Soil Microbiome of Abandoned Plaggic Podzol of Different-Aged Fallow Lands and Native Podzol in South Taiga (Leningrad Region)
Abstract
1. Introduction
- -
- To study the morphometric features of soil transformation under the conditions of the post-agrogenic development of ecosystems;
- -
- To determine the physico-chemical parameters of different-aged fallow soils;
- -
- To analyze the taxonomic and functional diversity of the microbiome of different-aged (40-, 80-, and 120-year-old) fallow and native soils using modern methods including high-throughput sequencing and bioinformatic analysis.
2. Materials and Methods
2.1. The Study Area
2.2. Laboratory Analyses
3. Results and Discussion
3.1. Features of the Transformation of the Soils as a Result of Their Transfer to the Fallow State
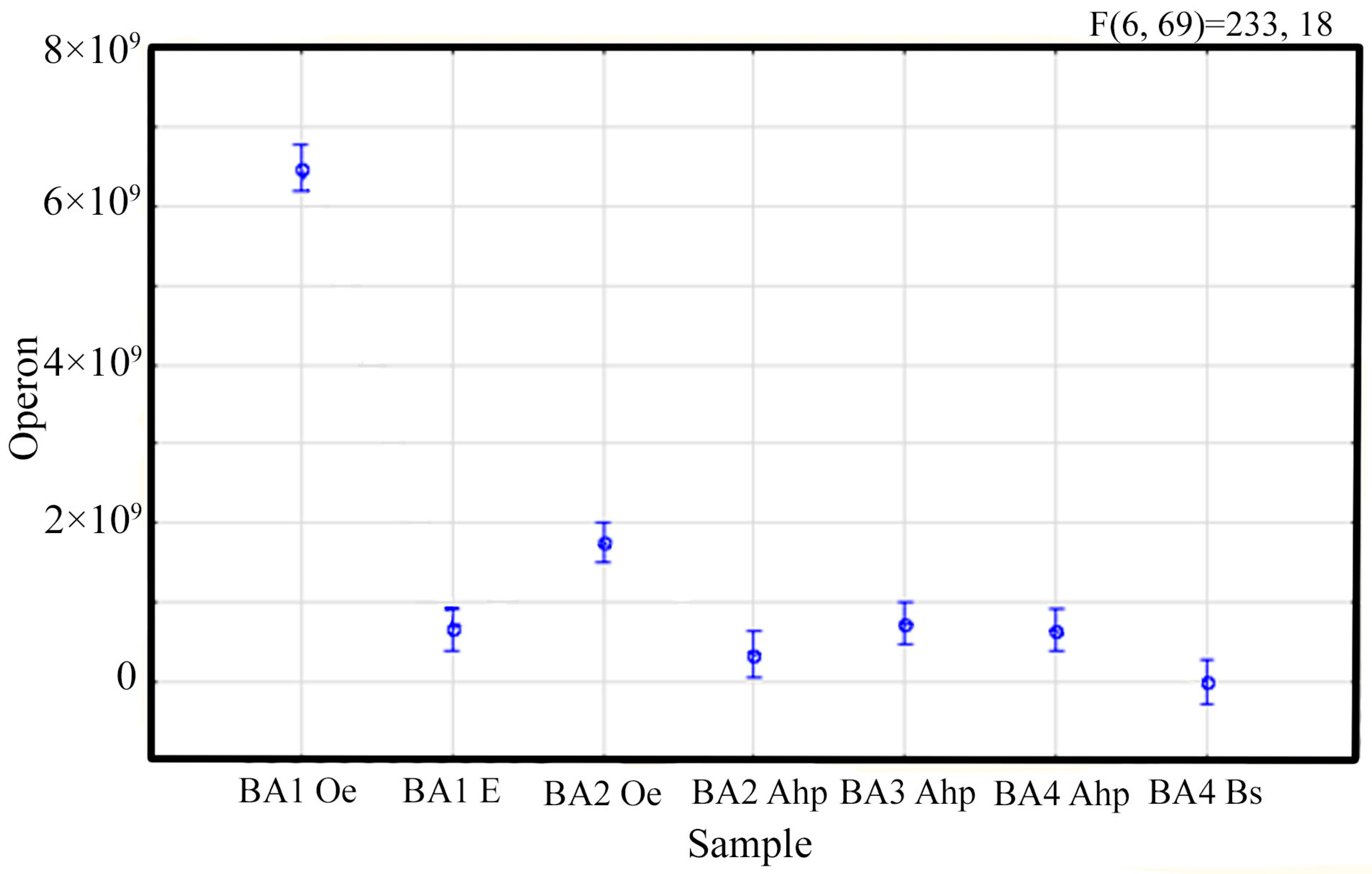
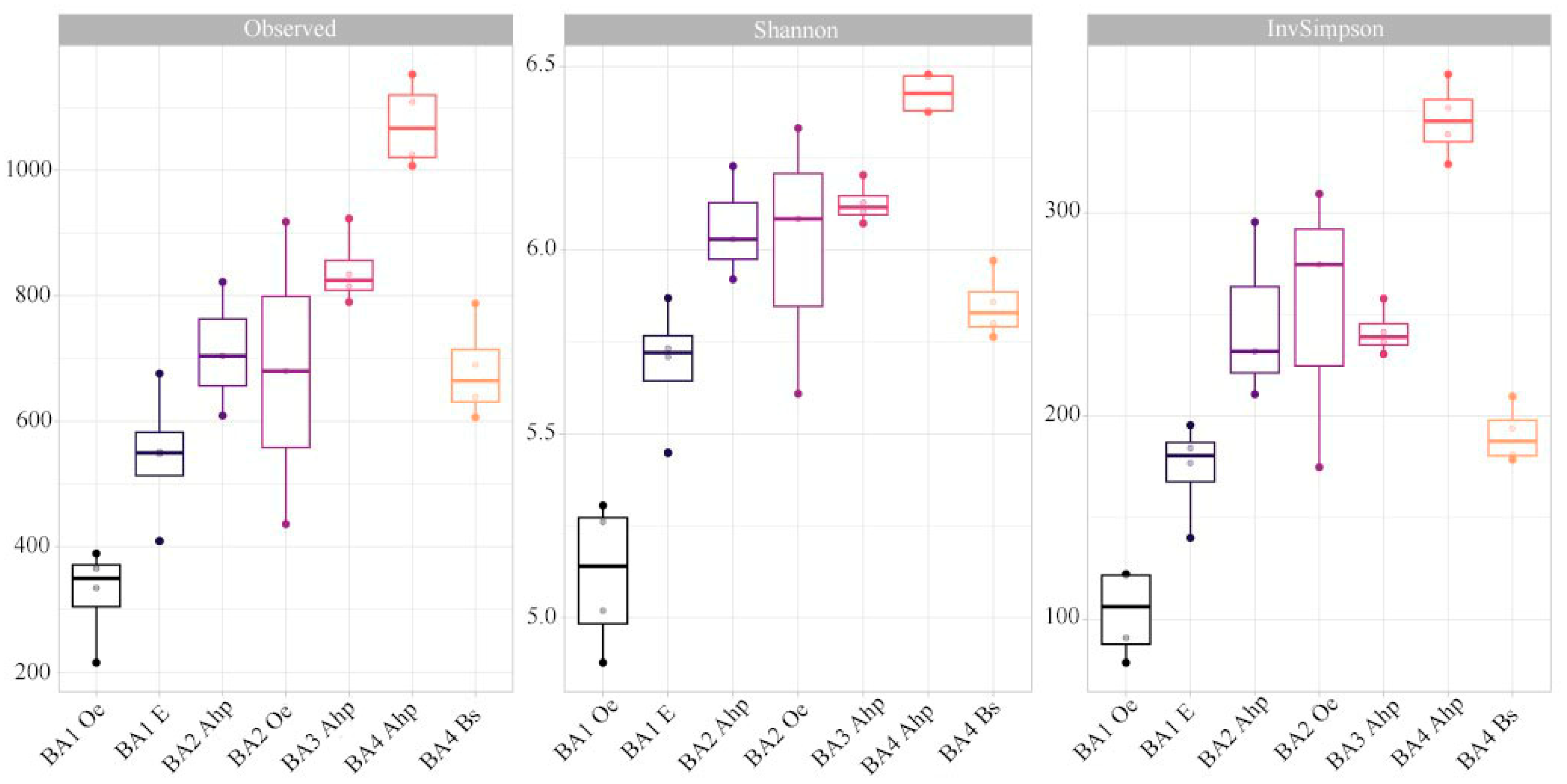
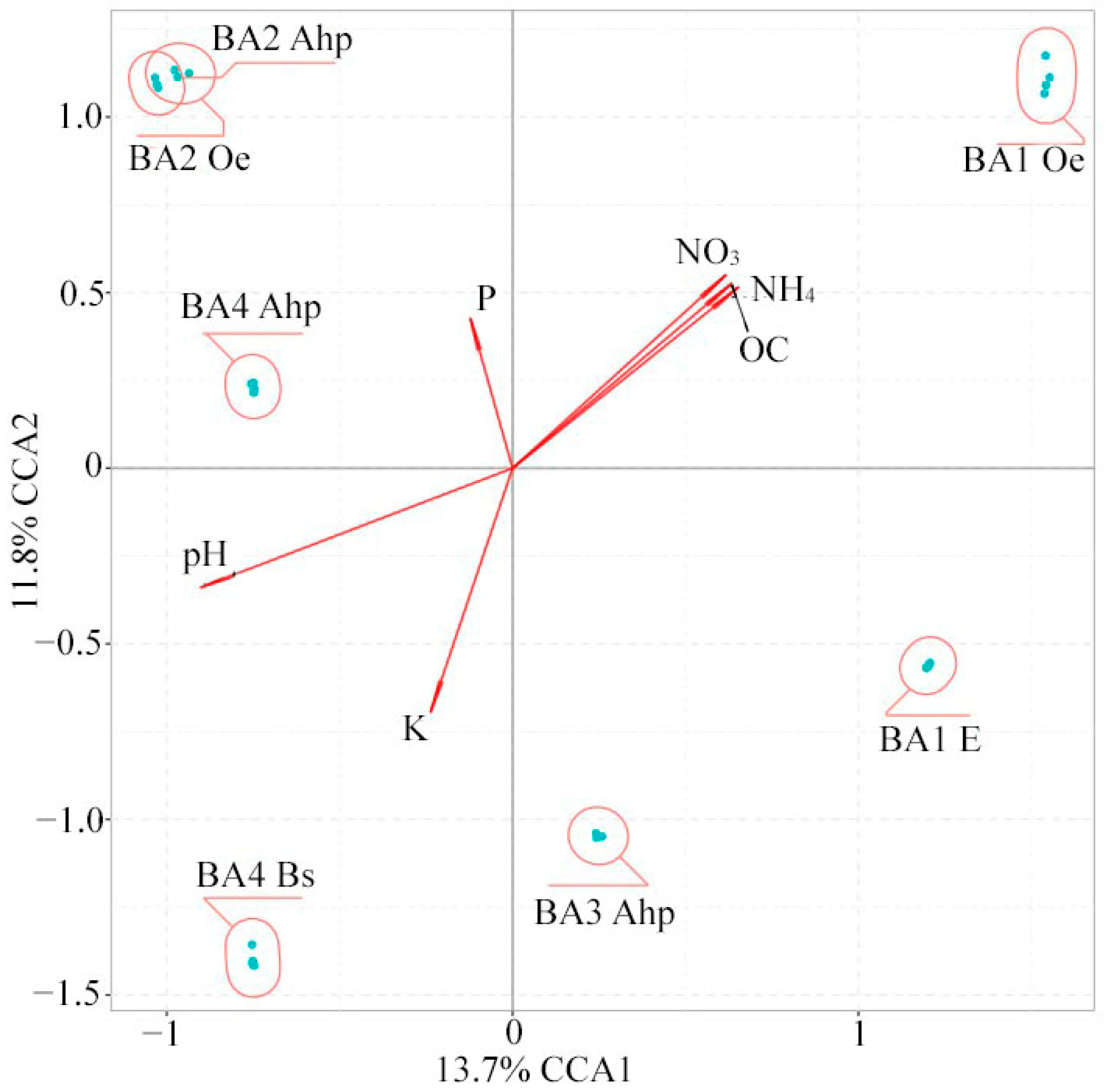
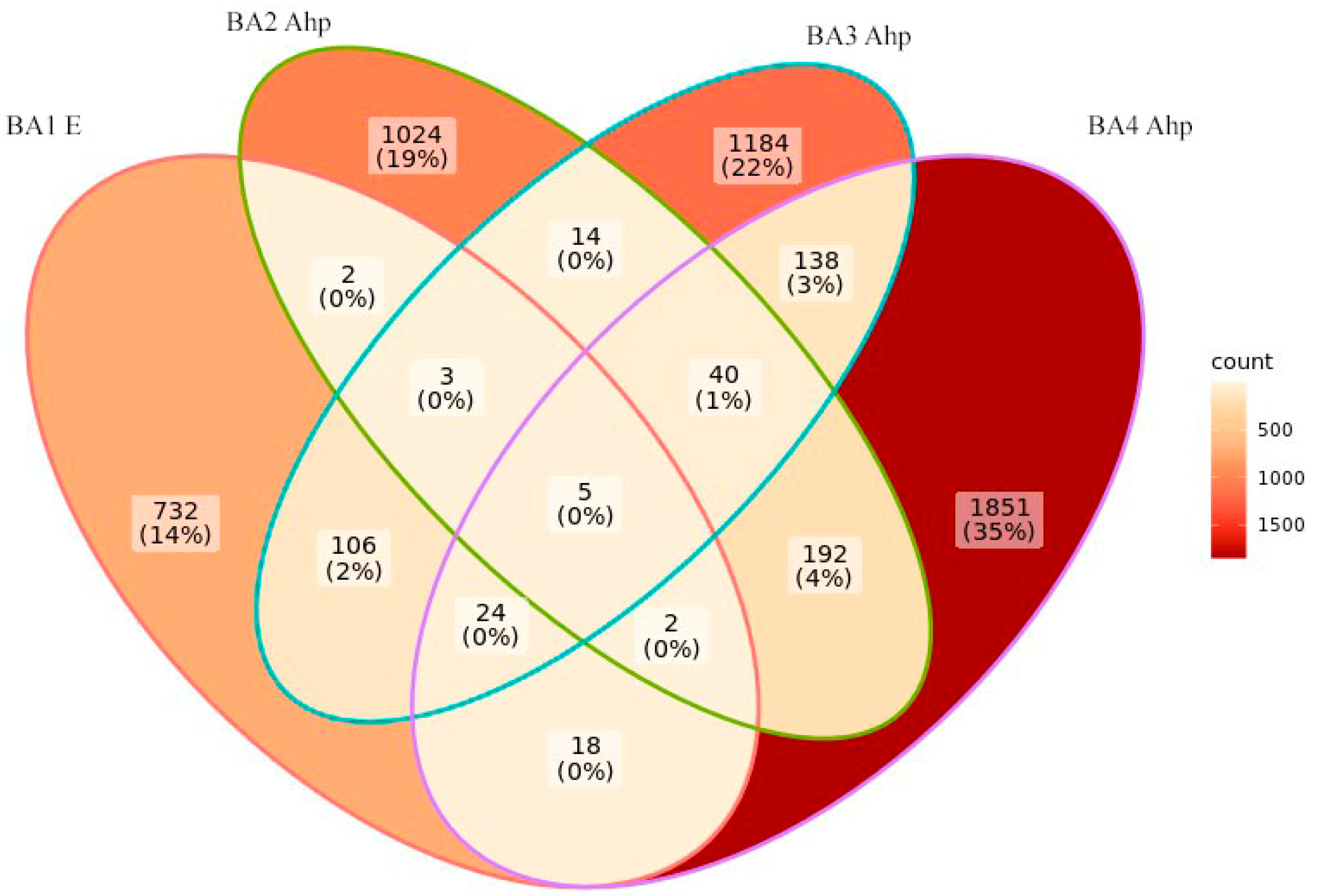
3.2. The Soil Microbiome of Different-Aged Fallow Lands in the Vicinity of Ban’kovo Village, Leningrad Region
3.3. Limitation of This Research
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A Paradigm Shift in the Application of Microorganisms for Sustainable Agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.F.; Olivares, F.L. Plant microbiome structure and benefits for sustainable agriculture. Curr. Plant Biol. 2021, 26, 100198. [Google Scholar] [CrossRef]
- Kughur, G.; Audu, O. Effects of Intensive Agricultural Production on the Environment in Benue State, Nigeria. CAB Rev. 2015, 8, 7–11. [Google Scholar]
