1. Introduction
The availability of carbohydrates in grapevines has been reported to impact inflorescence primordia initiation and development [
1,
2,
3], bud viability [
4], inflorescence count and flower numbers in each inflorescence [
2] and their growth and survival [
3,
4,
5,
6]. Ultimately, these factors impact on the following season’s vine fruitfulness and yield.
Total non-structural carbohydrate (TNC) reserves are depleted to support the growth of the current season shoot from budburst to flowering and fruit set, a period in which the leaf canopy is forming. TNC availability during this period is also considered to influence the initiation and development of inflorescence in latent buds that produce fruit in the following season [
3]. In a subtropical grape-growing environment, the budburst to flowering period is a short period compared to temperate climates. Further, temperatures above 30 °C, as commonly experienced in subtropical production environments such as in central Queensland Australia, can adversely impact photosynthesis by impairment of Rubisco carboxylation activity. For example, the optimum temperature for photosynthesis of the Semillon grapevine leaves was recorded at 25 °C in ambient light, and 30 °C while in a light saturation condition [
7]. Also, a higher root zone temperature (23 °C), compared to a lower root zone temperature (13 °C), was associated with a decrease in vine starch concentration by 20% and 40% in trunk and root tissues, respectively [
8]. Competing demands for reserve carbohydrates for canopy re-establishment and fruiting could impact inflorescence initiation and the differentiation process, and thus impact on yield in the following season.
Over 90% of vine TNC is attributable to root and trunk reserves [
9], with root tissues often contributing more to the TNC reserves than wood and shoot tissues [
9,
10,
11]. For example, the TNC content in the roots and trunk was 338 and 181 g, respectively, in a 14-year-old Pinot Noir vine [
9], and 404 g and 1792 g, respectively, in a 15-year-old Cabernet Franc vine [
12].
The TNC concentration within vines varies seasonally, with a large decrease associated with the onset of spring and re-establishment of canopy. In a review of carbohydrate dynamics in grapevine, Holzapfel et al. [
13] reported that the TNC of grapevine roots declined from ca. 22–25% w/dw at budburst to ca. 5–16% w/dw during flowering, and trunk TNC decreased from ca. 18–20% w/dw at budburst to ca. 10–12% w/dw at flowering. The TNC is gradually replenished in the roots and trunk during the period from flowering until leaf fall in cooler climates [
13,
14]. Holzapfel et al. [
10] reported that TNC (1.36 kg/vine) started to decrease 26 days before budburst and declined by more than half 8 days after flowering (<680 g) in 12-year-old grapevines (6.43 kg dry mass of vine during leaf fall) in Wagga Wagga, NSW, Australia. Bates et al. [
15] reported that up to 78% of starch in roots was utilised before flowering.
The mean biomass of 12-year-old own-rooted Thompson Seedless (table grape) vines growing in a subtropical environment (Emerald, Queensland, Australia) ranged from 9.9 kg to 13.7 kg during leaf fall [
16]. For a grapevine of 10 kg dry mass and average TNC of 12% w/dw before budburst and 6% w/dw at the flowering stage, a decrease in reserves from 1200 g to 600 g per vine is inferred.
Several methods could be employed to improve source–sink relationships and mitigate such a depletion of reserves in subtropical table grapes. Pressurised xylem injection techniques have been used to introduce minerals, soluble sugars and pesticides into tree crops. Commercial microinjectors (for example, Arborjet
® Inc., Woburn, MA, USA; Chemjet
® Tree injector, Banyo, QLD, Australia) are used to infuse small volumes (typically 10–20 mL) of pesticide formulation. Navarro et al. [
17] developed a low-pressure (60–80 kPa) injection system where a latex tube connected a reservoir to a drill hole in the tree trunk. This was used to deliver a marker of apoplastic flux and rubidium chloride into young olive trees, with an uptake of only 20 mL achieved over 10 to 95 min. Iglesias et al. [
18] used the same system to inject 10%
w/
v sucrose over 75 days into citrus trees. However, a high variation in solution uptake between trees was reported with a maximum injection of only 50–150 g in the five maximum uptake trees. The causes of low uptake and variation were not discussed. Martínez-Trinidad et al. [
19] employed a commercial injector using multiple injections to deliver sugar solutions. Approximately 10 L of solution (12%
w/
v) was injected into an oak tree over two seasons, and a significant increase in trunk growth over a non-treated tree was reported.
This study monitored the seasonal carbohydrate dynamics in the trunk and roots of table grape vines grown in the subtropical environment of Central Queensland, Australia, in context of following season inflorescence count over two seasons. A constant low-pressure injection method for the delivery of sucrose solution into the trunks of individual vines was employed to replenish depleted carbohydrates during the high demand period (i.e., budburst to flowering), aiming to improve the vine’s fruitfulness and yield in the ensuing season.
