Microbial Nutrient Limitation of Different Tea Cultivars: Evidence from Five Representative Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Plant Sampling
2.4. Assay of Soil Properties
2.5. Assay of Soil Ecoenzymatic Activity
2.6. Quantification of Microbial Nutrient Limitation
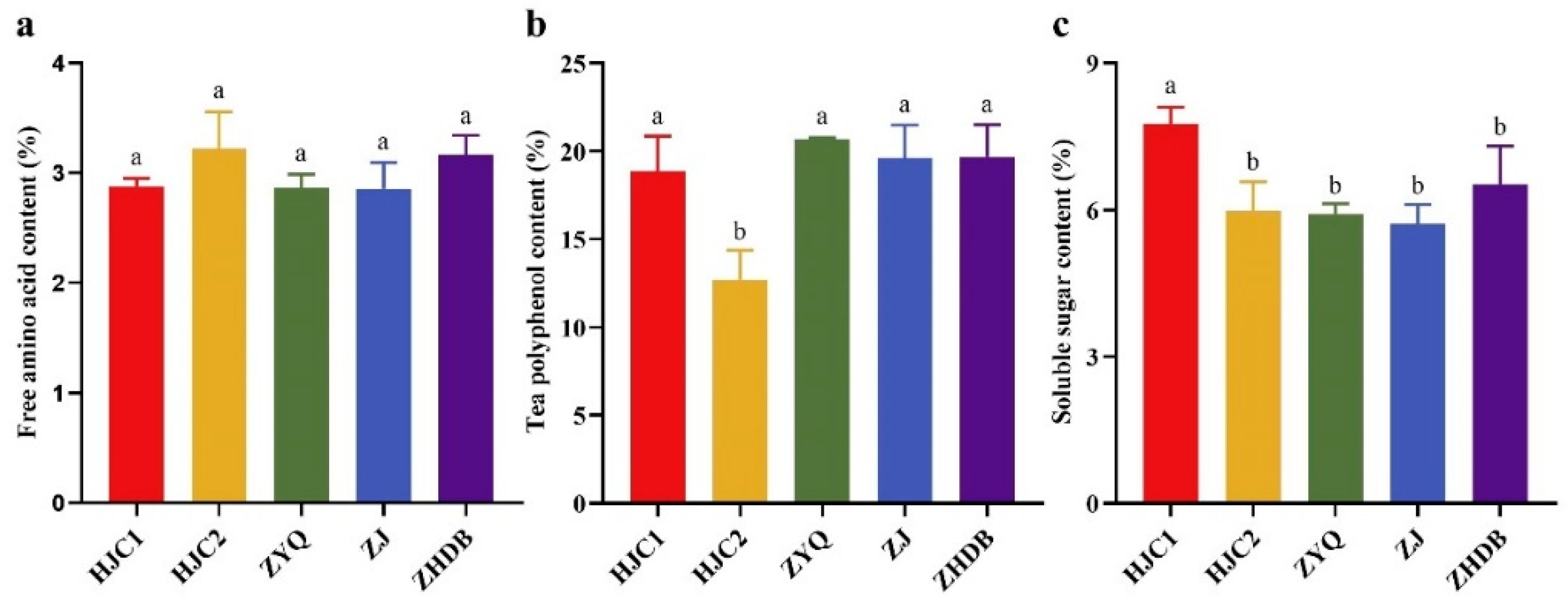
2.7. Determination of Tea Quality
2.8. Statistical Analysis
3. Results
3.1. Soil Properties and Microbial Biomass
3.2. Soil Ecoenzymatic Activity
3.3. Soil Microbial Nutrient Limitation
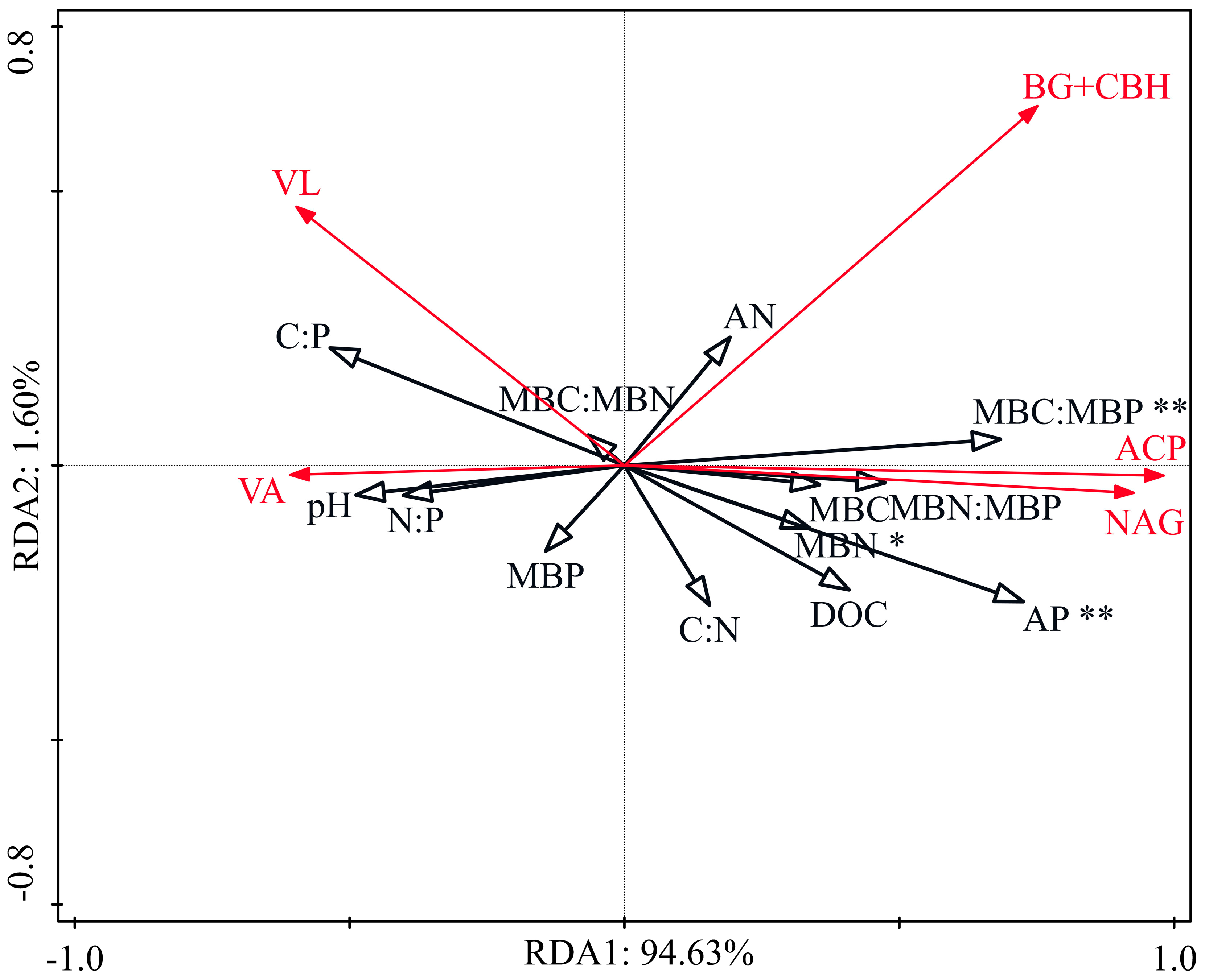
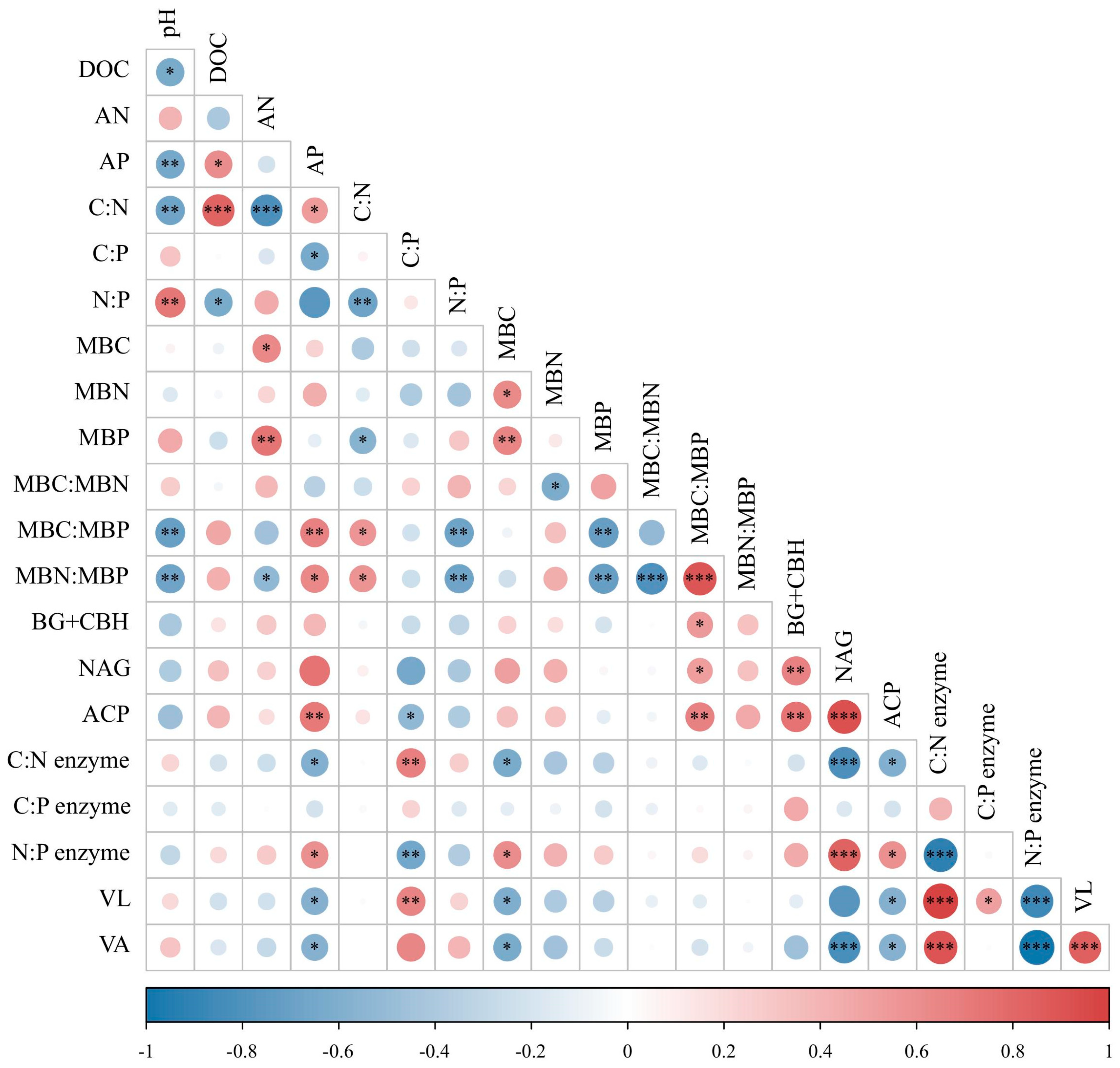
3.4. Relationships among Soil Properties, Ecoenzymatic Activities, and Microbial Nutrient Limitation
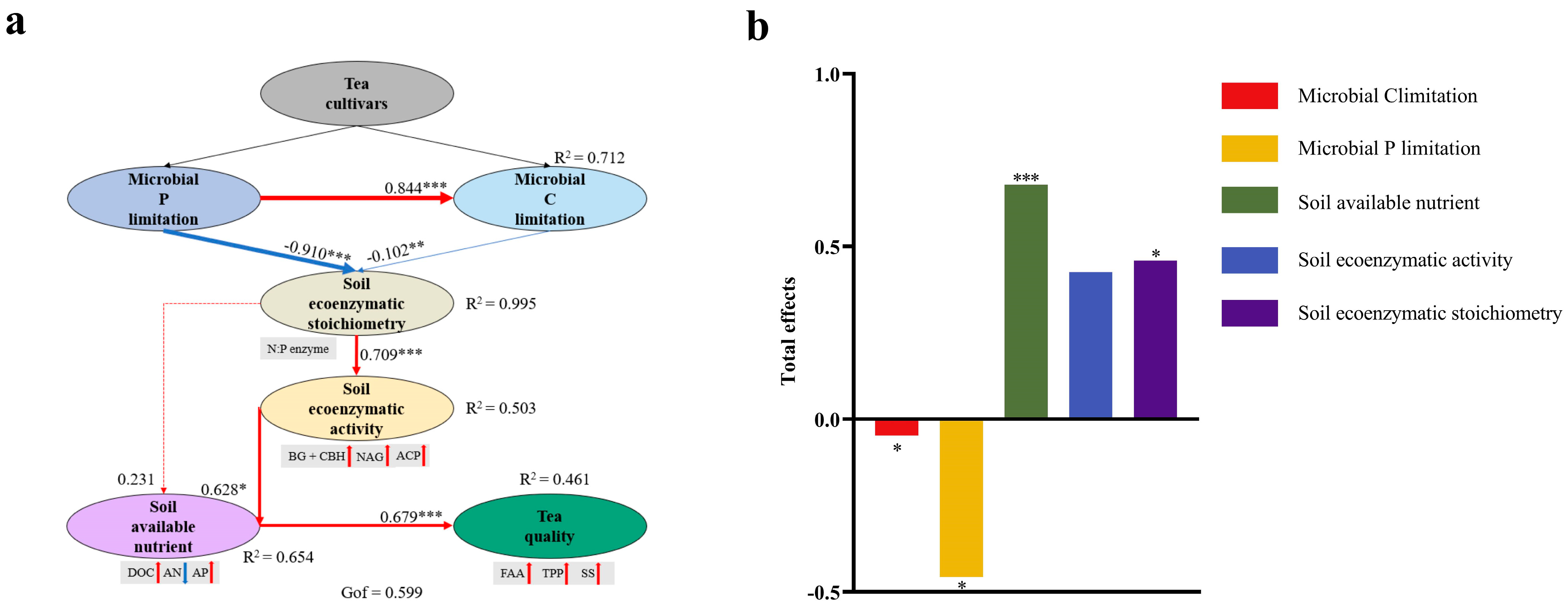
3.5. Relationship between Soil Microbial Nutrient Limitation and Tea Quality
4. Discussion
4.1. Differences in Soil Ecoenzymatic Activity in Different Tea Cultivars
4.2. Microbial Nutrient Limitation in Different Tea Cultivars
4.3. Effects of Soil Microbial Nutrient Limitation on Tea Quality in Different Tea Cultivars
4.4. Limitation of Current Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, M.V. Soil–Plant Nutrient Balance of Tea Crops in the Northern Mountainous Region, Vietnam. Agric. Ecosyst. Environ. 2005, 105, 413–418. [Google Scholar] [CrossRef]
- Arafat, Y.; Tayyab, M.; Khan, M.U.; Chen, T.; Amjad, H.; Awais, S.; Lin, X.; Lin, W.; Lin, S. Long-Term Monoculture Negatively Regulates Fungal Community Composition and Abundance of Tea Orchards. Agronomy 2019, 9, 466. [Google Scholar] [CrossRef]
- Gu, S.; Hu, Q.; Cheng, Y.; Bai, L.; Liu, Z.; Xiao, W.; Gong, Z.; Wu, Y.; Feng, K.; Deng, Y.; et al. Application of Organic Fertilizer Improves Microbial Community Diversity and Alters Microbial Network Structure in Tea (Camellia sinensis) Plantation Soils. Soil Tillage Res. 2019, 195, 104356. [Google Scholar] [CrossRef]
- Rothenberg, D.O.; Abbas, F.; Mei, X.; Yan, C.; Zeng, Z.; Mo, X.; Chen, S.; Zhang, L.; Huang, Y. Metabarcoding of Organic Tea (Camellia sinensis L.) Chronosequence Plots Elucidates Soil Acidification-Induced Shifts in Microbial Community Structure and Putative Function. Appl. Soil Ecol. 2022, 178, 104580. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, X.; Su, X.; Yang, Z. Chinese Oolong Tea: An Aromatic Beverage Produced under Multiple Stresses. Trends Food Sci. Technol. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.; Jin, S.; Chen, S.; Yue, C.; Wang, W.; Gao, S.; Cao, H.; Zheng, Y.; Gu, M.; et al. Genetic Basis of High Aroma and Stress Tolerance in the Oolong Tea Cultivar Genome. Hortic. Res. 2021, 8, 107. [Google Scholar] [CrossRef]
