Abstract
Biochar and plant growth-promoting rhizobacteria (PGPR) are widely used as an amendment for soil physicochemical properties and soil bacterial community diversity. In Guangxi, China, we carried out a study to determine how PGPR and biochar influence the soil’s environmental stability in an Eucalypt plantation. We applied biochar and PGPR in a contrasting application manner to an acidic red loam soil. Thus, three treatments were set up as 5 × 1010 CFU·mL−1 PGPR-only (MB0), 20 t·hm−2 biochar-only (B20), and co-application of 20 t·hm−2 biochar and 5 × 1010 CFU·mL−1 PGPR (MB20), as well as no biochar and no PGPR (M0B0). Our results indicated that MB20 significantly decreased the soil total nitrogen (TN) and increased the soil total phosphorus (Soil TP), soil ammonium nitrogen (NH4+), and soil water content (SWC) when compared with the control. The MB20 also significantly increased the Simpson, ACE, and Chao indices of the soil bacterial community’s diversity relative to the control. We also observed a significant effect of the Soil TN on both the bacterial community and the functional diversity in soil. These findings may indicate that assessing the soil N status is expected to be an essential indicator of the soil microenvironment’s response to biochar and PGPR applications.
1. Introduction
Plant growth-promoting rhizobacteria (PGPR) are a diverse group of beneficial bacteria that inhabit either plant root systems or rhizosphere soil [1]. PGPR could improve plant growth by either directly supplying the necessary nutrients via phosphorus (P) solubilization, potassium (K) dissolution, and nitrogen (N) fixation in soils, or indirectly accelerating the secretion of phytohormones (e.g., growth hormones, cytokinins, and erythromycin) in plants [2,3,4]. In addition, PGPR has the potential to change soil bacterial compositions and further influence the soil nutrient status as a result of extrinsic microbe inputs. For example, the application of PGPR in soils changed the soil-dominant bacterial genera in a eucalyptus plantation and further influenced the uptake of soil inorganic N by plants [5]. However, the mechanism for how biochar and PGPR influence the soil nutrient status mediated by soil microbial diversity remains less studied.
Biochar has been identified as a high-temperature pyrolysis material with a high carbon content in oxygen-free or oxygen-limited conditions [6]. Biochar has been widely used as a soil amendment to achieve beneficial impacts on soils, including the improvement of soil physicochemical properties and microbial carbon utilization, and the diversity of soil microbial communities [7,8]. Recent studies have indicated that the application of biochar could directly increase soil carbon (C) sequestration, soil water content (SWC), and soil nutrient retention [9,10]. Understanding the responses of soil nutrients to biochar treatments remains crucial because of the significant role nutrients play in the soil microenvironment [11]. In addition, biochar provides a novel niche for soil microbes with increased soil aeration due to the large porosity and nutrient-rich surfaces [12,13]. Therefore, the application of biochar may improve microbial activity and further increase microbial diversity in soils [14,15].
Eucalyptus belongs to the family Myrtaceae and is one of the fastest-growing and most productive afforestation species in the world. However, the increased application of chemical fertilizer on eucalyptus plantations not only increases the sylvicultural cost but also leads to negative impacts on the soil’s microenvironmental stability [16,17,18]. A potential solution to this problem is the use of biofertilizers consisting of biochar and PGPR to maintain ecologically sound forestry.
The microbial community and functional diversities reflect the changes in the soil nutrient status after amendment, which are an important indicator of soil microenvironmental stability and play an indicative role in soil ecosystems. Soil microbes have also been reported to prefer the specific carbon source, and this pattern may reflect the soil functional diversity of soil microbes [19]. While we acknowledge that using biochar or PGPR alone has the potential to improve soil fertility and microbial diversity [20,21], studies on the effects of the co-application of biochar and PGPR on soils’ microenvironmental stability remain relatively limited. In this study, we sought to determine the microbial diversity and physicochemical property responses to PGPR and biochar applications. Specifically, we investigated the (1) soil physicochemical properties; (2) soil bacterial community diversity and microbial functional diversity; and (3) the relationship between the soil N and soil microenvironment. We hypothesized that evaluating the soil N dynamics would as serve as an important indicator of soil microenvironmental stability.
2. Materials and Methods
2.1. Research Site
We conducted our study at the Gaofeng Forest Center, Changke Branch, Guangxi, China (108°21′ E, 22°58′ N). The annual temperature of the research site was 21.6 °C, with an average annual precipitation of 800–1600 mm, and air humidity of 70–80%. The soil type was classified as acidic red loam and metabolic red loam. The basic physicochemical properties of the soil were a pH 4.5–5.5, soil organic matter content of 2–3%, and soil colloidal fraction silica-alumina rate between 1.5–1.8. The soil’s nutrient contents (especially for N, P, and K) were classified as extremely lacking based on the Chinese Grade of Soil Nutrient Content. The herbaceous layer was dominated by native Chinese herbs, including Solanum nigrum L. (black nightshade), and Stellaria media (L.) Vill. (chickweed) and Bidens pilosa L. (beggarticks).
2.2. PGPR and Biochar Characterizations
The biochar in the experiment was made from wheat straw, which was produced via pyrolysis at 550–600 °C with a continuous carbonization time of 3 h. The basic properties of the biochar used in our study indicated that the biochar was N-free (Table S1).
The PGPR (Strain DU07) used in our experiment was isolated from the eucalyptus rhizosphere soil by Dr. Baoling Huang in the laboratory of environmental microbiology, Forestry College, Guangxi University in 2011, and identified as Bacillus megaterium [5].
2.3. Experimental Design
The topsoil of our research site was plowed on 13 January 2019, and then divided into two 11 m × 10 m experimental units, with a 2 m buffer strip between each unit. Each experimental unit was further divided into 5 columns. Each column was divided into 2 m × 2 m plots with 1 m buffer strips between plots (Figure S1). Three plots were randomly selected within each column, for n = 3 replicates per treatment and the control. The Eucalyptus urophylla × E. grandis (clone DH32−29) were generously provided by the Dongmen Forestry Center, Guangxi, China, and planted in our treated plots with a planting space of 2 m × 2 m on 14 January 2019. We followed standard eucalypt planting practices which included measuring the height (25.21 ± 4.12 cm) and basal diameter (3.77 ± 0.83 mm) of the seedlings before planting.
