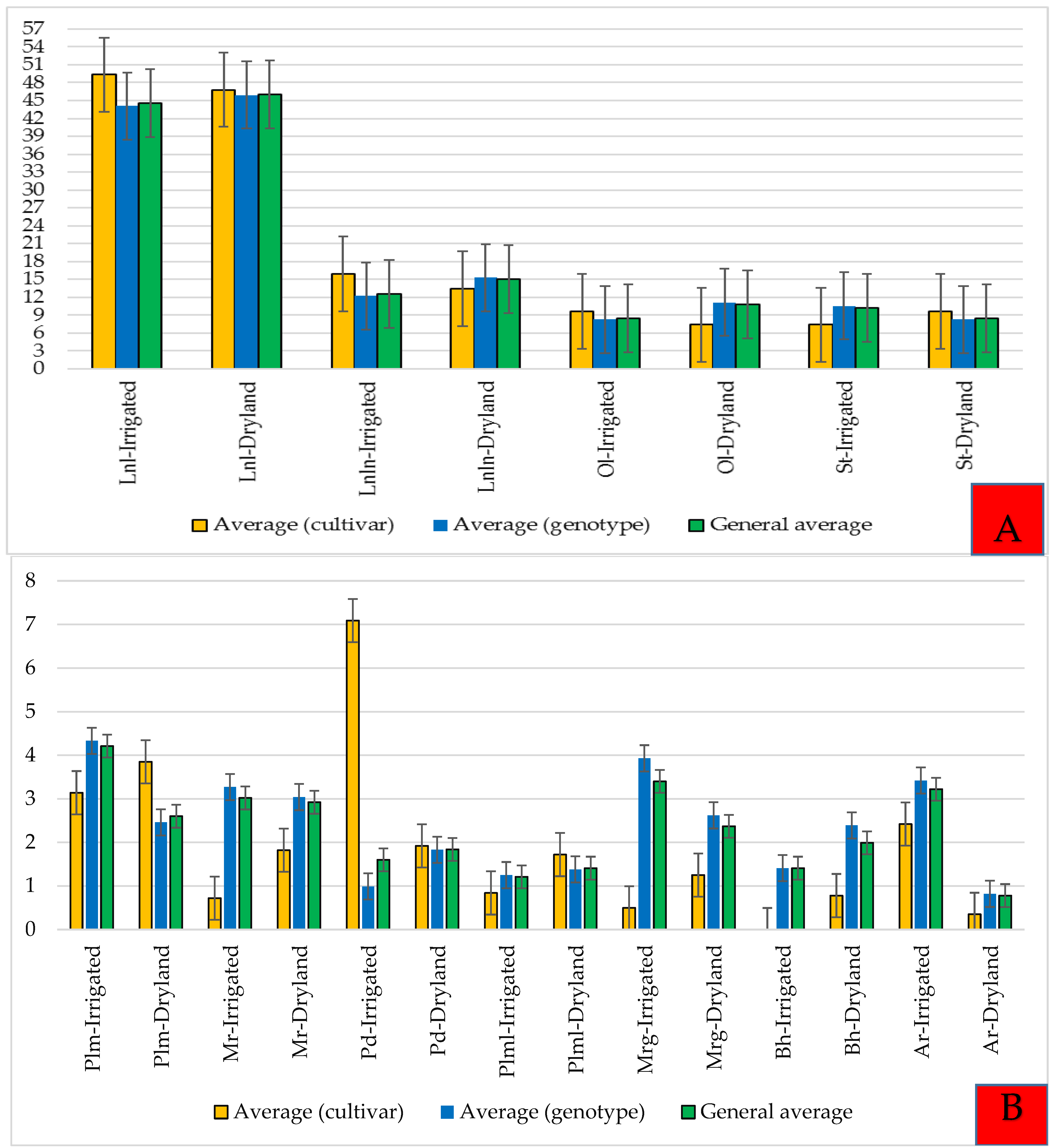
3.1. Plant Height, Branch Number, and Seed Yield
3.2. Total Alkaloid and Trigonelline Contents
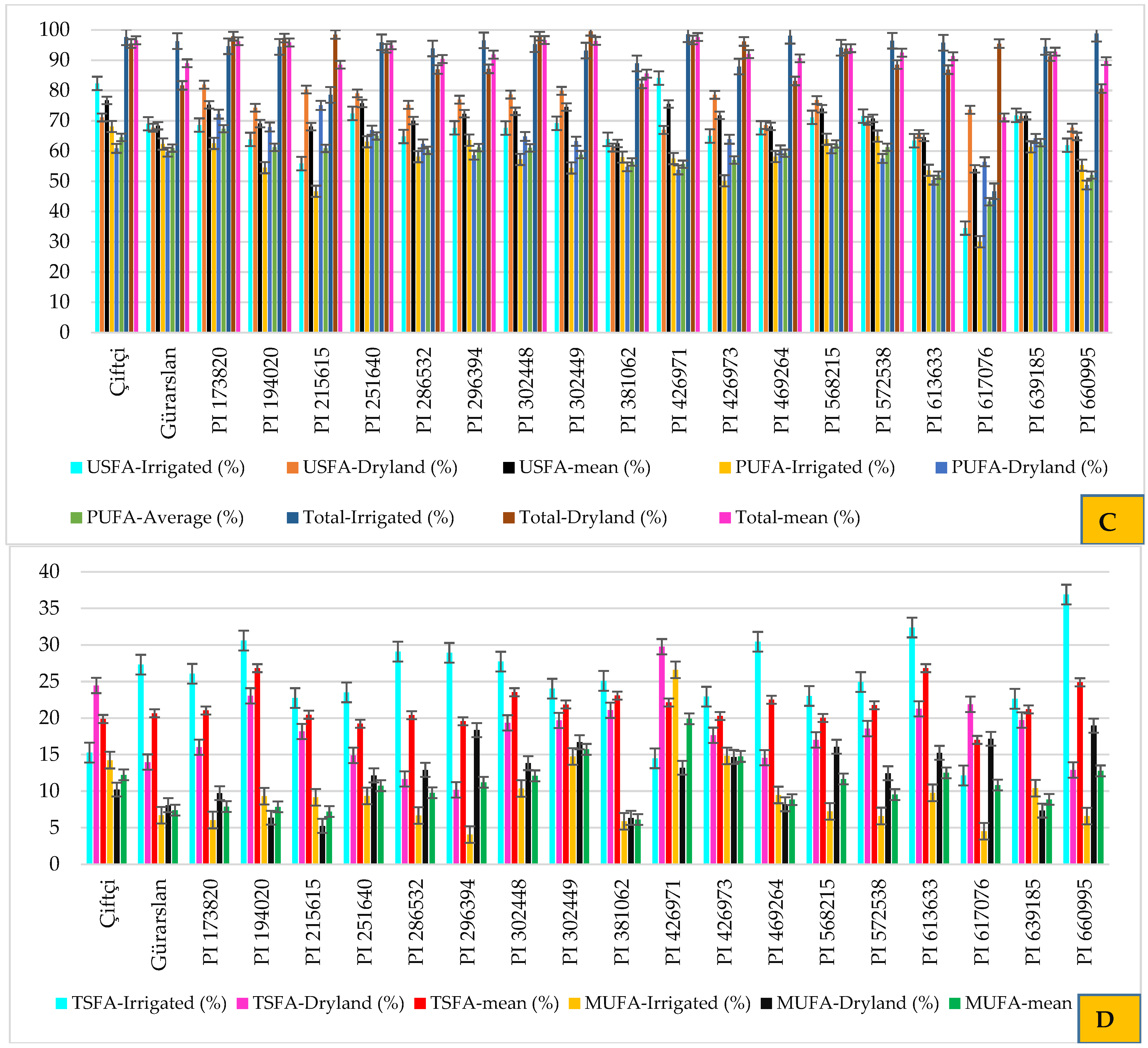
3.3. Fixed Oil Content and Fatty Acid Profiles
3.4. Antioxidant Properties
3.5. Total Phenolic and Flavonoid Contents
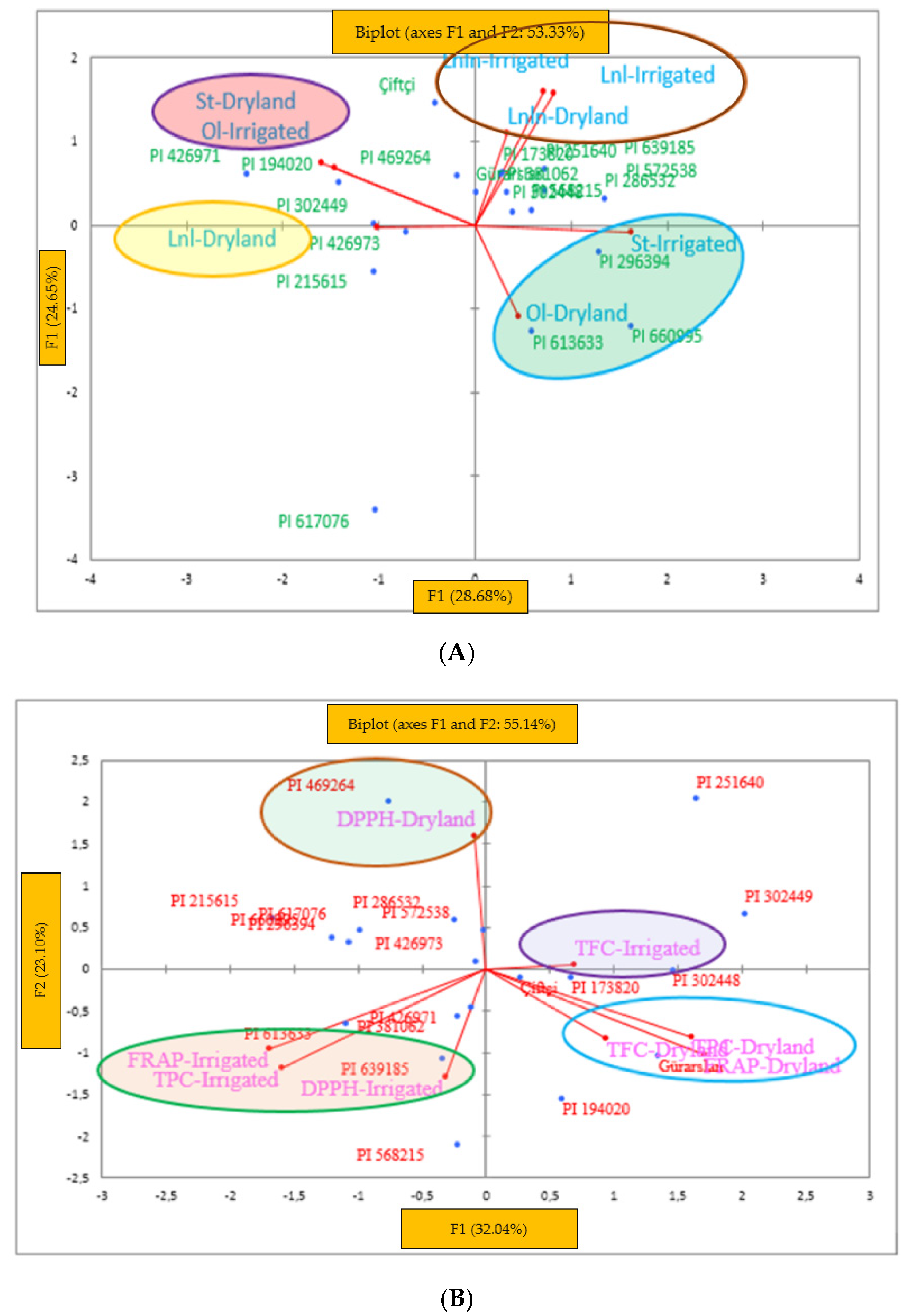
3.6. Biplot Analysis
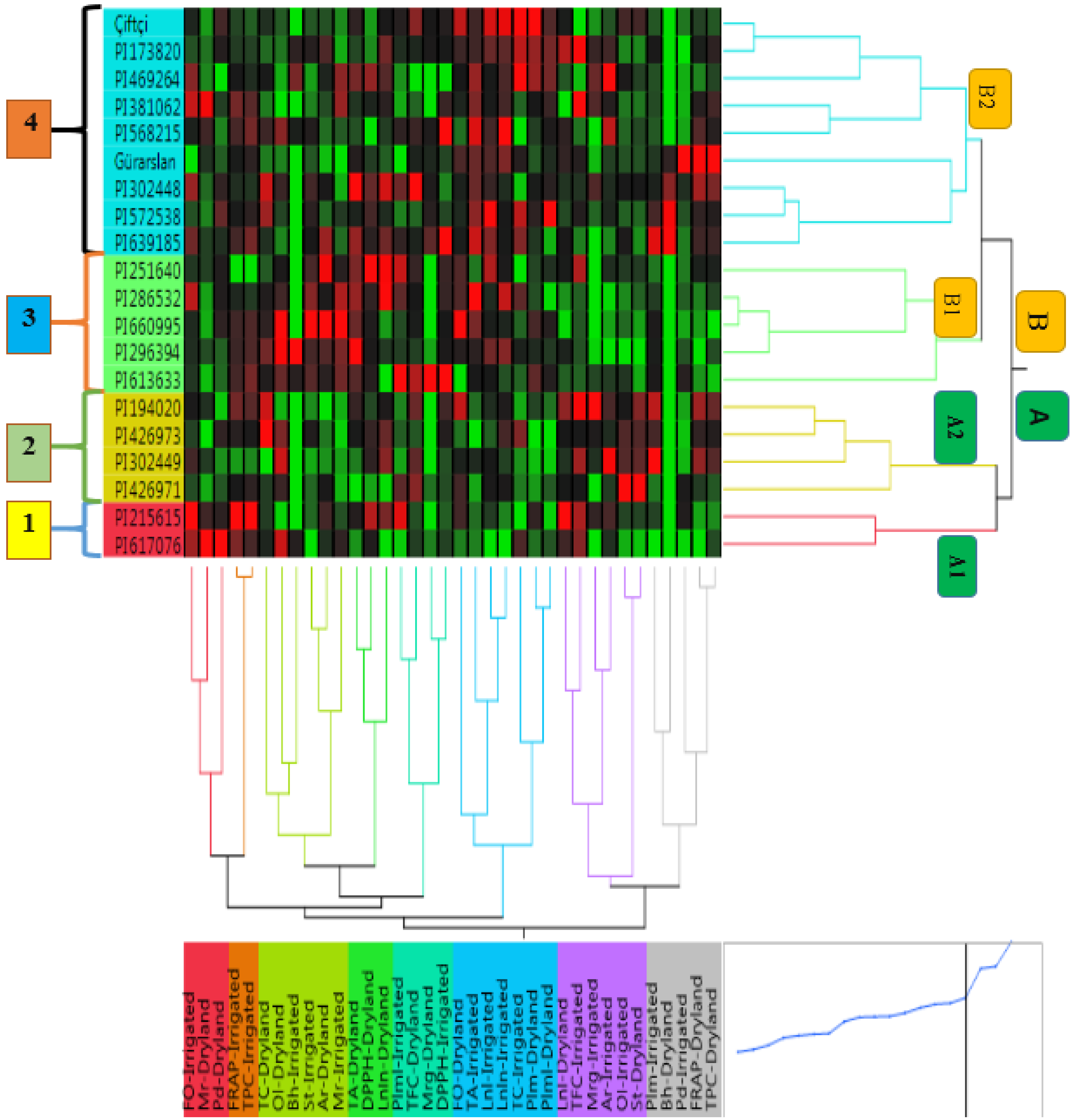
3.7. Cluster Analysis Results
4. Discussion
4.1. Plant Height, Branch Number, and Seed Yield
4.2. Total Alkaloid and Trigonelline Contents
4.3. Fixed Oil Content and Fatty Acid Profiles
4.4. Antioxidant Properties
4.5. Total Phenolic and Flavonoid Contents
4.6. Cluster Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camlica, M.; Yaldiz, G. Gum yield, optimization of gum isolation, diosgenin and crude protein contents of fenugreek genotypes and cultivars grown under irrigated and dryland conditions. J. Food Compos. Anal. 2022, 110, 104571. [Google Scholar] [CrossRef]
- Soriano, M.A.; Orgaz, F.; Villalobos, F.J.; Fereres, E. Efficiency of water use of early plantings of sunflower. Eur. J. Agron. 2004, 21, 465–467. [Google Scholar] [CrossRef]
- Sankar, B.; Abdul Jaleel, C.; Manivannan, P.; Kishorekumar, A.; Somasundaram, R.; Panncerselvam, R. Relative efficiency of water use in five varieties of Abelmoschus esculentus (L.) Moench. under water limeted conditions. Colloids Surf. B Biointerfaces 2008, 62, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh-Ahari, D.; Hassandokht, M.R.; Kashi, A.K.; Amri, A.; Alizadeh, K.H. Genetic variability of some agronomic traits in the Iranian Fenugreek landraces under drought stress and non-stress conditions. Afr. J. Plant Sci. 2010, 4, 12–20. [Google Scholar]
- Zandi, P.; Basu, S.K.; Khatibani, L.B.; Balogun, M.O.; Aremu, M.O.; Sharma, M.; Cetzal-Ix, W. Fenugreek (Trigonella foenum-graecum L.) seed: A review of physiological and biochemical properties and their genetic improvement. Acta Physiol. Plant. 2015, 37, 1714. [Google Scholar] [CrossRef]
- Al-Jasass, F.M.; Al-Jasser, M.S. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. Sci. World J. 2012, 2012, 859892. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.S.; Saxena, S.N.; Kakani, R.K.; Sharma, L.K.; Agrawal, D.; Singh, B. Genetic variation in fatty acid composition of fenugreek (Trigonella foenum-graecum L.) seed oil. Legume Res. 2017, 40, 609–617. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Ali, A.O.; Hemavathy, J. Lipid content and fatty acid composition of fenugreek (Trigonella foenum-graecum L.) seeds grown in Sudan. Int. J. Food Sci. Technol. 2008, 43, 380–382. [Google Scholar] [CrossRef]
- Heller, L. Fenugreek. A Noteworthy Hypoglycemic, Pacific College of Oriental Medicine. 2001. Available online: http://www.ormed.Edu/newsletters/fenugreek.html (accessed on 28 February 2024).