- Huang, X.; Tang, Q.; Li, Q.; Lin, H.; Li, J.; Zhu, M.; Liu, Z.; Wang, K. Integrative Analysis of Transcriptome and Metabolome Reveals the Mechanism of Foliar Application of Bacillus Amyloliquefaciens to Improve Summer Tea Quality (Camellia sinensis). Plant Physiol. Biochem. 2022, 185, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale: Stoichiometry of Soil Enzyme Activity. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Bárta, J.; Šlajsová, P.; Tahovská, K.; Picek, T.; Šantrůčková, H. Different Temperature Sensitivity and Kinetics of Soil Enzymes Indicate Seasonal Shifts in C, N and P Nutrient Stoichiometry in Acid Forest Soil. Biogeochemistry 2014, 117, 525–537. [Google Scholar] [CrossRef]
- Veres, Z.; Kotroczó, Z.; Fekete, I.; Tóth, J.A.; Lajtha, K.; Townsend, K.; Tóthmérész, B. Soil Extracellular Enzyme Activities Are Sensitive Indicators of Detrital Inputs and Carbon Availability. Appl. Soil Ecol. 2015, 92, 18–23. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic Stoichiometry of Microbial Nutrient Acquisition in Tropical Soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Schimel, J. The Implications of Exoenzyme Activity on Microbial Carbon and Nitrogen Limitation in Soil: A Theoretical Model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic Enzyme Activities in Agricultural and Forest Soils. Some Implications for Their Use as Indicators of Soil Quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Benedetti, A.; Marinari, S.; Pompili, L.; Moscatelli, M.C.; Roggero, P.P.; Lai, R.; Ledda, L.; Grego, S. Soil Organic C Variability and Microbial Functions in a Mediterranean Agro-Forest Ecosystem. Biol. Fertil. Soils 2011, 47, 283–291. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector Analysis of Ecoenzyme Activities Reveal Constraints on Coupled C., N and P Dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Z.; Tan, W. Plant Litter Quality Regulates Soil Eco-Enzymatic Stoichiometry and Microbial Nutrient Limitation in a Citrus Orchard. Plant Soil 2021, 466, 179–191. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic Relationships between Microbial Biomass, Respiration, Inorganic Nutrients and Enzyme Activities: Informing Enzyme-Based Decomposition Models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Torres, Y.; Elser, J.J.; Souza, V.; García-Oliva, F. Ecoenzymatic Stoichiometry at the Extremes: How Microbes Cope in an Ultra-Oligotrophic Desert Soil. Soil Biol. Biochem. 2015, 87, 34–42. [Google Scholar] [CrossRef]
- Fanin, N.; Moorhead, D.; Bertrand, I. Eco-Enzymatic Stoichiometry and Enzymatic Vectors Reveal Differential C, N, P Dynamics in Decaying Litter along a Land-Use Gradient. Biogeochemistry 2016, 129, 21–36. [Google Scholar] [CrossRef]
- Jones, D.L.; Kielland, K.; Sinclair, F.L.; Dahlgren, R.A.; Newsham, K.K.; Farrar, J.F.; Murphy, D.V. Soil Organic Nitrogen Mineralization across a Global Latitudinal Gradient. Glob. Biogeochem. Cycles 2009, 23, 2008GB003250. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Kolka, R.K.; Lehto, L.L.P.; Sebestyen, S.D.; Seifert-Monson, L.R. Ecoenzymatic Stoichiometry and Microbial Processing of Organic Matter in Northern Bogs and Fens Reveals a Common P-Limitation between Peatland Types. Biogeochemistry 2014, 120, 203–224. [Google Scholar] [CrossRef]
- Auwal, M.; Sun, H.; Adamu, U.K.; Meng, J.; Van Zwieten, L.; Pal Singh, B.; Luo, Y.; Xu, J. The Phosphorus Limitation in the Post-Fire Forest Soils Increases Soil CO2 Emission via Declining Cellular Carbon Use Efficiency and Increasing Extracellular Phosphatase. Catena 2023, 224, 106968. [Google Scholar] [CrossRef]
- Abay, P.; Gong, L.; Luo, Y.; Zhu, H.; Ding, Z. Soil Extracellular Enzyme Stoichiometry Reveals the Nutrient Limitations in Soil Microbial Metabolism under Different Carbon Input Manipulations. Sci. Total Environ. 2024, 913, 169793. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.B.; Hoosbeek, M.R.; Taylor, C.R.; Miglietta, F.; Phoenix, G.K.; Hartley, I.P. Soil C, N and P Cycling Enzyme Responses to Nutrient Limitation under Elevated CO2. Biogeochemistry 2020, 151, 221–235. [Google Scholar] [CrossRef]
- Feyissa, A.; Gurmesa, G.A.; Yang, F.; Long, C.; Zhang, Q.; Cheng, X. Soil Enzyme Activity and Stoichiometry in Secondary Grasslands along a Climatic Gradient of Subtropical China. Sci. Total Environ. 2022, 825, 154019. [Google Scholar] [CrossRef]