The experimental and control groups were set up as follows: no biochar and no PGPR (M0B0, referred to as the control), PGPR-only (5 × 1010 cfu·mL−1, MB0), biochar-only (20 t·hm−2, B20), and the co-application of 20 t·hm−2 biochar and 5 × 1010 CFU·mL−1 PGPR (MB20).
The biochar was applied by firstly digging 40 pits of 30 cm × 30 cm × 30 cm for the control and treatments before planting the eucalyptus seedlings, and then mixing the corresponding concentration of biochar with the excavated soil and backfilling the pits.
The PGPR was applied into the soil by inoculating strain DU07 into a liquid Luria-Bertani (LB) medium at 28 °C for 3 d at 180 r·min−1 and was diluted to 5 × 1010 cfu·mL−1 with sterile water before application [5]. The inoculation process was carried out on a clean bench under strict sterile conditions. At the same time as the eucalyptus seedlings were planted into the soil, 2 mL of PGPR at the logarithmic phase of growth was applied to each seedling in the MB0 and MB20. Our experiment was started on 14 January 2019, with a nine-month experimental period.
2.4. Field Sampling and Lab Measurements
Nine months after the planting of the eucalyptus seedlings, three soil samples (n = 3) were collected from the 0–20 cm top layer. Part of the soil samples were then stored at 4 °C for soil microbial abundance and the utilization of microbial carbon sources analysis. The other portion of the soil was air-dried and sieved through a 100-mesh after grinding to determine the soil nutrients.
The nutrient content of the soil samples included the soil nitrate N (NO3−), soil ammonium N (NH4+), soil total N (Soil TN), soil total phosphorus (Soil TP), and total potassium (Soil TK). The NO3− and NH4+ were determined using 0.01 mol·L−1 of CaCl2 extracted on a fully automated flow analyzer (AA3, SEAL, Hamburg, Germanly) [22]. The Soil TN was determined using H2SO4 and accelerators (CuSO4 and tin powder) for decoction, followed by machine determination on a flow analyzer (AA3, SEAL, Hamburg, Germanly). The Soil TP was colorimetrically measured at 700 nm on a Biotek Synergy H1 microplate reader (Winooski, VT, USA). The Soil TK was measured on the flame photometer (Shuangxu, FP6430, Shanghai, China) following digestion with H2SO4 and HNO3 [5]. The Soil pH and electronic conductivity (EC) were determined by a conductivity meter (HI 8733, HANNA Instruments, Kehl am Rhein, Germany) and pH meter (PB-10, Sapeen, Shanghai, China), respectively.
The carbon-usage capacity and functional diversity indices of the soil microbes were measured with the Biolog EcoPlate method, which was carried out based on the methods described in our previous study [23]. The average well color development (AWCD, Formula (S1)), Simpson index of soil microbial functional diversity (SimpsonMF, Formula (S2)), Shannon index of soil microbial functional diversity (ShannonMF, Formula (S3)), and McIntosh (Formula (S4)) characterized the functional diversity of soil microorganisms and were used to assess the dominance, diversity, and evenness of species, respectively.
2.5. Illumina High-Throughput Sequencing
We extracted genomic DNA from each soil sample using the E.Z.N.A. Soil DNA kit (Omega Bio-Tek, Norcross, GA, USA). The DNA concentration was determined using the NanoDrop2000 micro spectrometer (Thermo Scientific, Wilmington, DE, USA). DNA integrity was assessed by electrophoresis with a 1% agarose gel. Primers were used to amplify the V3–V4 hypervariable regions of the bacterial 16S rRNA (Table S2). PCR amplification was conducted using a PCR instrument (GeneAmp 9700, Applied Biosystems, Foster City, CA, USA). Samples from each of the four treatment groups were amplified three times in duplicate, and the products from multiple amplifications were mixed well to eliminate bias from a single amplification. The PCR products were then recovered from the agarose gels (2%). DNA was further purified using the AxyPrep DNA Gel Extraction Kit (AxyPrep Biosciences, Union City, CA, USA) and quantified using QuantiFluorTM-ST (Promega Madison, WI, USA). The sequencing of the purified amplicon was referred to Ren et al. [5]. The Shannon index of soil bacterial community diversity (ShannonBC, Formula (S5)), Simpson index of soil bacterial community diversity (SimpsonBC, Formula (S6)), ACE (Formulas (S7)–(S11)), and Chao (Formula (S12)) of samples were assayed to reveal the community structure.
2.6. Statistical Analysis of Data
The effects of the biochar and PGPR on the soil physicochemical properties, microbial diversity index, carbon source utilization, and abundance of dominant bacterial populations were evaluated using a one-way analysis of variance (ANOVA) by SPSS 26.0. ORIGIN 2022 was used for the principal component analysis (PCA) including the soil microbial carbon source utilization, and CANOCO 5.0 to investigate the role of the soil–microbial correlation between the soil’s physical and chemical properties, microbial carbon use, and microbial diversity index. The Mantel test was performed using the mantel function in R 4.3.1 to assess the correlation between the bacterial community and the environmental dissimilarity matrices. It is important to note that we determined 0.05 < p < 0.1 as marginal significance.
3. Results
3.1. Soil Physicochemical Properties
There were no observed significant differences between treatments in the NO3−, Soil TK, pH, and EC. The Soil TN significantly (p < 0.01) decreased by 29.35%, 42.27%, and 31.11%, for the MB0, B20, and MB20 compared to the control, respectively. The NH4+ was marginally significantly (p < 0.1) increased by 5.81%, 4.27%, and 7.96%, respectively, for the MB0, B20, and MB20 compared to the control. A similar pattern occurred in the Soil TP, with increases of 33.02%, 67.96%, and 128.9% in the MB0, B20, and MB20 compared to the control. The MB0, B20, and MB20 significantly (p < 0.05) increased the soil’s SWC by 44.07%, 7.48%, and 38.88%, respectively, relative to the control (Figure 1a).
