The Importance of Considering Levels of P and N Fertilization to Promote Beneficial Interaction between Rapeseed and Phosphate-Solubilizing Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. PSB Screening and Application in Plants
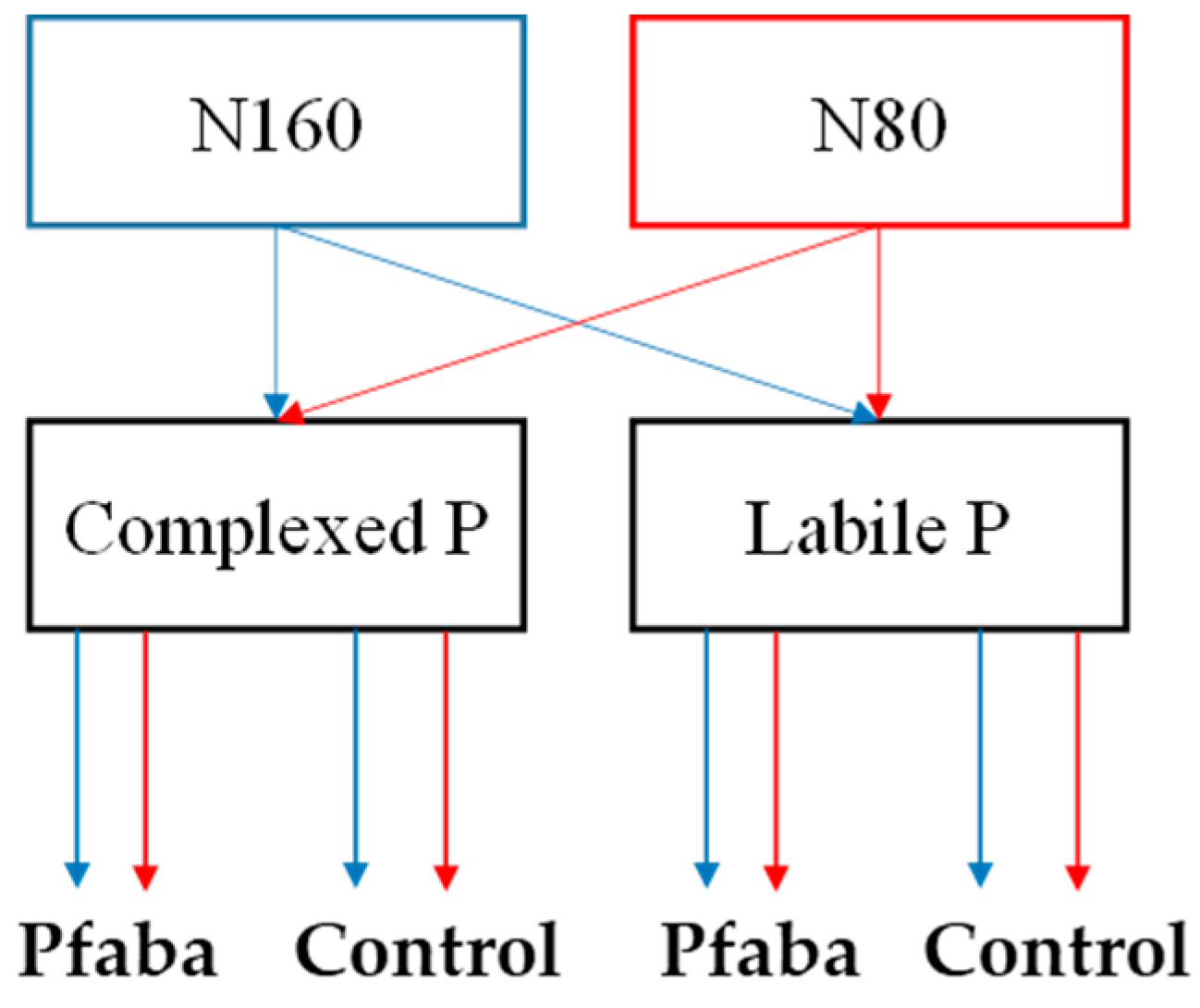
2.2. Greenhouse Experiment
2.3. Elemental Analyses in Plants
2.4. Determination of Root Traits Using WinRhizo™
2.5. Statistical Analyses
3. Results
3.1. Characterization of PGPR and PSB Traits of the Strain Pfaba
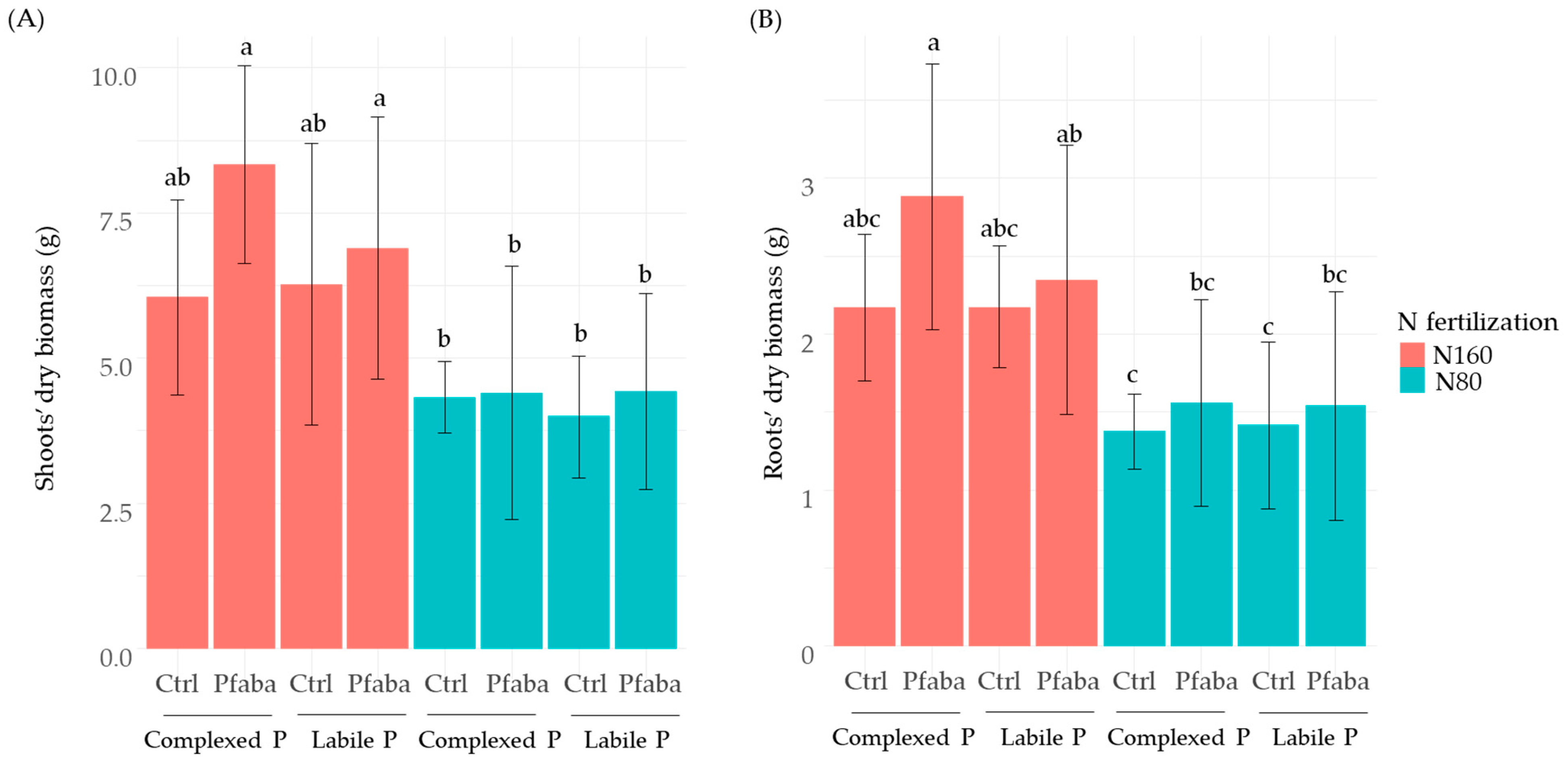
3.2. Morphometric Traits of Rapeseed Plants According to N Fertilization, P Forms, and Pfaba Inoculation
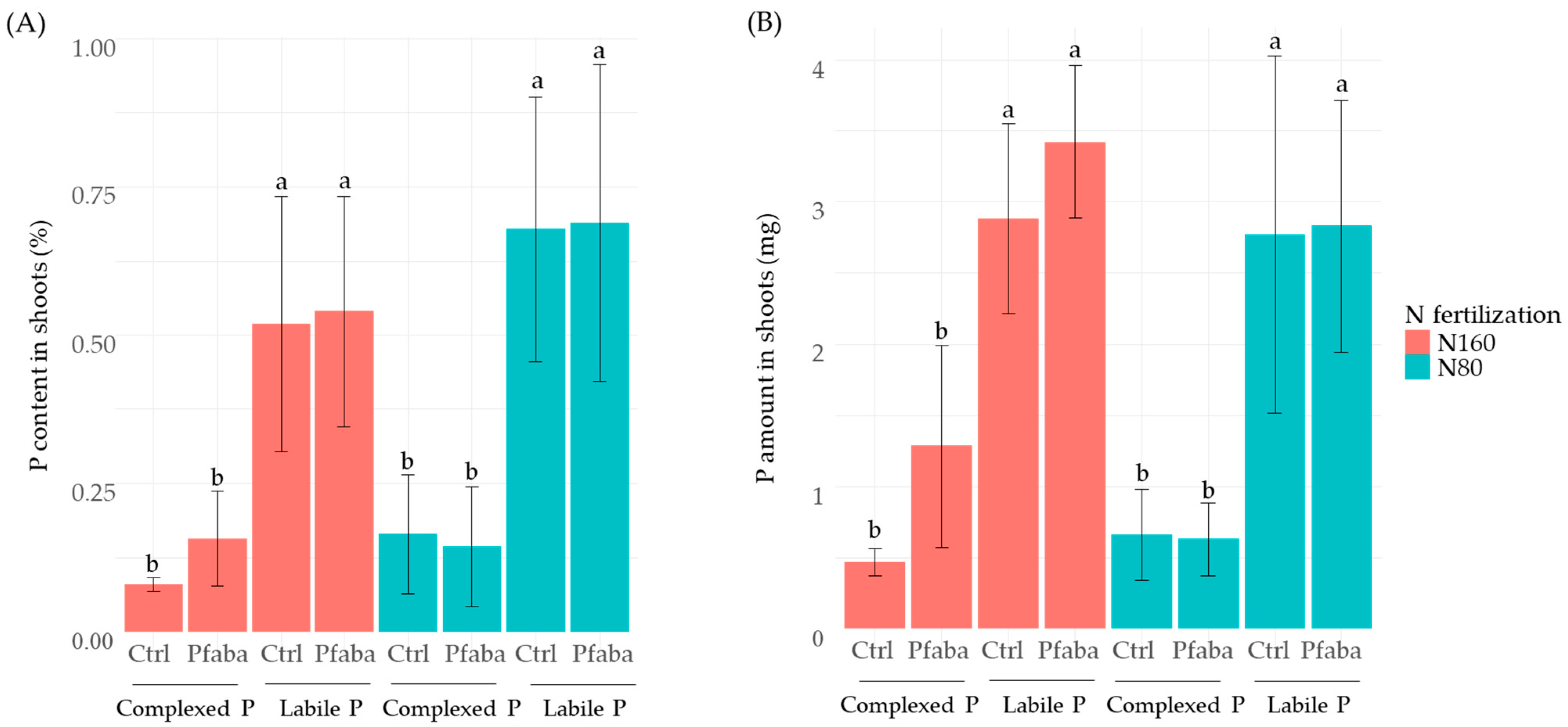
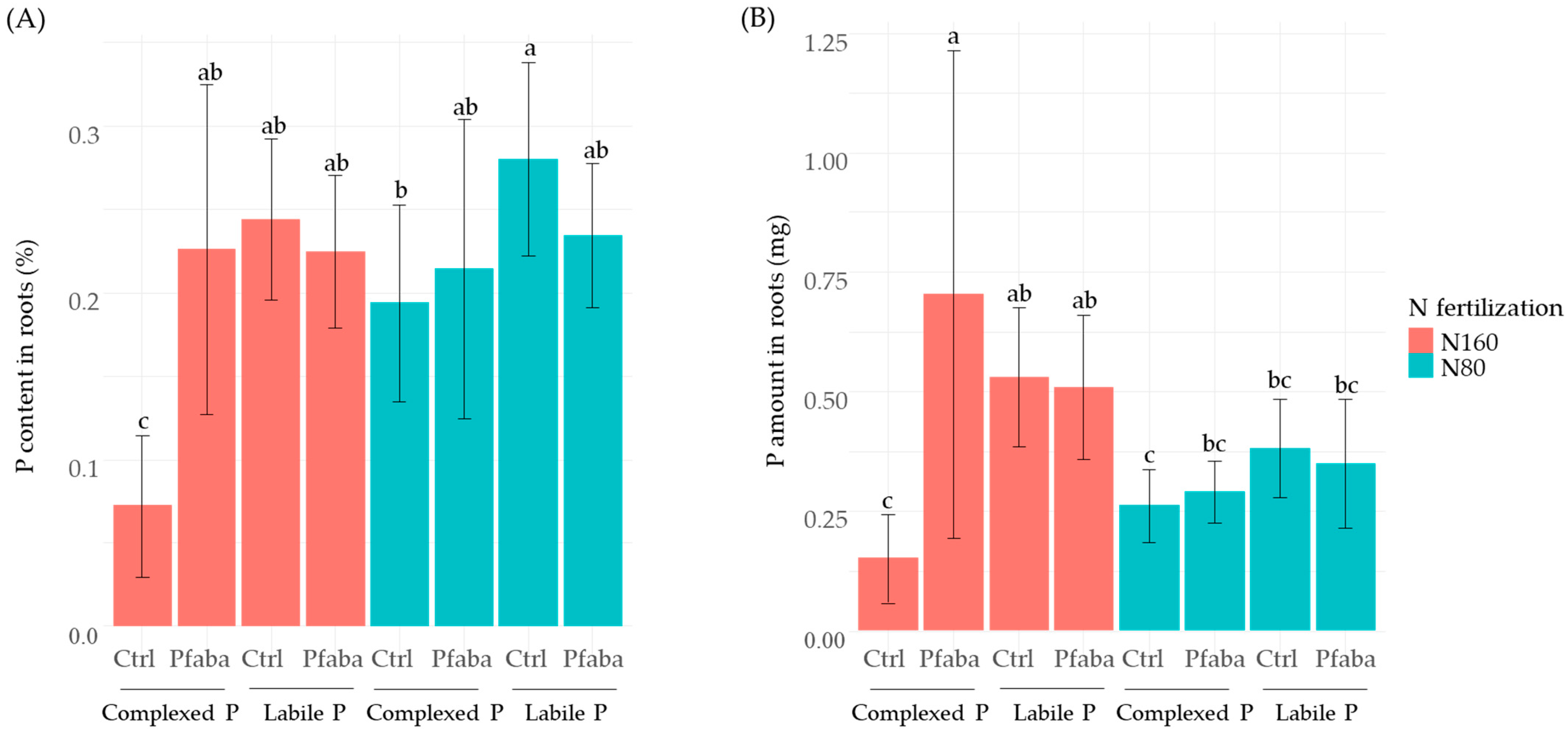
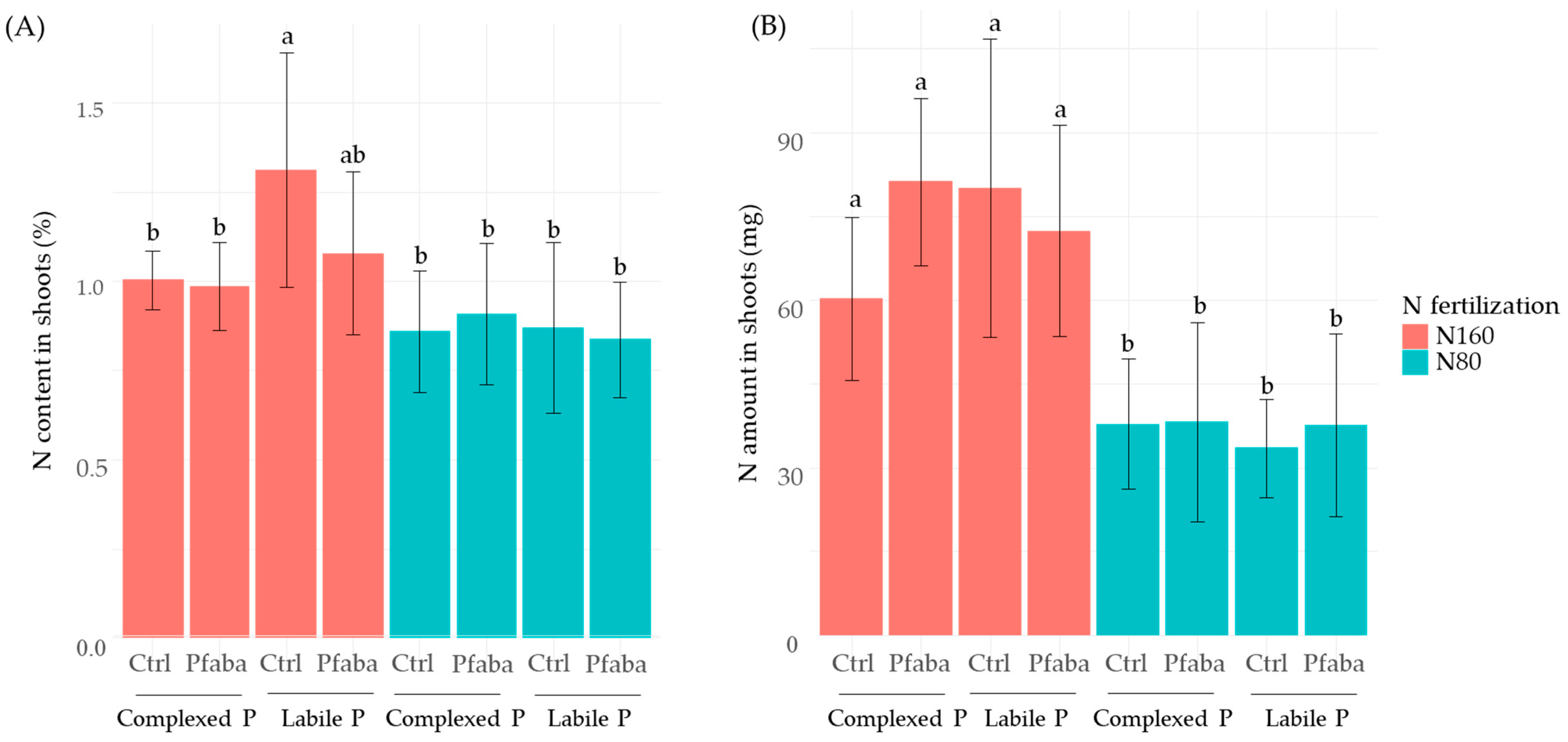
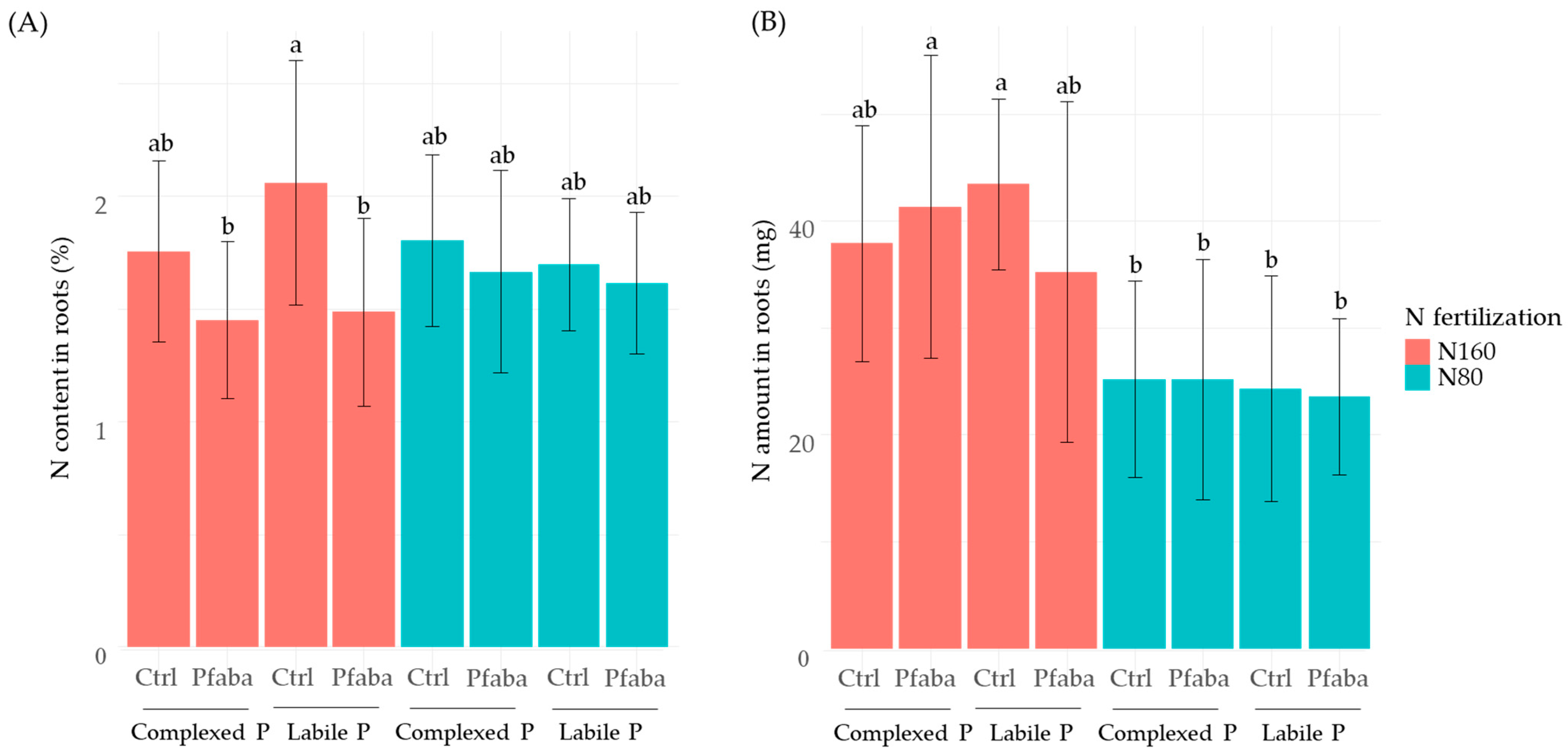
3.3. P and N Status of Rapeseed Plants according to N Fertilization, P Forms, and the Pfaba Inoculation
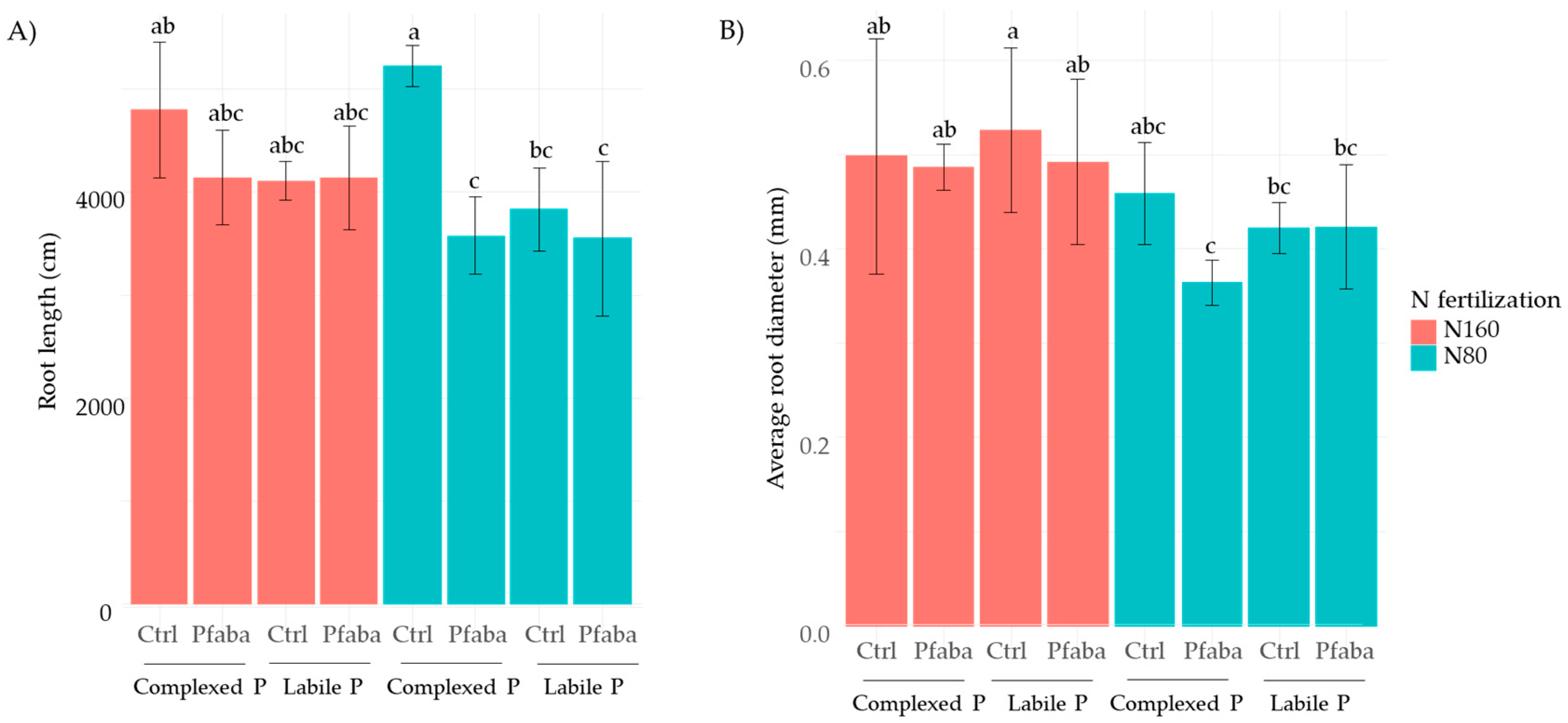
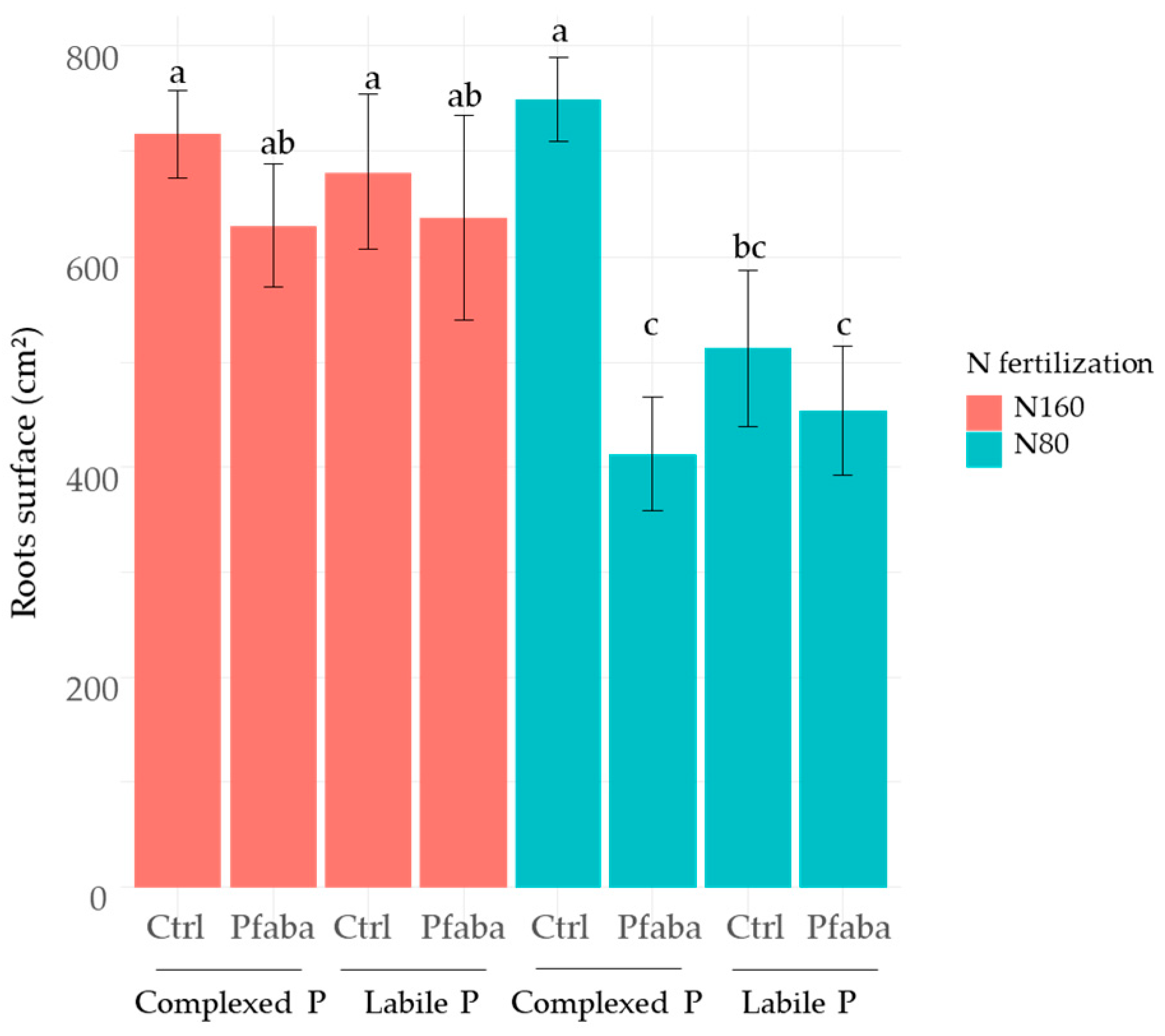
3.4. Rapeseed Root Traits according to N Fertilization, P Forms, and the Pfaba Inoculation
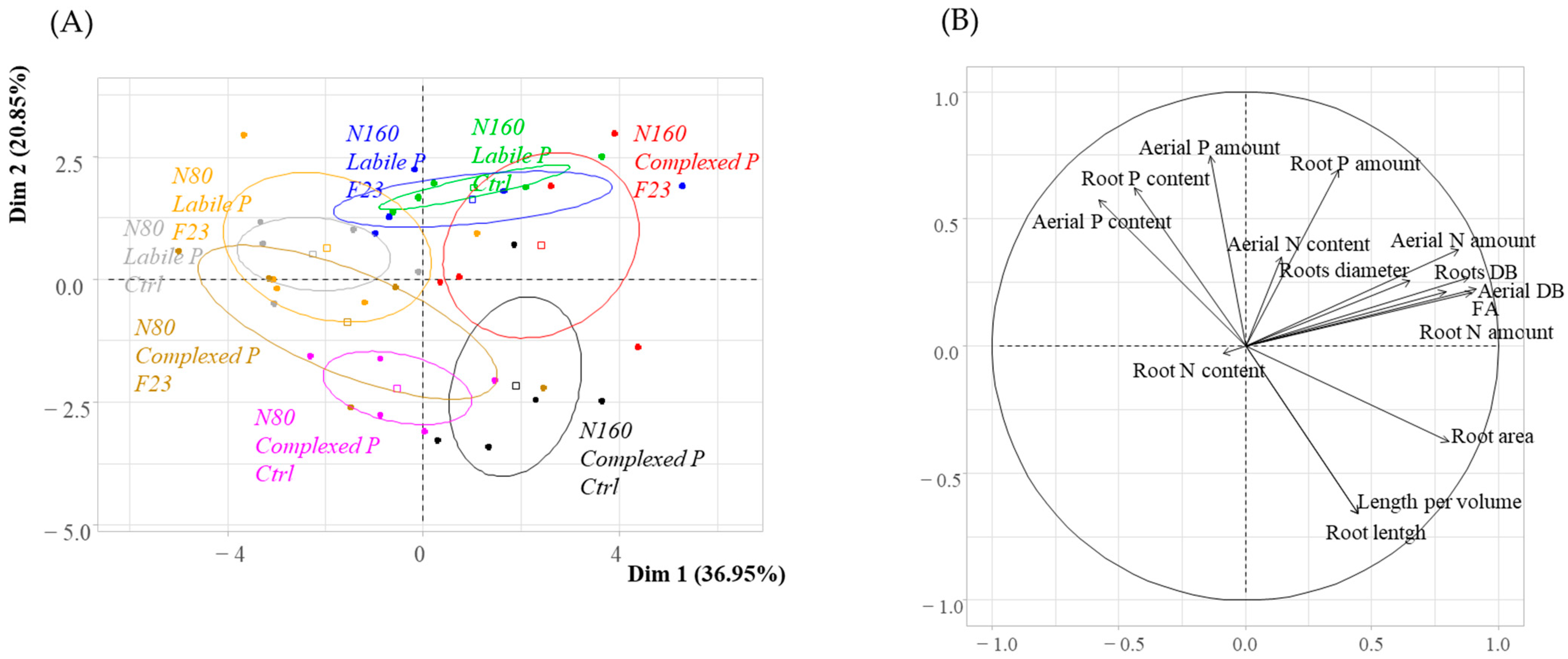
3.5. General Plant Profiles
4. Discussion
N Availability as a Key Factor of Success of Plant-PSB Association
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pankhurst, C.E.; Doube, B.M.; Gupta, V.V.S.R. (Eds.) Biological indicators of soil health: Synthesis. In Biological Indicators of Soil Health; CAB International: New York, NY, USA, 1997; pp. 419–435. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the Soil Microbiome to Increase Soil Health and Plant Fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research Priorities for Harnessing Plant Microbiomes in Sustainable Agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant Health: Feedback Effect of Root Exudates-Rhizobiome Interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Orozco-Mosqueda, M.; del Carmen Rocha-Granados, M.; Glick, B.R.; Santoyo, G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, M.P.; Santhoshkannada, A.N. Role of Rhizomicrobiome in Maintaining Soil Fertility and Crop Production. In Soil Health; Giri, B., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 373–401. ISBN 978-3-030-44364-1. [Google Scholar]
