Effect of Bacillus amyloliquefaciens QST713 on Inter-Root Substrate Environment of Cucumber under Low-Calcium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Experimental Design
2.2. Determination of Experimental Indexes
2.3. Data Analysis
3. Results
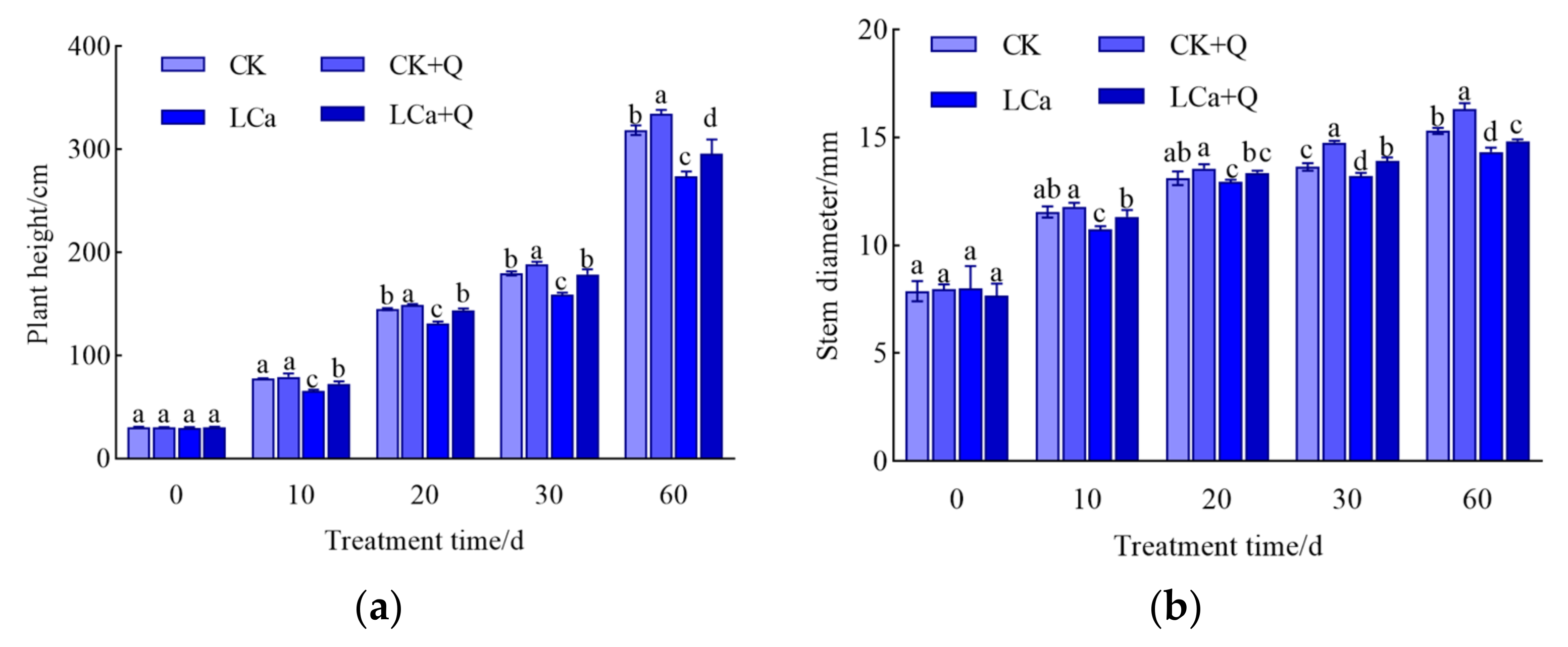
3.1. Effect of Bacillus amyloliquefaciens QST713 on Cucumber Growth under Low-Calcium Stress
3.2. Effect of Bacillus amyloliquefaciens QST713 on the Physicochemical Properties of Cucumber Inter-Root Matrix under Low-Calcium Stress
3.3. Effect of Bacillus amyloliquefaciens QST713 on Nutrient Content in Cucumber Inter-Root Matrix under Low-Calcium Stress
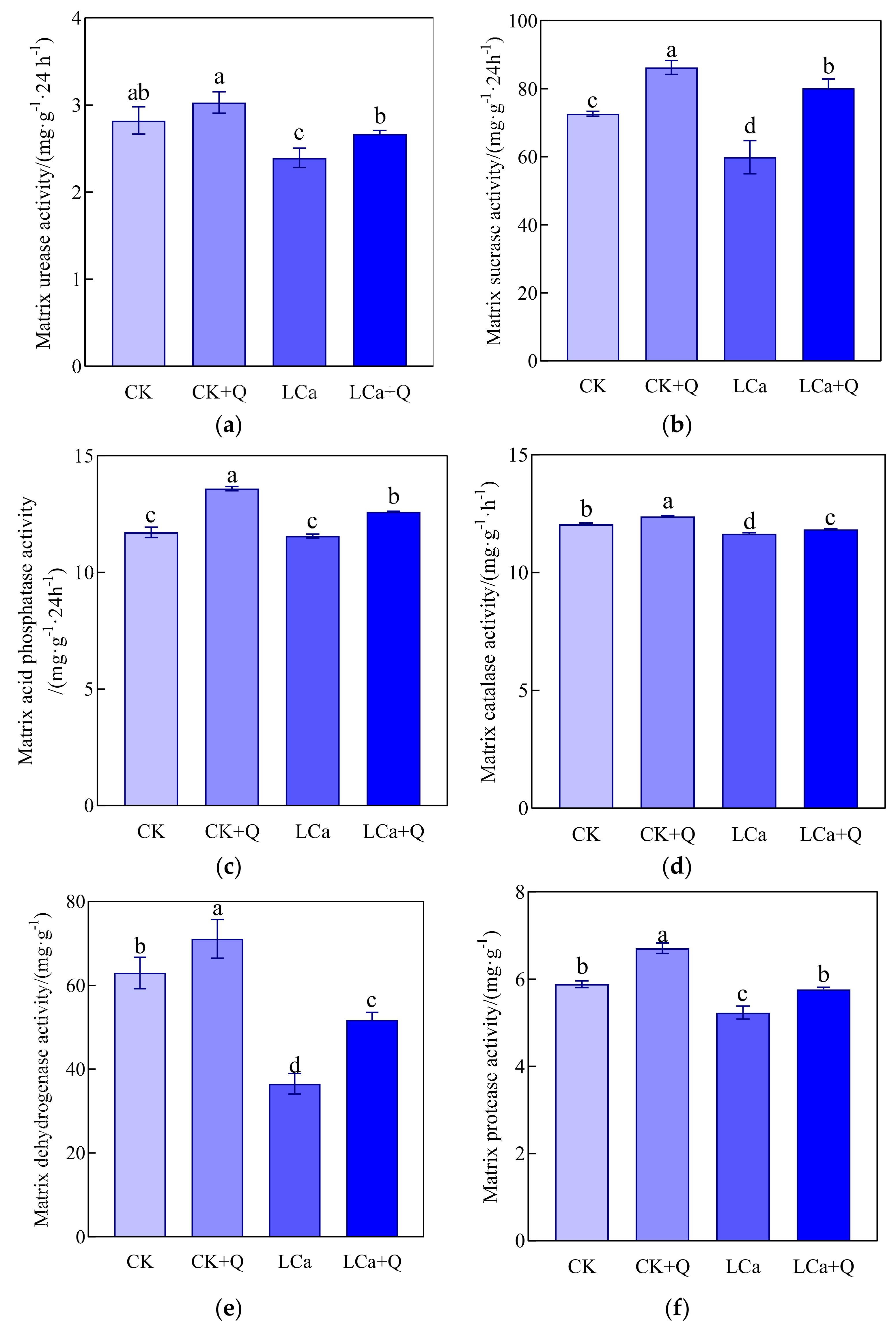
3.4. Effect of Bacillus amyloliquefaciens QST713 on Inter-Root Matrix Enzyme Activities of Cucumber under Low-Calcium Stress
3.5. Effect of Bacillus amyloliquefaciens QST713 on the Inter-Root Bacterial Community of Cucumber under Low-Calcium Stress
3.5.1. Evaluation of Illumina Sequencing Results of Cucumber Inter-Root Substrates with Different Treatments
3.5.2. Analysis of Alpha Diversity Index of Cucumber Inter-Root Substrate Bacterial Community in Different Treatments
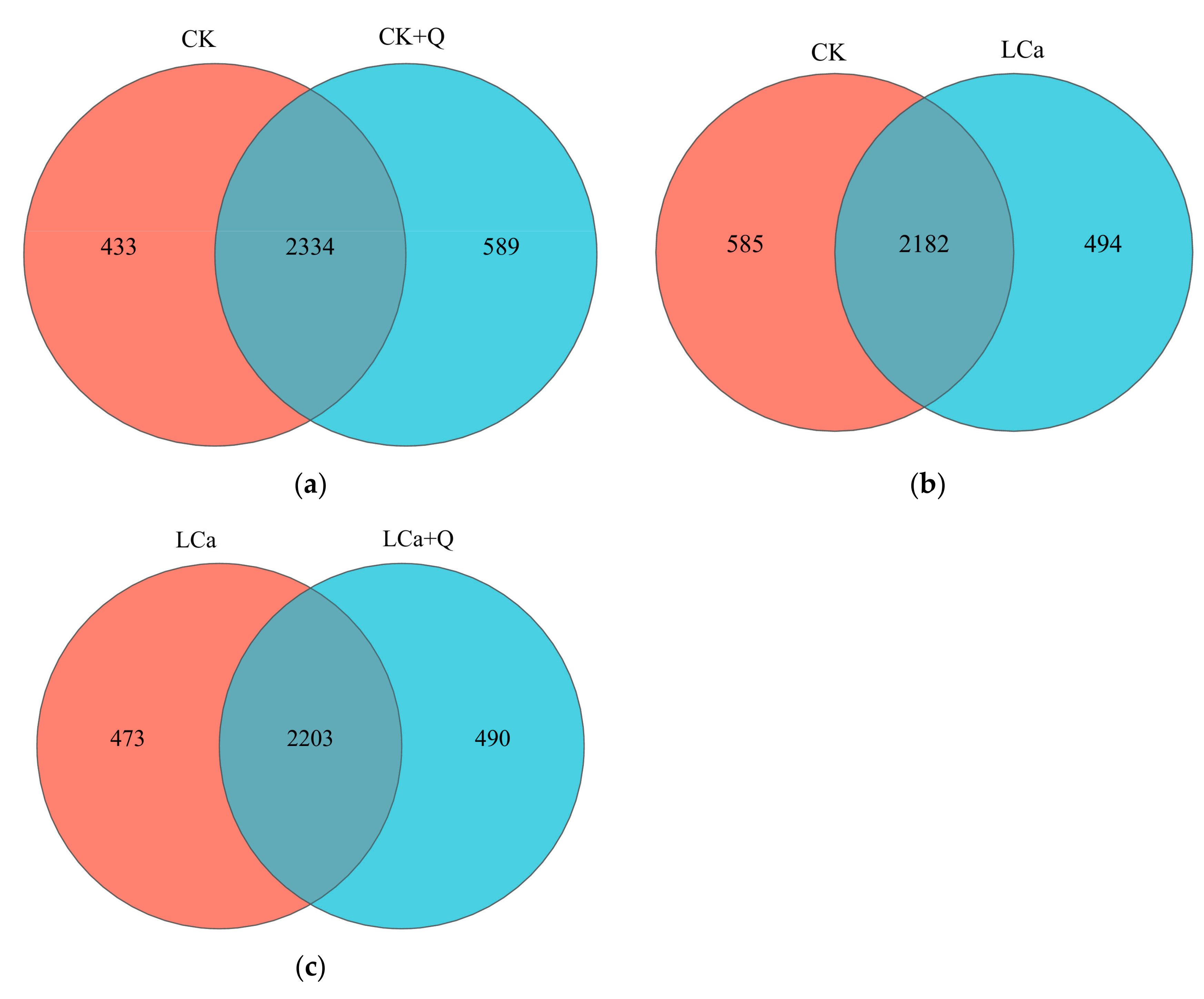
