Biocontrol Effect of Bacillus subtilis against Cnaphalocrocis medinalis (Guenèe) (Lepidoptera: Pyralidae): A Sustainable Approach to Rice Pest Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Cnaphalocrocis medinalis Culture
2.3. Screening Bioassay
2.4. Preparation of EB01 Bacterial Suspension
2.5. Concentration-Dependent Mortality and Development Bioassay
2.6. Enzyme Activity
2.7. Histological Analysis
2.8. In Vitro Seed Treatments
2.9. In Vivo Seed Treatment under Greenhouse
2.10. Statistical Analysis
3. Results
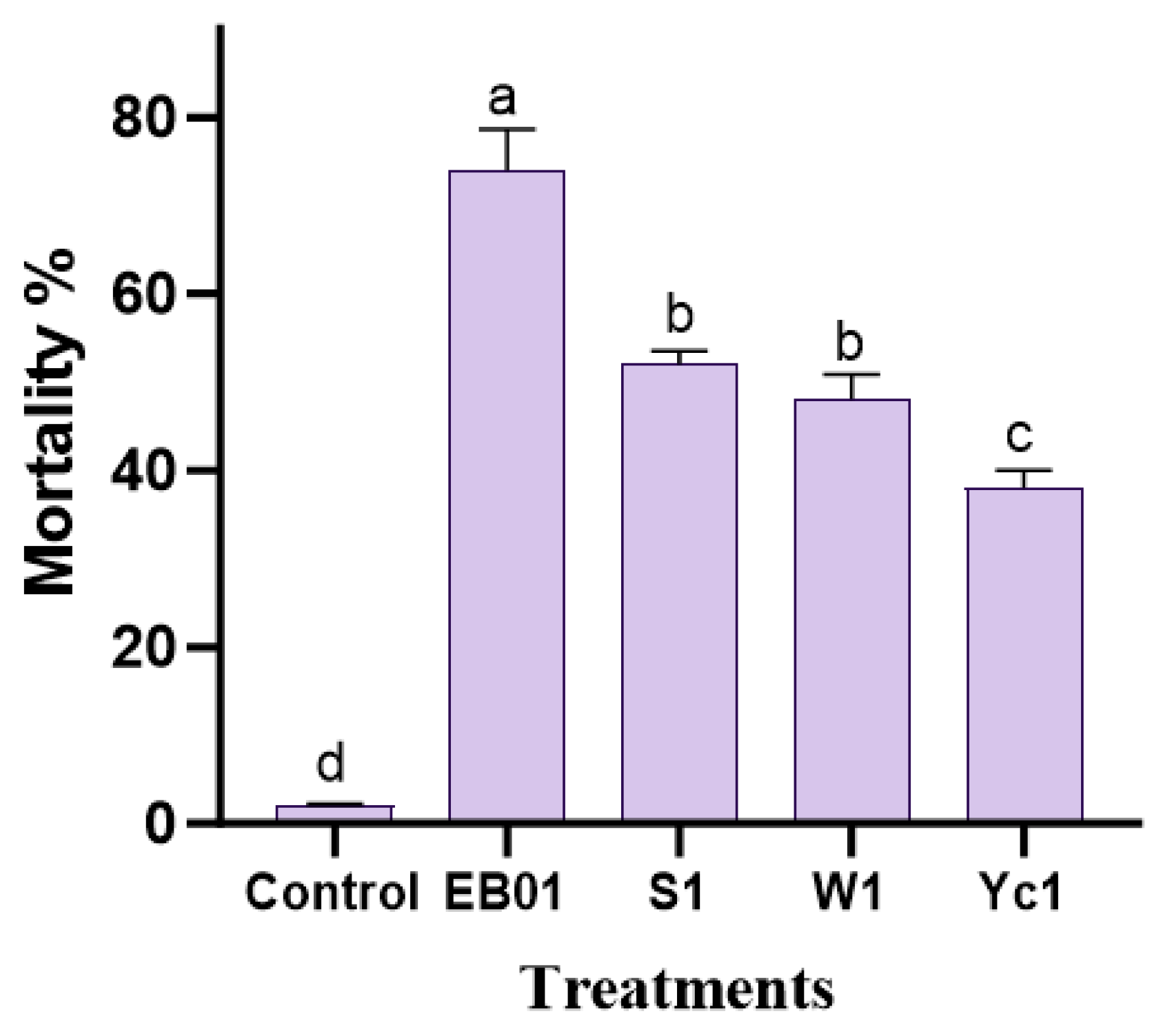
3.1. Insecticidal Bioassay
3.2. Concentration-Dependent Bioassay
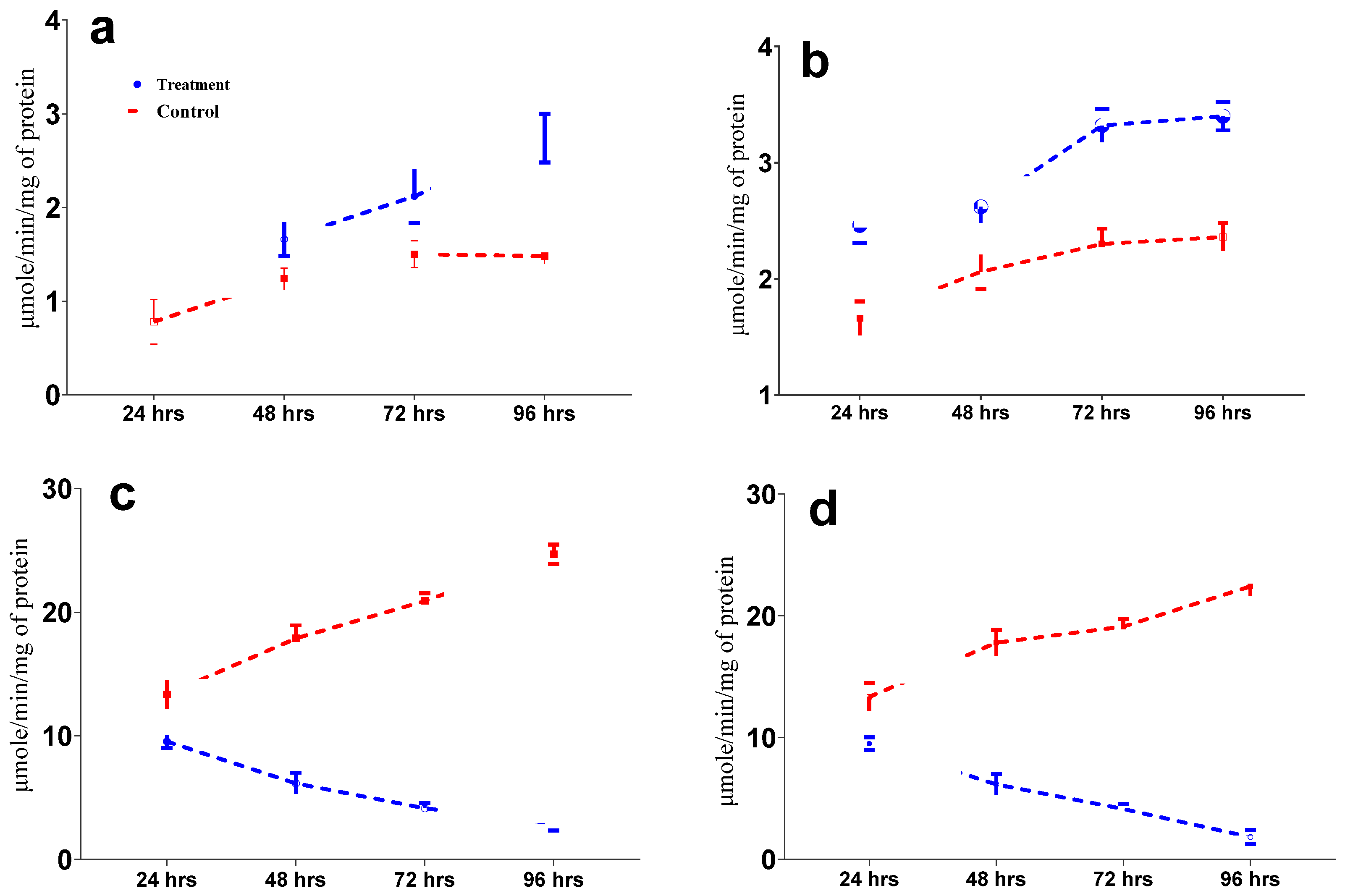
3.3. Enzyme Activity
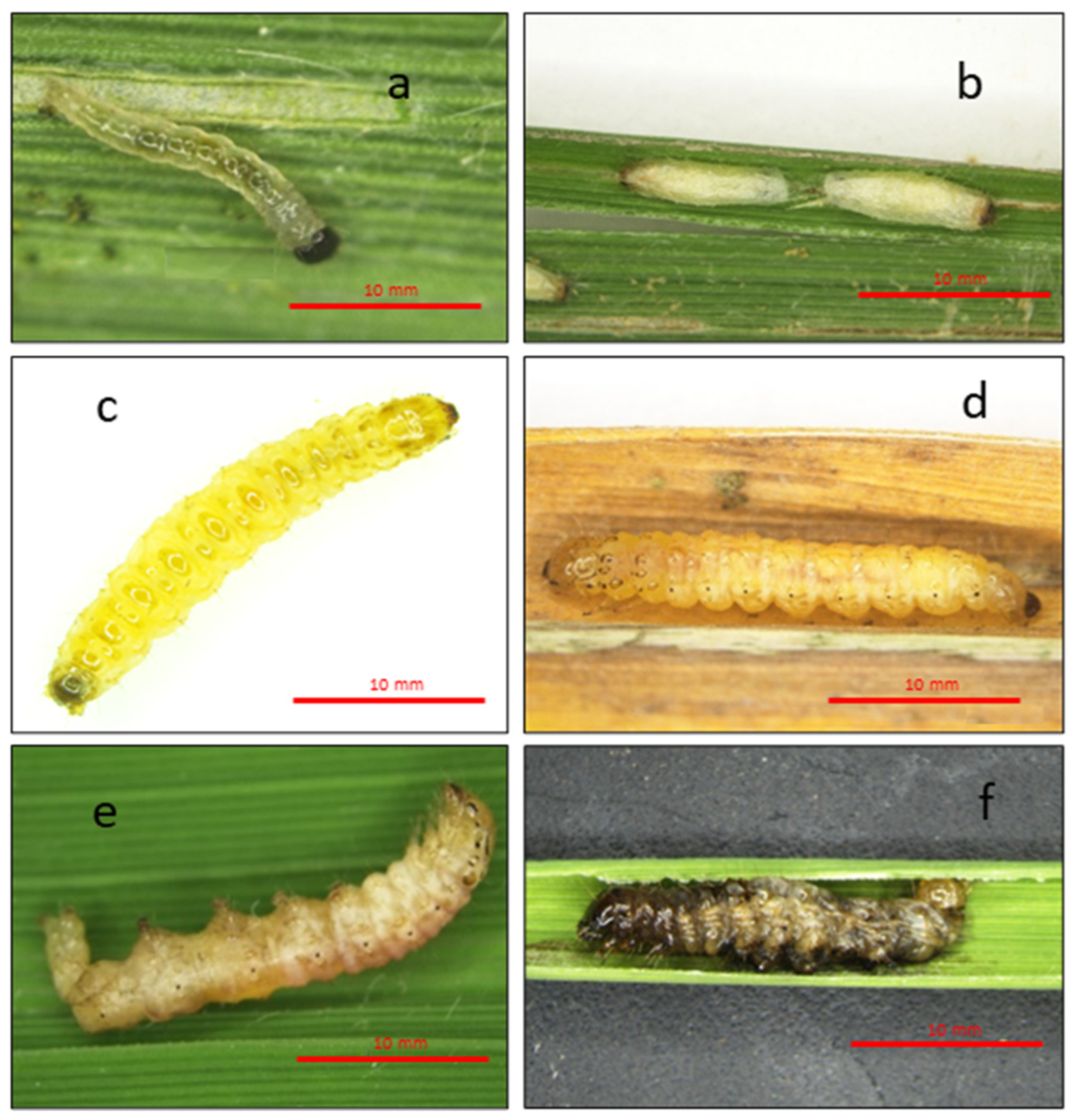
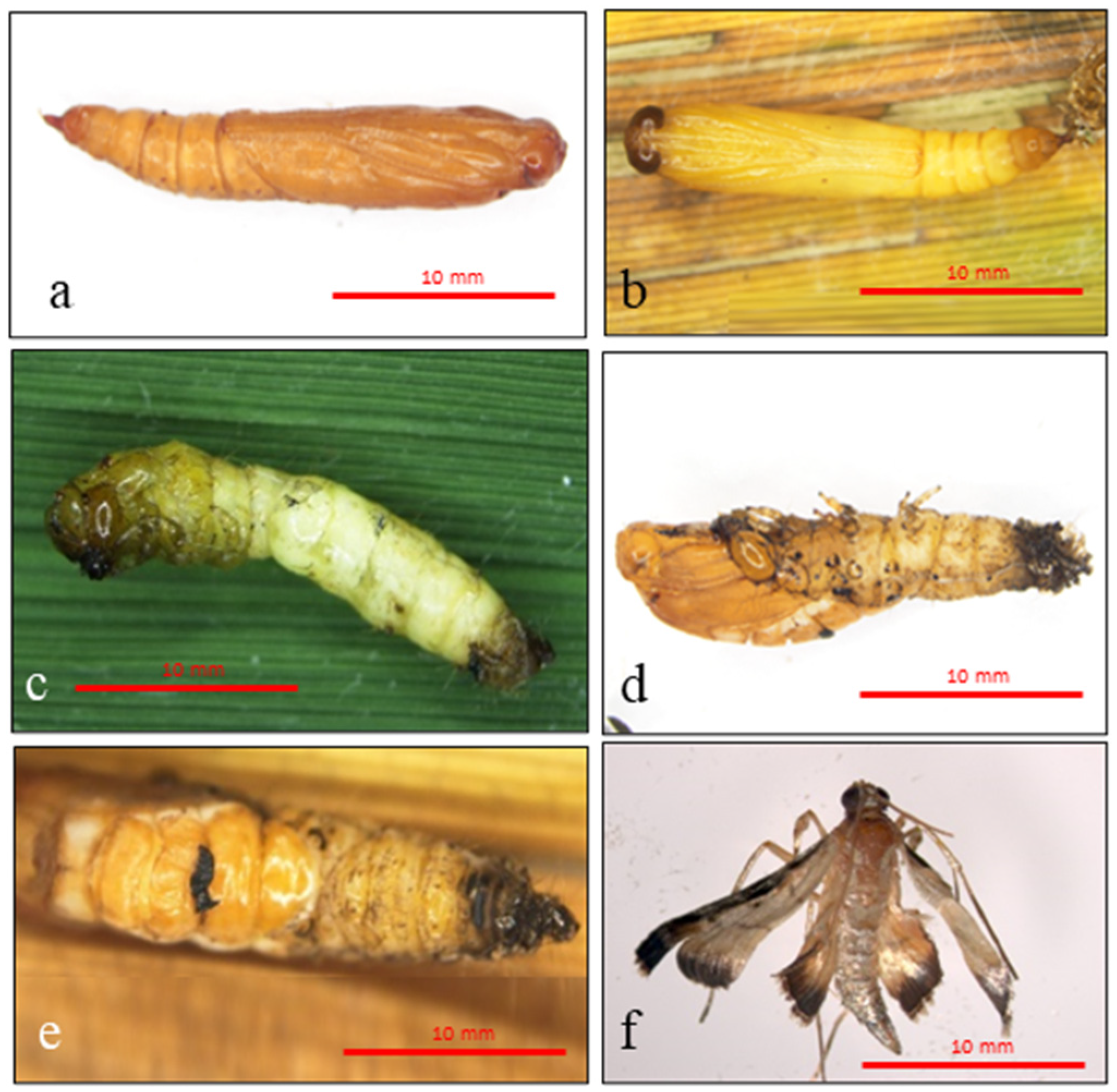
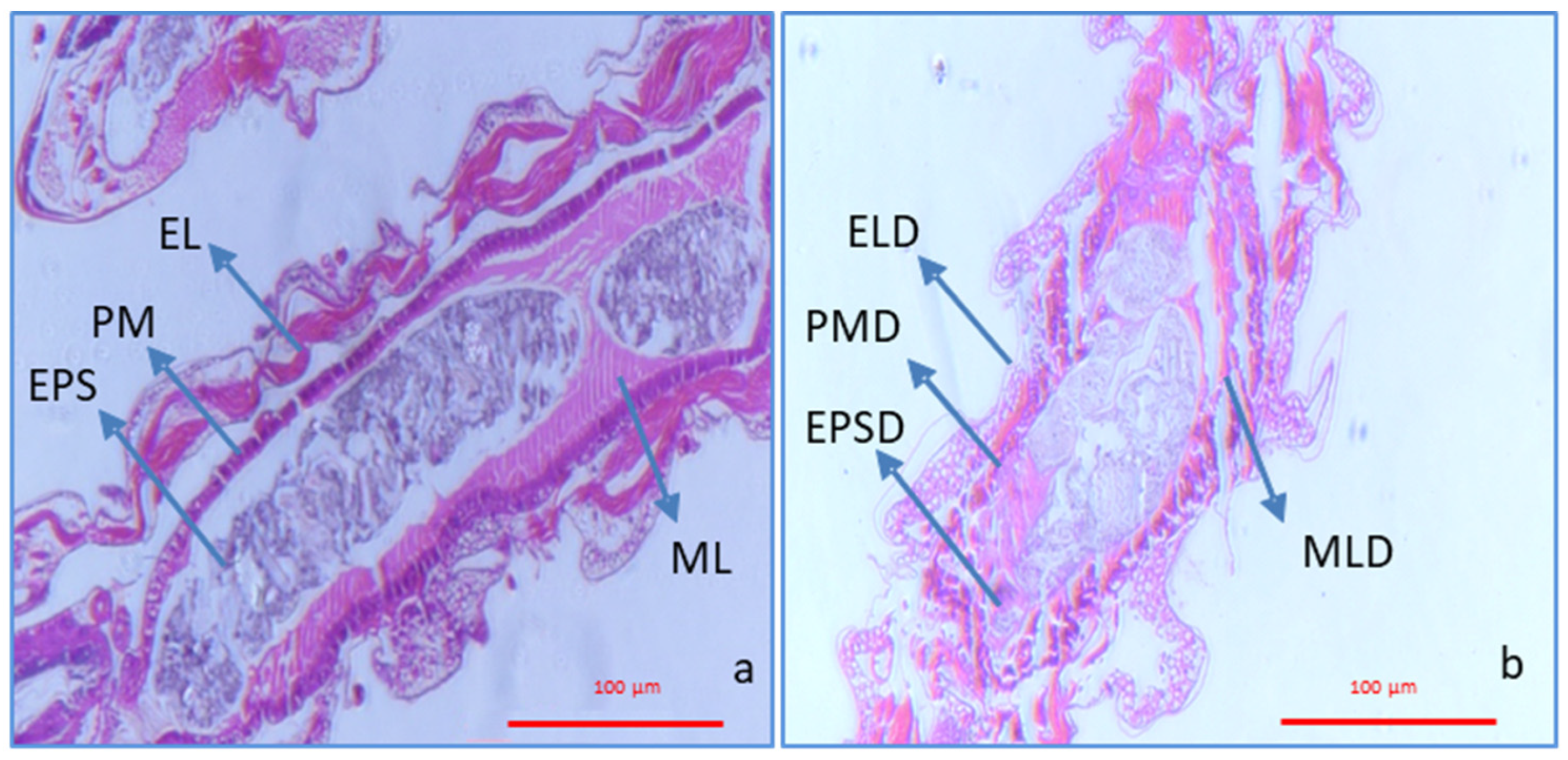
3.4. Histological Analysis
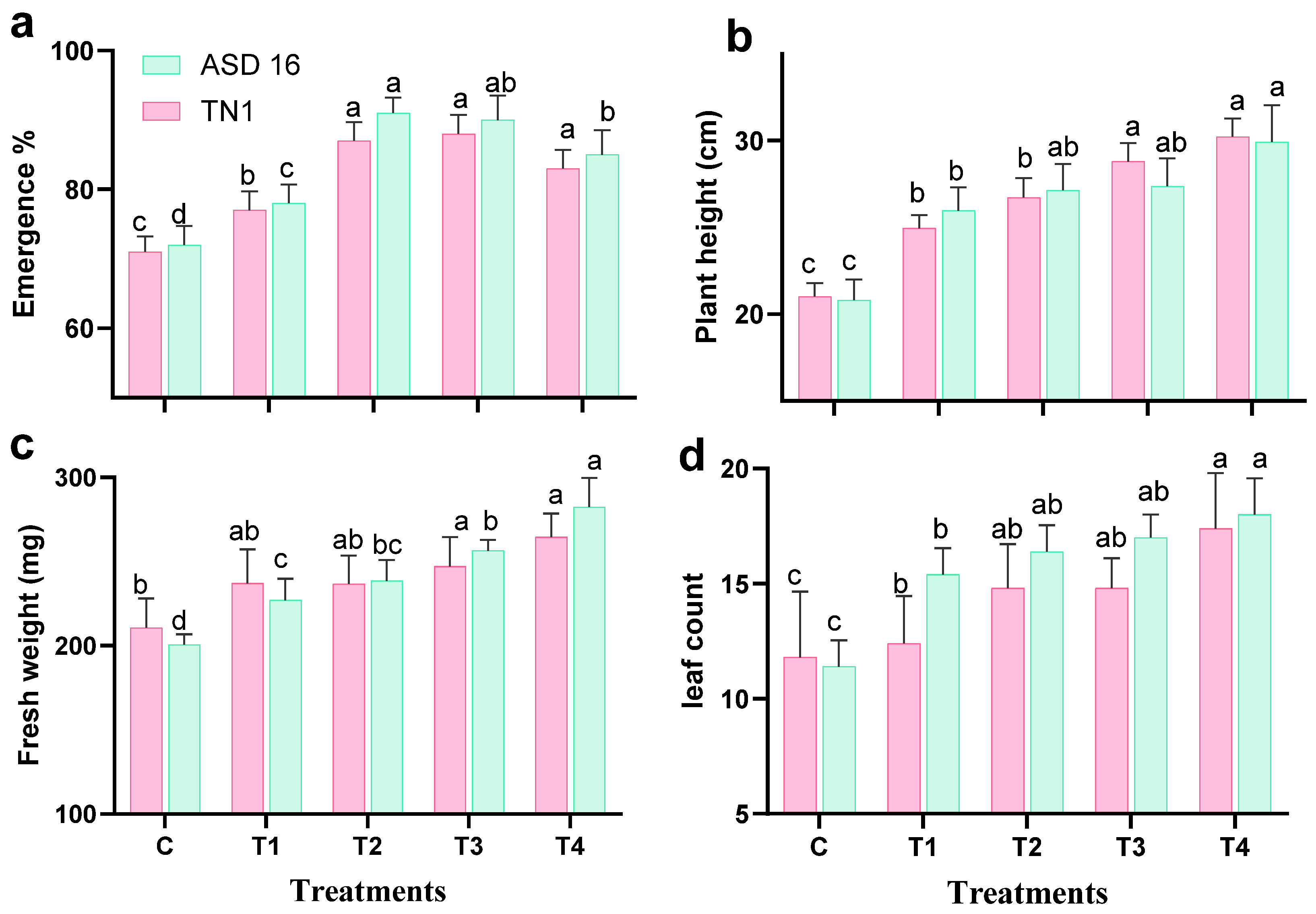
3.5. Plant–Bacteria Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Birla, D.S.; Malik, K.; Sainger, M.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Progress and challenges in improving the nutritional quality of rice (Oryza sativa L.). Crit. Rev. Food Sci. Nutri. 2017, 57, 2455–2481. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S. Biology, Behavioral and Population Dynamics of the Rice Leaffolder Complex: Dynamics of Insect Behavior; Scientific Publishers: Jodphur, India, 2011. [Google Scholar]
- Fahad, S.; Adnan, M.; Noor, M.; Arif, M.; Alam, M.; Khan, I.A.; Ullah, H.; Wahid, F.; Mian, I.A.; Jamal, Y.; et al. Major constraints for global rice production. In Advances in Rice Research for Abiotic Stress Tolerance; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–22. [Google Scholar]
- Senthil-Nathan, S. Effects of elevated CO2 on resistant and susceptible rice cultivar and its primary host, brown planthopper (BPH), Nilaparvata lugens (Stål). Sci. Rep. 2021, 11, 8905. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.V.; Zvereva, E.L. Background insect herbivory: Impacts, patterns and methodology. Prog. Bot. 2018, 79, 313–355. [Google Scholar]