- Küçük, M.; Gürbüz, B. A research on oil content and fatty acid com-position of some fenugreek (Trigonella foenum-graecum) lines. J. Food 1999, 24, 99–101. [Google Scholar]
- Duke, J.A. Handbook of Medicinal Spices; CRC Press: New York, NY, USA, 2001. [Google Scholar]
- Moorthy, R.; Prabhu, K.M.; Murthy, P.S. Anti-hyperglycemic compound (GII) from Fenugreek seeds, its purification and effect in diabetes mellitus. Indian J. Exp. Biol. 2010, 48, 1111–1118. [Google Scholar]
- Raheleh, A.; Hasanloo, T.; Khosroshahli, M. Evaluation of trigonelline production in Trigonella foenum-graecum hairy root cultures of two Iranian masses. Plant Omics J. 2011, 4, 408–412. [Google Scholar]
- Chaturvedi, S.; Hemamalini, R.; Khare, S.K. Effect of processing conditions on saponin content and antioxidant activity of Indian varieties of soybean (Glycine max Linn). Ann. Phytomed. 2012, 1, 62–68. [Google Scholar]
- Kim, R.P.T.; Bihud, V.; Bin Mohamad, K.; Leong, K.H.; Bin Mohamad, J.; Bin Ahmad, F.; Hazni, H.; Kasim, N.; Halim, S.N.A.; Awang, K. Cytotoxic and antioxidant compounds from the stem bark of Goniothalamus tapisoides mat salleh. Molecules 2013, 18, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic extraction of flavonoids, phenolics and antioxidants from Vernonia amygdalina leaf using two-level factorial design. J. King Saud Univ.-Sci. 2017, 32, 7–16. [Google Scholar] [CrossRef]
- Seif, H.S.A. Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 134–146. [Google Scholar]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Saxena, S.N.; Kakani, R.K.; Sharma, L.K.; Agarwal, D.; John, S. Genetic variation in seed quality and fatty acid composition of fenugreek (Trigonella foenum-graecum L.) genotypes grown under limited moisture conditions. Acta Physiol. Plant. 2017, 39, 218. [Google Scholar] [CrossRef]
- Camlica, M.; Yaldiz, G. Characterization of morphological and yield variation of fenugreek (Trigonella foenum-graecum L.) genotypes. Legume Res. 2019, 42, 500–504. [Google Scholar]
- Yaldiz, G.; Camlica, M. Performance of fenugreek (Trigonella foenum-graecum L.) genotypes towards growth, yield and UPOV properties. Legume Res. 2022, 45, 10–17. [Google Scholar] [CrossRef]
- BMGD. Bolu Meteorology General Directorate of Türkiye. Available online: https://www.mgm.gov.tr/tahmin/il-ve-ilceler.aspx?il=Bolu (accessed on 15 April 2022).
- Pandey, H.; Awasthi, P. Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. J. Food Sci. Technol. 2015, 52, 1054–1060. [Google Scholar] [CrossRef]
- Mohamed, H.H. Determination of the moisture-dependent physical and aerodynamic properties for fenugreek seeds to predict the best cleaning system. Misr J. Agric. Eng. 2013, 30, 831–844. [Google Scholar] [CrossRef]
- Benziane, M.N.A.; Acem, K.; Aggad, H.; Abdali, M. Phytochemistry, HPLC profile and antioxidant activity of aqueous extracts of fenugreek (Trigonella foenum-graecum L.) seeds grown in arid zones of Algeria. Acta Sci. Nat. 2019, 6, 71–87. [Google Scholar] [CrossRef]
- Hassanzadeh, E.; Reza Chaic, M.; Mazaheri, D.; Rezazadeh, S.; Naghdi Bad, H.A. Physical and chemical variabilities among domestic Iranian fenugreek (Trigonella foenum-graecum) seeds. Asian J. Plant Sci. 2011, 10, 323–330. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M. Variation in the fruit phytochemical and mineral composition, and phenolic content and antioxidant activity of the fruit extracts of different fennel (Foeniculum vulgare L.) genotypes. Ind. Crops Prod. 2019, 142, 111852. [Google Scholar] [CrossRef]
- Camlica, M.; Yaldiz, G. Analyses and evaluation of the main chemical components indifferent tobacco (Nicotiana tabacum L.) genotypes. Grasas Y Aceites 2021, 72, e389. [Google Scholar] [CrossRef]
- Gikas, E.; Bazoti, F.N.; Papadopoulos, N.; Alesta, A.; Economou, G.; Tsarbopoulos, A. Quantitation of the flavanols quercetin and kaempherol in the leaves of Trigonella foenum-graecum by high-performance liquid chromatography-diode array detection. Anal. Lett. 2011, 44, 1463–1472. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M. Essential oils content, composition and antioxidant activity of selected basil (Ocimum basilicum L.) genotypes. S. Afr. J. Bot. 2022, 151, 675–694. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): Effect of extraction techniques and solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Geçit, H.H.; Çiftçi, C.Y.; Emeklier, Y.; İkincikarakaya, S.; Adak, M.S.; Ekiz, H.; Altınok, S.; Sancak, C.; Sevimay, C.S.; Kendir, H. Field Crops; Ankara University, Agriculture Faculty: Ankara, Türkiye, 2009; No:1569; p. 521. [Google Scholar]
- Hussein, F.; Janat, M.; Yakoub, A. Assessment of yield and water use efficiency of drip-irrigated cotton (Gossypium hirsutum L.) as affected by deficit irrigation. Turk. J. Agric. For. 2011, 35, 611–621. [Google Scholar] [CrossRef]
- İsotçu, Ç. Determination of Full and Deficit Irrigation Effects on Yield Components and Fiber Quality Traits of Cotton (Gossypium hirsutum L.) at f3:5 Generation. Master’s Thesis, Department of Field Crop Sciences, Agriculture Faculty, Adnan Menderes University, Aydın, Türkiye, 2016. [Google Scholar]
- Sharma, K.C.; Sastry, E.V.D. Path analysis for seed yield and its component characters in fenugreek (Trigonella foenum-graecum L.). J. Spices Aromat. Crops 2008, 17, 69–74. [Google Scholar]
- Aşkın, H. Determination of Agricultural and Some Quality Characteristics of Different Fenugreek (Trigonella foenum-graecum L.) Genotypes. Master’s Thesis, Graduate School of Bolu Abant Izzet Baysal University, Department of Field Crops, Bolu, Türkiye, 2021. [Google Scholar]