3.5.3. Distribution and Comparison of OTUs in Cucumber Inter-Root Matrix Communities of Different Treatments’ Venn Diagram
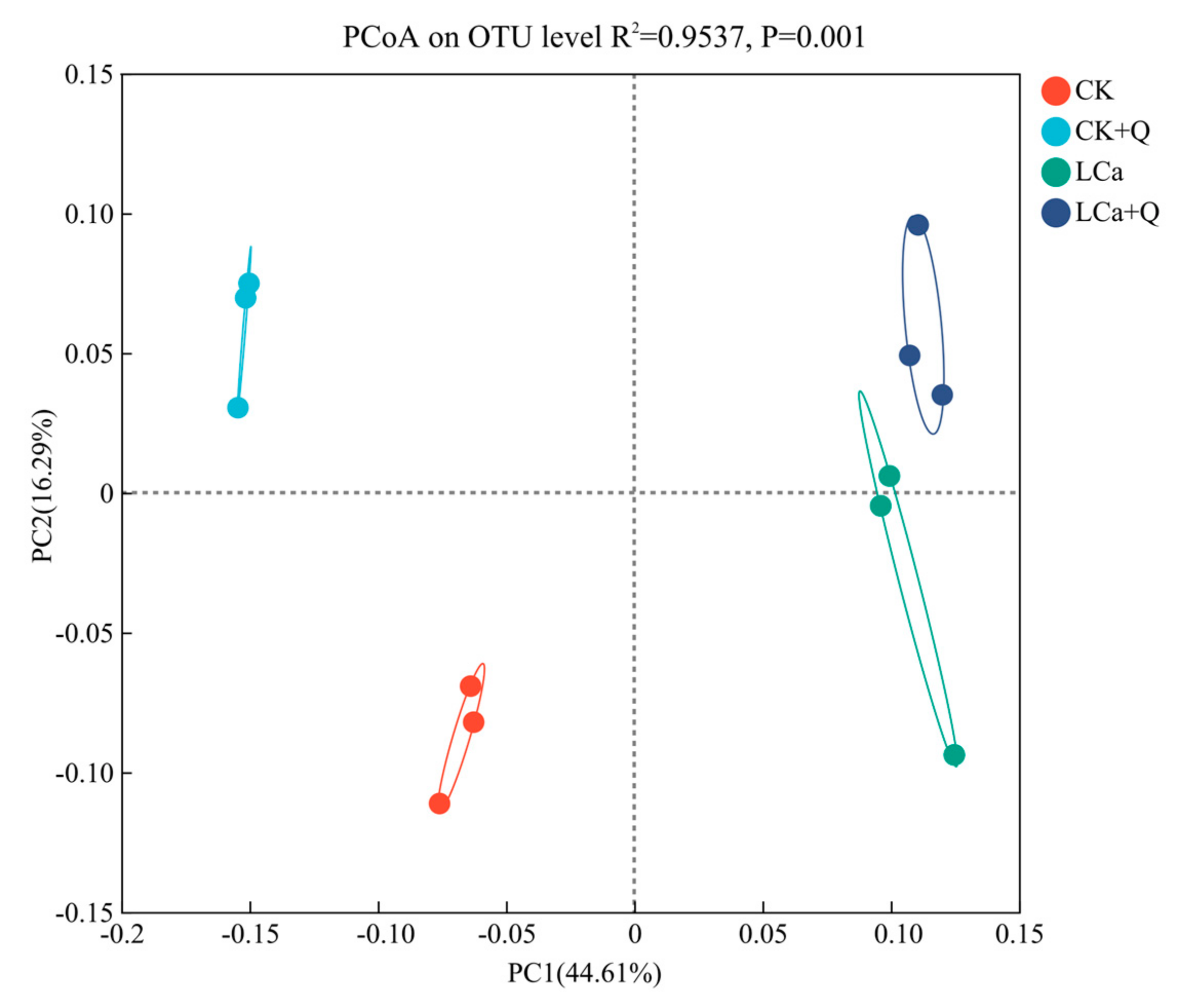
3.5.4. Principal Coordinate Analysis (PCoA) of Cucumber Inter-Root Matrix Communities in Different Treatments
3.5.5. Diversity Analysis of Dominant Bacterial Communities in the Inter-Root Matrix Based on Different Taxonomic Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.L.; Yang, X.; Zhang, M.C.; Li, D.Y.; Jiang, Y.; Yao, W.H.; Zhang, Z. Yield, Quality, and Water and Fertilizer Partial Productivity of Cucumber as Influenced by the Interaction of Water, Nitrogen, and Magnesium. Agronomy 2023, 13, 772. [Google Scholar] [CrossRef]
- Barker, J.C.; Sonneveld, C. Calcium deficiency of glasshouse cucumber as affected by environmental humidity and mineral nutrition. J. Hortic. Sci. 1988, 63, 241–246. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Iqbal, A. Phosphate solubilizing rhizobacteria as alternative of chemical fertilizer for growth and yield of Triticum aestivum (Var. Galaxy 2013). Saudi J. Biol. Sci. 2019, 26, 1400–1410. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Ma, Y.; Rocha, I.; Carvalho, M.F.; Vosátka, M.; Freitas, H. Arbuscular mycorrhizal fungi are an alternative to the application of chemical fertilizer in the production of the medicinal and aromatic plant Coriandrum sativum L. J. Toxicol. Environ. Health Part A 2016, 79, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hassen, A.I.; Bopape, F.L.; Sanger, L.K. Microbial Inoculants as Agents of Growth Promotion and Abiotic Stress Tolerance in Plants; Springer: New Delhi, India, 2016; pp. 23–36. [Google Scholar] [CrossRef]
- Stamenkovic, S.; Beskoski, V.; Karabegovic, I. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Stamford, N.P.; Felix, F.; Oliveira, W.; Silva, E.; Carolina, S.; Arnaud, T.; Freitas, A.D. Interactive effectiveness of microbial fertilizer enriched in N on lettuce growth and on characteristics of an Ultisol of the rainforest region. Sci. Hortic. 2019, 247, 242–246. [Google Scholar] [CrossRef]
- Cong, P.O.; Zhu, O.Y.; Hou, R.X.; Han, D.R. Effects of application of microbial fertilizer on aggregation and aggregate-associated carbon in saline soils. Soil Tillage Res. 2017, 168, 33–41. [Google Scholar] [CrossRef]