- Mahesh, P.; Srikanth, J.; Chandran, K.; Singaravelu, B.; Salin, K.P.; Jayabose, C.; Balan, S. Occurrence, damage pattern and status of the rice leaf folder Cnaphalocrocis ruralis Walker (Lepidoptera: Crambidae) in Erianthus spp. in India. Exp. Agric. 2019, 55, 471–483. [Google Scholar] [CrossRef]
- Hajjar, M.J.; Ahmed, N.; Alhudaib, K.A.; Ullah, H. Integrated Insect Pest Management Techniques for Rice. Sustainability 2023, 15, 4499. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Effect of methyl jasmonate (MeJA)-induced defenses in rice against the rice leaffolder Cnaphalocrocis medinalis (Guenèe) (Lepidoptera: Pyralidae). Pest Manag. Sci. 2019, 75, 460–465. [Google Scholar] [CrossRef]
- Kaiwart, P.K.; Kumar, A.; Khan, H.H.; Sahu, R. Field Efficacy of Certain Chemical Insecticides against Rice Leaf Folder, Cnaphalocrocis medinalis Guenee. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1692–1696. [Google Scholar] [CrossRef][Green Version]
- Poudel, S.; Poudel, B.; Acharya, B.; Poudel, P. Pesticide use and its impacts on human health and environment. Environ. Ecosyst. Sci. 2020, 4, 47–51. [Google Scholar] [CrossRef]
- Siegwart, M.; Graillot, B.; Blachere Lopez, C.; Besse, S.; Bardin, M.; Nicot, P.C.; Lopez-Ferber, M. Resistance to bio-insecticides or how to enhance their sustainability: A review. Front. Plant Sci. 2015, 6, 381. [Google Scholar] [CrossRef]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as promising alternatives to chemical pesticides: A review of their current and future status. Online J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. [Google Scholar]
- Ruiu, L. Insect pathogenic bacteria in integrated pest management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Bamisile, B.S.; Siddiqui, J.A.; Akutse, K.S.; Ramos Aguila, L.C.; Xu, Y. General limitations to endophytic entomopathogenic fungi use as plant growth promoters, pests and pathogens biocontrol agents. Plants 2021, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.; Baruah, C.; Babu, A. Entomopathogenic microorganisms: Their role in insect pest management. Egypt. J. Biol. Pest Control 2021, 31, 121. [Google Scholar] [CrossRef]