- Howard, M.M.; Kaminsky, L.M.; Kessler, A.; Bell, T.H. Merging microbial and plant profiling to understand the impact of human-generated extreme environments on natural and agricultural systems. In Advanced Techniques for Studying Microorganisms in Extreme Environments; Yergeau, É., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 57–92. [Google Scholar]
- Blundell, R.; Schmidt, J.E.; Igwe, A.; Cheung, A.L.; Vannette, R.L.; Gaudin, A.C.M.; Casteel, C.L. Organic management promotes natural pest control through altered plant resistance to insects. Nat. Plants 2020, 6, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.-C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Hannula, S.E.; Zhu, F.; Heinen, R.; Bezemer, T.M. Foliar-feeding insects acquire microbiomes from the soil rather than the host plant. Nat. Commun. 2019, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, O.; Cherkinsky, A.; Chertov, O.; Goryachkin, S.; Kurganova, I.; Lopes de Gerenyu, V.; Lyuri, D.; Kuzyakov, Y.; Giani, L. Post-agricultural restoration: Implications for dynamics of soil organic matter pools. Catena 2019, 181, 104096. [Google Scholar] [CrossRef]
- Lyuri, D.I.; Goryachkin, S.V.; Karavaeva, N.A.; Denisenko, E.A.; Nefedova, T.G. Dynamics of Agricultural Lands of Russia in XX Century and Postagrogenic Restoration of Vegetation and Soils; GEOS: Moscow, Russia, 2010. [Google Scholar]
- Kalinina, O.; Goryachkin, S.V.; Karavaeva, N.A.; Lyuri, D.I.; Najdenko, L.; Giani, L. Self-restoration of post-agrogenic sandy soils in the southern Taiga of Russia: Soil development, nutrient status, and carbon dynamics. Geoderma 2009, 152, 35–42. [Google Scholar] [CrossRef]