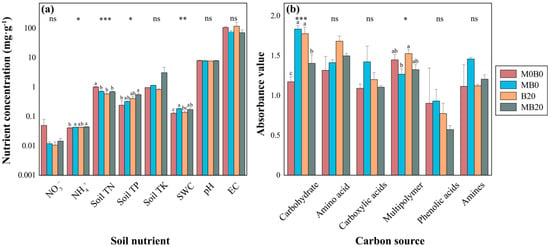
Figure 1.
Mean (±standard error) of soil nutrients and soil microbial carbon utilization capacity measured in biochar and PGPR−treated soils (n = 3). *, **, *** indicate statistically significant differences between treatments and the control at 0.1 < p < 0.05, 0.01 < p < 0.05, and 0.001 < p < 0.01, and “ns” indicates “not significant”. Significant statistical differences between the treatments and the control are indicated by lowercase letters at the α = 0.05.
3.2. Soil Microbial Functional Diversity
The AWCD value of all treatments increased with the prolongation of the treatment time and continuously during the incubation period from 24 to 96 h, the trend of increase slowed down and stabilized at 96 h (Figure S2a). The MB0 resulted in the highest AWCD during the incubation process. In contrast, the AWCD of MB20 was the lowest of all the treatments. The microbial metabolism of the incubation at 120 h was in a stable stage according to the trend of carbon source utilization; therefore, the AWCD at 120 h was analyzed for its soil microbial functional diversity. All treatments had no significant effects on the ShannonMF, SimpsonMF, and McIntosh at 120 h (Table S4).
The utilization of two major carbon sources, carbohydrate and multipolymer, was significantly impacted by the application of biochar, but not the amino acids, carboxylic acids, phenolic acids, and amines (Figure 1b). For the ability of the soil microorganisms to utilize carbohydrates, a significant (p < 0.05) increase was evident in the MB0 (56.35%), B20 (51.54%) and MB20 (19.69%) compared to the control. The B20 significantly enhanced the soil microbial utilization of multipolymers by 5.39% compared to the control.
The carbon utilization capability of the soils treated with PGPR and biochar was analyzed using a principal component analysis (PCA). The results showed that the first principal component (PC1) and the second principal component (PC2) contributed 25.0% and 20.7% of the variance, respectively, with a cumulative variance contribution of 45.7% (Figure S2b, Table S5). The PC1 separated the control and the treatments, the MB0 and MB20 clustered upward, and the B20 clustered downward. The biochar and PGPR generally shifted the treatments to the right, indicating that the carbohydrate and amino acid carbon source utilization rates of the biochar and PGPR treatment were higher. A positive correlation was observed between the PC1 and the D-cellose, β-menthyl D-glycoside, glucose-1-phosphate, and D-galactose. The PC2 showed negative correlations with the D-xylose, r-hydroxybutyric acid, a-ketobutyric acid, and phenylethylamine, and positive correlations with the L-phenylalanine. An apparent division was observed between the biochar and PGPR-treated groups and the control, indicating that biochar and PGPR treatments affect microbial functional diversity.
A canonical redundancy analysis (RDA) was used to analyze the relationship between the selected soil’s physicochemical properties and soil microbial carbon utilization capacity (Figure 2). Two ordination axes accounted for 61.49% of the variability. With 31.63% of the variability being explained by the first ordination axis (RDA1), it had a primarily positive correlation with the Soil TN. With 29.86% of the variability being explained by the second ordination axis (RDA2), it was primarily positively correlated with theSWC. In general, the results indicated that Soil TN (p = 0.068) may have a marginally significant impact on soil microbial carbon use.
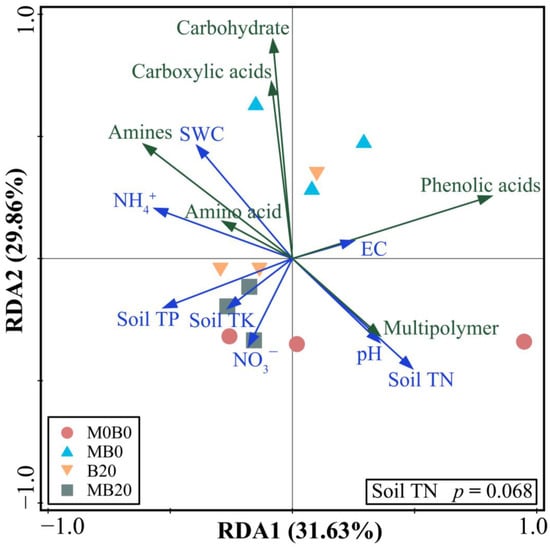
Figure 2.
Redundancy analysis (RDA) of soil physicochemical properties and soil carbon usage in soils treated with PGPR and biochar. Arrows of different colors represent the explanatory variables, green lines represent the soil’s carbon use, and blue lines represent the soil’s physicochemical properties.
3.3. Soil Bacterial Community Diversity
There were no significant differences between all treatments on the ShannonBC compared to the control. The MB0, B20, and MB20 significantly increased the SimpsonBC by 18.16%, 27.21%, and 13.80%, the ACE by 7.36%, 13.78%, and 13.51%, and the Chao by 7.49%, 13.06%, and 12.44%, respectively, compared to the control (Table 1).

Table 1.
Mean values (±standard error) of soil bacterial community diversity after biochar and PGPR treatments. Simspon index for soil bacterial community diversity (SimpsonBC). Shannon index of soil bacterial community diversity (ShannonBC). Four treatments were set up as no biochar and no PGPR (M0B0, referred to as the control), 5 × 1010 CFU·mL−1 PGPR-only (MB0), 20 t·hm−2 biochar-only (B20), and co-application of 20 t·hm−2 biochar and 5 × 1010 CFU·mL−1 PGPR (MB20). Differences in lowercase letters within column indicate statistically significant differences at α = 0.05 level.
The results indicated that the top 12 dominant phyla in soil samples, among which Proteobacteria performed the highest in all treatments, ranged from 27.34% to 39.15%. The order of the other dominant bacterial phyla was Actinobacteria, Chloroflexi, Acidobacteria, Gemmatimonadetes, Bacteroidetes, Rokubacteria, Cyanobacteria, Planctomycetes, Firmicutes, Patescibacteria, and Verrucomicrobia (Figure 3). The relative abundance of each bacterial phylum level varied significantly (p < 0.05) across all treatments. The MB20 significantly increased the relative abundance of Proteobacteria by 29.01%, Bacteroidetes by 43.64%, and Gemmatimonadetes by 32.8%, in comparison to the control. In general, the composition of the microbial community was significantly influenced by the co-application of PGPR and biochar.