- Sarkhandia, S.; Devi, M.; Sharma, G.; Mahajan, R.; Chadha, P.; Saini, H.S.; Kaur, S. Larvicidal, growth inhibitory and biochemical effects of soil bacterium, Pseudomonas sp. EN4 against Spodoptera litura (Fab). (Lepidoptera: Noctuidae). BMC Microbiol. 2023, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Paul, B.; Ahmad, W.; Paul, S.; Aggarwal, C.; Khan, Z.; Akhtar, M.S. Potential of Bacillus thuringiensis in the management of pernicious lepidopteran pests. In Plant, Soil and Microbes: Volume 2: Mechanisms and Molecular Interactions; Springer: Cham, Switzerland, 2016; pp. 277–301. [Google Scholar]
- Khewa Subba, S. Survey, Isolation and Characterization of Entomopathogenic Bacteria of Some Sporadic lepidopteran Pests of Tea Foliage from Darjeeling Foothills and Plains. Doctoral Dissertation, University of North Bengal, Siliguri, India, 2017. [Google Scholar]
- Senthil-Nathan, S.; Kalaivani, K.; Murugan, K. Behavioural responses and changes in biology of rice leaffolder following treatment with a combination of bacterial toxins and botanical insecticides. Chemosphere 2006, 64, 1650–1658. [Google Scholar] [CrossRef]
- Thakur, A.; Dhammi, P.; Saini, H.S.; Kaur, S. Pathogenicity of bacteria isolated from gut of Spodoptera litura (Lepidoptera: Noctuidae) and fitness costs of insect associated with consumption of bacteria. J. Invertebr. Pathol. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Hiebert, N.; Kessel, T.; Skaljac, M.; Spohn, M.; Vilcinskas, A.; Lee, K.Z. The gram-positive bacterium Leuconostoc pseudomesenteroides shows insecticidal activity against drosophilid and aphid pests. Insects 2020, 11, 471. [Google Scholar] [CrossRef]
- Shyam-Sundar, N.; Ramasubramanian, R.; Karthi, S.; Senthil-Nathan, S.; Chanthini, K.M.; Sivanesh, H.; Stanley-Raja, V.; Ramkumar, G.; Narayanan, K.R.; Mahboob, S.; et al. Effects of phytocompound Precocene 1 on the expression and functionality of the P450 gene in λ-cyhalothrin-resistant Spodoptera litura (Fab.). Front. Physiol. 2022, 13, 900570. [Google Scholar] [CrossRef]
- Kranthi, K.R. Insecticide Resistance-Monitoring. Mechanisms and Management Manual; Central Institute for Cotton Research: Nagpur, India, 2005. [Google Scholar]