- Isachenko, G.A.; Isachenko, T.E. Approaches to cultural landscape zoning. Proc. Russ. Geogr. Soc. 2016, 148, 1–17. [Google Scholar]
- Lyuri, D.I.; Karelin, D.V.; Kudikov, A.V.; Goryachkin, S.V. Changes in soil respiration in the course of the postagrogenic succession on sandy soils in the southern taiga zone. Eurasian Soil Sci. 2013, 46, 935–947. [Google Scholar] [CrossRef]
- Abakumov, E. Rendzinas of the Russian Northwest: Diversity, Genesis, and Ecosystem Functions: A Review. Geosciences 2023, 13, 216. [Google Scholar] [CrossRef]
- Sommer, R.; de Pauw, E. Organic carbon in soils of Central Asia—Status quo and potentials for sequestration. Plant Soil 2011, 338, 273–288. [Google Scholar] [CrossRef]
- Giani, L.; Chertov, O.; Gebhardt, C.; Kalinina, O.; Nadporozhskaya, M.; Tolkdorf-Lienemann, E. Plagganthrepts in northwest Russia? Genesis, properties and classification. Geoderma 2004, 121, 113–122. [Google Scholar] [CrossRef]
- LaganiÈRe, J.; Angers, D.A.; ParÉ, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Malakhovsky, D.B. On the role of the latest tectonics in the relief formation of glacial areas of North-West Russia. Izv. Russ. Geogr. Soc. 2000, 132, 45–52. [Google Scholar]
- Gagarina, E.I. Lithological Factor of Soil Formation (by the Example of the North-West Russian Plain); Saint-Petersburg State University: Saint-Petersburg, Russia, 2004; p. 260. [Google Scholar]
- Jahn, R.; Blume, H.P.; Spaargaren, O.; Schad, P. Guidelines for Soil Description; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; p. 98. [Google Scholar]
- World Reference Base for Soil Resourses; Food and Agriculture Organization of the United Nations; IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015; FAO: Rome, Italy, 2015; 195p. [Google Scholar]
- Kroetsch, D.; Wang, C. Particle Size Distribution. In Soil Sampling and Methods of Analysis; Angers, D.A., Larney, F.J., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 713–725. [Google Scholar]
- GOST. Determination of Mobile Phosphorus and Potassium Compounds by Kirsanov Method Modified by ClNAO; Pryanishnikov Institute of Agrochemistry: Moscow, Russia, 2011; p. 8. [Google Scholar]
- ISO/TS 14256-1-2003:2003; Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Field Moist Soils by Extraction with Potassium Chloride Solution—Part 1: Manual Method. International Organization for Standardization: Geneva, Switzerland, 2003; p. 14.
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Gladkov, G.; Kimeklis, A.; Zverev, A.; Pershina, E.; Ivanova, E.; Kichko, A.; Andronov, E.; Abakumov, E. Soil microbiome of the postmining areas in polar ecosystems in surroundings of Nadym, Western Siberia, Russia. Open Agric. 2019, 4, 684–696. [Google Scholar] [CrossRef]
- Girden, E.R. ANOVA: Repeated Measures; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1992. [Google Scholar]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.2-1. 2015, Volume 2, pp. 1–2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 20 December 2023).
- Litvinovich, A.V. Postagrogenic Evolution of Well Cultivated Soddy-Podzolic Soils in the Northwestern Nonchernozemic Zone. Agronomy 2009, 7, 85–93. [Google Scholar]
- Litvinovich, A.; Pavlova, O.; Lavrishchev, A.; Bure, V. Study of Indicators of Soil Fertility of Cultivated Sod-Podzolic Sandy Soil at Different Stages of Formation of Natural Ecosystems. Agrochemistry 2022, 6, 14–27. [Google Scholar]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Howard, M.M.; Kao-Kniffin, J.; Kessler, A. Shifts in plant–microbe interactions over community succession and their effects on plant resistance to herbivores. New Phytol. 2020, 226, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.-M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Y.; Li, Y.; Cheng, X.; Huang, S.; Yang, X.; Qin, Y. Genotype-Specific Recruitment of Rhizosphere Bacteria From Sandy Loam Soil for Growth Promotion of Cucumis sativus var. hardwickii. Front. Microbiol. 2022, 13, 910644. [Google Scholar] [CrossRef] [PubMed]
- Orlova, O.V.; Kichko, A.A.; Chirak, E.L.; Zverev, A.O.; Lisina, T.O.; Andronov, E.E. Soil bacterial community during straw decomposition depending on the amount of available organic matter. Eurasian Soil Sci. 2023, 5, 626–639. [Google Scholar]
- Choma, M.; Šamonil, P.; Kaštovská, E.; Bárta, J.; Tahovská, K.; Valtera, M.; Šantrůčková, H. Soil Microbiome Composition along the Natural Norway Spruce Forest Life Cycle. Forests 2021, 12, 410. [Google Scholar] [CrossRef]
- Górska, E.B.; Stępień, W.; Hewelke, E.; Lata, J.-C.; Gworek, B.; Gozdowski, D.; Sas-Paszt, L.; Bazot, S.; Lisek, A.; Gradowski, M.; et al. Response of soil microbiota to various soil management practices in 100-year-old agriculture field and identification of potential bacterial ecological indicator. Ecol. Indic. 2024, 158, 111545. [Google Scholar] [CrossRef]
- Wang, L.; Ye, X.; Hu, H.; Du, J.; Xi, Y.; Shen, Z.; Lin, J.; Chen, D. Soil bacterial communities triggered by organic matter inputs associates with a high-yielding pear production. Soil 2022, 8, 337–348. [Google Scholar] [CrossRef]
- Lladó, S.; Žifčáková, L.; Větrovský, T.; Eichlerová, I.; Baldrian, P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol. Fertil. Soils 2016, 52, 251–260. [Google Scholar] [CrossRef]
- Colette, M.; Guentas, L.; Patrona, L.D.; Ansquer, D.; Callac, N. Dynamic of active microbial diversity in rhizosphere sediments of halophytes used for bioremediation of earthen shrimp ponds. Environ. Microbiome 2023, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, G.; Tang, W.; Zhu, Q.; Li, X.; Du, C.; Li, C.; Li, J.; Zhao, C.; Zhang, L. Role of Sedum alfredii and soil microbes in the remediation of ultra-high content heavy metals contaminated soil. Agric. Ecosyst. Environ. 2022, 339, 108090. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and Its Role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef]
- Swędrzyńska, D.; Grześ, S. Microbiological Parameters of Soil under Sugar Beet as a Response to the Long-Term Application of Different Tillage Systems. Pol. J. Environ. Stud. 2015, 24, 285–294. [Google Scholar] [CrossRef]
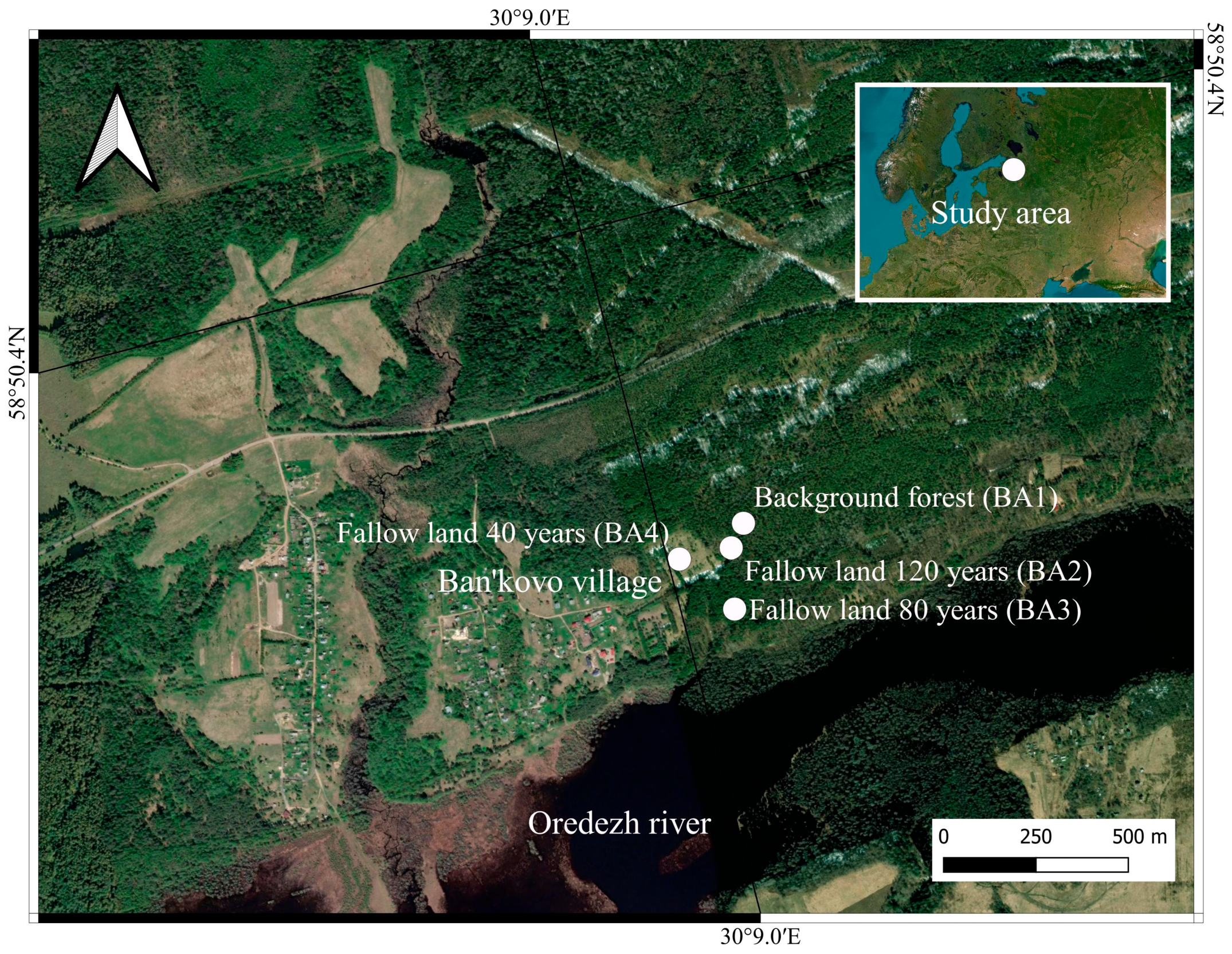
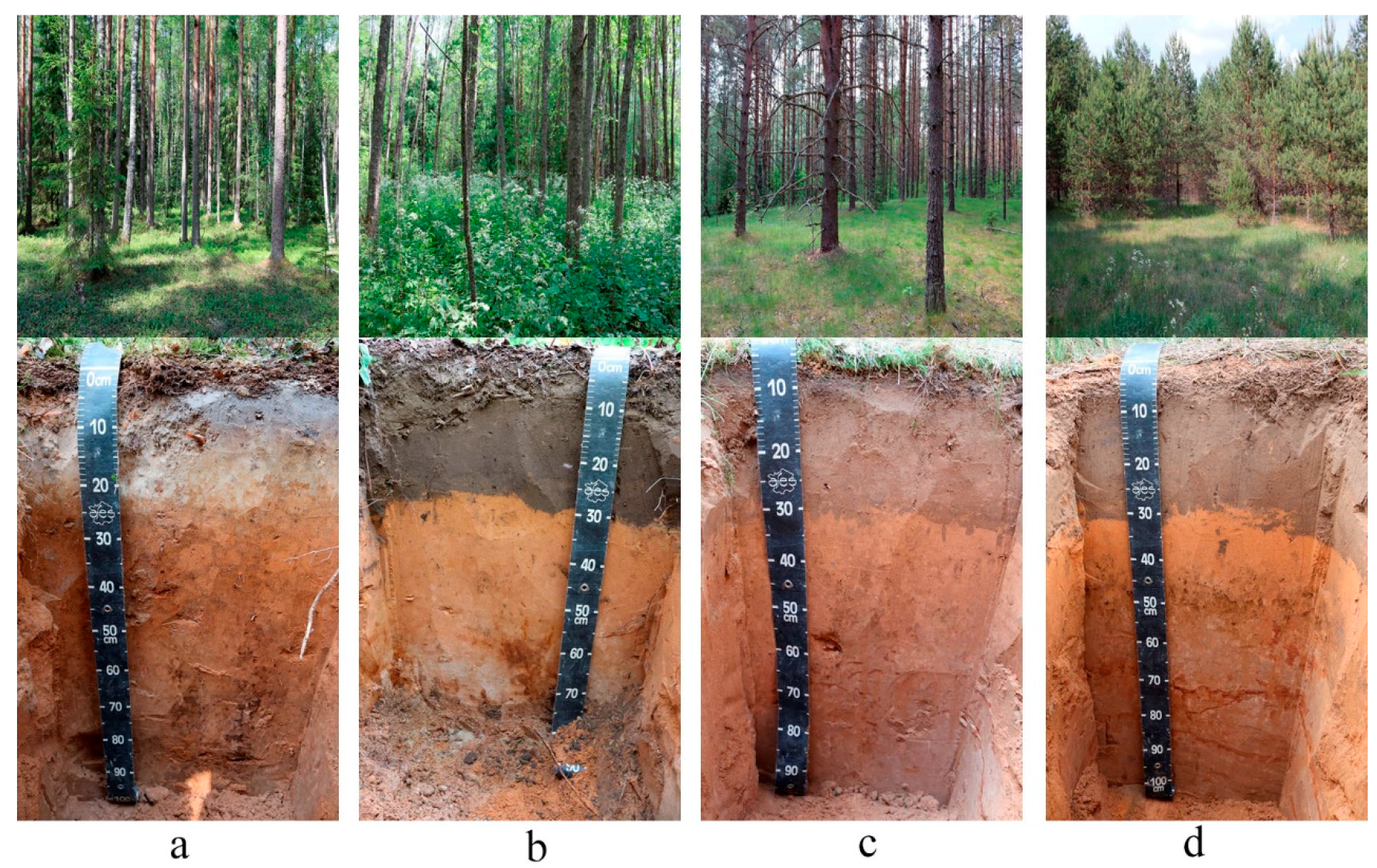


| Soil ID | Horizon * | Depth, cm | Description | Color | Location | Coordinates | Soil Name ** |
|---|---|---|---|---|---|---|---|
| BA1 | Oe | 0–4 | Moss cover, poorly decomposed | - | Benchmark forest | N 58.832090 E 30.153881 | Stagnic podzol (arenic) |
| E | 4–10 | Mineral horizon characterized by the loss of silicate clay, iron, and aluminum | 2.5YR 7/1 | ||||
| BA2 | O | 0–3 | Moss cover, slightly decomposed | - | Fallow land, 120 years old | N 58.831588 E 30.153031 | Plaggic podzol (arenic) |
| Ahp | 3–28 | Postagrogenic horizon, sandy loam, accumulation of leached mineral grains, abundance of roots, border uneven in color | 5YR 5/4 | ||||
| BA3 | Ahp | 0–30 | Postagrogenic horizon, sandy loam, the accumulation of leached mineral grains, abundance of roots, border uneven in color | 5YR 6/4 | Fallow land, 80 years old | N 58.830129 E 30.152454 | Plaggic podzol (arenic) |
| BA4 | Ahp | 0–30 | Postagrogenic horizon, sandy loam, abundance of roots, the accumulation of leached mineral grains, border uneven in color | 5YR 6/4 | Fallow land, 40 years old | N 58.831647 E 30.150548 | Plaggic podzol (arenic) |
| Bs | 30–70 | Horizon with illuvial accumulation of sesquioxides | 5YR 6/6 |
| Soil ID | Horizon | pHH2O | pHKCl | OC | N-NH4+ | N-NO3− | P | K | Particle Size Distribution | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Available Form, mg·kg−1 | Clay, % | Silt, % | Sand, % | |||||||
| BA1 | Oe | 4.22 | 2.24 | 43.7 | 90.07 | 11.18 | 250 | 158 | - | - | - |
| E | 4.98 | 2.43 | 1.04 | 3.88 | 0.36 | 29 | 25 | 4 | 4 | 92 | |
| BA2 | Oe | 5.85 | 4.76 | 4.70 | 9.43 | 2.12 | 205 | 28 | - | - | - |
| Ahp | 5.64 | 5.17 | 1.39 | 2.46 | 0.45 | 140 | 23 | 5 | 7 | 88 | |
| BA3 | Ahp | 5.68 | 4.17 | 0.62 | 1.36 | 0.36 | 222 | 505 | 3 | 4 | 93 |
| BA4 | Ahp | 5.89 | 4.33 | 1.44 | 1.1 | 0.36 | 276 | 397 | 4 | 7 | 89 |
| Bs | 5.85 | 4.55 | 0.38 | 0.68 | 0.18 | 106 | 522 | 3 | 7 | 90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrishchev, A.; Litvinovich, A.; Abakumov, E.; Kimeklis, A.; Gladkov, G.; Andronov, E.; Polyakov, V. Soil Microbiome of Abandoned Plaggic Podzol of Different-Aged Fallow Lands and Native Podzol in South Taiga (Leningrad Region). Agronomy 2024, 14, 429. https://doi.org/10.3390/agronomy14030429
Lavrishchev A, Litvinovich A, Abakumov E, Kimeklis A, Gladkov G, Andronov E, Polyakov V. Soil Microbiome of Abandoned Plaggic Podzol of Different-Aged Fallow Lands and Native Podzol in South Taiga (Leningrad Region). Agronomy. 2024; 14(3):429. https://doi.org/10.3390/agronomy14030429
Chicago/Turabian StyleLavrishchev, Anton, Andrey Litvinovich, Evgeny Abakumov, Anastasia Kimeklis, Grigory Gladkov, Evgeny Andronov, and Vyacheslav Polyakov. 2024. "Soil Microbiome of Abandoned Plaggic Podzol of Different-Aged Fallow Lands and Native Podzol in South Taiga (Leningrad Region)" Agronomy 14, no. 3: 429. https://doi.org/10.3390/agronomy14030429
APA StyleLavrishchev, A., Litvinovich, A., Abakumov, E., Kimeklis, A., Gladkov, G., Andronov, E., & Polyakov, V. (2024). Soil Microbiome of Abandoned Plaggic Podzol of Different-Aged Fallow Lands and Native Podzol in South Taiga (Leningrad Region). Agronomy, 14(3), 429. https://doi.org/10.3390/agronomy14030429