- Kanté, M.; Riah-Anglet, W.; Cliquet, J.-B.; Trinsoutrot-Gattin, I. Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition. Agronomy 2021, 11, 2131. [Google Scholar] [CrossRef]
- Yi, J.; Zeng, Q.; Mei, T.; Zhang, S.; Li, Q.; Wang, M.; Tan, W. Disentangling Drivers of Soil Microbial Nutrient Limitation in Intensive Agricultural and Natural Ecosystems. Sci. Total Environ. 2022, 806, 150555. [Google Scholar] [CrossRef]
- Guan, H.L.; Fan, J.W.; Lu, X. Soil Specific Enzyme Stoichiometry Reflects Nitrogen Limitation of Microorganisms under Different Types of Vegetation Restoration in the Karst Areas. Appl. Soil Ecol. 2022, 169, 104253. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Kou, Y.; Liu, D.; Liu, Q.; Zhang, Z.; Jiang, Z.; Yin, H. Differential Effects of N Addition on the Stoichiometry of Microbes and Extracellular Enzymes in the Rhizosphere and Bulk Soils of an Alpine Shrubland. Plant Soil 2020, 449, 285–301. [Google Scholar] [CrossRef]
- Pokharel, P.; Chang, S.X. Biochar Decreases and Nitrification Inhibitor Increases Phosphorus Limitation for Microbial Growth in a Wheat-Canola Rotation. Sci. Total Environ. 2023, 858, 159773. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, S.; Li, T.; Han, L. Response of Soil Faunal Communities to Tea Tree Cultivars in the Hilly Region of Western Sichuan, China. Sci. Hortic. 2021, 275, 109701. [Google Scholar] [CrossRef]
- Du, L.; Zheng, Z.; Li, T.; Wang, Y.; Huang, H.; Yu, H.; Ye, D.; Liu, T.; Yao, T.; Zhang, X. Variations of Fungal Communities within the Soils of Different Tea Varieties (Camellia sinensis L.) Following Long-Term Plantation. Plant Soil 2022, 477, 665–677. [Google Scholar] [CrossRef]
- Zhu, D.; Hui, D.; Wang, M.; Yang, Q.; Yu, S. Light and Competition Alter Leaf Stoichiometry of Introduced Species and Native Mangrove Species. Sci. Total Environ. 2020, 738, 140301. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wu, J.; Brookes, P.C. Measuring Soil Microbial Biomass Using an Automated Procedure. Soil Biol. Biochem. 2011, 43, 873–876. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Seifert, L.R.; May, A.A.; Tarquinio, E. Microbial Enzyme Stoichiometry and Nutrient Limitation in US Streams and Rivers. Ecol. Indic. 2012, 18, 540–551. [Google Scholar] [CrossRef]