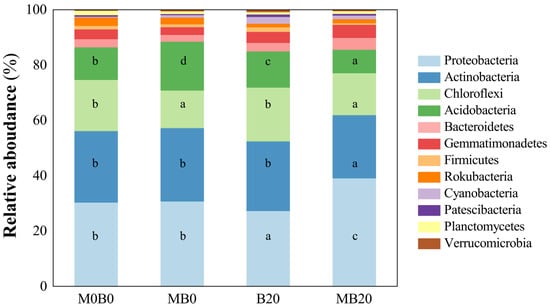
Figure 3.
Relative abundance of the bacterial community in the soils treated with biochar and PGPR. Significant statistical differences between treatments and the control are indicated by lowercase letters within panel.
To explore the main effects on bacterial community composition, we conducted the Mantel test, the bacterial community composition (based on the Bray–Curtis distance matrix) was compared with the soil’s physicochemical properties (based on the Euclidean distance matrix) (Figure 4, Table S6). The N03− (r = 0.250, p = 0.002), and Soil TN (r = 0.726, p = 0.001) were the main factors affecting the bacterial community composition in the MB20. The primary factor influencing the makeup of the B20 bacterial community were the Soil TN (r = 0.541, p = 0.002), Soil TP (r = 0.291, p = 0.029), and the N03− (r = 0.127, p = 0.038). The Soil TK (r = 0.244, p = 0.039) was the main factor affecting the MB0.
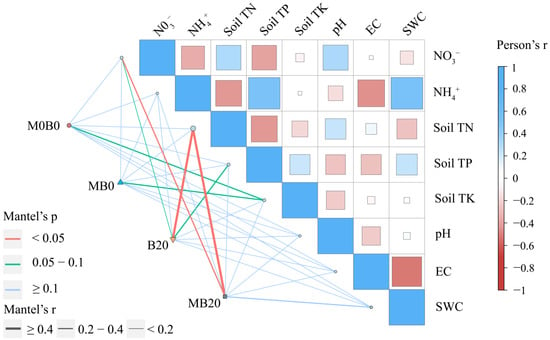
Figure 4.
Pearson’s correlation coefficients of relative abundance and community composition of dominant bacteria and impact factors based on Mantel tests.
We then conducted the RDA to gain a deep understanding of the relationship between the soil’s physicochemical properties and soil bacterial community diversity. The results revealed that the RDA1 was primarily related to the Soil TN, accounting for 91.23% of the total variability, and the RDA2 was primarily related to the Soil TP, accounting for 0.07% of the total variability (Figure 5). The diversity of the soil bacterial community was negatively correlated with the Soil TN (p = 0.002), which was the main effective environmental factor influencing soil bacteria. The soil bacterial community diversity under different treatments showed significant spatial differences, which positively correlated with the NH4+, Soil TP, Soil TK, and SWC and negatively correlated with the Soil TN, NO3−, and pH.
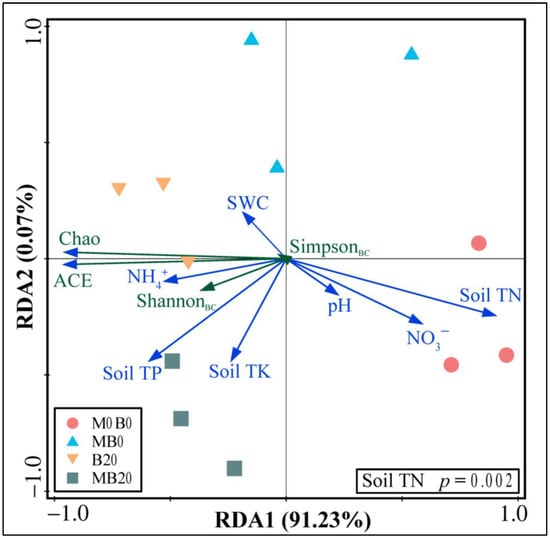
Figure 5.
Redundancy analysis (RDA) of the soil’s physicochemical properties and bacterial community diversity in soils treated with PGPR and biochar. Arrows of different colors represent the explanatory variables, green lines represent the soil bacterial community diversity, and blue lines represent the soil’s physicochemical properties.
4. Discussion
4.1. Soil Nutrient Concentration
Our study investigated the effects of biochar and PGPR on a soil’s physicochemical properties, soil community, and functional diversities important for soil microenvironment evaluation. The decreased Soil TN was in line with other studies. The absorption and use of N from soil may be improved by biochar [24,25,26]. PGPR-converted active N could be used for plant chlorophyll synthesis for plant growth. The NO3− decreased following all treatments, significant differences were not observed, despite numerical variations. NO3− was easily leached from soil colloids because of its negative charge, leading to the decreased soil NO3− in our study [27]. Studies have shown that biochar could enhance the abundance of denitrifying bacteria and upregulate the denitrifying narG, nirS, nirK, and nosZ genes in the soil [28,29]. The increase in NH4+ may also be due to the N-fixation of PGPR, which is the most important biological N supply in the terrestrial ecosystem [24,30]. Some PGPRs, such as Bacillus megaterium, had the potential ability to fix N and played an important role in the supply of non-artificial N [31]. Biochar had the potential to accumulate soil NH4+ [32]. In addition, biochar could facilitate the retention of nutrients and provide a suitable ecological niche for PGPR, resulting in more soil NH4+ in the MB20 [13]. The co-application of biochar and PGPR further improved the Soil TP content, which was more significant than either alone. Nutrient availability can be further improved by the co-application of biochar and PGPR [33]. The Bacillus megaterium phosphorus solvent has been reported to break down organic P and immobilize inorganic P in the soil for plant uptake and utilization [34]. Studies have shown that biochar has a high P retention capacity, which also reduce soil P leaching. Biochar and PGPR significantly increased the SWC, which could be due to changes in the soil’s physical properties, such as the aggregation of soil particles, and the relatively large specific surface area of biochar. The increase in the SWC may also be due to the influence of the extracellular polymeric substances (EPSs) released by PGPR. The results are consistent with Hossain et al. [35]. PGPR has been usually embedded in biofilms formed by the EPSs secreted by bacteria when added to soils [36]. Studies have shown that Bacillus sp. could release EPSs, which have a large water-holding capacity [37,38]. Adding even a small amount of 1% (w/w) of an EPS to the soil could significantly increase the water-holding capacity of the soil [39]. This may also explain the elevated SWC in the MB0 and MB20 (Figure 1a).