- Zibaee, A. Digestive enzymes of large cabbage white butterfly, Pieris brassicae L. (Lepidoptera: Pieridae) from developmental and site of activity perspectives. Ital. J. Zool. 2012, 79, 13–26. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Further evidence that enzymes involved in the final stages of digestion by Rhynchosciara do not enter the endoperitrophic space. Insect Biochem. 1983, 13, 143–150. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Chung, P.G.; Murugan, K. Effect of botanical insecticides and bacterial toxins on the gut enzyme of the rice leaffolder Cnaphalocrocis medinalis. Phytoparasitica 2004, 32, 433–443. [Google Scholar] [CrossRef]
- Dutta, T.K.; Santhoshkumar, K.; Mathur, C.; Mandal, A.; Sagar, D. A Photorhabdus akhurstii toxin altered gut homeostasis prior conferring cytotoxicity in Spodoptera frugiperda, S. litura and Helicoverpa armigera. Phytoparasitica 2021, 49, 943–958. [Google Scholar] [CrossRef]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological activity of chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci. Rep. 2021, 11, 20488. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, A.M.E.; Nemat, A.M.; Magdy, A. Potency of Bacillus thuringiensis and Bacillus subtilis against the cotton leafworm, Spodoptera littoralis (Bosid.) larvae. Arch. Phytopathol. Plant Prot. 2011, 44, 204–215. [Google Scholar] [CrossRef]
- Rizwan, M.; Atta, B.; Sabir, A.M.; Yaqub, M.; Qadir, A. Evaluation of the entomopathogenic fungi as a non-traditional control of the rice leaf roller, Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) under controlled conditions. Egypt. J. Biol. Pest Control 2019, 29, 10. [Google Scholar] [CrossRef]
- Maciel-Vergara, G.; Jensen, A.B.; Eilenberg, J. Cannibalism as a possible entry route for opportunistic pathogenic bacteria to insect hosts, exemplified by Pseudomonas aeruginosa, a pathogen of the giant mealworm Zophobas morio. Insects 2018, 9, 88. [Google Scholar] [CrossRef]
- Eski, A.; Demir, I.; Güllü, M.; Demirbağ, Z. Biodiversity and pathogenicity of bacteria associated with the gut microbiota of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Microb. Pathog. 2018, 121, 350–358. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Jackson, T.A. Bacterial entomopathogens. In Insect Pathology; Academic Press: Cambridge, MA, USA, 2012; pp. 265–349. [Google Scholar]