- Song, C.; Zhu, F.; Carrión, V.J.; Cordovez, V. Beyond Plant Microbiome Composition: Exploiting Microbial Functions and Plant Traits via Integrated Approaches. Front. Bioeng. Biotechnol. 2020, 8, 896. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Arif, I.; Batool, M.; Schenk, P.M. Plant Microbiome Engineering: Expected Benefits for Improved Crop Growth and Resilience. Trends Biotechnol. 2020, 38, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Kumar, P.; Pandey, P.; Dubey, R.C.; Maheshwari, D.K. Bacteria Consortium Optimization Improves Nutrient Uptake, Nodulation, Disease Suppression and Growth of the Common Bean (Phaseolus vulgaris) in Both Pot and Field Studies. Rhizosphere 2016, 2, 13–23. [Google Scholar] [CrossRef]
- Peix, A.; Lang, E.; Verbarg, S.; Spröer, C.; Rivas, R.; Santa-Regina, I.; Mateos, P.F.; Martínez-Molina, E.; Rodríguez-Barrueco, C.; Velázquez, E. Acinetobacter Strains IH9 and OCI1, Two Rhizospheric Phosphate Solubilizing Isolates Able to Promote Plant Growth, Constitute a New Genomovar of Acinetobacter calcoaceticus. Syst. Appl. Microbiol. 2009, 32, 334–341. [Google Scholar] [CrossRef]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis Strain SJ-101 as a Bioinoculant for Concurrent Plant Growth Promotion and Nickel Accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Shen, Z.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Pre-Colonization of PGPR Triggers Rhizosphere Microbiota Succession Associated with Crop Yield Enhancement. Plant Soil 2019, 439, 553–567. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Illmer, P.; Schinner, F. Solubilization of Inorganic Calcium Phosphates—Solubilization Mechanisms. Soil Biol. Biochem. 1995, 27, 257–263. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, Noxious, and Not Readily Available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for Mobilizing Recalcitrant Phosphorus from Agricultural Soils: A Review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef]