- Cui, L.; Yao, S.; Dai, X.; Yin, Q.; Liu, Y.; Jiang, X.; Wu, Y.; Qian, Y.; Pang, Y.; Gao, L.; et al. Identification of UDP-Glycosyltransferases Involved in the Biosynthesis of Astringent Taste Compounds in Tea (Camellia sinensis ). J. Exp. Bot. 2016, 67, 2285–2297. [Google Scholar] [CrossRef]
- Ho, C.-T.; Zheng, X.; Li, S. Tea Aroma Formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- GB/T 8314-2013; Tea–Determination of Free Amino Acids Content. China Standards Press: Beijing, China, 2013; pp. 1–8.
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea. China Standards Press: Beijing, China, 2018; pp. 1–8.
- Wang, H.; Ouyang, W.; Yu, Y.; Wang, J.; Yuan, H.; Hua, J.; Jiang, Y. Analysis of Non-Volatile and Volatile Metabolites Reveals the Influence of Second-Drying Heat Transfer Methods on Green Tea Quality. Food Chem. X 2022, 14, 100354. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Turner, B.L.; Talbot, J.M.; Waring, B.G.; Powers, J.S.; Kuske, C.R.; Moorhead, D.L.; Follstad Shah, J.J. Stoichiometry of Microbial Carbon Use Efficiency in Soils. Ecol. Monogr. 2016, 86, 172–189. [Google Scholar] [CrossRef]
- Wallenius, K.; Rita, H.; Mikkonen, A.; Lappi, K.; Lindström, K.; Hartikainen, H.; Raateland, A.; Niemi, R.M. Effects of Land Use on the Level, Variation and Spatial Structure of Soil Enzyme Activities and Bacterial Communities. Soil Biol. Biochem. 2011, 43, 1464–1473. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clément, J.-C.; Lavorel, S. Linkages between Extracellular Enzyme Activities and the Carbon and Nitrogen Content of Grassland Soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef]
- Atala, C.; Acuña-Rodríguez, I.S.; Torres-Díaz, C.; Molina-Montenegro, M.A. Fungal Endophytes Improve the Performance of Host Plants but Do Not Eliminate the Growth/Defence Trade-off. New Phytol. 2022, 235, 384–387. [Google Scholar] [CrossRef]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of Long-Term Nitrogen Addition on Microbial Enzyme Activity in Eight Forested and Grassland Sites: Implications for Litter and Soil Organic Matter Decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Yan, P.; Shen, C.; Zou, Z.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Dong, C.; Fu, J.; Han, W.; et al. Increased Soil Fertility in Tea Gardens Leads to Declines in Fungal Diversity and Complexity in Subsoils. Agronomy 2022, 12, 1751. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Wang, Y.; Sun, Y.; Xia, X.; Su, Y.; Liao, W. Combined Application of Biochar and Pruned Tea Plant Litter Benefits Nitrogen Availability for Tea and Alters Microbial Community Structure. Agronomy 2023, 13, 1465. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-Carbon Response to Warming Dependent on Microbial Physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Sihi, D.; Gerber, S.; Inglett, P.W.; Inglett, K.S. Comparing Models of Microbial–Substrate Interactions and Their Response to Warming. Biogeosciences 2016, 13, 1733–1752. [Google Scholar] [CrossRef]
- Yu, J.; Bing, H.; Chang, R.; Cui, Y.; Shen, G.; Wang, X.; Zhang, S.; Fang, L. Microbial Metabolic Limitation Response to Experimental Warming along an Altitudinal Gradient in Alpine Grasslands, Eastern Tibetan Plateau. Catena 2022, 214, 106243. [Google Scholar] [CrossRef]
- Chen, H.; Luo, P.; Wen, L.; Yang, L.; Wang, K.; Li, D. Determinants of Soil Extracellular Enzyme Activity in a Karst Region, Southwest China. Eur. J. Soil Biol. 2017, 80, 69–76. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Han, F.; Ju, W.; Ye, L.; Wang, X.; Tan, W.; Zhang, X. Natural Grassland as the Optimal Pattern of Vegetation Restoration in Arid and Semi-Arid Regions: Evidence from Nutrient Limitation of Soil Microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Gao, D.; Wang, X.; Liu, W.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Ecoenzymatic Stoichiometry and Nutrient Dynamics along a Revegetation Chronosequence in the Soils of Abandoned Land and Robinia Pseudoacacia Plantation on the Loess Plateau, China. Soil Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Wei, S.; Liu, B.; Ni, K.; Ma, L.; Shi, Y.; Leng, Y.; Zheng, S.; Gao, S.; Yang, X.; Ruan, J. Rhizosphere Microbial Community Shows a Greater Response Than Soil Properties to Tea (Camellia sinensis L.) Cultivars. Agronomy 2023, 13, 221. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Wang, Y.; Duan, C.; Niu, Y.; Sun, R.; Shen, Y.; Guo, X.; Fang, L. Ecoenzymatic Stoichiometry Reveals Phosphorus Addition Alleviates Microbial Nutrient Limitation and Promotes Soil Carbon Sequestration in Agricultural Ecosystems. J Soils Sediments 2022, 22, 536–546. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Zhang, W.; Xiao, K.; Wang, K. Nitrogen Addition Aggravates Microbial Carbon Limitation: Evidence from Ecoenzymatic Stoichiometry. Geoderma 2018, 329, 61–64. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.; Cheng, H.; An, S.; Chang, S.X. Soil Extracellular Enzyme Stoichiometry Reflects the Shift from P- to N-Limitation of Microorganisms with Grassland Restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
- Arai, Y.; Sparks, D.L. Phosphate Reaction Dynamics in Soils and Soil Components: A Multiscale Approach. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2007; Volume 94, pp. 135–179. [Google Scholar] [CrossRef]
- Sun, D.; Yang, X.; Wang, C.; Hao, X.; Hong, J.; Lin, X. Dynamics of Available and Enzymatically Hydrolysable Soil Phosphorus Fractions during Repeated Freeze-Thaw Cycles. Geoderma 2019, 345, 1–4. [Google Scholar] [CrossRef]
- Sistla, S.A.; Appling, A.P.; Lewandowska, A.M.; Taylor, B.N.; Wolf, A.A. Stoichiometric Flexibility in Response to Fertilization along Gradients of Environmental and Organismal Nutrient Richness: Woodstoich III. Oikos 2015, 124, 949–959. [Google Scholar] [CrossRef]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; et al. Growth Rate–Stoichiometry Couplings in Diverse Biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P Stoichiometry in Soil: Is There a “Redfield Ratio” for the Microbial Biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.; Lu, X.; Yan, J.; Biswas, A.; Zou, W. Long-term Continuous Cropping Affects Ecoenzymatic Stoichiometry of Microbial Nutrient Acquisition: A Case Study from a Chinese Mollisol. J. Sci. Food Agric. 2021, 101, 6338–6346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, J.; Manuel, D.-B.; Op De Beeck, M.; Shahbaz, M.; Chen, Y.; Deng, X.; Xu, Z.; Li, J.; Liu, Z. Rotation Cropping and Organic Fertilizer Jointly Promote Soil Health and Crop Production. J. Environ. Manag. 2022, 315, 115190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Luo, G. Regulation of Soil C–N–P Stoichiometry by Intercropping Mitigates Microbial Resource Limitations and Contributes to Maize Productivity. Plant Soil 2023. [Google Scholar] [CrossRef]


| HJC1 | HJC2 | ZYQ | ZJ | ZHDB | |
|---|---|---|---|---|---|