- Sun, Y.M.; Zhang, N.N.; Wang, E.T.; Yuan, H.L.; Yang, J.S.; Chen, W.X. Influence of intercropping and intercropping plus rhizobial inoculation on microbial activity and community composition in rhizosphere of alfalfa (Medicago sativa L.) and Siberian wild rye (Elymus sibiricus L.). FEMS Microbiol. Ecol. 2009, 70, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.P.; Xue, Z.Q.; Yang, Z.Y.; Chai, Z.; Niu, J.P.; Shi, Z.Y. Effects of microbial organic fertilizers on Astragalus membranaceus growth and rhizosphere microbial community. Ann. Microbiol. 2021, 71, 11. [Google Scholar] [CrossRef]
- Hu, J.L.; Lin, X.G.; Wang, J.H.; Cui, X.C.; Dai, J.; Chu, H.Y.; Zhang, J.B. Arbuscular mycorrhizal fungus enhances P acquisition of wheat (Triticum aestivum L.) in a sandy loam soil with long-term inorganic fertilization regime. Appl. Microbiol. Biotechnol. 2010, 3, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Veresoglou, S.D.; Halley, J.M.; Rillig, M.C. Extinction risk of soil biota. Nat. Commun. 2015, 6, 8862. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Si, G.C.; Wang, J.; Luo, T.X.; Zhang, G.X. Bacterial community in alpine grasslands along an altitudinal gradient on the Tibetan Plateau. FEMS Microbiol. Ecol. 2014, 87, 121–132. [Google Scholar] [CrossRef]