- Amy, C.; Avice, J.-C.; Laval, K.; Bressan, M. Are Native Phosphate Solubilizing Bacteria a Relevant Alternative to Mineral Fertilizations for Crops? Part I. When Rhizobacteria Meet Plant P Requirements. Rhizosphere 2022, 21, 100476. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of Plant Growth by Bacterial ACC Deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-Acetic Acid in Microbial and Microorganism-Plant Signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Isidra-Arellano, M.C.; Delaux, P.-M.; Valdés-López, O. The Phosphate Starvation Response System: Its Role in the Regulation of Plant–Microbe Interactions. Plant Cell Physiol. 2021, 62, 392–400. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Triviño, V.; Suárez, J. Holobionts: Ecological Communities, Hybrids, or Biological Individuals? A Metaphysical Perspective on Multispecies Systems. Stud. Hist. Philos. Sci. Stud. Hist. Philos. Biol. Biomed. Sci. 2020, 84, 101323. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef]
- Dutta, S.; Podile, A.R. Plant Growth Promoting Rhizobacteria (PGPR): The Bugs to Debug the Root Zone. Crit. Rev. Microbiol. 2010, 36, 232–244. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Wang, H.; Ding, N.; Xu, J. Does History Matter? Temperature Effects on Soil Microbial Biomass and Community Structure Based on the Phospholipid Fatty Acid (PLFA) Analysis. J. Soils Sediments 2010, 10, 223–230. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Rodler, A.; Kuffner, M.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S. Experimental Warming Effects on the Microbial Community of a Temperate Mountain Forest Soil. Soil Biol. Biochem. 2011, 43, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Booth, I.R.; Cash, P.; O’Byrne, C. Sensing and Adapting to Acid Stress. Antonie Van Leeuwenhoek 2002, 81, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in Vitro Characterization and Comparative Efficacy of Plant Growth-Promoting Rhizobacteria for Improving Cucumber Salt Tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef]
- Demoling, F.; Figueroa, D.; Bååth, E. Comparison of Factors Limiting Bacterial Growth in Different Soils. Soil Biol. Biochem. 2007, 39, 2485–2495. [Google Scholar] [CrossRef]
- Aldén, L.; Demoling, F.; Bååth, E. Rapid Method of Determining Factors Limiting Bacterial Growth in Soil. Appl. Environ. Microbiol. 2001, 67, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Martínez, O.A.; Jorquera, M.A.; Crowley, D.E.; de la Luz Mora, M. Influence of Nitrogen Fertilisation on Pasture Culturable Rhizobacteria Occurrence and the Role of Environmental Factors on Their Potential PGPR Activities. Biol. Fertil. Soils 2011, 47, 875–885. [Google Scholar] [CrossRef]
- Öztürk, Ö. Effects of Source and Rate of Nitrogen Fertilizer on Yield, Yield Components and Quality of Winter Rapeseed (Brassica napus L.). Chil. J. Agric. Res. 2010, 70, 132–141. [Google Scholar] [CrossRef]
- Rashid, A.; Bughio, N. Evaluating Internal Phosphorus Requirement of Rapeseed, Chickpea, Lentil, and Wheat by Seed Analysis. Commun. Soil Sci. Plant Anal. 1993, 24, 1359–1369. [Google Scholar] [CrossRef]
- Grizzetti, B.; Billen, G.; Davidson, E.A.; Winiwarter, W.; de Vries, W.; Fowler, D.; Howard, C.M.; Bleeker, A.; Sutton, M.A.; Lassaletta, L.; et al. Global Nitrogen and Phosphorus Pollution. In Just Enough Nitrogen: Perspectives on How to Get There for Regions with Too Much and Too Little Nitrogen; Sutton, M.A., Mason, K.E., Bleeker, A., Hicks, W.K., Masso, C., Raghuram, N., Reis, S., Bekunda, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 421–431. ISBN 978-3-030-58065-0. [Google Scholar]
- Helfenstein, J.; Tamburini, F.; Sperber, C.; Massey, M.; Chiara, P.; Chadwick, O.; Vitousek, P.; Kretzschmar, R.; Frossard, E. Combining Spectroscopic and Isotopic Techniques Gives a Dynamic View of Phosphorus Cycling in Soil. Nat. Commun. 2018, 9, 3226. [Google Scholar] [CrossRef]
- Cai, F.; Pang, G.; Miao, Y.; Li, R.; Li, R.; Shen, Q.; Chen, W. The Nutrient Preference of Plants Influences Their Rhizosphere Microbiome. Appl. Soil Ecol. 2017, 110, 146–150. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, e4917256. [Google Scholar] [CrossRef]
- Amy, C.; Avice, J.-C.; Laval, K.; Bressan, M. Are Native Phosphate-Solubilizing Bacteria a Relevant Alternative to Mineral Fertilizations for Crops? Part II: PSB Inoculation Enables a Halving of P Input and Improves the Microbial Community in the Rapeseed Rhizosphere. Rhizosphere 2022, 21, 100480. [Google Scholar] [CrossRef]