2. Materials and Methods
2.1. Stem Injection Method Development
Commercial root and trunk injectors are intended for the injection of a small volume of pesticides. To facilitate injection of a large volume of sucrose, a number of exercises were performed starting from intravenous drip bags (CompoSelect, Selangor, Malaysia) as a reservoir, supplying 2.5–10%
w/
v sucrose under a 10–15 kPa gravity head to vine trunks of potted vines in a glasshouse. Sucrose solution was prepared using commercial cane sugar (100% sucrose, Woolworths, Sydney, Australia) and reverse osmosis water. However, uptake rates were low, and therefore a constantly pressurised low-pressure injection system (69 kPa) delivering 5%
w/
v sucrose was developed (
Supplementary Materials S1). In essence, a 2HP 21 L ProjectAir (Bunnings, Hawthorn East, Australia) compressor was fitted with two regulators and was used to deliver air via pneumatic tubing (6 mm outer diameter) to each vine ‘feed’ bottle (
Supplementary Materials S1). The autoclaved 2.5 L brown glass ‘feed’ bottles were filled with 2 L of sucrose solution prepared in the laboratory. Bottles were covered with aluminium foil to prevent direct exposure of the solution to light (
Supplementary Materials S1). A tubing inlet was fitted above the level of solution and used to pressurise the bottle. An outlet port had tubing extending to the bottom of the bottle, with tubing continuing to the injection port on the vine. The drill tool and the trunk surface were sterilised with 70% ethanol before injecting the trunk. Pressure was optimised at 69 kPa, as lower pressures resulted in a lower injection rate, and higher pressures resulted in fluid leakage from stem cuts and petiole scars.
The initial liquid level of each bottle was marked with a white permanent marker and solution was added to maintain this level. An isolation tap allowed individual bottles to be handled without affecting the pressure of the whole pressure system. Top-up volumes for each vine were measured by the cylinder and recorded. Once the target injection volume was achieved, the vine bottle was isolated from the pressure source.
The vine trunk was drilled 10–30 cm above the graft union to 30 mm depth with a 6 mm diameter bit while constantly wetting the cut surface. The holes were drilled parallel to the ground surface using a Ryobi 12 V 1.3 Ah drill driver (Bunnings, Hawthorn East, Australia). A two-end brass coupler (outer diameter 6.35 mm, 0.31 × 0.31 mm inner tube diameter, equal union; IFS standard brass No. 401, Norosco, Rockhampton, Australia) was then inserted into the drill hole without delay and connected with a bolt and delivery tubing (
Supplementary Materials S1—Figure SII) to the feed bottle. The coupler was compatible with a 3 mm diameter flexible pneumatic tube (Dixon, Chesterton, MD, USA; working pressure rated to 150 psi). Implements were washed with 70% ethanol to maintain sterile conditions. After injection was terminated, a wooden dowel was used to plug the cavity following removal of the injector.
The process of vine drilling was repeated twice during each injection period in each year.
2.2. Description of Site and Vines
Field experiments were conducted in a uniform well-managed (average annual yield of 7.1 ± 3.1 kg/vine over 12 years to 2014) commercial table grape vineyard (Menindee Seedless grafted on 5BB Kober rootstock established in 1999) located at latitude 23°35′ S, longitude 148°12′ E and altitude 199 masl, Central Highlands, Queensland, Australia. The soil at this site is a vertosol (a black cracking clay). Vines were 15 years old at the start of experimentation in 2014. Vines were cane-pruned during weeks 3–4 of June in each year, trained to a 1.8 m wide sloping T-trellis system and spaced at 2.4 m in rows with 3.4 m between rows. Major growth stages are summarised in
Table 1.
A consistent method for vine pruning was followed throughout the trial period. The number of nodes per vine in fruiting canes was maintained at 96 (8 canes/vine and 12 nodes/cane) for both the 2015 and 2016 seasons. Similarly, 6–8 renewal spurs per vine (4 nodes/spur) were also maintained to balance vegetative growth.