4.2. Soil Microbial Functional Diversification
Soil microorganisms were involved in the cycling of soil nutrients and the transformation of root secretions [40,41]. Therefore, soil microorganisms played an important role in maintaining the dynamic balance of soil nutrients as the processing plant and transportation medium of soil nutrients. However, the diversity of soil microorganisms may be in dynamic equilibrium due to factors such as the soil’s organic matter and mineral nutrients. Soil microorganisms were important components of terrestrial ecosystems, and microbial functional diversity was a sensitive indicator of changes in the soil quality. The AWCD, ShannonMF, SimpsonMF, and McIntosh indices of the soil microbial communities were not significantly different between all treatments, indicating that the soil microbial species and quantity were similar in the 3rd quarter after the amendment (Table S4).
The capacity of soil microorganisms to utilize various carbon sources was a key indicator of soil fertility, as the carbon source metabolism played an important role in soil nutrient transformation [42]. The ability of soil microbes to use carbohydrates was greatly increased in all treatments, and either the sole application of biochar or PGPR was significantly higher than the co-application of biochar and PGPR (Figure 1b). The preferred carbon source for soil microorganisms in our study was carbohydrates, consistent with studies showing carbohydrates as the preferred carbon source for soil microorganisms in forest ecosystems [43]. Carbohydrate and amino acid-based carbon sources have been strongly associated with plant root exudates (REs) [44]. The plant–PGPR interactions were mainly mediated by REs, which are the main source of nutrients for the PGPR and promote PGPR colonization and EPS formation in the soil [45]. Studies have shown that the application of PGPR significantly improves plant root growth and the production of REs [46]. The adsorption of REs by biochar allowed soil microorganisms to better utilize carbohydrate carbon sources. The abundant soil organic matter (SOM) in the soil provided sufficient carbohydrates [44]. In our study, the fallen leaves of Eucalyptus plantations provide abundant SOM, and Microorganisms can act on more substrates thereby enhancing their ability to utilize carbohydrates, which may explain the preference of soil microorganisms for carbohydrates as a carbon source.
In the present study, we used a PCA to reveal the global associations between different addition modalities and microbial carbon source utilizations. The treatments and control groups segregated mainly on PC1, explaining 25% of the variance, suggesting that the PGPR and biochar alone and synergistically had some degree of enhancement of carbohydrate utilization. The outcomes align with the findings presented in Figure 1b in which the microbial utilization of carbohydrate carbon sources was enhanced by each treatment group, where the clustering of the treatment groups was mainly based on the D-cellose, β-menthyl-D-glycoside, and Glucose-1-phosphate variables (Figure S2a).
The soil’s physicochemical properties were a key factor influencing soil microbial functional diversity, on which soil microbial diversity was more significant than that of above-ground plants [47]. In our study, different treatments could affect the soil microbial utilization of specific carbon sources, as well as the microbial community diversity by influencing the soil’s physicochemical properties. It was found that different soil physicochemical factors affected the soil’s microbial carbon source utilization to different degrees, and the Soil TN mainly positively affected multipolymer and phenolic acids, and negatively affected the amines, carboxylic acids, carbohydrates, and amino acids (Figure 2). These findings suggest that the changes in microbial function in biochar and PGPR-amended soils may be driven by soil physicochemical properties, especially the Soil TN.
4.3. Soil Microbial Evaluation
Soil bacteria make up the majority of the three major groups of soil microorganisms that influence the metabolism and reproduction of soil fungi and actinomycetes [48]. The characteristics of the community structure reflected the status of the soil microbial community [49]. The soil bacterial community diversity could be used as an indicator for the evaluation of soil ecological functions [50]. There was a significant increase in soil bacterial community diversity in our study. Specifically, the B20 had the highest ShannonBC, SimpsonBC, ACE, and Chao indices. The SimpsonBC, ACE, and Chao indices of all the treatments were higher than the corresponding indicators for the native soil bacterial community (Table 1). It has been shown that biochar could change the physicochemical properties, such as loosening the soil structure, inputting inorganic and organic nutrients, and increasing the SWC, which could improve the microbial metabolism and increase soil bacterial community diversity [42,51,52]. Previous studies have shown that inoculation with PGPR increased the bacterial community diversity in the inter-root soil of bananas and promoted plant growth [53]. Specifically, our results show that the application of biochar and PGPR increases soil bacterial community diversity, which is essential for maintaining soil stability.
Our results showed that the relative abundance of bacterial communities varied greatly at the phylum level. This phylum level resembles other soil conditions [54,55]. Proteobacteria and Actinobacteria were the most prevalent phyla regardless of the treatment used, which was consistent with the observation that soils often include two ubiquitous types of bacteria [56,57]. N-fixing microorganisms were mainly distributed in more than 60 genera in seven phyla, including Proteobacteria, Actinobacteria, Cyanobacteria, Chlorobi, Chloroflexi, Firmicutes, and Euryarchaeota [58]. Proteobacteria dominated with the highest percentage of abundance across treatments, and this phylum included some N-fixing bacteria [59]. Acidobacteria have been one of the important members of soil ecosystems and widely distributed in different ecosystems, which could contribute to plant performance and productivity by participating in the N cycle [60,61]. The community diversity of bacteria directly affects the conversion of N and eucalypt uptake and the utilization of nitrogen [62]. The higher abundance of Proteobacteria, Bacteroidetes, and Gemmatimonadetes in the MB20 indicates that the co-application of biochar and PGPR moderated the dominant species in the Eucalyptus soil through soil N regulation. Previous studies have shown that the application of biochar could affect the community composition and the abundance of the associated species of soil microorganisms, which may drive nutrient cycling, and subsequently affect plant growth directly or indirectly.