- Al-Maamari, I.T.; Khan, M.M.; Ali, A.; Al-Sadi, A.M.; Waly, M.I.; Al-Saady, N.A. Diversity in phytochemical composition of omani fenugreek (Trigonella foenum-graecum L.) accessions. Pak. J. Agric. Res. 2016, 53, 851–862. [Google Scholar]
- Yaldiz, G.; Camlica, M. Yield, yield components and some quality properties of fenugreek cultivar and lines. Banat’s J. Biotechnol. 2020, 11, 40–47. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.M.; Norton, R.M.; Eagles, H.A. Phenotypic variation within a fenugreek (Trigonella foenum-graecum L.) germplasm collection. II. Cultivar selection based on traits associated with seed yield. Genet. Resour. Crop Evol. 2009, 56, 651–661. [Google Scholar] [CrossRef]
- Pavlista, A.D.; Santra, D.K. Planting and harvest dates, and irrigation on fenugreek in the semi-arid high plains of the USA. Ind. Crops Prod. 2016, 94, 65–71. [Google Scholar] [CrossRef]
- Mandegary, A.; Pournamdari, M.; Sharififar, F.; Pournourmohammadi, S.; Reza Fardiar, R.; Shooli, S. Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti-inflammatory effects. Food Chem. Toxicol. 2012, 50, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
- Sharara, M.S. Effect of germination and heat treatment on chemical composition and bioactive components of fenugreek seeds. World J. Dairy Food Sci. 2017, 12, 33–41. [Google Scholar]
- Mahmood, M.N.; Yahya, I.K. Nutrient and phytochemical of fenugreek (Trigonella foenum-graecum) seeds. Int. J. Sci. Basic Appl. Res. 2017, 36, 203–213. [Google Scholar]
- Afshar, R.K.; Chaichi, M.R.; Ansari Jovini, M.; Jahanzad, E.; Hashemi, M. Accumulation of phenolic compounds in milk thistle seeds under drought stress. Planta 2015, 242, 2265–2269. [Google Scholar]
- Dadrasana, M.; Chaichi, M.R.; Pourbabaee, A.A.; Yazdani, D.; Keshavarz-Afshar, R. Deficit irrigation and biological fertilizer influence on yield and trigonelline production of fenugreek. Ind. Crops Prod. 2015, 77, 156–162. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Salehi, A.; Mehdi, B.; Fallah, S.; Kaul, H.P.; Neugschwandtner, R.W. Productivity and nutrient use efficiency with integrated fertilization of buckwheat-fenugreek intercrops. Nutr. Cycl. Agroecosyst. 2018, 110, 407–425. [Google Scholar] [CrossRef]
- Facchini, P.J. Alkaloid biosynthesis in plants: Biochemistry cell biology, molecular regulation, and metabolic engineering applications. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 29–66. [Google Scholar] [CrossRef]
- Mutlu, S. Determination of Some Phenological, Morphological, Yield and Quality Characteristics of Fenugreeks (Trigonella foenum-graceum L.) from Different Origins. Master’s Thesis, Graduate School of Ondokuz Mayıs University, Department of Field Crops, Samsun, Türkiye, 2011. [Google Scholar]
- Beyzi, E.; Şafak, E.K.; Gürbüz, P.; Koşar, M.; Gürbüz, B. Fatty acid composition, diosgenin and trigonelline contents of fenugreek (Trigonella foenum-graecum): Effects of phosphorus fertilizer. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 663–667. [Google Scholar] [CrossRef]
- Guzel, Y.; Ozyazici, G. Adoption of promising fenugreek (Trigonella foenum-graceum L.) genotypes for yield and quality characteristics in the Semiarid Climate of Turkey. Atmosphere 2021, 12, 1199. [Google Scholar] [CrossRef]
- Anwar, F.; Zafar, S.N.; Rashid, U. Characterization of Moringa oleifera seed oil from drought and irrigated regions of Punjab, Pakistan. Grasas Aceites 2006, 57, 160–168. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M.; Anwar, F. Physico-chemical attributes of seed oil from drought stressed sunflower (Helianthus annuus L.) plants. Grasas Aceites 2009, 60, 475–481. [Google Scholar]
- Mori, T.A.; Hodgson, J.M. Fatty acids: Health effects of omega-6 polyunsaturated fatty acid. In Reference Module in Biomedical Sciences, Encyclopedia of Human Nutrition, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 209–214. [Google Scholar]
- Skakovskii, E.D.; Tychinskaya, L.Y.; Mauchanava, V.A.; Karankevich, E.G.; Lamotkin, S.A.; Ahabalayeva, A.D.; Reshetnikov, V.N. Combining NMR spectroscopy and gas-liquid chromatography for analysis of the fatty acid composition of fenugreek seed oil (Trigonella foenum graecum L.). J. Spectrosc. 2013, 80, 779–782. [Google Scholar] [CrossRef]
- Bienkowski, T.; Zuk-Golaszewska, K.; Kaliniewicz, J.; Golaszewski, J. Content of biogenic elements and fatty acid compositions of fenugreek seeds cultivated under different conditions. Chil. J. Agric. Res. 2017, 77, 134–141. [Google Scholar] [CrossRef]
- Baccou, J.C.; Sauvaire, Y.; Olle, M.; Petit, J. L’huile de fenugreek: Composition, properties, possibilities d’utilisationdsans I’indust rie des peintures et vernis. Rerue Fr. Corps Gars 1978, 25, 353–359. [Google Scholar]
- Thakur, M.; Nanda, V. Assessment of physico-chemical properties, fatty acid, amino acid and mineral profile of bee pollen from India with a multivariate perspective. J. Food Nutr. Res. 2020, 57, 328–340. [Google Scholar]
- Beyzi, E. PCA analysis on postharvest quality characterization of fenugreek depending on seed weight. Int. J. Agric. Environ. Food Sci. 2020, 4, 356–361. [Google Scholar] [CrossRef]
- Hilditch, T.P.; Williams, P.N. The Chemical Constitution of Natural Fats, 4th ed.; Chapman & Hall: London, UK, 1964; p. 614. [Google Scholar]