- Chu, H.Y.; Sun, H.B.; Tripathi, B.M.; Adams, J.M.; Huang, R.; Zhang, Y.J.; Shi, Y. Bacterial community dissimilarity between the surface and subsurface soils equals horizontal differences over several kilometers in the western Tibetan Plateau. Environ. Microbiol. 2016, 18, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.P.; Li, J.B.; Wang, S.P.; Wang, S.P.; An, J.X.; Liu, W.T.; Liu, Q.Y.; Yang, Y.F.; He, Z.L.; Li, X.Z. Responses of Bacterial Communities to Simulated Climate Changes in Alpine Meadow Soil of the Qinghai-Tibet Plateau. Appl. Environ. Microbiol. 2015, 81, 6070–6077. [Google Scholar] [CrossRef] [PubMed]
- Pérez Castro, S.; Cleland, E.E.; Wagner, R.; Sawad, R.A.; Lipson, D.A. Soil microbial responses to drought and exotic plants shift carbon metabolism. ISME J. 2019, 13, 1776–1787. [Google Scholar] [CrossRef]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Chu, H.Y.; Lin, X.G.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J.L.; Zhang, J.B. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol. Biochem. 2007, 39, 2971–2976. [Google Scholar] [CrossRef]
- Xiang, X.J.; Gibbons, S.M.; He, J.S.; Wang, C.; He, D.; Li, Q.; Ni, Y.Y.; Chu, H.Y. Rapid response of arbuscular mycorrhizal fungal communities to short-term fertilization in an alpine grassland on the Qinghai-Tibet Plateau. PeerJ 2016, 4, e2226. [Google Scholar] [CrossRef]
- Xiang, X.J.; He, D.; He, J.S.; Myrold, D.D.; Chu, H.Y. Ammonia-oxidizing bacteria rather than archaea respond to short-term urea amendment in an alpine grassland. Soil Biol. Biochem. 2017, 107, 218–225. [Google Scholar] [CrossRef]
- Zapata-Sifuentes, G.; Hernandez-Montiel, L.G.; Saenz-Mata, J.; Fortis-Hernandez, M.; Blanco-Contreras, E.; Chiquito-Contreras, R.J.; Preciado-Rangel, P. Plant growth-promoting rhizobacteria improve growth and fruit quality of cucumber under greenhouse conditions. Plants 2022, 11, 1612. [Google Scholar] [CrossRef]
- Dilfuza, E.; Zulfiya, K.; Kakhramon, D. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fertil. Soils 2011, 47, 197–205. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Pineda, A.; Zheng, S.J.; Van Loon, J. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Guo, Q.; Li, Y.Z.; Sun, Y.F.; Xue, Q.H.; Lai, H.X. Streptomyces pactum Act12 controls tomato yellow leaf curl virus disease and alters rhizosphere microbial communities. Biol. