Doubled Haploid Lines Derived from a European Maize Flint Landrace Contrast in Recovery from Cold Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growth Conditions
2.3. Phenotyping
2.4. Statistical Analysis
3. Results
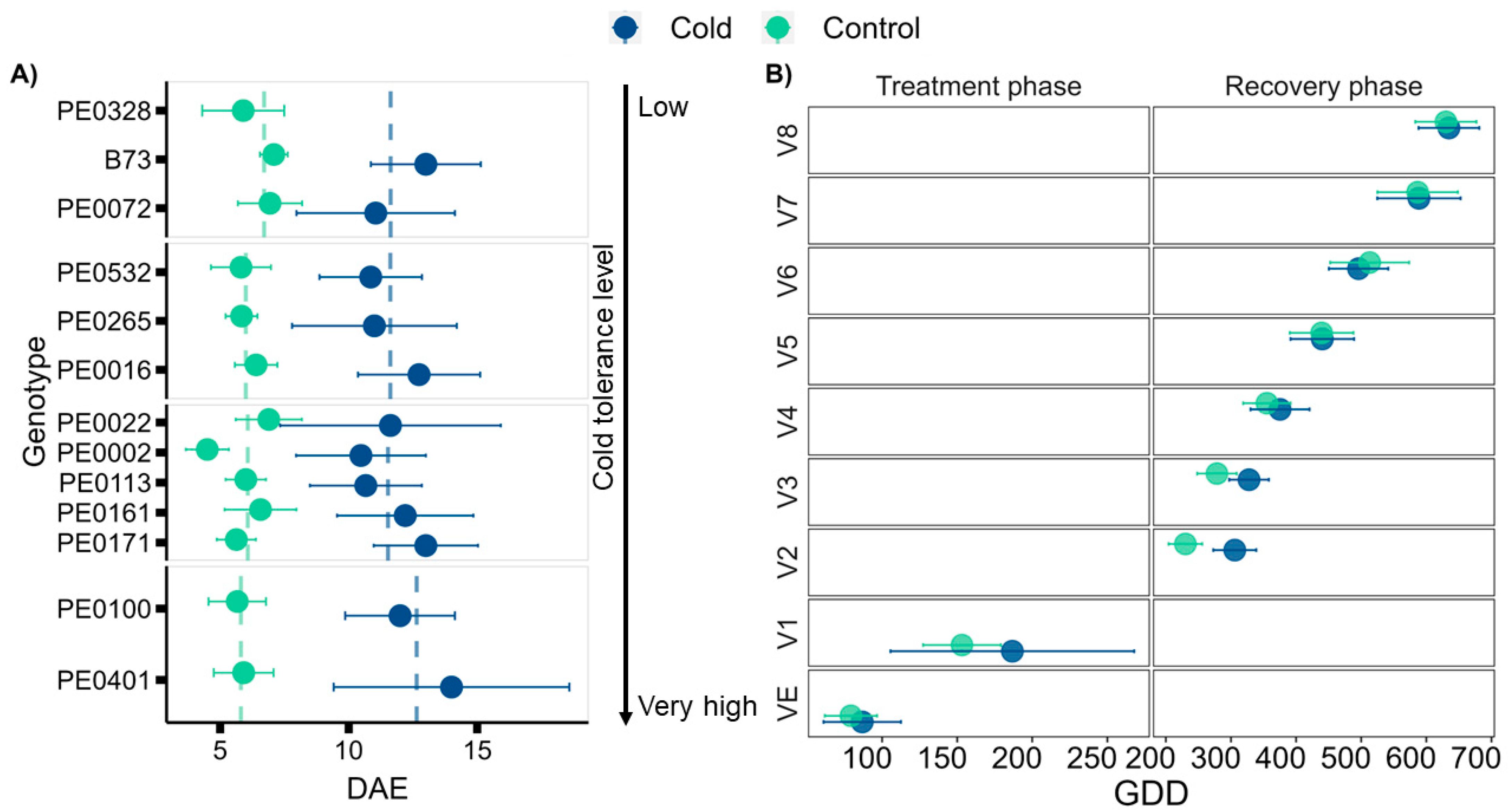
3.1. Seedling Cold Tolerance Depends on the Genotype
3.2. Early Cold Stress Delays Vegetative Development during and after Exposure
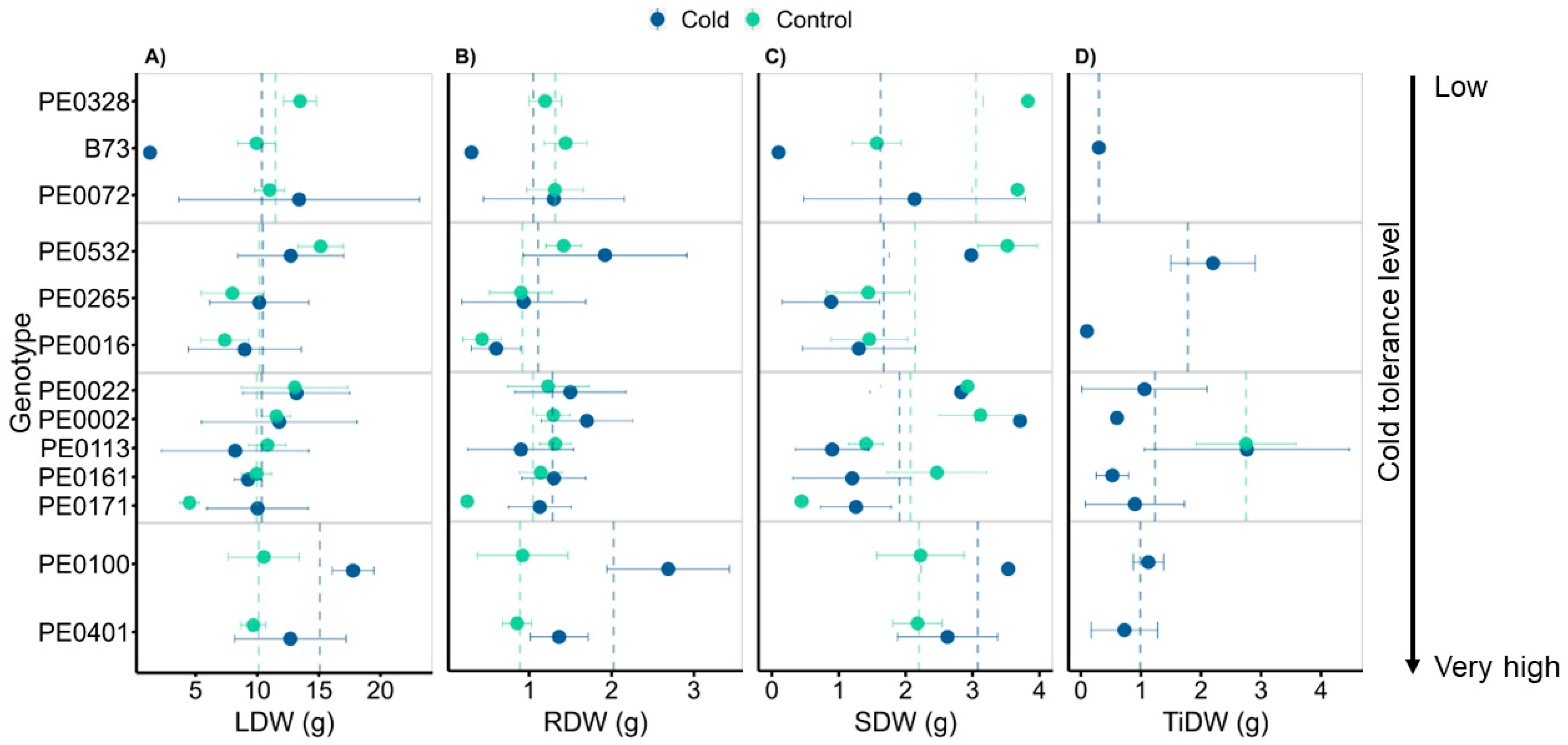
3.3. Genotypes Differed in Biomass following Cold Exposure
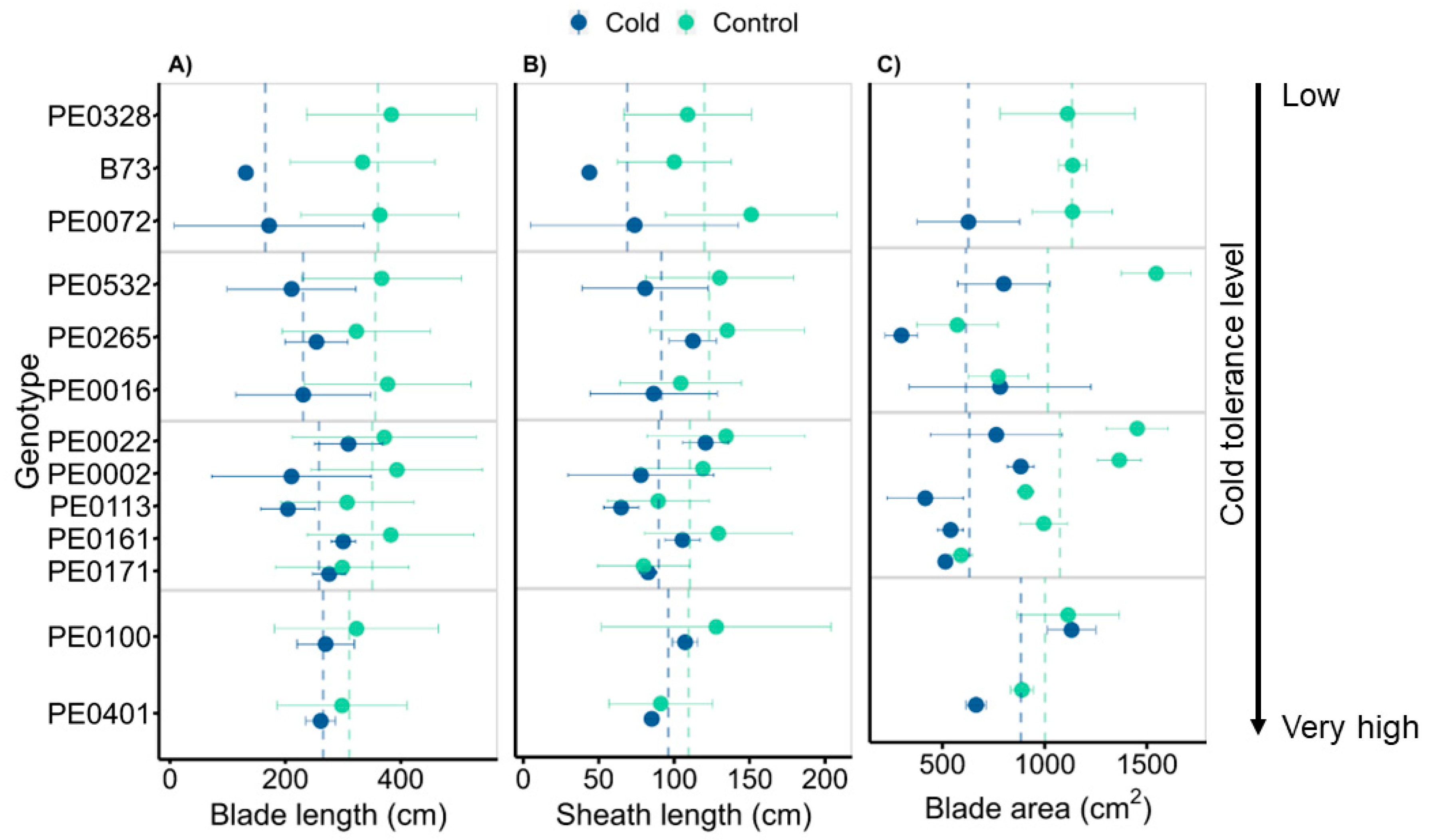
3.4. Genotypes Vary in Leaf Morphology Adjustment
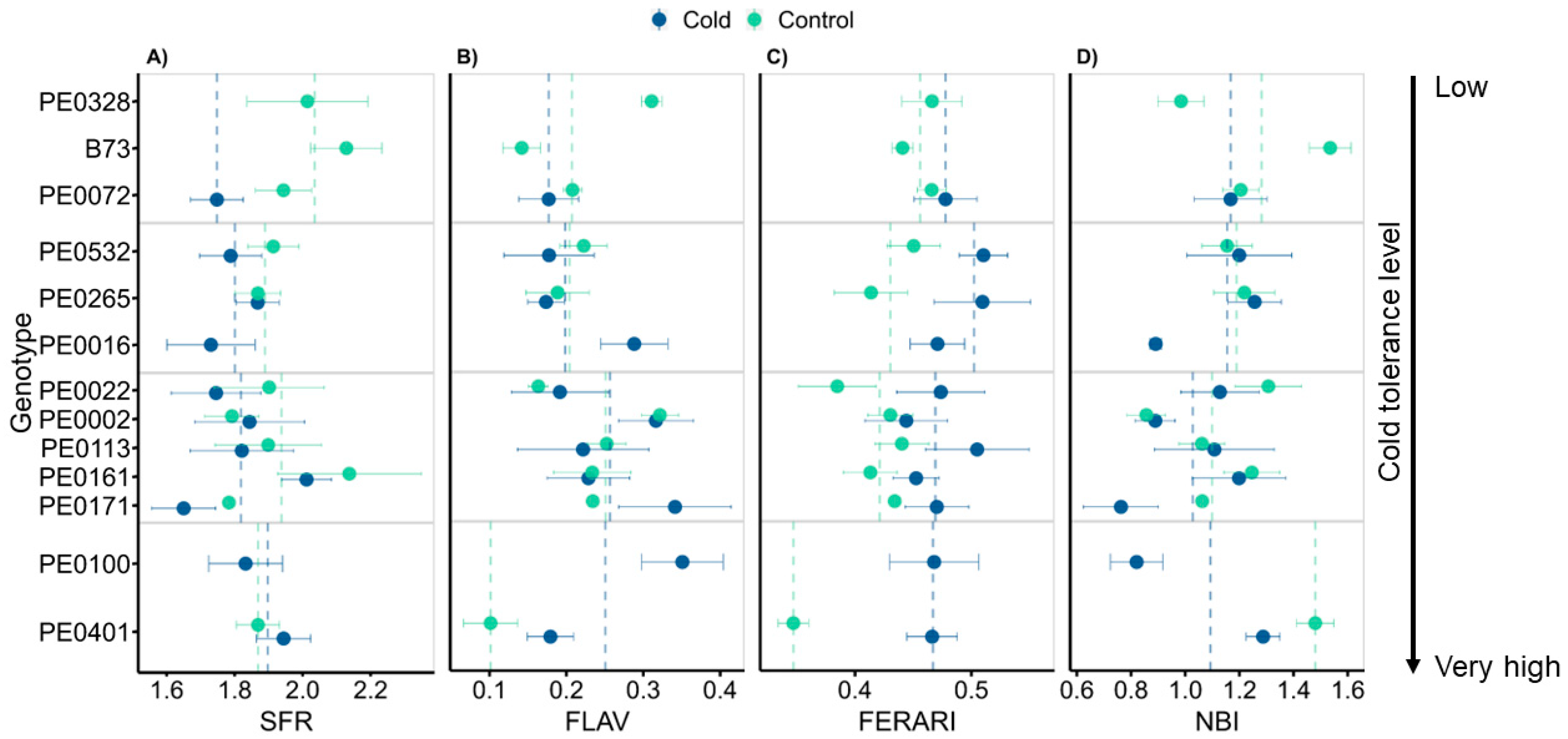
3.5. Leaf Optical Properties Changed Only Marginally between Growth Conditions
4. Discussion
4.1. Genotype Cold Tolerance
4.2. Plant Development
4.3. Relationship between Leaf Acclimation and Biomass Allocation following Cold and Control Treatments
4.4. Leaf Optical Traits Relate to Cold Recovery
4.5. The Effect of Early Low-Temperature Exposure on Plant Performance during Recovery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | air temperature (°C) |
| DAE | days after emergence |
| DH | doubled haploid |
| DLI | daily light integral (mol m−2 d−1) |
| ER | emergence rates recorded 30 days after sowing under cold and after 2 weeks under control (%) |
| FERARI | Fluorescence Excitation Ratio Anthocyanin Relative Index (−log (FRF_R735); [37]) |
| FLAV | Flavonol index (log (FRF_R/FRF_UV); [38]) |
| GDD | Growing degree days (°Cd) |
| Il | internode length (cm) |
| L8_wd | leaf 8 width (cm) |
| LA | leaf area (cm2) |
| Lbl | leaf blade length (cm) |
| LDW | leaf dry weight (g) |
| LMA | leaf dry mass per unit area (g/cm2) |
| LMF | leaf mass fraction (%) |
| Lshl | leaf sheath length (in cm) |
| maxID | maximum internode diameter (cm) |
| NBI | Nitrogen Balance Index (NBI_R = FRF_UV/RF_R, [40]) |
| PDW | total plant dry weight (g) |
| PH | plant height from the first node to the ligule of leaf 8 (cm) |
| PPFD | photosynthetic photon flux density (µmol m−2 s−1) |
| RDW | total root dry weight (g) |
| RMF | root mass fraction (%) |
| SDW | total stem dry weight (g) |
| SeDs | seed density (g/cm3) |
| SenescL | number of senescent leaves |
| SeW | seed weight (g) |
| SFR | Simple chlorophyll Fluorescence Ratio (SFR_R) of far-red emission divided by red emission (SFR_R=FRF_R735/RF_R700; [39]) |
| ShDW | total shoot dry weight (g) |
| SLA | specific leaf area (cm2/g) |
| SMF | stem mass fraction (%) |
| TassFW | tassel fresh weight (g) |
| TiDW | tiller dry weight (g) |
| TiMF | tiller mass fraction (%) |
| WU | water use of the whole plant (g biomass/l water between V1 & V8) |
References
- Hansen, J.; Sato, M.; Ruedy, R. Perception of climate change. Proc. Natl. Acad. Sci. USA 2012, 109, E2415–E2423. [Google Scholar] [CrossRef] [PubMed]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse weather conditions for European wheat production will become more frequent with climate change. Nat. Clim. Change 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Parker, P.S.; Shonkwiler, J.S.; Aurbacher, J. Cause and Consequence in Maize Planting Dates in Germany. J. Agro. Crop Sci. 2017, 203, 227–240. [Google Scholar] [CrossRef]
- Rebourg, C.; Chastanet, M.; Gouesnard, B.; Welcker, C.; Dubreuil, P.; Charcosset, A. Maize introduction into Europe: The history reviewed in the light of molecular data. Theor. Appl. Genet. 2003, 106, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Spence, A.K. Toward cool C(4) crops. Annu. Rev. Plant Biol. 2013, 64, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Di, H.; Huang, J.; Yang, X.; Wang, Z.; Zhang, L.; Dong, L.; et al. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seqapproaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- Xuhui, L.; Weiwei, C.; Siqi, L.; Junteng, F.; Hang, Z.; Xiangbo, Z.; Yongwen, Q. Full-length transcriptome analysis of maize root tips reveals the molecular mechanism of cold stress during the seedling stage. BMC Plant Biol. 2022, 22, 398. [Google Scholar] [CrossRef] [PubMed]
- Frey, F.P.; Pitz, M.; Schön, C.-C.; Hochholdinger, F. Transcriptomic diversity in seedling roots of European flint maize in response to cold. BMC Genom. 2020, 21, 300. [Google Scholar] [CrossRef]