- Charles, J.F.; Silva-Filha, M.H.; Nielsen-LeRoux, C. Mode of action of Bacillus sphaericus on mosquito larvae: Incidence on resistance. In Entomopathogenic Bacteria: From Laboratory to Field Application; Springer: Dordrecht, The Netherlands, 2000; pp. 237–252. [Google Scholar]
- Chen, W.J.; Hsieh, F.C.; Hsu, F.C.; Tasy, Y.F.; Liu, J.R.; Shih, M.C. Characterization of an insecticidal toxin and pathogenicity of Pseudomonas taiwanensis against insects. PLoS Pathol. 2014, 10, e1004288. [Google Scholar] [CrossRef]
- Dieppois, G.; Opota, O.; Lalucat, J.; Lemaitre, B. Pseudomonas entomophila: A versatile bacterium with entomopathogenic properties. In Pseudomonas: Volume 7: New Aspects of Pseudomonas Biology; Springer: Dordrecht, The Netherlands, 2014; pp. 25–49. [Google Scholar]
- Devi, S.; Saini, H.S.; Kaur, S. Insecticidal and growth inhibitory activity of gut microbes isolated from adults of Spodoptera litura (Fab.). BMC Microbiol. 2022, 22, 71. [Google Scholar] [CrossRef]
- Andrejko, M.; Zdybicka-Barabas, A.; Cytryńska, M. Diverse effects of Galleria mellonella infection with entomopathogenic and clinical strains of Pseudomonas aeruginosa. J. Invertebr. Pathol. 2014, 115, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ruffner, B.; Péchy-Tarr, M.; Ryffel, F.; Hoegger, P.; Obrist, C.; Rindlisbacher, A.; Keel, C.; Maurhofer, M. Oral insecticidal activity of plant-associated Pseudomonads. Environ. Microb. 2013, 15, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Okuda, J.; Hayashi, N.; Okamoto, M.; Sawada, S.; Minagawa, S.; Yano, Y.; Gotoh, N. Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na, K-ATPase regulator, FXYD3. Infect. Immun. 2010, 78, 4511–4522. [Google Scholar] [CrossRef]
- Chieda, Y.; Iiyama, K.; Lee, J.M.; Kusakabe, T.; Yasunaga-Aoki, C.; Shimizu, S. Virulence of an exotoxin A-deficient strain of Pseudomonas aeruginosa toward the silkworm, Bombyx mori. Microb. Pathol. 2011, 51, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Loper, J.E.; Henkels, M.D.; Rangel, L.I.; Olcott, M.H.; Walker, F.L.; Bond, K.L.; Kidarsa, T.A.; Hesse, C.N.; Sneh, B.; Stockwell, V.O.; et al. Rhizoxin analogs, orfamide A and chitinase production contribute to the toxicity of Pseudomonas protegens strain Pf-5 to Drosophila melanogaster. Environ. Microb. 