2.3. Experimental Design and Treatments
Initially, a randomised complete block design was employed using three replications and five treatments with three vines in an experimental unit, i.e., a total of 45 vines in three rows. The treatments consisted of a control (no injection), injection of 3 L of water and injection of 5%
w/
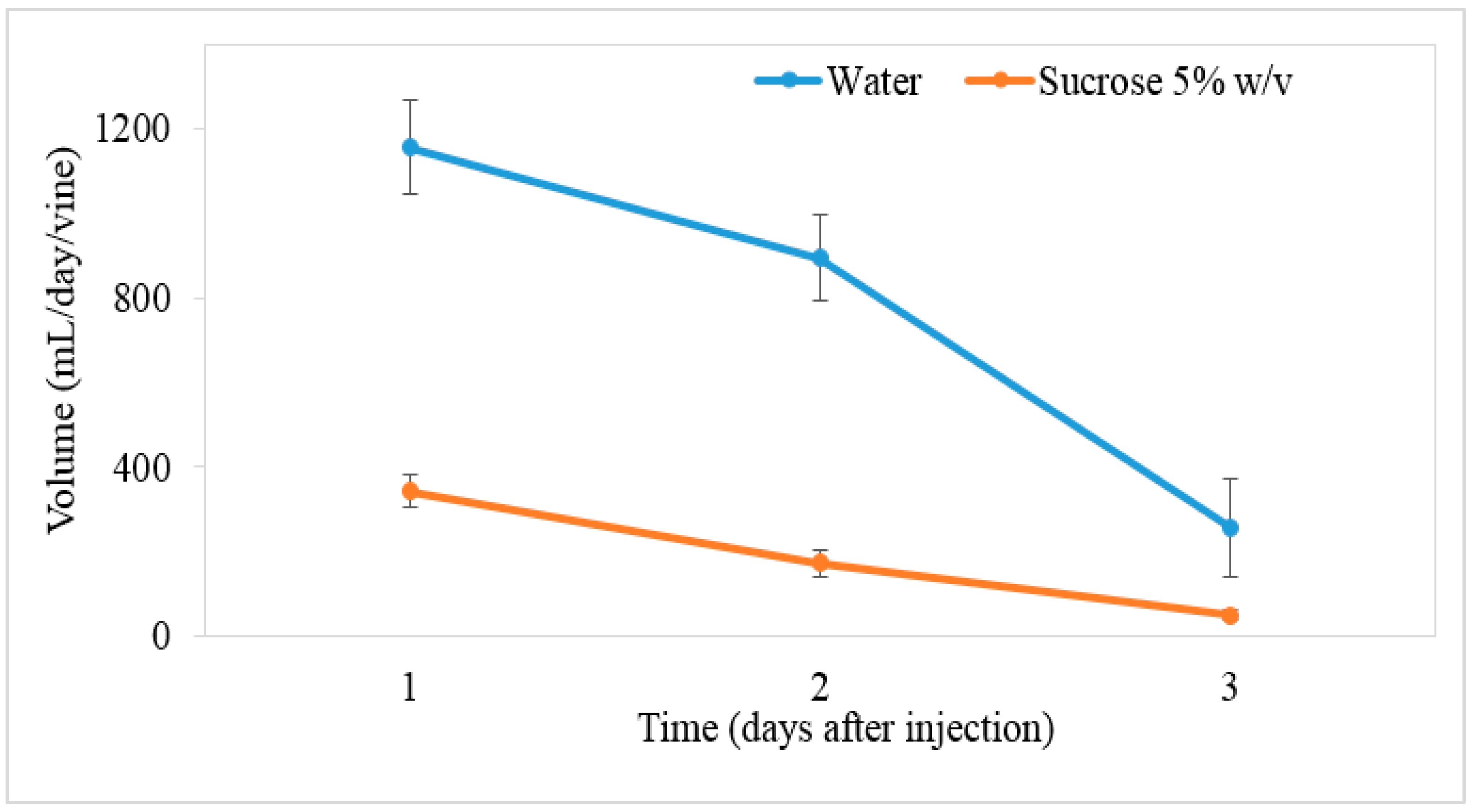
v sucrose to deliver three amounts of sucrose: 150, 300 and 600 g per vine. Solution injection was undertaken between budburst and flowering in each season (7 August to 20 September in 2014 and 5 August to 18 September in 2015). A high level of variation in solution uptake between vines was noted and the injected volume declined over the injection period (
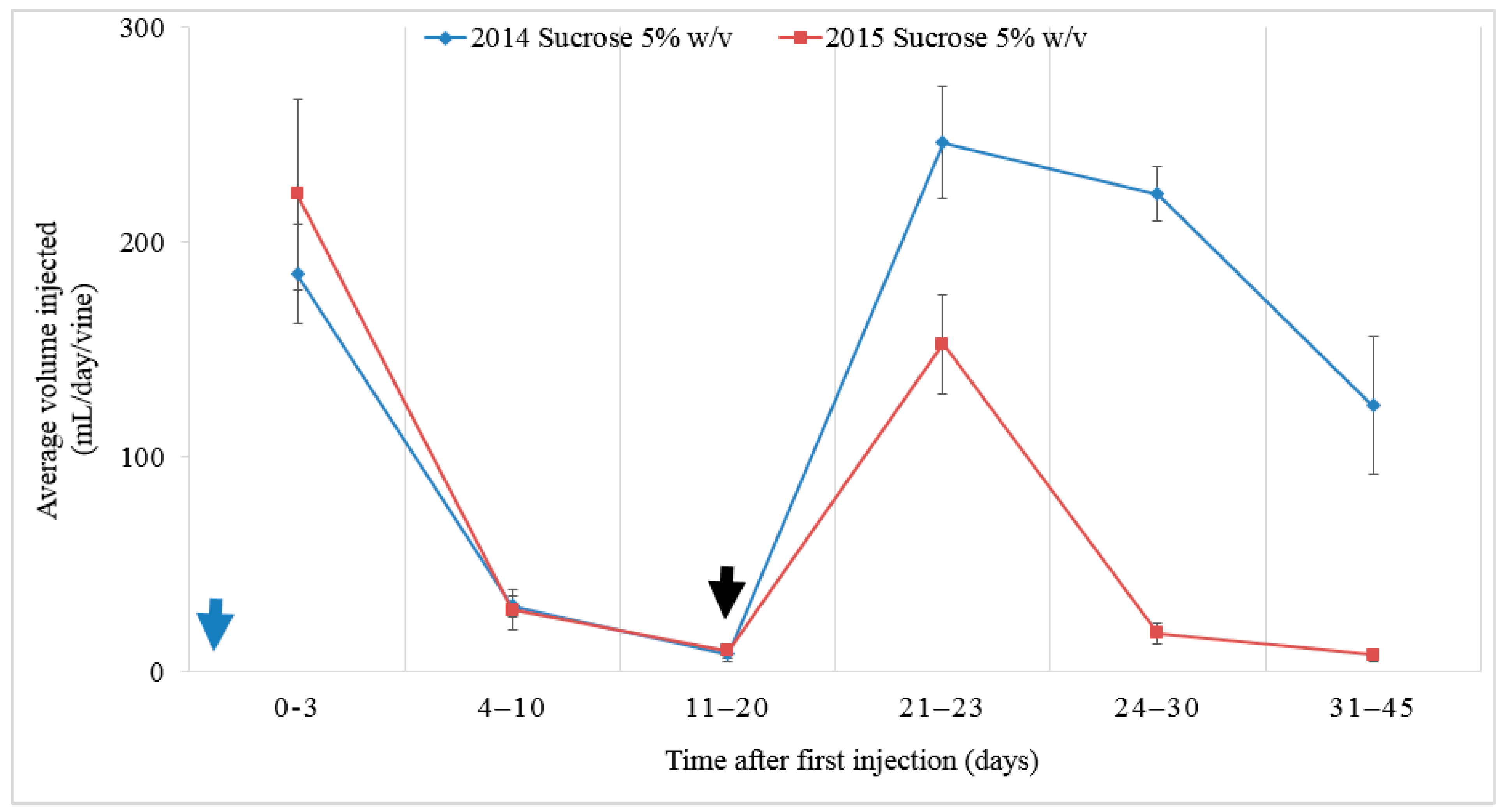
Figure 1). The desired injection volume of sucrose solution per vine was not achieved. Twenty days from the start of injection, 50% of water-injected vines had reached the targeted input volume (3 L); however, sucrose-injected vines had quite a low uptake (50 to 2150 mL/vine, with an average of 777 mL/vine) and no vines reached the targets of 3, 6, or 12 L (150, 300, or 600 g sucrose/vine). A second drill was undertaken 20 days after the first drill in an attempt to increase injection volume.
The experiment was therefore redesigned, adopting the nine vines with the highest sucrose input as replicates for the injection treatment and the nine no-injection vines as the control treatment for the tissue sampling and fruitfulness study.
2.4. Vine Characterisation and Tissue Sampling Procedure
For the assessment of seasonal variation in carbohydrate reserves, samples were taken from different vine tissues at major growth stages over years from budburst (August) to flowering (September) (
Table 2) from 18 vines (9 control and 9 sucrose-injected). A 5.15 mm diameter trunk core was sampled using a Haglof increment borer (Haglof, Sweden) approximately >40 cm above the injection point, as preliminary experiments determined that the sampling positions of the trunk core did not differ in carbohydrate concentrations between the graft union and trunk head. Root samples (2–5 mm diameter) were collected to 15–20 cm around the trunk at 5–30 cm soil depth. Some stem cores had necrotic tissues and that tissue was removed from the core before TNC analysis, as TNC concentration in the gross trunk core was greatly influenced by the proportion of necrotic tissue (
Supplementary Materials S2). Samples were collected during the budburst and flowering stages, before and after sucrose injection. Core and root samples were cut into small pieces and oven-dried at 65 °C until constant weight (60–72 h). Additionally, for assessment of the tissue’s natural stable carbon isotope signature (δ
13C), young shoots were collected at flowering and berries were collected at harvest in 2014, and then were dried and ground as described below.