- Choulis, N.H. Miscellaneous drugs, materials, medical devices, and techniques. A worldwide yearly survey of new data in adverse drug reactions. In Side Effects of Drugs Annual; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 33, pp. 1009–1029. [Google Scholar]
- De Caterina, R.; Basta, G. n-3 Fatty acids and the inflammatory response-Biological background. Eur. Heart J. Suppl. 2001, 3, 42–49. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Vinson, J.A.; Hao, Y.; Su, C.; Zubic, L. Phenol antioxidant quantity and quality in foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Ali, A.M.A.; ElNour, M.E.M. Antioxidant activity, total phenolic, flavonoid and tannin contents of callus and seeds extracts of fenugreek (Trigonella foenum-graecum L.). Int. J. Sci. Res. 2014, 3, 1268–1272. [Google Scholar]
- Mashkor, I.M.A.A. Phenolic content and antioxidant activity of fenugreek seeds extract. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 841–844. [Google Scholar]
- Uras Güngör, Ş.S.; Güzel, S.; İlçim, A.; Kökdil, G. Total phenolic and flavonoid content, mineral composition and antioxidant potential of Trigonella monspeliaca. Turk. J. Pharm. Sci. 2014, 11, 255–262. [Google Scholar]
- Dixit, P.; Ghaskadbi, S.; Mohan, H.; Devasagayam, T.P. Antioxidant properties of germinated fenugreek seeds. Phytother. Res. 2005, 19, 977–983. [Google Scholar] [CrossRef]
- Lopez-Amoros, M.L.; Hernandez, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Wissal, A.; Fatouma, M.A.L.; Jalludin, M.; Manar, O.; Adnane, E.Y.; Ayoub, A.; Tarik, A. Antimicrobial and antioxidant activities of Trigonella foenum-graecum essential oil from the region of Settat (Morocco). Pharmacologyonline 2021, 435, 434–442. [Google Scholar]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.B.; Bhanger, M.I.; Memon, S. Antioxidative activity of extracts from fenugreek seeds (Trigonella foenum-graecum). Pak. J. Anal. Environ. Chem. 2008, 9, 78–83. [Google Scholar]
- Rahmani, M.; Hamel, L.; Toumi-Benali, F.; Dif, M.M.; Moumen, F.; Rahmani, H. Determination of antioxidant activity, phenolic quantification of four varieties of fenugreek Trigonella foenum graecum L. seed extract cultured in west Algeria. J. Mater. Environ. Sci. 2018, 9, 1656–1661. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 4, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Dastan, S.; Turker, İ.; Isleroglu, H. An optimization study on the extraction of phenolic compounds from fenugreek seeds. J. Food 2021, 46, 959–970. [Google Scholar]
- Palma-Tenango, M.; Soto-Hernández, M.; Aguirre-Hernández, E. Flavonoids in Agriculture. Flavonoids-Biosynth. Hum. Health 2017, 189–201. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M. Breeding improvement of fennel genotypes of different origins (Foeniculum vulgare L.) using morphological and yield parameters. Int. J. Agric. Nat. Resour. 2022, 49, 97–111. [Google Scholar]
| No | Accession Code | Country | Collection Site | No | Accession Code | Country | Collection Site |
|---|---|---|---|---|---|---|---|
| 1 | Çiftçi * | Türkiye | Türkiye | 11 | PI 381062 | Iran | Ghazvin |
| 2 | Gürarslan * | Türkiye | Türkiye | 12 | PI 426971 | Pakistan | Gujjo, Karachi |
| 3 | PI 173820 | Türkiye | Malatya | 13 | PI 426973 | Pakistan | Mirpur Batoro |
| 4 | PI 194020 | Ethiopia | Debra Markos | 14 | PI 469264 | Egypt | Nile Delta |
| 5 | PI 215615 | India | Sirsa, Punjab | 15 | PI 568215 | Türkiye | - |
| 6 | PI 251640 | Ethiopia | - | 16 | PI 572538 | Egypt | Nubaria, North Delta |
| 7 | PI 286532 | India | Kulu bazaar | 17 | PI 613633 | Australia | - |
| 8 | PI 296394 | Iran | - | 18 | PI 617076 | Bulgaria | - |
| 9 | PI 302448 | India | Delhi, India | 19 | PI 639185 | Armenia | Yerevan |
| 10 | PI 302449 | India | - | 20 | PI 660995 | Armenia | Yerevan |
| No | Genotypes/ Cultivars | Plant Height (cm) | Branch Number | Seed Yield (kg/da) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||||||||
| IR | DRY | IR | DRY | IR | DRY | IR | DRY | IR | DRY | IR | DRY | ||
| 1 | Çiftçi | 53.50 cde | 48.87 d–ı | 67.80 a–d | 53.43 e–m | 3.73 abc | 3.03 a–f | 4.07 b–e | 2.93 g–m | 58.77 b–f | 58.92 b–f | 81.26 a–ı | 79.78 a–ı |
| 2 | Gürarslan | 49.43 d–ı | 42.33 h–l | 56.13 c–ı | 45.23 ı–p | 3.50 a–d | 2.77 b–f | 3.87 b–f | 2.40 l–o | 63.64 b–f | 69.60 b–f | 62.36 a–ı | 78.93 a–ı |
| 3 | PI 173820 | 44.03 f–k | 34.23 lm | 54.73 d–k | 42.30 j–q | 3.10 a–f | 2.53 c–f | 4.03 b–f | 2.27 mno | 63.01 b–f | 42.06 def | 43.79 f–ı | 38.55 ı |
| 4 | PI 194020 | 46.30 e–k | 43.97 f–k | 55.13 c–j | 37.13 pq | 3.23 a–f | 3.07 a–f | 3.33 d–j | 2.30 mno | 37.74 ef | 71.24 b–f | 55.27 c–ı | 44.68 f–ı |
| 5 | PI 215615 | 55.10 bcd | 51.03 d–g | 63.27 b–f | 47.27 h–p | 3.03 a–f | 2.47 c–f | 4.13 a–d | 1.87 no | 85.39 b–f | 67.00 b–f | 93.59 a–e | 76.42 a–ı |
| 6 | PI 251640 | 56.57 bcd | 49.17 d–ı | 64.27 b–e | 50.87 e–o | 3.53 a–d | 3.50 a–d | 4.63 ab | 2.67 ı–o | 51.99 b–f | 65.09 b–f | 64.45 a–ı | 79.34 a–ı |
| 7 | PI 286532 | 48.17 d–j | 38.20 klm | 55.77 c–j | 38.90 n–q | 3.50 a–d | 3.03 a–f | 3.27 e–k | 2.40 l–o | 59.73 b–f | 34.82 ef | 58.00 b–ı | 61.80 a–ı |
| 8 | PI 296394 | 50.23 d–h | 41.73 h–l | 62.07 b–g | 45.10 ı–p | 4.03 ab | 3.20 a–f | 3.53 d–h | 2.40 l–o | 63.75 b–f | 43.94 def | 54.97 d–ı | 86.38 a–h |
| 9 | PI 302448 | 54.40 cde | 42.80 g–l | 50.80 e–o | 43.57 ı–q | 3.37 a–e | 3.37 a–e | 4.93 a | 2.07 no | 73.80 b–f | 58.46 b–f | 62.88 a–ı | 42.65 ghı |
| 10 | PI 302449 | 38.42 klm | 34.73 lm | 42.43 j–q | 40.10 m–q | 4.10 a | 3.13 a–f | 4.47 abc | 2.70 h–n | 62.73 b–f | 59.34 b–f | 47.09 f–ı | 48.14 e–ı |