2016, 18, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Flury, P.; Vesga, P.; Dominguez-Ferreras, A.; Tinguely, C.; Ullrich, C.I.; Kleespies, R.G.; Keel, C.; Maurhofer, M. Persistence of root-colonizing Pseudomonas protegens in herbivorous insects throughout different developmental stages and dispersal to new host plants. ISME J. 2019, 13, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Fite, T.; Tefera, T.; Negeri, M.; Damte, T.; Sori, W. Evaluation of Beauveria bassiana, Metarhizium anisopliae, and Bacillus thuringiensis for the management of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) under laboratory and field conditions. Biocontrol Sci. Technol. 2020, 30, 278–295. [Google Scholar] [CrossRef]
- Mohan, M.; Sushil, S.N.; Bhatt, J.C.; Gujar, G.T.; Gupta, H.S. Synergistic interaction between sublethal doses of Bacillus thuringiensis and Campoletis chlorideae in managing Helicoverpa armigera. BioControl 2008, 53, 375–386. [Google Scholar] [CrossRef]
- Olcott, M.H.; Henkels, M.D.; Rosen, K.L.L.; Walker, F.; Sneh, B.; Loper, J.E.; Taylor, B.J. Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PLoS ONE 2010, 5, e12504. [Google Scholar] [CrossRef]
- Aggarwal, C.; Paul, S.; Tripathi, V.; Paul, B.; Khan, M.A. Chitinolytic activity in Serratia marcescens (strain SEN) and potency against different larval stages of Spodoptera litura with effect of sublethal doses on insect development. BioControl 2015, 60, 631–640. [Google Scholar] [CrossRef]
- Karthi, S.; Shivakumar, M.S. Time-of-day specific changes in pesticide detoxification ability of Spodoptera litura (Lepidoptera: Noctuidae). Biol. Rhythm Res. 2016, 47, 303–314. [Google Scholar] [CrossRef]
- Kilani-Morakchi, S.; Bezzar-Bendjazia, R.; Ferdenache, M.; Aribi, N. Preimaginal exposure to azadirachtin affects food selection and digestive enzymes in adults of Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 140, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S.; Chung, P.G.; Murugan, K. Combined effect of biopesticides on the digestive enzymatic profiles of Cnaphalocrocis medinalis (Guenée)(the rice