To elucidate the cause of the large variation in injection volume observed between the vines, five sucrose-injected and non-injected vines were destructively sampled after harvesting in 2016. Vine trunks were cut into 3–5 cm thick slices at 10, 50 and 100 cm above the graft union and transported overnight in an insulated bag to the National Wine and Grape Industry Centre, Charles Sturt University, Australia, for pathogen detection following the protocol of Pitt et al. [
20]. Briefly, a surface-sterilised wood sample was transferred to potato dextrose agar supplemented with streptomycin sulphate and incubated at room temperature until fungal colonies were observed. Isolates were examined and identified according to conidial size, shape, colour and the presence or absence of septation.
2.5. Carbohydrate and δ13C Assessment
Dried tissue cores and roots were ground in a heavy-duty cutting mill (Retsch® ZM1000, Haan, Germany) fitted with a 0.12 mm sieve and operated at 10,000 rpm. Samples were stored at −20 °C in air-tight Eppendorf sample tubes (stem core and shoot samples) or zipped plastic bags (root samples) until analysis.
Total non-structural carbohydrates were assessed with a sequential enzymatic degradation method using enzymatic assay kits (Megazyme International, Bray, Ireland). Sub-samples of ground tissue were taken (20 ± 2 mg), placed in 10 mL glass tubes, and soluble sugars were extracted twice with 1.25 mL of 80% v/v aqueous ethanol in a 80 °C water bath for 10 min and then extracted with a 1.25 mL wash of 80% v/v ethanol at room temperature. Extracts were centrifuged for 5 min at 1811× g (3000 rpm using rotor 18 cm in a 5810 R centrifuge, Eppendorf, Hamburg, Germany) and were combined into a 15 mL centrifuge tube. After diluting the combined aliquots with pure water to make a final volume of 10 mL, the tubes were centrifuged again for 5 min. The concentration of soluble sugars (D-glucose, D-fructose and sucrose) was determined according to the procedure as outlined in K-SURFG (Megazyme International, Bray, Ireland). This method involved the conversion of sugar to glucose-6-phosphate (G6P) and the quantification of NADPH in the presence of NADP+ and the enzyme G6P-dehydrogenase.
The remaining insoluble material was resuspended in 200 μL dimethylsulfoxide (DMSO), stirred and heated in a boiling water bath for 10 min following the DMSO format outlined in K-TSTA (Megazyme International, Bray, Ireland). Thermostable α-amylase in sodium acetate buffer (600 μL) was then added, mixed and incubated for 15 min in a 98 °C water bath, where they were stirred vigorously three times at 3–5 min intervals. The incubation tube was then transferred to a 50 °C water bath for 15 min, and 20 μL of amyloglucosidase was added, mixed and held for 30 min in the water bath. The tube volume was then adjusted to 10 mL for trunk wood samples and 15 mL for root samples using pure water, and the tube was centrifuged at 1811× g for 10 min. An aliquot (50 μL) was transferred to a culture tube and 1.5 mL of GOPOD reagent (a mixture of glucose oxidase, peroxidase and 4-aminoantipyrine in a potassium phosphate and ρ-hydroxybenzoic acid buffer) was added. The tube was covered with film and incubated for 20 min at 50 °C. Absorbance was assessed using 2.5 mL semi-micro cuvettes (Starna, Castle Hill, NSW, Australia) at 510 nm using a Varian Cary 50 UV-VIS spectrophotometer. The amount of starch in the original sample was calculated following the procedure outlined in the assay kit. Repeatability was assessed as the standard deviation (0.96% w/dw) of replicate (n = 4) assessments of regular maize starch (supplied with kit). A standard starch sample was included in each batch of determinations.
About 6–8 mg of samples (young shoots collected at flowering, and trunk core, root and berry samples collected at harvest in 2014) of the nine sucrose-injected vines and three control vines were subjected to δ13C (‰) analysis using a SerCon Hydra 20–22 isotope ratio mass spectrometer (Stable Isotope Laboratory, Griffith University, Gold Coast, QLD, Australia). The reproducibility (sd, n = 13) of the standard (NCSPT15D; −11.6‰) was 0.03‰.
2.6. Budburst and Fruitfulness Assessment
The number of canes and buds per vine was controlled and standardised by pruning to maintain a consistent vegetative and reproductive load across all experimental vines. Three weeks after budburst, each node was counted and described following the method of Antcliff et al. [
21] as (i) no budburst, (ii) budburst with shoot only, or (iii) budburst with at least one inflorescence. Percentage budburst was defined as the percent of nodes which had burst (Equation (1)) and percentage observed fruitfulness was defined as the percent of nodes with at least one inflorescence (Equation (2)).
Inflorescences arising from tissues other than cane (e.g., spurs) were termed ‘extra inflorescences’ and counted in the estimate of the total number of inflorescences per vine. Stunted/arrested-growth inflorescences and tendril inflorescences were not counted as they were unsuitable for the table grape market.