- Li, M.; Sui, N.; Lin, L.; Yang, Z.; Zhang, Y. Transcriptomic profiling revealed genes involved in response to cold stress in maize. Funct. Plant Biol. 2019, 46, 830–844. [Google Scholar] [CrossRef]
- Tardieu, F.; Reymond, M.; Hamard, P.; Granier, C.; Muller, B. Spatial distributions of expansion rate, cell division rate and cell size in maize leaves: A synthesis of the effects of soil water status, evaporative demand and temperature. J. Exp. Bot. 2000, 51, 1505–1514. [Google Scholar] [CrossRef]
- Granier, C.; Inzé, D.; Tardieu, F. Spatial distribution of cell division rate can be deduced from that of p34(cdc2) kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol. 2000, 124, 1393–1402. [Google Scholar] [CrossRef]
- Rymen, B.; Fiorani, F.; Kartal, F.; Vandepoele, K.; Inzé, D.; Beemster, G.T.S. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol. 2007, 143, 1429–1438. [Google Scholar] [CrossRef]
- Riva-Roveda, L.; Escale, B.; Giauffret, C.; Périlleux, C. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biol. 2016, 16, 212. [Google Scholar] [CrossRef]
- Leipner, J.; Stamp, P. Chilling Stress in Maize Seedlings. In Handbook of Maize: Its Biology; Bennetzen, J.L., Hake, S.C., Eds.; Springer: New York, NY, USA, 2009; pp. 291–310. ISBN 978-0-387-79417-4. [Google Scholar]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Silk, W.K.; Schurr, U. Environmental effects on spatial and temporal patterns of leaf and root growth. Annu. Rev. Plant Biol. 2009, 60, 279–304. [Google Scholar] [CrossRef]
- Molnar, P. Differences between soil and air temperatures: Implications for geological reconstructions of past climate. Geosphere 2022, 18, 800–824. [Google Scholar] [CrossRef]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Stamp, P. Cold tolerance of maize seedlings as determined by root morphology and photosynthetic traits. Eur. J. Agron. 2008, 28, 178–185. [Google Scholar] [CrossRef]
- Melkonian, J.; Yu, L.-X.; Setter, T.L. Chilling responses of maize (Zea mays L.) seedlings: Root hydraulic conductance, abscisic acid, and stomatal conductance. J. Exp. Bot. 2004, 55, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Chenu, K.; Fournier, C.; Andrieu, B.; Giauffret, C. An Architectural Approach to Investigate Maize Response to Low Temperature. In Scale and Complexity in Plant Systems Research; Spiertz, J., Struik, P.C., van Laar, H.H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 203–212. ISBN 978-1-4020-5904-9. [Google Scholar]
- Aroca, R.; Amodeo, G.; Fernández-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef]
- Schils, R.; van den Berg, W.; van der Schoot, J.R.; Groten, J.; Rijk, B.; van de Ven, G.; van Middelkoop, J.C.; Holshof, G.; van Ittersum, M.K. Disentangling genetic and non-genetic components of yield trends of Dutch forage crops in the Netherlands. Field Crops Res. 2020, 249, 107755. [Google Scholar] [CrossRef]
- Taube, F.; Vogeler, I.; Kluß, C.; Herrmann, A.; Hasler, M.; Rath, J.; Loges, R.; Malisch, C.S. Yield Progress in Forage Maize in NW Europe-Breeding Progress or Climate Change Effects? Front. Plant Sci. 2020, 11, 1214. [Google Scholar] [CrossRef]
- Greaves, J.A. Improving suboptimal temperature tolerance in maize- the search for variation. J. Exp. Bot. 1996, 47, 307–323. [Google Scholar] [CrossRef]
- Sowiński, P.; Rudzińska-Langwald, A.; Dalbiak, A.; Sowińska, A. Assimilate export from leaves of chilling-treated seedlings of maize. The path to vein. Plant Physiol. Biochem. 2001, 39, 881–889. [Google Scholar] [CrossRef]
- Bhosale, S.U.; Rymen, B.; Beemster, G.T.S.; Melchinger, A.E.; Reif, J.C. Chilling tolerance of Central European maize lines and their factorial crosses. Ann. Bot. 2007, 100, 1315–1321. [Google Scholar] [CrossRef]
- Unterseer, S.; Pophaly, S.D.; Peis, R.; Westermeier, P.; Mayer, M.; Seidel, M.A.; Haberer, G.; Mayer, K.F.X.; Ordas, B.; Pausch, H.; et al. A comprehensive study of the genomic differentiation between temperate Dent and Flint maize. Genome Biol. 2016, 17, 137. [Google Scholar] [CrossRef]
- Wędzony, M.; Forster, B.P.; Żur, I.; Golemiec, E.; Szechyńska-Hebda, M.; Dubas, E.; Gotębiowska, G. Progress in Doubled Haploid Technology in Higher Plants. In Advances in Haploid Production in Higher Plants; Touraev, A., Forster, B.P., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–33. ISBN 978-1-4020-8854-4. [Google Scholar]
- Chaikam, V.; Molenaar, W.; Melchinger, A.E.; Boddupalli, P.M. Doubled haploid technology for line development in maize: Technical advances and prospects. Theor. Appl. Genet. 2019, 132, 3227–3243. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.; Eschholz, T.W.; Stamp, P.; Liedgens, M. Swiss Flint maize landraces—A rich pool of variability for early vigour in cool environments. Field Crops Res. 2009, 110, 157–166. [Google Scholar] [CrossRef]
- Aroca, R.; Irigoyen, J.J.; Sánchez-Díaz, M. Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Sci. 2001, 161, 719–726. [Google Scholar] [CrossRef]
- Hölker, A.C.; Mayer, M.; Presterl, T.; Bolduan, T.; Bauer, E.; Ordas, B.; Brauner, P.C.; Ouzunova, M.; Melchinger, A.E.; Schön, C.-C. European maize landraces made accessible for plant breeding and genome-based studies. Theor. Appl. Genet. 2019, 132, 3333–3345. [Google Scholar] [CrossRef] [PubMed]
- Gioia, T.; Galinski, A.; Lenz, H.; Müller, C.; Lentz, J.; Heinz, K.; Briese, C.; Putz, A.; Fiorani, F.; Watt, M.; et al. GrowScreen-PaGe, a non-invasive, high-throughput phenotyping system based on germination paper to quantify crop phenotypic diversity and plasticity of root traits under varying nutrient supply. Funct. Plant Biol. 2017, 44, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Pariyar, S.R.; Nagel, K.A.; Lentz, J.; Galinski, A.; Wilhelm, J.; Putz, A.; Adels, S.; Heinz, K.; Frohberg, C.; Watt, M. Variation in Root System Architecture among the Founder Parents of Two 8-way MAGIC Wheat Populations for Selection in Breeding. Agronomy 2021, 11, 2452. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A Closed-form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil. Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Gilmore, E.C., Jr.; Rogers, J.S. Heat Units as a Method of Measuring Maturity in Corn1. Agron. J. 1958, 50, 611–615. [Google Scholar] [CrossRef]
- Ben Ghozlen, N.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Cerovic, Z.G.; Pinelli, P.; Tattini, M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 2011, 73, 3–9. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of the Chlorophyll Content in Plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Diago, M.-P.; Rey Caramés, C.; Moigne, M.; Fadaili, E.; Tardaguila, J.; Cerovic, Z.G. Calibration of non-invasive fluorescence-based sensors for the manual and on-the-go assessment of grapevine vegetative status in the field: Non-invasive sensing of grapevine vegetative status. Aust. J. Grape Wine Res. 2016, 22, 12228. [Google Scholar] [CrossRef]
- Jahnke, S.; Roussel, J.; Hombach, T.; Kochs, J.; Fischbach, A.; Huber, G.; Scharr, H. phenoSeeder - A Robot System for Automated Handling and Phenotyping of Individual Seeds. Plant Physiol. 2016, 172, 1358–1370. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 19 January 2024).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion (accessed on 19 January 2024).
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package Version 0.7.1. 2022. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 19 January 2024).
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package Version 1.7.2. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 19 January 2024).
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous. R package Version 4.7-0. 2022. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 19 January 2024).
- Wei, T.; Simko, V. R package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 19 January 2024).
- Garcia, O.; Debuisson, S.; Morlet, M.; Germain, C.; Panigai, L.; Le Moigne, M.; Fadaili, E.M.; Ghozlen, N.B.; Cerovic, Z.G. Using Multiplex® to manage nitrogen variability in champagne vineyards. In Proceedings of the 11th International Conference on Precision Agriculture (ICPA), Indianapolis, Indiana, 15–18 July 2012; International Society of Precision Agriculture: Monticello, IL, USA, 2012; pp. 15–18. [Google Scholar]
- Lainé, C.M.S.; AbdElgawad, H.; Beemster, G.T.S. A meta-analysis reveals differential sensitivity of cold stress responses in the maize leaf. Plant Cell Environ. 2023, 46, 2432–2449. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Butrón, A.; Rady, M.O.A.; Soengas, P.; Revilla, P. Identification of quantitative trait loci involved in the response to cold stress in maize (Zea mays L.). Mol. Breed. 2014, 33, 363–371. [Google Scholar] [CrossRef]