leaffolder)(Insecta: Lepidoptera: Pyralidae). Ecotoxicol. Environ. Saf. 2006, 64, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.N.B.; DaMatta, R.A.; Samuels, R.I.; Silva, C.P. Effects of entomopathogenic bacterium Photorhabdus temperata infection on the digestive enzymes of Diatraea saccharalis (Lepidoptera: Crambidae) larvae. Protein Pept. Lett. 2008, 15, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Zibaee, I.; Bandani, A.R.; Sendi, J.J.; Talaei-Hassanloei, R.; Kouchaki, B. Effects of Bacillus thuringiensis var. kurstaki and medicinal plants on Hyphantria cunea Drury (Lepidoptera: Arctiidae). Invertebr. Surv. J. 2010, 7, 251–261. [Google Scholar]
- Pandey, S.; Joshi, B.D.; Tiwari, L.D. Histopathological changes in the midgut of Spodoptera litura larvae on ingestion of Bacillus thuringiensis delta endotoxin. Arch. Phytopathol. Plant Prot. 2009, 42, 376–383. [Google Scholar] [CrossRef]
- Song, F.; Lin, Y.; Chen, C.; Shao, E.; Guan, X.; Huang, Z. Insecticidal activity and histopathological effects of Vip3Aa protein from Bacillus thuringiensis on Spodoptera litura. J. Microbiol. Biotechnol. 2016, 26, 1774–1780. [Google Scholar] [CrossRef]
- Castro, B.M.D.C.E.; Martinez, L.C.; Barbosa, S.G.; Serrão, J.E.; Wilcken, C.F.; Soares, M.A.; da Silva, A.A.; de Carvalho, A.G.; Zanuncio, J.C. Toxicity and cytopathology mediated by Bacillus thuringiensis in the midgut of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 6667. [Google Scholar] [CrossRef]
- Rekha, K.; Baskar, B.; Srinath, S.; Usha, B. Plant-growth-promoting rhizobacteria Bacillus subtilis RR4 isolated from rice rhizosphere induces malic acid biosynthesis in rice roots. Can. J. Microbiol. 2018, 64, 20–27. [Google Scholar] [CrossRef]
- Javed, K.; Humayun, T.; Humayun, A.; Shaheen, S.; Wang, Y.; Javed, H. Biocontrol Potential of PeBL2, a Novel Entomopathogenic Bacterium from Brevibacillus laterosporus A60, Induces Systemic Resistance against Rice Leaf Folder Cnaphalocrocis exigua (Butler) in Rice (Oryza sativa L.). Plants 2022, 11, 3350. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.R.; Jung, B.K.; Khan, A.L.; Lee, I.J.; Shin, J.H. Gibberellins synthesized by the entomopathogenic bacterium, Photorhabdus temperata M1021 as one of the factors of rice plant growth promotion. J. Plant Interact. 2014, 9, 775–782. [Google Scholar] [CrossRef]
- Niu, H.; Sun, Y.; Zhang, Z.; Zhao, D.; Wang, N.; Wang, L.; Guo, H. The endophytic bacterial entomopathogen Serratia marcescens promotes plant growth and improves resistance against Nilaparvata lugens in rice. Microbiol. Res. 2022, 256, 126956. [Google Scholar] [CrossRef] [PubMed]
- Sutio, G.; Afifah, A.N.; Maharani, R.; Basri, M. Serratia marcescens strain NPKC3 2 21 as endophytic phosphate solubilizing bacteria and entomopathogen: Promising combination approach as rice biofertilizer and biopesticide. Biodiversitas J. Biol. Divers. 2023, 24, 901–909. [Google Scholar]

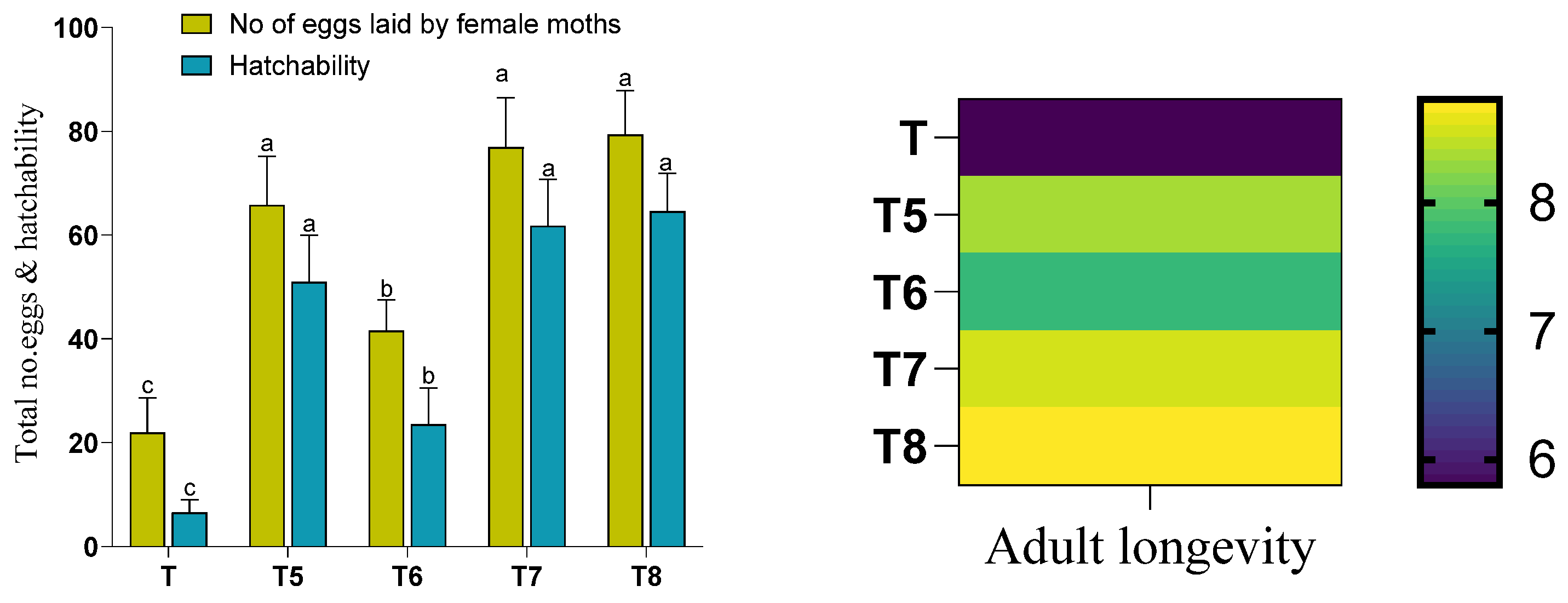
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janaki, M.; Sivadasan Unni, P.K.; Stanley-Raja, V.; Senthil-Nathan, S.; Almutairi, B.O.; Abdel-Megeed, A. Biocontrol Effect of Bacillus subtilis against Cnaphalocrocis medinalis (Guenèe) (Lepidoptera: Pyralidae): A Sustainable Approach to Rice Pest Management. Agronomy 2024, 14, 310. https://doi.org/10.3390/agronomy14020310
Janaki M, Sivadasan Unni PK, Stanley-Raja V, Senthil-Nathan S, Almutairi BO, Abdel-Megeed A. Biocontrol Effect of Bacillus subtilis against Cnaphalocrocis medinalis (Guenèe) (Lepidoptera: Pyralidae): A Sustainable Approach to Rice Pest Management. Agronomy. 2024; 14(2):310. https://doi.org/10.3390/agronomy14020310
Chicago/Turabian StyleJanaki, Muthusamy, Pavana K. Sivadasan Unni, Vethamonickam Stanley-Raja, Sengottayan Senthil-Nathan, Bader O. Almutairi, and Ahmed Abdel-Megeed. 2024. "Biocontrol Effect of Bacillus subtilis against Cnaphalocrocis medinalis (Guenèe) (Lepidoptera: Pyralidae): A Sustainable Approach to Rice Pest Management" Agronomy 14, no. 2: 310. https://doi.org/10.3390/agronomy14020310
APA StyleJanaki, M., Sivadasan Unni, P. K., Stanley-Raja, V., Senthil-Nathan, S., Almutairi, B. O., & Abdel-Megeed, A. (2024). Biocontrol Effect of Bacillus subtilis against Cnaphalocrocis medinalis (Guenèe) (Lepidoptera: Pyralidae): A Sustainable Approach to Rice Pest Management. Agronomy, 14(2), 310. https://doi.org/10.3390/agronomy14020310