| 11 | PI 381062 | 40.27 j–m | 34.77 lm | 55.03 c–j | 37.27 opq | 3.50 a–d | 2.93 a–f | 3.77 c–g | 2.43 k–o | 64.62 b–f | 41.62 def | 89.47 a–f | 46.88 f–ı |
| 12 | PI 426971 | 53.10 cde | 41.43 ı–l | 48.80 g–p | 41.23 k–q | 2.77 b–f | 2.53 c–f | 2.93 g–m | 1.83 o | 87.95 a–e | 50.58 c–f | 44.84 f–ı | 70.16 a–ı |
| 13 | PI 426973 | 59.70 abc | 54.00 cde | 79.50 a | 52.27 e–n | 3.50 a–d | 3.03 a–f | 4.47 abc | 2.33 mno | 105.78 ab | 61.39 b–f | 87.48 a–g | 86.10 a–h |
| 14 | PI 469264 | 51.67 c–f | 44.03 f–k | 56.40 c–ı | 40.87 l–q | 2.87 a–f | 3.17 a–f | 3.53 d–h | 1.97 no | 50.42 c–f | 51.93 b–f | 61.73 a–ı | 56.46 c–ı |
| 15 | PI 568215 | 68.03 a | 54.30 cde | 72.23 ab | 46.37 ı–p | 3.17 a–f | 2.93 a–f | 3.77 c–g | 2.20 mno | 142.02 a | 85.62 b–f | 99.85 a–d | 107.17 a |
| 16 | PI 572538 | 49.70 d–ı | 39.10 klm | 48.20 h–p | 44.63 ı–q | 2.70 c–f | 2.20 ef | 2.53 j–o | 2.67 ı–o | 39.79 def | 31.14 f | 41.13 hı | 42.28 ghı |
| 17 | PI 613633 | 46.17 e–k | 39.27 klm | 60.77 b–h | 39.03 n–q | 2.37 def | 2.00 f | 3.20 f–l | 2.00 no | 77.07 b–f | 32.93 ef | 54.22 d–ı | 45.10 f–ı |
| 18 | PI 617076 | 53.60 cde | 32.57 m | 50.83 e–o | 31.23 q | 2.36 def | 2.33 def | 2.37 l–o | 2.13 mno | 101.78 abc | 141.37 a | 84.58 a–ı | 77.72 a–ı |
| 19 | PI 639185 | 67.67 a | 40.20 j–m | 68.60 abc | 50.23 f–p | 2.57 c–f | 2.13 ef | 3.40 d–ı | 2.50 j–o | 77.14 b–f | 50.05 c–f | 101.37 abc | 105.52 a |
| 20 | PI 660995 | 63.40 ab | 55.57 bcd | 73.20 ab | 54.50 d–l | 3.67 abc | 2.77 b–f | 3.73 c–g | 2.53 j–o | 94.82 a–d | 79.92 b–f | 103.83 ab | 95.58 a–d |
| Cultivar mean | 51.47 | 45.60 | 61.97 | 49.33 | 3.62 | 2.90 | 3.97 | 2.67 | 61.21 | 64.26 | 71.81 | 79.36 | |
| Genotype mean | 52.58 | 42.84 | 59.00 | 43.49 | 3.19 | 2.80 | 3.67 | 2.29 | 74.42 | 59.36 | 69.36 | 67.27 | |
| General mean | 52.47 a | 43.12 b | 59.30 a | 44.08 b | 3.23 a | 2.81 b | 3.70 a | 2.33 b | 73.10 a | 59.85 b | 69.61 ns | 68.48 ns | |
| Fgenotype | 10.56 * | 4.69 * | 1.93 * | 3.49 * | 2.44 * | 3.00 * | |||||||
| Fapplication | 97.35 * | 101.62 * | 9.08 * | 215.97 * | 4.71 * | 0.05 ns | |||||||
| Fgenotype×application | 1.98 * | 0.94 * | 0.28 * | 2.66 * | 0.81 * | 0.51 * | |||||||
| No | Genotype/Cultivar | Total Alkaloid Content (%) | Trigonelline Content (%) | ||
|---|---|---|---|---|---|
| Irrigated | Dryland | Irrigated | Dryland | ||
| 1 | Çiftçi | 2.33 O | 2.14 V | 0.46 abc | 0.34 b–m |
| 2 | Gürarslan | 2.43 K | 2.10 Y | 0.34 c–m | 0.29 g–m |
| 3 | PI 173820 | 2.33 O | 1.75 h | 0.43 a–e | 0.30 f–m |
| 4 | PI 194020 | 1.80 e | 2.16 U | 0.25 j–m | 0.46 abc |
| 5 | PI 215615 | 1.80 e | 2.10 Y | 0.23 m | 0.33 d–m |
| 6 | PI 251640 | 1.76 g | 2.34 N | 0.23 m | 0.31 e–m |
| 7 | PI 286532 | 2.70 A | 2.40 L | 0.38 a–ı | 0.37 a–j |
| 8 | PI 296394 | 2.27 R | 2.60 D | 0.32 d–m | 0.42 a–f |
| 9 | PI 302448 | 2.43 J | 2.60 D | 0.24 klm | 0.46 abc |
| 10 | PI 302449 | 2.10 Z | 1.50 l | 0.26 ı–m | 0.24 klm |
| 11 | PI 381062 | 2.47 H | 1.85 d | 0.28 g–m | 0.29 g–m |
| 12 | PI 426971 | 1.60 k | 1.13 o | 0.27 h–m | 0.33 d–m |
| 13 | PI 426973 | 1.43 m | 2.03 b | 0.40 a–g | 0.48 a |
| 14 | PI 469264 | 2.52 F | 2.33 O | 0.46 abc | 0.36 a–k |
| 15 | PI 568215 | 2.64 B | 1.70 j | 0.23 m | 0.39 a–h |
| 16 | PI 572538 | 2.50 G | 1.97 c | 0.43 a–d | 0.44 a–d |
| 17 | PI 613633 | 2.21 T | 2.25 S | 0.28 h–m | 0.37 a–j |
| 18 | PI 617076 | 1.73 ı | 1.41 n | 0.37 a–j | 0.35 b–l |
| 19 | PI 639185 | 2.61 C | 2.40 L | 0.40 a–g | 0.34 b–m |
| 20 | PI 660995 | 2.46 I | 2.30 Q | 0.28 g–m | 0.38 a–ı |
| Cultivar mean | 2.38 | 2.12 | 0.40 | 0.32 | |
| Genotype mean | 2.19 | 2.04 | 0.32 | 0.37 | |
| General mean | 2.21 a | 2.05 b | 0.33 b | 0.36 a | |
| Fgenotype | 3742560.42 * | 3.10 * | |||
| Fapplication | 4156380.01 * | 6.93 * | |||
| Fgenotype×application | 1633692.48 * | 2.65 * | |||
| No | Genotype/Cultivar | Fixed Oil (%) | No | Genotype/Cultivar | Fixed Oil (%) | ||
|---|---|---|---|---|---|---|---|
| Irrigated | Dryland | Irrigated | Dryland | ||||
| 1 | Çiftçi | 8.06 a–h | 8.14 a–g | 11 | PI 381062 | 9.08 ab | 6.92 c–ı |
| 2 | Gürarslan | 6.58 e–ı | 7.53 a–h | 12 | PI 426971 | 7.64 a–h | 7.47 a–h |
| 3 | PI 173820 | 7.60 a–h | 7.42 a–ı | 13 | PI 426973 | 7.74 a–h | 6.08 ghı |
| 4 | PI 194020 | 8.25 a–f | 8.16 a–g | 14 | PI 469264 | 8.83 a–d | 7.11 b–ı |
| 5 | PI 215615 | 9.29 a | 7.46 a–h | 15 | PI 568215 | 8.11 a–h | 6.11 f–ı |
| 6 | PI 251640 | 7.85 a–h | 7.56 a–h | 16 | PI 572538 | 7.60 a–h | 6.87 d–ı |
| 7 | PI 286532 | 9.05 abc | 7.55 a–h | 17 | PI 613633 | 7.86 a–h | 5.27 ı |
| 8 | PI 296394 | 7.96 a–h | 7.24 a–ı | 18 | PI 617076 | 8.94 a–d | 5.98 hı |
| 9 | PI 302448 | 8.91 a–d | 6.98 b–ı | 19 | PI 639185 | 8.73 a–d | 7.03 b–ı |
| 10 | PI 302449 | 8.51 a–e | 6.98 b–ı | 20 | PI 660995 | 8.00 a–h | 8.42 a–e |
| Mean (cultivar–irrigated) | 7.32 | ||||||
| Mean (cultivar–dryland) | 7.83 | ||||||
| Mean (genotype–irrigated) | 8.33 | ||||||
| Mean (genotype–dryland) | 7.03 | ||||||
| General mean (irrigated) | 8.23 a | ||||||
| General mean (dryland) | 7.11 b | ||||||
| Fgenotype | 0.88 * | ||||||
| Fapplication | 21.97 * | ||||||
| Fgenotype×application | 1.01 * | ||||||
| No | Genotype/ Cultivar | Linoleic Acid (%) | Linolenic Acid (%) | Oleic Acid (%) | Stearic Acid (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Dryland | Irrigated | Dryland | Irrigated | Dryland | Irrigated | Dryland | ||
| 1 | Çiftçi | 51.34 a–d | 45.71 a–e | 16.77 a–g | 15.17 c–ı | 12.91 b–ı | 8.04 e–m | 7.44 g–o | 12.91 c–j |
| 2 | Gürarslan | 47.28 a–e | 47.94 a–e | 15.04 c–ı | 11.69 h–n | 6.35 h–m | 6.83 g–m | 7.39 g–o | 6.35 k–o |