- Amy, C. Optimiser La Nutrition Azotée et Phosphorée Du Colza Pour Une Production Durable via L’utilisation de Biointrants Améliorant Le Fonctionnement Du Phytobiome. Ph.D. Thesis, Normandie Université, Caen, France, 2021. [Google Scholar]
- Cleveland, C.; Liptzin, D. C:N:P Stoichiometry in Soil: Is There a “Redfield Ratio” for the Microbial Biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of Phosphorus in Soil in Connection with Vital Activity of Some Microbial Species. Microbiology 1948, 17, 362–370. [Google Scholar]
- Maignan, V.; Géliot, P.; Avice, J.-C. Glutacetine® Biostimulant Applied on Wheat under Contrasting Field Conditions Improves Grain Number Leading to Better Yield, Upgrades N-Related Traits and Changes Grain Ionome. Plants 2021, 10, 456. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent Insights into the Metabolic Adaptations of Phosphorus-Deprived Plants. J. Exp. Bot. 2021, 72, 199–223. [Google Scholar] [CrossRef]
- de Groot, C.C.; Marcelis, L.F.M.; van den Boogaard, R.; Kaiser, W.M.; Lambers, H. Interaction of Nitrogen and Phosphorus Nutrition in Determining Growth. Plant Soil 2003, 248, 257–268. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Imran, Q.M.; Yun, B.-W.; Lee, I.-J. Osmoprotective Functions Conferred to Soybean Plants via Inoculation with Sphingomonas sp. LK11 and Exogenous Trehalose. Microbiol. Res. 2017, 205, 135–145. [Google Scholar] [CrossRef]
- Islam, S.; Akanda, A.M.; Prova, A.; Islam, M.T.; Hossain, M.M. Isolation and Identification of Plant Growth Promoting Rhizobacteria from Cucumber Rhizosphere and Their Effect on Plant Growth Promotion and Disease Suppression. Front. Microbiol. 2015, 6, 1360. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Freitas, H. Inoculation of Plant Growth Promoting Bacterium Achromobacter xylosoxidans Strain Ax10 for the Improvement of Copper Phytoextraction by Brassica juncea. J. Environ. Manag. 2009, 90, 831–837. [Google Scholar] [CrossRef]
- Makarova, L.E.; Dudareva, L.V.; Petrova, I.G.; Vasil’eva, G.G. Secretion of Phenolic Compounds into Root Exudates of Pea Seedlings upon Inoculation with Rhizobium leguminosarum Bv. Viceae or Pseudomonas siringae Pv. Pisi. Appl. Biochem. Microbiol. 2016, 52, 205–209. [Google Scholar] [CrossRef]