The mantel test also confirmed that Soil TN was the main environmental factor affecting the bacterial communities of the B20 and MB20, indicating that PGPR and biochar amendment may alter bacteria abundance indirectly through changes in the Soil TN (Figure 4). Similar observations have been reported in previous studies [63,64].
The RDA of the soil’s physicochemical properties and bacterial community diversity showed that the SimpsonBC, ShannonBC, ACE, and Chao indices were significantly (p < 0.01) correlated with the Soil TN negatively (Figure 5). The increase in the soil N suppressed the ability of microorganisms to metabolize carbon sources [65]. This could be the reason why the decline in the Soil TN in this study increased soil bacterial community diversity. In addition, the decrease in Soil TN led to the soil C/N increase. Sufficient soil microbial nutrients were conducive to the increase of soil bacterial community diversity [66].
5. Conclusions
All treatments significantly improved the microbial utilization of carbohydrates. MB20 significantly increased SWC, soil TP, NH4+, the SimpsonBC, ACE, and Chao indices, but decreased soil TN compared with the control. The RDA results showed that a strong negative correlation between the Soil TN and the diversity index of the soil bacterial community occurred. In conclusion, biochar and PGPR had positive effects on the Soil TN, Soil TP, NH4+, SWC, soil bacterial community diversity index, and carbohydrate utilization by soil microbial communities. Our study represents the nine months after biochar and PGPR application and is important for N reduction and soil improvement in regional eucalyptus plantation forest cultivation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14030428/s1. Figure S1: Experimental design at the Gaofeng Forest Center, Changke Branch; Figure S2: (a) Microbial metabolic activity of carbon sources in soil under M0B0, MB0, B20 and MB20 treatments. (b) Principal component Analysis (PCA) of selected parameters measured on a grown under MB0, B20 MB20 and the control; Table S1: Main technical specifications of the biochar under study; Table S2: PCR primer of bacteria; Table S3: PCR reaction system and amplification program of bacteria. Table S4: Mean values (± standard error) of diversity of soil microbial functional after biochar and PGPR treatments; Table S5: Carbon sources with contribution rates for principal component 1 (PC1) and principal component 2 (PC2) in soils treated by MB0, B20 MB20 and the control; Table S6: Pearson’s correlation coefficients of relative abundance and community composition of dominant bacteria and impact factors based on Mantel tests; Formula S1: AWCD index; Formula S2: SimpsonMF index; Formula S3: ShannonMF index; Formula S4: McIntosh index; Formula S5: ShannonBC index; Formula S6: SimpsonBC index; Formulas S7–S11: ACE index; Formula S12: Chao index.
Author Contributions
C.D.: Software, Formal analysis, Writing—original draft; C.L.: Methodology, Validation, Resources; H.C.: Investigation, Supervision; J.Z.: Validation, Visualization; H.R.: Methodology, Resources, Data curation, Writing—original draft, Writing—review & editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32201326), the Scientific Research Fund of Zhejiang Provincial Education Department (Y202352538), and the Graduate Scientific Research Foundation of Wenzhou University (3162023003052).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Siddique, M.H.; Anwar, F.; Shahzad, T. Unlocking the Potential of Co-Applied Biochar and Plant Growth-Promoting Rhizobacteria (PGPR) for Sustainable Agriculture under Stress Conditions. Chem. Biol. Technol. Agric. 2022, 9, 58. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Dardanelli, M.S.; Manyani, H.; González-Barroso, S.; Rodríguez-Carvajal, M.A.; Gil-Serrano, A.M.; Espuny, M.R.; López-Baena, F.J.; Bellogín, R.A.; Megías, M.; Ollero, F.J. Effect of the Presence of the Plant Growth Promoting Rhizobacterium (PGPR) Chryseobacterium Balustinum Aur9 and Salt Stress in the Pattern of Flavonoids Exuded by Soybean Roots. Plant Soil 2010, 328, 483–493. [Google Scholar] [CrossRef]
- Tkacz, A.; Poole, P. Role of Root Microbiota in Plant Productivity. J. Exp. Bot. 2015, 66, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Huang, B.; Fernandez-Garcia, V.; Miesel, J.; Yan, L.; Lv, C. Biochar and Rhizobacteria Amendments Improve Several Soil Properties and Bacterial Diversity. Microorganisms 2020, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, Y.; Chang, E.; Wang, R.; Hong, Z.; Cui, J.; Zhang, F.; Jiang, J.; Xu, R. Effect of Biochar Incorporation on Phosphorus Supplementation and Availability in Soil: A Review. J. Soils Sediments 2023, 23, 672–686. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar Reduces Soil Heterotrophic Respiration in a Subtropical Plantation through Increasing Soil Organic Carbon Recalcitrancy and Decreasing Carbon Degrading Microbial Activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Awad, Y.M.; Ok, Y.S.; Abrigata, J.; Beiyuan, J.; Beckers, F.; Tsang, D.C.W.; Rinklebe, J. Pine Sawdust Biomass and Biochars at Different Pyrolysis Temperatures Change Soil Redox Processes. Sci. Total Environ. 2018, 625, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-Stimulated Plant Performance Is Strongly Linked to Microbial Diversity and Metabolic Potential in the Rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Warnock, D.D.; Tiemann, L.K.; Quigley, K.; Miesel, J.R. Evaluating Foliar Characteristics as Early Indicators of Plant Response to Biochar Amendments. For. Ecol. Manag. 2021, 489, 119047. [Google Scholar] [CrossRef]
- Nasiri, S.; Andalibi, B.; Tavakoli, A.; Delavar, M.A.; El-Keblawy, A.; Zwieten, L.V.; Mastinu, A. The Mineral Biochar Alters the Biochemical and Microbial Properties of the Soil and the Grain Yield of Hordeum vulgare L. under Drought Stress. Land 2023, 12, 559. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial Utilisation of Biochar-Derived Carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef]
- Choppala, G.; Bolan, N.; Kunhikrishnan, A.; Bush, R. Differential Effect of Biochar upon Reduction-Induced Mobility and Bioavailability of Arsenate and Chromate. Chemosphere 2016, 144, 374–381. [Google Scholar] [CrossRef]
- Wong, J.T.F.; Chen, X.; Deng, W.; Chai, Y.; Ng, C.W.W.; Wong, M.H. Effects of Biochar on Bacterial Communities in a Newly Established Landfill Cover Topsoil. J. Environ. Manag. 2019, 236, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gong, T.; Wang, J.; Li, G.; Liu, Y.; Zhen, J.; Ning, M.; Yue, D.; Du, Z.; Chen, G. Effects of Compound Microbial Fertilizer on Soil Characteristics and Yield of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Shi, P.; Bi, Z.; Shan, Z.; Ren, L. The Deep Challenge of Nitrate Pollution in River Water of China. Sci. Total Environ. 2021, 770, 144674. [Google Scholar] [CrossRef] [PubMed]
- Lazaratou, C.V.; Vayenas, D.V.; Papoulis, D. The Role of Clays, Clay Minerals and Clay-Based Materials for Nitrate Removal from Water Systems: A Review. Appl. Clay Sci. 2020, 185, 105377. [Google Scholar] [CrossRef]
- Wang, R.-C.; Wang, H.-M.; Xiang, X.; Gao, Y.; Song, Q.-W.; Gong, L.-F. Temporal and Spatial Variations of Microbial Carbon Utilization in Water Bodies from the Dajiuhu Peatland, Central China. J. Earth Sci. 2018, 29, 969–976. [Google Scholar] [CrossRef]
- Kim, Y.C.; Anderson, A.J. Rhizosphere Pseudomonads as Probiotics Improving Plant Health. Mol. Plant Pathol. 2018, 19, 2349–2359. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of Biochar on Soil Available Inorganic Nitrogen: A Review and Meta-Analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- D’Elia, C.F.; Steudler, P.A.; Corwin, N. Determination of Total Nitrogen in Aqueous Samples Using Persulfate Digestion1. Limnol. Oceanogr. 1977, 22, 760–764. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.; Chen, H.; Zhou, J.; Lv, C. Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability. Sustainability 2022, 14, 10922. [Google Scholar] [CrossRef]
- Ker, K.; Seguin, P.; Driscoll, B.T.; Fyles, J.W.; Smith, D.L. Evidence for Enhanced N Availability during Switchgrass Establishment and Seeding Year Production Following Inoculation with Rhizosphere Endophytes. Arch. Agron. Soil Sci. 2014, 60, 1553–1563. [Google Scholar] [CrossRef]
- Sahin, O.; Taskin, M.B.; Kaya, E.C.; Atakol, O.; Emir, E.; Inal, A.; Gunes, A. Effect of Acid Modification of Biochar on Nutrient Availability and Maize Growth in a Calcareous Soil. Soil Use Manag. 2017, 33, 447–456. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Lu, X.; Yin, Q.; Liu, D. Utilization of Plant Litter to Enhance the Effect of Lakebank on Nitrogen Interception/Removal from Runoff: A Microcosm Simulation Study. Environ. Eng. Sci. 2022, 39, 235–247. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Liu, S. HMGB1 Induced Inflammatory Effect Is Blocked by CRISPLD2 via MiR155 in Hepatic Fibrogenesis. Mol. Immunol. 2016, 69, 1–6. [Google Scholar] [CrossRef]
- Sun, B.; Gu, L.; Bao, L.; Zhang, S.; Wei, Y.; Bai, Z.; Zhuang, G.; Zhuang, X. Application of Biofertilizer Containing Bacillus subtilis Reduced the Nitrogen Loss in Agricultural Soil. Soil Biol. Biochem. 2020, 148, 107911. [Google Scholar] [CrossRef]
- Song, Z.; Su, X.; Li, P.; Sun, F.; Dong, W.; Zhao, Z.; Wen, Z.; Liao, R. Facial Fabricated Biocompatible Homogeneous Biocarriers Involving Biochar to Enhance Denitrification Performance in an Anoxic Moving Bed Biofilm Reactor. Bioresour. Technol. 2021, 341, 125866. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant Growth-Promoting Rhizobacteria Inoculation to Enhance Vegetative Growth, Nitrogen Fixation and Nitrogen Remobilisation of Maize under Greenhouse Conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef]
- Yousuf, J.; Thajudeen, J.; Rahiman, M.; Krishnankutty, S.; Alikunj, A.P.; Abdulla, M.H.A. Nitrogen Fixing Potential of Various Heterotrophic Bacillus Strains from a Tropical Estuary and Adjacent Coastal Regions. J. Basic Microbiol. 2017, 57, 922–932. [Google Scholar] [CrossRef]
- Nelissen, V.; Rutting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize Biochars Accelerate Short-Term Soil Nitrogen Dynamics in a Loamy Sand Soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Uzinger, N.; Takács, T.; Szili-Kovács, T.; Radimszky, L.; Füzy, A.; Draskovits, E.; Szűcs-Vásárhelyi, N.; Molnár, M.; Farkas, É.; Kutasi, J.; et al. Fertility Impact of Separate and Combined Treatments with Biochar, Sewage Sludge Compost and Bacterial Inocula on Acidic Sandy Soil. Agronomy 2020, 10, 1612. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Rodrigues, M.; Coelho, M.J.A.; Gasperini, A.M.; Sartor, L.R.; Pavinato, P.S. Changes in Soil Phosphorus Lability Promoted by Phosphate Sources and Cover Crops. Soil Tillage Res. 2018, 179, 20–28. [Google Scholar] [CrossRef]
- Hossain, M.A.; Islam, S.M.S.; Hasan, M.M. Changes in Soil Properties with Combined Use of Probiotic Cultures and Organic Farming Practices in Degraded Soils of Bangladesh. Sustainability 2023, 15, 4430. [Google Scholar] [CrossRef]
- Zheng, W.; Zeng, S.; Bais, H.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L.; Jin, Y. Plant Growth-Promoting Rhizobacteria (PGPR) Reduce Evaporation and Increase Soil Water Retention. Water Resour. Res. 2018, 54, 3673–3687. [Google Scholar] [CrossRef]