| 3 | PI 173820 | 48.13 a–e | 54.12 ab | 14.40 c–j | 18.08 a–d | 4.98 klm | 7.19 f–m | 11.44 c–l | 4.98 l–o |
| 4 | PI 194020 | 41.69 b–e | 52.59 abc | 12.82 f–l | 15.36 c–ı | 8.65 e–m | 5.50 j–m | 5.71 k–o | 17.65 bc |
| 5 | PI 215615 | 39.85 cde | 56.82 a | 6.84 op | 18.26 a–d | 6.77 g–m | 4.77 lm | 6.85 j–o | 6.77 j–o |
| 6 | PI 251640 | 50.35 a–d | 45.80 a–e | 12.73 f–l | 21.09 a | 7.07 f–m | 10.93 b–l | 13.88 c–g | 7.07 h–o |
| 7 | PI 286532 | 41.92 b–e | 41.12 b–e | 16.23 b–h | 21.23 a | 4.92 klm | 12.47 b–j | 17.10 bcd | 5.92 k–o |
| 8 | PI 296394 | 48.43 a–e | 45.39 a–e | 15.17 c–ı | 13.25 e–l | 3.08 m | 17.18 b | 13.57 c–h | 3.08 o |
| 9 | PI 302448 | 46.18 a–e | 44.51 a–e | 11.06 ı–o | 20.31 ab | 8.55 e–m | 12.15 b–k | 13.42 c–ı | 8.55 f–o |
| 10 | PI 302449 | 44.50 a–e | 44.56 a–e | 9.92 j–o | 18.66 abc | 14.20 b–f | 16.18 bc | 4.85 mno | 15.20 cde |
| 11 | PI 381062 | 44.14 a–e | 38.91 def | 13.84 d–k | 15.88 b–h | 5.35 j–m | 4.46 lm | 6.04 k–o | 5.35 k–o |
| 12 | PI 426971 | 44.79 a–e | 45.99 a–e | 12.72 f–l | 7.77 m–p | 24.88 a | 12.21 b–k | 6.89 ı–o | 24.88 a |
| 13 | PI 426973 | 36.31 ef | 46.06 a–e | 13.85 d–k | 17.83 a–e | 13.99 b–g | 14.25 b–f | 10.97 d–m | 13.99 c–f |
| 14 | PI 469264 | 44.21 a–e | 43.10 b–e | 13.98 c–k | 17.28 a–f | 8.86 d–m | 5.68 ı–m | 5.17 l–o | 6.86 j–o |
| 15 | PI 568215 | 46.76 a–e | 47.66 a–e | 17.13 a–g | 13.10 f–l | 5.83 ı–m | 14.56 b–e | 11.08 d–m | 6.64 j–o |
| 16 | PI 572538 | 52.42 abc | 43.60 b–e | 12.51 g–l | 13.95 c–k | 5.93 ı–m | 8.80 e–m | 11.77 c–k | 5.79 k–o |
| 17 | PI 613633 | 44.51 a–e | 42.78 b–e | 9.12 l–o | 7.62 nop | 7.26 f–m | 13.63 b–h | 14.70 c–f | 4.16 no |
| 18 | PI 617076 | 26.57 f | 48.72 a–e | 3.46 p | 7.74 nop | 4.09 lm | 16.14 bcd | 3.48 o | 3.66 o |
| 19 | PI 639185 | 48.93 a–e | 46.16 a–e | 12.45 g–m | 18.01 a–d | 8.97 c–m | 6.74 g–m | 10.27 e–n | 4.78 mno |
| 20 | PI 660995 | 43.69 a–e | 39.13 def | 11.64 h–n | 9.64 k–o | 5.69 ı–m | 17.32 b | 22.28 ab | 4.36 no |
| Cultivar mean | 49.31 | 46.82 | 15.91 | 13.43 | 9.63 | 7.43 | 7.42 | 9.63 | |
| Genotype mean | 44.08 | 45.94 | 12.22 | 15.28 | 8.28 | 11.12 | 10.53 | 8.31 | |
| General mean | 44.60 ns | 46.03 ns | 12.58 b | 15.10 a | 8.42 b | 10.75 a | 10.22 a | 8.45 b | |
| Fgenotype | 1.07 * | 7.08 * | 3.52 * | 3.21 * | |||||
| Fapplication | 0.97 ns | 23.25 * | 8.38 * | 5.96 * | |||||
| Fgenotype×application | 1.49 * | 4.15 * | 3.25 * | 7.11 * | |||||
| No | Genotype/ Cultivar | Palmitic Acid (%) | Myristic Acid (%) | Pentadecanoic Acid (%) | Palmitoleic Acid (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Dryland | Irrigated | Dryland | Irrigated | Dryland | Irrigated | Dryland | ||
| 1 | Çiftçi | 2.84 e–k | 5.99 a–d | 1.19 e–h | 1.20 e–h | 0.85 bc | 2.48 bc | 1.33 b–l | 2.18 b–f |
| 2 | Gürarslan | 3.45 d–k | 1.70 h–k | 0.25 h | 2.44 d–h | 13.33 a | 1.37 bc | 0.35 l | 1.26 b–l |
| 3 | PI 173820 | 4.28 d–h | 4.74 d–g | 0.81 fgh | 1.62 d–h | 0.29 c | 2.04 bc | 1.07 e–l | 2.54 ab |
| 4 | PI 194020 | 7.78 abc | 1.93 f–k | 0.77 gh | 2.49 d–h | 0.60 bc | 0.87 bc | 0.67 g–l | 0.87 g–l |
| 5 | PI 215615 | 3.22 d–k | 3.17 d–k | 1.99 d–h | 5.03 c–h | 2.40 bc | 2.16 bc | 2.38 a–d | 0.49 ı–l |
| 6 | PI 251640 | 2.31 f–k | 1.92 f–k | 3.61 c–h | 2.25 d–h | 0.83 bc | 1.99 bc | 2.27 b–e | 1.23 c–l |
| 7 | PI 286532 | 3.34 d–k | 1.81 g–k | 6.88 a–d | 1.14 e–h | 1.01 bc | 1.99 bc | 1.74 b–ı | 0.45 jkl |
| 8 | PI 296394 | 4.03 d–h | 2.29 f–k | 5.82 b–g | 2.08 d–h | 1.09 bc | 1.34 bc | 0.98 e–l | 1.17 d–l |
| 9 | PI 302448 | 3.97 d–ı | 2.52 f–k | 0.77 gh | 1.72 d–h | 1.38 bc | 2.24 bc | 1.82 b–h | 1.68 b–k |
| 10 | PI 302449 | 8.85 a | 0.74 jk | 0.96 fgh | 2.31 d–h | 0.50 bc | 1.11 bc | 0.55 h–l | 0.51 ı–l |
| 11 | PI 381062 | 3.06 d–k | 2.60 e–k | 6.44 a–e | 11.02 ab | 0.52 bc | 1.55 bc | 0.53 h–l | 1.89 b–g |
| 12 | PI 426971 | 2.29 f–k | 1.05 ıjk | 0.70 gh | 1.04 fgh | 1.98 bc | 1.40 bc | 1.72 b–j | 0.99 e–l |
| 13 | PI 426973 | 5.51 b–e | 0.47 k | 0.42 gh | 0.57 gh | 0.44 bc | 2.22 bc | 0.83 g–l | 0.43 jkl |
| 14 | PI 469264 | 2.96 e–k | 4.82 c–f | 6.20 a–f | 1.41 e–h | 0.54 bc | 0.77 bc | 0.62 g–l | 2.53 ab |
| 15 | PI 568215 | 2.80 e–k | 2.01 f–k | 1.83 d–h | 5.02 c–h | 0.59 bc | 1.17 bc | 1.41 b–l | 1.51 b–l |
| 16 | PI 572538 | 5.54 b–e | 3.08 d–k | 2.35 d–h | 1.44 e–h | 1.10 bc | 2.85 bc | 0.68 g–l | 3.65 a |
| 17 | PI 613633 | 4.34 d–h | 3.98 d–ı | 5.58 c–h | 0.88 fgh | 2.85 bc | 1.31 bc | 2.52 abc | 1.61 b–l |
| 18 | PI 617076 | 0.88 jk | 1.44 h–k | 3.89 c–h | 11.45 a | 0.35 c | 4.18 b | 0.42 kl | 1.02 e–l |
| 19 | PI 639185 | 8.36 ab | 2.10 f–k | 1.58 d–h | 2.25 d–h | 0.82 bc | 1.65 bc | 1.43 b–l | 0.60 g–l |
| 20 | PI 660995 | 4.37 d–h | 3.59 d–j | 8.35 abc | 1.03 fgh | 0.58 bc | 2.20 bc | 0.91 f–l | 1.63 b–l |
| Cultivar mean | 3.14 | 3.85 | 0.72 | 1.82 | 7.09 | 1.92 | 0.84 | 1.72 | |
| Genotype mean | 4.33 | 2.46 | 3.27 | 3.04 | 0.99 | 1.83 | 1.25 | 1.38 | |
| General mean | 4.21 a | 2.60 b | 3.02 ns | 2.92 ns | 1.60 ns | 1.84 ns | 1.21 ns | 1.41 ns | |
| Fgenotype | 2.35 * | 2.64 * | 2.31 * | 2.26 * | |||||
| Fapplication | 23.85 * | 0.03 ns | 0.34 ns | 1.97 * | |||||
| Fgenotype×application | 3.54 * | 1.89 * | 2.74 * | 3.43 * | |||||
| No | Genotype/ Cultivar | Margaric Acid (%) | Behenic Acid (%) | Arachidic Acid (%) | |||
|---|---|---|---|---|---|---|---|
| Irrigated | Dryland | Irrigated | Dryland | Irrigated | Dryland | ||
| 1 | Çiftçi | 0.67 de | 1.35 de | - | - | 2.29 d–j | 0.52 g–j |
| 2 | Gürarslan | 0.32 e | 1.15 de | - | 0.78 bcd | 2.56 d–j | 0.19 ıj |