2.7. Statistical Analysis
Temporal variation in carbohydrate concentration dynamics was subjected to a repeated measures analysis over the growth stages within seasons (budburst 2014 to dormancy 2015 for season 2015 and budburst 2015 to dormancy 2016 for season 2016) using residual maximum likelihood (REML) and modelling the variance–covariance matrix as an antedependence structure of order 1 to account for the correlation structure induced by the repeated sampling. The model included the fixed effects of treatment (sucrose injected or control), growth stage, season (2015 and 2016, as described above) and their interactions, and the random effect of vines over months. The model was simplified by sequentially removing non-significant terms but always retaining the main effects.
Multiple linear regression was employed to investigate the relationship between carbohydrate reserve concentrations at different stages of inflorescence primordia growth and development and the following season’s vine inflorescence count. Regression analysis was performed to select the most influential terms to be included in the final regression model.
Analysis of variance and a t-test were employed for the analysis of δ13C between the control and sucrose-injected vines. All analyses were performed using Genstat 18 (VSN International, Hemel Hempstead, UK). Mean separation was performed by Tukey’s honest significance test (hst) (α = 0.05).
4. Discussion
4.1. Factors Limiting Sucrose Injection in Grapevines
The injected volume of sucrose (5% w/v) greatly varied between vines within seasons and between seasons, and the rate declined over the injection period. Three hypotheses for these variations were considered: (i) tylose formation; (ii) pectin swelling; and (iii) air embolisms associated with xylem tissue necrosis.
There was no evidence of physical vessel blockage (e.g., tyloses or microbiota) in the hand sections of control or sucrose 5% w/v-injected vines.
A decrease in the hydraulic conductance of the xylem has been noted during infusion lacking cations, presumably associated with hydrogel swelling of pectins [
22]. This decrease was ameliorated by the inclusion of 10 mM KCl in sugar solution for conductance of sucrose through cuttings of Menindee Seedless grapevine [
23]. This was achieved using a healthy shoot cutting model where sucrose was supplied in 10 mM KCl via the transpiration stream over four days to the equivalent of 37% of the total dry weight of the cutting. The failure to include K in the injection solutions was a flaw in the current study; however, this limitation applied equally to the control and sucrose treatments.
A weak positive relationship has been reported between the concentration of TNC and the degree of xylem embolism in two grapevine cultivars [
24]. The induced embolisms may have influenced the amount of injected sucrose in the present study if sucrose alone were responsible for the embolisms.
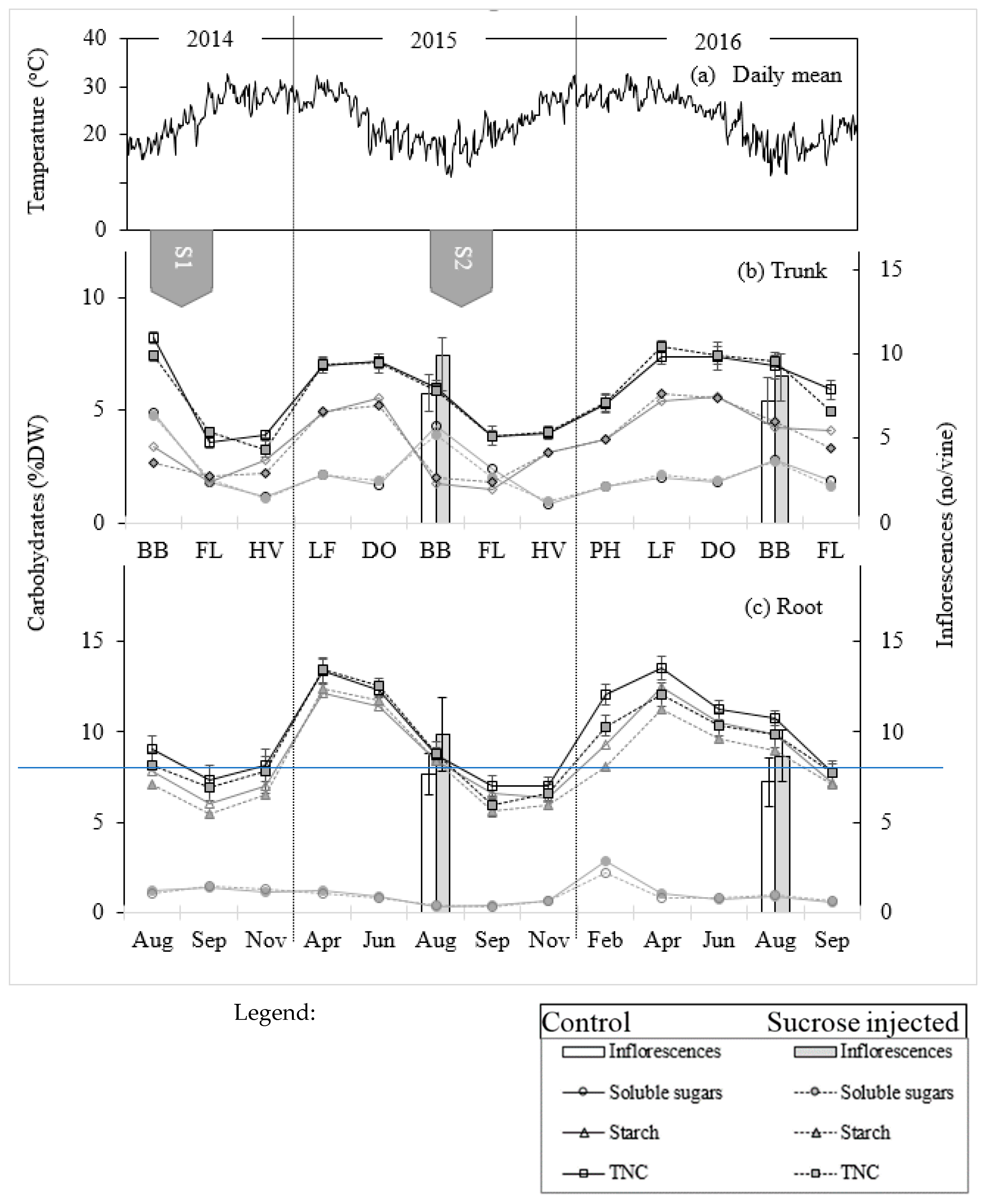
All five vines (three injected and two controls) that were destructively sampled contained necrotic xylem tissue, increasing from 11 to 47% of cross-sectional area from the base to the top of the trunk (