- Sowiński, P.; Rudzińska-Langwald, A.; Adamczyk, J.; Kubica, I.; Fronk, J. Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. J. Plant Physiol. 2005, 162, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhao, W.; Ren, T.; Zhang, X.; Ning, T.; Liu, P.; Li, G. Low Light Increases the Abundance of Light Reaction Proteins: Proteomics Analysis of Maize (Zea mays L.) Grown at High Planting Density. Int. J. Mol. Sci. 2022, 23, 3015. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, U.; Walter, A.; Fiorani, F.; Schurr, U. A method to construct dose-response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data. J. Exp. Bot. 2010, 61, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Walne, C.H.; Reddy, K.R. Temperature Effects on the Shoot and Root Growth, Development, and Biomass Accumulation of Corn (Zea mays L.). Agriculture 2022, 12, 443. [Google Scholar] [CrossRef]
- Windt, C.W.; van Hasselt, P.R. Development of Frost Tolerance in Winter Wheat as Modulated by Differential Root and Shoot Temperature. Plant Biol. 1999, 1, 573–580. [Google Scholar] [CrossRef]
- Rotili, D.H.; Sadras, V.O.; Abeledo, L.G.; Ferreyra, J.M.; Micheloud, J.R.; Duarte, G.; Giron, P.; Ermácora, M.; Maddonni, G.A. Impacts of vegetative and reproductive plasticity associated with tillering in maize crops in low-yielding environments: A physiological framework. Field Crops Res. 2021, 265, 108107. [Google Scholar] [CrossRef]
- Urrutia, M.; Blein-Nicolas, M.; Prigent, S.; Bernillon, S.; Deborde, C.; Balliau, T.; Maucourt, M.; Jacob, D.; Ballias, P.; Bénard, C.; et al. Maize metabolome and proteome responses to controlled cold stress partly mimic early-sowing effects in the field and differ from those of Arabidopsis. Plant Cell Environ. 2021, 44, 1504–1521. [Google Scholar] [CrossRef] [PubMed]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Frascaroli, E.; Salvi, S.; Stamp, P. QTL controlling root and shoot traits of maize seedlings under cold stress. Theor. Appl. Genet. 2004, 109, 618–629. [Google Scholar] [CrossRef]
- Szalai, G.; Janda, T.; Páldi, E.; Szigeti, Z. Role of Light in the Development of Post-chilling Symptoms in Maize. J. Plant Physiol. 1996, 148, 378–383. [Google Scholar] [CrossRef]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Leipner, J.; Jompuk, C.; Camp, K.-H.; Stamp, P.; Fracheboud, Y. QTL studies reveal little relevance of chilling-related seedling traits for yield in maize. Theor. Appl. Genet. 2008, 116, 555–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Genotype | Survival Rate (%) | Leaf Colour Score | Cold Tolerance Level |
|---|---|---|---|
| PE0328 | - | 1 | Low |
| B73 | 10 | 1 | Low |
| PE0072 | 50 | 2 | Low |
| PE0532 | 60 | 2 | Medium |
| PE0265 | 70 | 3 | Medium |
| PE0016 | 80 | 3 | Medium |
| PE0022 | 80 | 4 | High |
| PE0002 | 90 | 3 | High |
| PE0113 | 90 | 3 | High |
| PE0161 | 90 | 5 | High |
| PE0171 | 90 | 5 | High |
| PE0100 | 100 | 3 | Very high |
| PE0401 | 100 | 5 | Very high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boughazi, K.; Wuyts, N.; Muller, O.; Windt, C.W.; Nagel, K.A.; Rascher, U.; Fiorani, F. Doubled Haploid Lines Derived from a European Maize Flint Landrace Contrast in Recovery from Cold Stress. Agronomy 2024, 14, 408. https://doi.org/10.3390/agronomy14030408
Boughazi K, Wuyts N, Muller O, Windt CW, Nagel KA, Rascher U, Fiorani F. Doubled Haploid Lines Derived from a European Maize Flint Landrace Contrast in Recovery from Cold Stress. Agronomy. 2024; 14(3):408. https://doi.org/10.3390/agronomy14030408
Chicago/Turabian StyleBoughazi, Khadija, Nathalie Wuyts, Onno Muller, Carel W. Windt, Kerstin A. Nagel, Uwe Rascher, and Fabio Fiorani. 2024. "Doubled Haploid Lines Derived from a European Maize Flint Landrace Contrast in Recovery from Cold Stress" Agronomy 14, no. 3: 408. https://doi.org/10.3390/agronomy14030408
APA StyleBoughazi, K., Wuyts, N., Muller, O., Windt, C. W., Nagel, K. A., Rascher, U., & Fiorani, F. (2024). Doubled Haploid Lines Derived from a European Maize Flint Landrace Contrast in Recovery from Cold Stress. Agronomy, 14(3), 408. https://doi.org/10.3390/agronomy14030408