| 3 | PI 173820 | 4.03 cde | 1.55 cde | 0.89 bcd | 4.31 cde | 1.08 f–j | |
| 4 | PI 194020 | 11.27 a | – | - | - | 4.46 bcd | 0.10 j |
| 5 | PI 215615 | 3.81 cde | – | - | - | 4.46 bcd | 1.03 g–j |
| 6 | PI 251640 | – | – | - | - | 2.88 d–h | 1.67 e–j |
| 7 | PI 286532 | – | – | - | - | 0.76 g–j | 0.81 g–j |
| 8 | PI 296394 | 0.12 e | 0.29 e | 3.62 a | - | 0.67 g–j | 1.10 f–j |
| 9 | PI 302448 | 5.27 bcd | 1.76 cde | - | 1.76 a–d | 2.90 d–h | 0.79 g–j |
| 10 | PI 302449 | 1.80 cde | – | - | - | 7.06 ab | 0.31 hıj |
| 11 | PI 381062 | 5.33 bcd | – | - | - | 3.69 c–f | 0.54 g–j |
| 12 | PI 426971 | – | 0.59 de | - | - | 2.63 d–j | 0.79 g–j |
| 13 | PI 426973 | 2.67 cde | – | - | - | 2.91 d–h | 0.39 g–j |
| 14 | PI 469264 | 6.32 bc | – | 2.07 abc | - | 7.17 a | 0.71 g–j |
| 15 | PI 568215 | – | 1.75 cde | 0.40 cd | - | 6.32 abc | 0.42 g–j |
| 16 | PI 572538 | 1.13 de | 2.29 cde | - | 2.68 ab | 3.03 d–g | 0.43 g–j |
| 17 | PI 613633 | 1.5 cde | 10.03 ab | 0.79 bcd | - | 2.62 d–j | 0.89 g–j |
| 18 | PI 617076 | – | 0.50 de | 0.71 cd | - | 2.83 d–ı | 0.65 g–j |
| 19 | PI 639185 | – | 4.82 cde | - | 2.74 ab | 1.60 f–j | 1.39 f–j |
| 20 | PI 660995 | – | – | - | - | 1.30 f–j | 1.72 e–j |
| Cultivar mean | 0.50 | 1.25 | 0.00 | 0.78 | 2.42 | 0.35 | |
| Genotype mean | 3.93 | 2.62 | 1.41 | 2.39 | 3.42 | 0.82 | |
| General mean | 3.40 a | 2.37 b | 1.41 b | 1.99 a | 3.22 a | 0.78 b | |
| Fgenotype | 2.04 * | 1.35 * | 1.73 * | ||||
| Fapplication | 2.77 ns | 0.01 ns | 75.79 * | ||||
| Fgenotype×application | 2.86 * | 2.10 * | 2.55 * | ||||
| No | Genotype/ Cultivar | DPPH (%) | FRAP (mg TE/100 g) | Total Phenolic (mg GAE/100 g) | Total Flavonoid (mg QE/100 g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Dryland | Irrigated | Dryland | Irrigated | Dryland | Irrigated | Dryland | ||
| 1 | Çiftçi | 48.88 abc | 27.86 def | 40.75 a–e | 37.88 b–e | 18.08 a–d | 15.72 bcd | 7.39 ab | 8.69 ab |
| 2 | Gürarslan | 43.18 a–f | 31.94 b–f | 45.28 a–e | 53.76 ab | 24.14 a–d | 28.65 ab | 8.27 ab | 8.01 ab |
| 3 | PI 173820 | 47.45 a–e | 28.56 c–f | 41.67 a–e | 39.93 a–e | 19.88 a–d | 20.01 a–d | 8.89 ab | 7.98 ab |
| 4 | PI 194020 | 50.94 ab | 31.40 b–f | 51.26 abc | 49.29 a–d | 25.87 a–d | 23.01 a–d | 8.88 ab | 8.56 ab |
| 5 | PI 215615 | 41.30 a–f | 40.53 a–f | 56.92 a | 33.71 cde | 30.38 a | 14.03 cd | 8.70 ab | 7.69 ab |
| 6 | PI 251640 | 50.46 ab | 42.64 a–f | 29.59 e | 39.78 a–e | 12.03 d | 18.04 a–d | 8.71 ab | 8.31 ab |
| 7 | PI 286532 | 50.20 ab | 35.67 b–f | 43.86 a–e | 35.05 cde | 20.39 a–d | 16.43 a–d | 7.83 ab | 8.11 ab |
| 8 | PI 296394 | 48.04 a–e | 34.46 b–f | 48.40 a–d | 31.77 de | 25.50 a–d | 16.12 a–d | 7.89 ab | 7.79 ab |
| 9 | PI 302448 | 38.76 b–f | 37.89 b–f | 43.08 a–e | 49.55 a–d | 20.75 a–d | 23.86 a–d | 8.04 ab | 9.10 a |
| 10 | PI 302449 | 42.36 a–f | 35.51 b–f | 33.96 cde | 48.68 a–d | 16.01 a–d | 22.22 a–d | 8.67 ab | 8.51 ab |
| 11 | PI 381062 | 50.46 ab | 33.42 b–f | 51.36 abc | 41.79 a–e | 25.88 a–d | 21.31 a–d | 8.90 ab | 7.57 ab |
| 12 | PI 426971 | 42.72 a–f | 27.61 ef | 45.70 a–e | 38.20 b–e | 24.18 a–d | 15.73 bcd | 8.21 ab | 8.68 ab |
| 13 | PI 426973 | 48.65 a–d | 35.83 b–f | 47.06 a–e | 42.66 a–e | 23.38 a–d | 19.43 a–d | 8.21 ab | 7.65 ab |
| 14 | PI 469264 | 33.58 b–f | 39.45 b–f | 45.96 a–e | 34.95 cde | 21.38 a–d | 16.39 a–d | 7.50 ab | 6.99 b |
| 15 | PI 568215 | 60.59 a | 25.59 f | 49.02 a–d | 45.16 a–e | 26.74 abc | 19.64 a–d | 7.75 ab | 8.33 ab |
| 16 | PI 572538 | 43.09 a–f | 36.54 b–f | 45.41 a–e | 42.57 a–e | 24.13 a–d | 19.43 a–d | 8.26 ab | 7.56 ab |
| 17 | PI 613633 | 60.55 a | 35.06 b–f | 48.94 a–d | 34.30 cde | 26.81 abc | 12.47 cd | 7.82 ab | 8.84 ab |
| 18 | PI 617076 | 43.54 a–f | 34.76 b–f | 51.76 abc | 31.88 de | 25.26 a–d | 16.22 a–d | 8.60 ab | 7.80 ab |
| 19 | PI 639185 | 60.45 a | 35.28 b–f | 48.87 a–d | 43.72 a–e | 26.73 abc | 20.90 a–d | 7.71 ab | 8.01 ab |
| 20 | PI 660995 | 47.60 a–e | 34.74 b–f | 48.71 a–d | 33.99 cde | 25.50 a–d | 11.76 d | 7.87 ab | 8.06 ab |
| Cultivar mean | 46.03 | 29.90 | 43.02 | 45.82 | 21.11 | 22.19 | 7.83 | 8.35 | |
| Genotype mean | 47.82 | 34.72 | 46.2 | 39.83 | 23.38 | 18.17 | 8.25 | 8.09 | |
| General mean | 47.64 a | 34.24 b | 45.88 a | 40.43 b | 23.15 a | 18.57 b | 8.21 ns | 8.11 ns | |
| Fgenotype | 0.45 * | 0.71 * | 0.58 * | 0.48 * | |||||
| Fapplication | 33.32 * | 7.09 * | 8.18 * | 0.18 ns | |||||
| Fgenotype×application | 0.84 * | 1.20 * | 0.80 * | 0.55 * | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camlica, M.; Yaldiz, G. Comparison of Twenty Selected Fenugreek Genotypes Grown under Irrigated and Dryland Conditions: Morphology, Yield, Quality Properties and Antioxidant Activities. Agronomy 2024, 14, 713. https://doi.org/10.3390/agronomy14040713
Camlica M, Yaldiz G. Comparison of Twenty Selected Fenugreek Genotypes Grown under Irrigated and Dryland Conditions: Morphology, Yield, Quality Properties and Antioxidant Activities. Agronomy. 2024; 14(4):713. https://doi.org/10.3390/agronomy14040713
Chicago/Turabian StyleCamlica, Mahmut, and Gulsum Yaldiz. 2024. "Comparison of Twenty Selected Fenugreek Genotypes Grown under Irrigated and Dryland Conditions: Morphology, Yield, Quality Properties and Antioxidant Activities" Agronomy 14, no. 4: 713. https://doi.org/10.3390/agronomy14040713
APA StyleCamlica, M., & Yaldiz, G. (2024). Comparison of Twenty Selected Fenugreek Genotypes Grown under Irrigated and Dryland Conditions: Morphology, Yield, Quality Properties and Antioxidant Activities. Agronomy, 14(4), 713. https://doi.org/10.3390/agronomy14040713