Figure 4;
Supplementary Materials S2). All 15 samples, taken from the five vines, were positive for
Botryosphaeria spp., i.e., the causal agent of Botryosphaeria dieback of grapevine. This pathogen has been associated with vineyard decline in the Hunter Valley, New South Wales, and in South Australia [
20]. This pathogen had not been reported in this vineyard before the experiment but is likely to have been present in the vineyard for many years, progressing slowly through the trunk and contributing to a general yield decline. Tissue necrosis, particularly in the second season of injection, is a likely cause of variability in rates of injection, and tissue death may be the cause of the decline in xylem conductance.
Areas of the xylem around the injection drill holes had also become necrotic by the following season. It is possible that the xylem damage (necrosis) exacerbated by two drills for injections and the number of trunk/arm core samples collected from each trunk for carbohydrate dynamics was also responsible for the decreased uptake in the second season.
There are various methods of detecting decay within trees or vines. For example, the Tree Radar Unit™ (TRU™) is a non-invasive method. Such technology could be used to quantify decay in the trunk and roots, and then utilised with a management plan for vine replacement or treatment of the causal agent if sucrose injection were to become a useful management practice.
4.2. Carbohydrate Concentration and Dynamics
The average TNC concentration in the root and trunk tissue of the control vines ranged from 13.5% and 7.5% w/dw at leaf fall to 7.2 and 3.7% w/dw at flowering, respectively. This range is somewhat less than that reported in wine grape cultivars grown in cooler climates. In a study of 34 vineyards across NSW, Australia, the TNC at leaf fall ranged from 9.9 to 41.8% w/dw in roots and from 3.4 to 20.7% w/dw in the trunk [
10]. In a similar management practice, the trunk starch concentration of Shiraz was high at different growth stages (i.e., 12–16% w/dw) compared to Cabernet Sauvignon (2–6% w/dw) and showed a strong genetic base related to carbohydrates [
25]. The lower carbohydrate concentration in our study could be a characteristic of this cultivar and growing environment.
In the current study, one of the tissue samples was obtained when 50% of buds had burst, and thus a decrease in TNC relative to the dormant period sample was evident. Hydrogen cyanamide was applied ~20 days before budburst, a treatment expected to enhance starch hydrolysis and a transient accumulation of soluble sugars in the bud [
26]. The observed rapid decrease in TNC concentration from budburst to flowering is similar to that reported for a range of grapevine cultivars and growing environments [
11,
14,
27]. Carbohydrate reserve recovery usually starts after flowering and continues until leaf fall in cooler-climate vineyards [
2,
11]. In the current study, this recovery was evident only after harvest, a result attributed to the carbon demands of accelerated berry growth and development in the subtropics. Thus, the TNC dynamics of grapevine in cooler and warmer climate are remarkably different.