| pH | 5.99 ± 0.33 ab | 6.12 ± 0.06 a | 6.40 ± 0.06 a | 5.45 ± 0.23 b | 4.92 ± 0.17 b |
| DOC (mg kg−1) | 56.54 ± 4.49 ab | 39.55 ± 4.83 b | 46.21 ± 2.73 b | 53.07 ± 7.60 ab | 66.38 ± 4.36 a |
| AN (mg kg−1) | 8.70 ± 0.70 a | 8.53 ± 0.42 a | 5.29 ± 0.47 b | 5.61 ± 0.45 b | 5.58 ± 0.35 b |
| AP (mg kg−1) | 45.66 ± 10.56 b | 43.02 ± 6.75 b | 27.32 ± 0.51 b | 43.85 ± 3.90 b | 75.90 ± 8.28 a |
| C:N | 6.62 ± 0.87 bc | 4.63 ± 0.45 c | 8.86 ± 0.92 b | 8.76 ± 1.19 b | 12.03 ± 1.34 a |
| C:P | 1.36 ± 0.28 ab | 0.71 ± 0.03 c | 1.69 ± 0.11 a | 1.21 ± 0.12 b | 0.89 ± 0.10 bc |
| N:P | 0.15 ± 0.00 b | 0.22 ± 0.04 a | 0.20 ± 0.02 ab | 0.13 ± 0.02 b | 0.08 ± 0.02 b |
| MBC (mg kg−1) | 241.22 ± 13.36 a | 202.9 ± 28.79 ab | 147.54 ± 12.1 ab | 152.66 ± 22.06 b | 180.93 ± 19.7 b |
| MBN (mg kg−1) | 32.13 ± 3.70 a | 28.95 ± 4.92 a | 20.49 ± 2.55 a | 32.43 ± 5.85 a | 31.06 ± 4.53 a |
| MBP (mg kg−1) | 9.92 ± 1.21 a | 8.87 ± 1.58 a | 4.67 ± 0.63 b | 4.61 ± 0.78 b | 2.75 ± 0.22 b |
| MBC:MBN | 7.63 ± 0.56 a | 7.11 ± 0.41 a | 7.28 ± 0.31 a | 4.94 ± 0.84 b | 5.98 ± 0.67 ab |
| MBC:MBP | 25.35 ± 4.42 b | 23.35 ± 2.52 b | 28.09 ± 1.86 b | 34.61 ± 5.73 b | 65.72 ± 4.58 a |
| MBN:MBP | 3.36 ± 0.62 b | 3.34 ± 0.54 b | 3.79 ± 0.30 b | 8.65 ± 1.55 a | 11.43 ± 1.96 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Shen, C.; Gao, K.; Feng, S.; Li, D.; Hu, Q.; Liu, Y.; Luo, Z. Microbial Nutrient Limitation of Different Tea Cultivars: Evidence from Five Representative Cultivars. Agronomy 2024, 14, 467. https://doi.org/10.3390/agronomy14030467
Yuan S, Shen C, Gao K, Feng S, Li D, Hu Q, Liu Y, Luo Z. Microbial Nutrient Limitation of Different Tea Cultivars: Evidence from Five Representative Cultivars. Agronomy. 2024; 14(3):467. https://doi.org/10.3390/agronomy14030467
Chicago/Turabian StyleYuan, Shijie, Chengwen Shen, Kun Gao, Shuzhen Feng, Dejun Li, Qiulong Hu, Yu Liu, and Ze Luo. 2024. "Microbial Nutrient Limitation of Different Tea Cultivars: Evidence from Five Representative Cultivars" Agronomy 14, no. 3: 467. https://doi.org/10.3390/agronomy14030467
APA StyleYuan, S., Shen, C., Gao, K., Feng, S., Li, D., Hu, Q., Liu, Y., & Luo, Z. (2024). Microbial Nutrient Limitation of Different Tea Cultivars: Evidence from Five Representative Cultivars. Agronomy, 14(3), 467. https://doi.org/10.3390/agronomy14030467