- Chung, K.-S.; Shin, J.-S.; Lee, J.-H.; Park, S.-E.; Han, H.-S.; Rhee, Y.K.; Cho, C.-W.; Hong, H.-D.; Lee, K.-T. Protective Effect of Exopolysaccharide Fraction from Bacillus subtilis against Dextran Sulfate Sodium-Induced Colitis through Maintenance of Intestinal Barrier and Suppression of Inflammatory Responses. Int. J. Biol. Macromol. 2021, 178, 363–372. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two Novel Exopolysaccharides from Bacillus Amyloliquefaciens C-1: Antioxidation and Effect on Oxidative Stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Shavit, U.; Furman, A. Water Retention Curves of Biofilm-Affected Soils Using Xanthan as an Analogue. Soil Sci. Soc. Am. J. 2012, 76, 61–69. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Yang, J.; Gao, C.; Wei, Y.; Chen, S.; Liesche, J. Arabidopsis Plants Engineered for High Root Sugar Secretion Enhance the Diversity of Soil Microorganisms. Biotechnol. J. 2022, 17, 2100638. [Google Scholar] [CrossRef]
- Adomako, M.O.; Roiloa, S.; Yu, F.-H. Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance. Microorganisms 2022, 10, 2399. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Shi, Y.; Zhang, Y.; Yang, D.; Guo, C. Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions. Diversity 2023, 15, 537. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Starr, E.; Karaoz, U.; Brodie, E.L.; Zhou, J.; Tringe, S.G.; Malmstrom, R.R.; Woyke, T.; Banfield, J.F.; Firestone, M.K.; et al. Niche Differentiation Is Spatially and Temporally Regulated in the Rhizosphere. ISME J. 2020, 14, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Hu, W.; Zheng, J.; Du, F.; Zhang, X. Estimating Soil Organic Carbon Storage and Distribution in a Catchment of Loess Plateau, China. Geoderma 2010, 154, 261–266. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of Plant Growth Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Dallaire, K.; Skousen, J. Early Tree Growth in Reclaimed Mine Soils in Appalachia USA. Forests 2019, 10, 549. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil Microbiomes and One Health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Zhong, W.; Bian, B.; Gao, N.; Min, J.; Shi, W.; Lin, X.; Shen, W. Nitrogen Fertilization Induced Changes in Ammonia Oxidation Are Attributable Mostly to Bacteria Rather than Archaea in Greenhouse-Based High N Input Vegetable Soil. Soil Biol. Biochem. 2016, 93, 150–159. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Verma, S.; Wang, Q.; Chen, H.; Ren, X.; Zhang, Z.; Awasthi, M.K. Positive Impact of Biochar Alone and Combined with Bacterial Consortium Amendment on Improvement of Bacterial Community during Cow Manure Composting. Bioresour. Technol. 2019, 280, 79–87. [Google Scholar] [CrossRef]
- Han, G.; Chen, Q.; Zhang, S.; Li, G.; Yi, X.; Feng, C.; Wang, X.; Yu, C.; Lan, J. Biochar Effects on Bacterial Community and Metabolic Pathways in Continuously Cotton-Cropped Soil. J. Soil Sci. Plant Nutr. 2019, 19, 249–261. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the Rhizosphere Microbiome by Biofertilizer Application to Suppress Banana Fusarium Wilt Disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Guo, J.; Liu, W.; Zhu, C.; Luo, G.; Kong, Y.; Ling, N.; Wang, M.; Dai, J.; Shen, Q.; Guo, S. Bacterial Rather than Fungal Community Composition Is Associated with Microbial Activities and Nutrient-Use Efficiencies in a Paddy Soil with Short-Term Organic Amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Z.; Mou, H.; Li, J.; Zhang, P.; Jia, Z. Impact of Farmland Mulching Practices on the Soil Bacterial Community Structure in the Semiarid Area of the Loess Plateau in China. Eur. J. Soil Biol. 2019, 92, 8–15. [Google Scholar] [CrossRef]
- Kim, H.M.; Jung, J.Y.; Yergeau, E.; Hwang, C.Y.; Hinzman, L.; Nam, S.; Hong, S.G.; Kim, O.-S.; Chun, J.; Lee, Y.K. Bacterial Community Structure and Soil Properties of a Subarctic Tundra Soil in Council, Alaska. FEMS Microbiol. Ecol. 2014, 89, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rui, J.; Mao, Y.; Yannarell, A.; Mackie, R. Dynamics of the Bacterial Community Structure in the Rhizosphere of a Maize Cultivar. Soil Biol. Biochem. 2014, 68, 392–401. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, Composition, Diversity and Novelty of Soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Trojan, D.; Roux, S.; Herbold, C.; Rattei, T.; Woebken, D. Genomic Insights into the Acidobacteria Reveal Strategies for Their Success in Terrestrial Environments. Environ. Microbiol. 2018, 20, 1041–1063. [Google Scholar] [CrossRef] [PubMed]
- Pajares, S.; Bohannan, B.J.M. Ecology of Nitrogen Fixing, Nitrifying, and Denitrifying Microorganisms in Tropical Forest Soils. Front. Microbiol. 2016, 7, 1045. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Wen, H.; Zhou, T.; Zhang, T.; Gao, X. 454 Pyrosequencing Analysis of Bacterial Diversity Revealed by a Comparative Study of Soils from Mining Subsidence and Reclamation Areas. J. Microbiol. Biotechnol. 2014, 24, 313–323. [Google Scholar] [CrossRef]
- Loganathachetti, D.S.; Venkatachalam, S.; Jabir, T.; Vipindas, P.V.; Krishnan, K.P. Total Nitrogen Influence Bacterial Community Structure of Active Layer Permafrost across Summer and Winter Seasons in Ny-Ålesund, Svalbard. World J. Microbiol. Biotechnol. 2022, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Sui, X.; Liu, Y.; Yang, L.; Zhang, R. Effect of Nitrogen Addition on the Carbon Metabolism of Soil Microorganisms in a Calamagrostis Angustifolia Wetland of the Sanjiang Plain, Northeastern China. Ann. Microbiol. 2022, 72, 18. [Google Scholar] [CrossRef]
- Kong, J.; He, Z.; Chen, L.; Yang, R.; Du, J. Efficiency of Biochar, Nitrogen Addition, and Microbial Agent Amendments in Remediation of Soil Properties and Microbial Community in Qilian Mountains Mine Soils. Ecol. Evol. 2021, 11, 9318–9331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).