- Tajini, F.; Trabelsi, M.; Drevon, J.-J. Combined Inoculation with Glomus intraradices and Rhizobium tropici CIAT899 Increases Phosphorus Use Efficiency for Symbiotic Nitrogen Fixation in Common Bean (Phaseolus vulgaris L.). Saudi J. Biol. Sci. 2012, 19, 157–163. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Yin, T.; Xu, S.; Zhao, W.; Wang, J.; Huang, Z. The Persistence and Performance of Phosphate-Solubilizing Gluconacetobacter liquefaciens Qzr14 in a Cucumber Soil. 3 Biotech 2017, 7, 294. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Tran, H.T. Metabolic Adaptations of Phosphate-Starved Plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef]
- Gerke, J. The Acquisition of Phosphate by Higher Plants: Effect of Carboxylate Release by the Roots. A Critical Review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Christensen, J.V.; Hennig, A.M.F.; Mckenzie, J.S.; Legge, W.G.; Depauw, R.M.; Siemens, B.; Thomas, J.B. Effect of Seeding Date, Nitrogen and Phosphate Fertilizer on Growth, Yield and Quality of Rapeseed in Northwest Alberta. Can. J. Plant Sci. 1985, 65, 275–284. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G. Alteration in Yield and Oil Quality Traits of Winter Rapeseed by Lodging at Different Planting Density and Nitrogen Rates. Sci. Rep. 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Leleu, O.; Vuylsteker, C.; Têtu, J.-F.; Degrande, D.; Champolivier, L.; Rambour, S. Effect of Two Contrasted N Fertilisations on Rapeseed Growth and Nitrate Metabolism. Plant Physiol. Biochem. 2000, 38, 639–645. [Google Scholar] [CrossRef]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J.; et al. Adaption of Roots to Nitrogen Deficiency Revealed by 3D Quantification and Proteomic Analysis. Plant Physiol. 2019, 179, 329–347. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Crawford, I.P.; Gunsalus, I.C. Inducibility of Tryptophan Synthetase in Pseudomonas putida. Proc. Natl. Acad. Sci. USA 1966, 56, 717–724. [Google Scholar] [CrossRef]