The use of δ13C (‰) analysis allows for an interpretation of the contribution of the injected sucrose to the total carbohydrate pool and the result is consistent with the utilisation of the injected sucrose in growing shoots rather than in trunk and roots. However, the correlation between the amount of loaded sucrose and δ13C in young shoot tissue was poor (r2 = 0.16). Either the proportion of injected sucrose partitioned to the developing shoot or the proportion of this sucrose lost from the young shoot as phloem export or respiration was variable.
4.3. Inflorescence Count, Budburst and Observed Fruitfulness
Previous studies [
2,
5,
27] have reported a relationship between TNC concentration in trunk and root tissue in the prior season and the inflorescence number per vine in the current season. Such a relationship, between TNC concentration in trunk and root tissue in the prior season and the inflorescence number per vine in the current season, was not observed in the current study. The previous studies involved temperate to warm temperate climate vineyards and defoliation treatments rather than fruit load management. Early de-fruiting practices can enhance the build-up of TNC reserves, especially in the roots, and yield in the following season(s) [
28], whereas continuous defoliation at harvest results in a decline in TNC concentrations and yield in the following season, and more so if the defoliation is repeated over two seasons.
The observed range in budburst (%) and node fruitfulness (%) was consistent with that reported by Marquez Cervantes et al. [
29] and Leão and Silva [
30] for Menindee Seedless. Leão and Silva [
30] observed 12% fruitfulness in Menindee Seedless as compared to 17%, 14% and 37% for Perlette, Thompson Seedless and Morro Seedless, respectively. However, these levels of fruitfulness are low compared to those reported by Sánchez and Dokoozlian [
31] for the table grape cultivars Thompson Seedless and Flame Seedless with more than one bunch per node in medium to high levels of light exposure. Budburst and node fruitfulness percents were dependent on node position, with the first three nodes recording lower rates than more distant nodes (where the budburst percent was between 60 and 80%) (
Supplementary Materials S3). Irrespective of treatment, i.e., control or sucrose-injected, there were no fruitful buds in node position 1; <5% of nodes were fruitful in nodes 2–4; and the highest fruitfulness was noted in nodes 10–12. The average node fruitfulness recorded for Menindee Seedless in the 10–12th node positions was more than 6 times higher than in the 2nd–4th node positions in both years. The fruitfulness was very low compared to that reported in other cultivars; however, a tendency for lower bud fruitfulness in basal nodes (1–4) and an increase with distal node positions have been reported in a number of studies [
30,
31]. Low bud fertility coupled with only one inflorescence in more than 97% of nodes resulted in low yield in this cultivar.
4.4. Influence of Reserve Carbohydrates on Yield Attributes
The variation in crop load (up to six-fold between vines) was much higher than the variation between vines in TNC concentration in the roots and trunk at harvest (1.5- and 2.5-fold, respectively). For example, in the 2015 season harvest, the coefficient of variation for vine yield (kg/vine) of 18 vines was 63%, while the coefficients of variation for root and trunk TNC concentration were 23 and 11%, respectively.
Studies such as those of Smith and Holzapfel [
28] and Pellegrino et al. [
25] show the effect of cultural practices on current season fruitfulness and yields, but the effects on the following season’s fruitfulness and yields have rarely been studied, particularly in the context of subtropical grape production. For example, the cultural practices of deficit irrigation in warm-climate vineyards of the Sunraysia region, Victoria, Australia [
25], resulted in lower leaf and trunk starch concentrations and current season yield, but the following season’s fruitfulness was not documented. An exception is the study in [
1], where carry-over effects of treatments designed to diminish springtime non-structural carbohydrate concentration (albeit measured in roots) were evident for berry mass and berries per cluster even into a third season.
5. Conclusions
A constant low-pressure, continuous, trunk injection system was developed with the potential for loading sucrose at amounts relevant to the carbon economy of the vine (c. 25–30% of total TNC available during flowering), such that injection might support better vine fruitfulness. However, a consistent decline in solution uptake was observed, related to the necrosis of tissue around the drill wound of the injection site. Further, the vines were discovered to be subject to a trunk pathogen, most likely contributing to variability in injection rates between injection sites. An assessment of xylem health is therefore necessary before trunk injection.
In this study, carbohydrate reserve recovery in both root and trunk tissue continued after harvest. It is therefore recommended that the vine management practices (e.g., continued application of nitrogen) also need to allow for the restoration of carbohydrate reserves after harvest. Carbon loading was not associated with increased crop load in the current or subsequent seasons, suggesting that the primary reason for yield variation lies elsewhere than TNC concentration in the trunk and roots. The lack of a significant response of fruitfulness and yield to sucrose injection could be due to the poor uptake of sucrose solution, and a relationship between TNC concentration and bud fruitfulness or primary bud necrosis might exist by looking at TNC in the bud rather than in the trunk and roots.
Further evaluation of sucrose injection in field vines without pathogen complications and using multicomponent injection solutions including KCl is recommended.