- Gasser, V.; Guillon, L.; Cunrath, O.; Schalk, I.J. Cellular Organization of Siderophore Biosynthesis in Pseudomonas aeruginosa: Evidence for Siderosomes. J. Inorg. Biochem. 2015, 148, 27–34. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of Organic C by Plants: Mechanisms and Controls. Agronomie 2003, 23, 375–396. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.-R.; Borriss, R.; von Wirén, N. Root Exudation of Sugars, Amino Acids, and Organic Acids by Maize as Affected by Nitrogen, Phosphorus, Potassium, and Iron Deficiency. J. Plant Nutr. Soil Sci. 2011, 174, 3–11. [Google Scholar] [CrossRef]
- Higgins, N.F.; Crittenden, P.D. Phytase Activity in Lichens. New Phytol. 2015, 208, 544–554. [Google Scholar] [CrossRef]
- Kamble, P.N.; Bååth, E. Comparison of Fungal and Bacterial Growth after Alleviating Induced N-Limitation in Soil. Soil Biol. Biochem. 2016, 103, 97–105. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Schjørring, J.K. Nitrate and Ammonium Absorption by Plants Growing at a Sufficient or Insufficient Level of Phosphorus in Nutrient Solutions. Plant Soil 1986, 91, 313–318. [Google Scholar] [CrossRef]
- Heitkötter, J.; Marschner, B. Soil Zymography as a Powerful Tool for Exploring Hotspots and Substrate Limitation in Undisturbed Subsoil. Soil Biol. Biochem. 2018, 124, 210–217. [Google Scholar] [CrossRef]
- Razavi, B.S.; Zhang, X.; Bilyera, N.; Guber, A.; Zarebanadkouki, M. Soil Zymography: Simple and Reliable? Review of Current Knowledge and Optimization of the Method. Rhizosphere 2019, 11, 100161. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Spatial and Temporal Dynamics of Hotspots of Enzyme Activity in Soil as Affected by Living and Dead Roots—A Soil Zymography Analysis. Plant Soil 2014, 379, 67–77. [Google Scholar] [CrossRef]
| PSB | P Form | N Fertilization | P Form × PSB | P × N | N × PSB | N × P × PSB | ||
|---|---|---|---|---|---|---|---|---|
| Shoot | Dry Biomass | 0.142 | 0.507 | <0.001 | 0.579 | 0.689 | 0.300 | 0.383 |
| P content | 0.420 | <0.001 | 0.050 | 0.583 | 0.600 | 1.000 | 0.929 | |
| P amount | 0.100 | <0.001 | 0.231 | 0.762 | 0.703 | 0.166 | 0.753 | |
| N content | 0.367 | 0.197 | 0.001 | 0.260 | 0.083 | 0.310 | 0.613 | |
| N amount | 0.412 | 0.777 | <0.001 | 0.256 | 0.469 | 0.685 | 0.142 | |
| C content | 0.641 | 0.154 | 0.249 | 0.576 | 0.150 | 0.289 | 0.503 | |
| C amount | 0.185 | 0.660 | <0.001 | 0.610 | 0.865 | 0.405 | 0.422 | |
| Roots | Dry Biomass | 0.145 | 0.531 | <0.001 | 0.465 | 0.490 | 0.474 | 0.557 |
| P content | 0.394 | 0.001 | 0.032 | 0.024 | 0.254 | 0.154 | 0.413 | |
| P amount | 0.124 | 0.082 | 0.067 | 0.056 | 0.661 | 0.120 | 0.142 | |
| N content | 0.037 | 0.728 | 0.966 | 0.676 | 0.334 | 0.204 | 0.521 | |
| N amount | 0.703 | 0.831 | <0.001 | 0.393 | 0.891 | 0.778 | 0.449 | |
| C content | 0.712 | 0.138 | 0.661 | 0.227 | 0.554 | 0.750 | 0.027 | |
| C content | 0.135 | 0.323 | <0.001 | 0.284 | 0.332 | 0.446 | 0.204 | |
| Length | 0.043 | 0.063 | 0.365 | 0.104 | 0.634 | 0.246 | 0.546 | |
| Area | 0.001 | 0.171 | 0.001 | 0.088 | 0.407 | 0.088 | 0.137 | |
| Diameter | 0.052 | 0.536 | 0.001 | 0.556 | 0.958 | 0.621 | 0.254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amy, C.; Avice, J.-C.; Laval, K.; Trinsoutrot-Gattin, I.; Bressan, M. The Importance of Considering Levels of P and N Fertilization to Promote Beneficial Interaction between Rapeseed and Phosphate-Solubilizing Bacteria. Agronomy 2024, 14, 334. https://doi.org/10.3390/agronomy14020334
Amy C, Avice J-C, Laval K, Trinsoutrot-Gattin I, Bressan M. The Importance of Considering Levels of P and N Fertilization to Promote Beneficial Interaction between Rapeseed and Phosphate-Solubilizing Bacteria. Agronomy. 2024; 14(2):334. https://doi.org/10.3390/agronomy14020334
Chicago/Turabian StyleAmy, Charlotte, Jean-Christophe Avice, Karine Laval, Isabelle Trinsoutrot-Gattin, and Mélanie Bressan. 2024. "The Importance of Considering Levels of P and N Fertilization to Promote Beneficial Interaction between Rapeseed and Phosphate-Solubilizing Bacteria" Agronomy 14, no. 2: 334. https://doi.org/10.3390/agronomy14020334
APA StyleAmy, C., Avice, J.-C., Laval, K., Trinsoutrot-Gattin, I., & Bressan, M. (2024). The Importance of Considering Levels of P and N Fertilization to Promote Beneficial Interaction between Rapeseed and Phosphate-Solubilizing Bacteria. Agronomy, 14(2), 334. https://doi.org/10.3390/agronomy14020334







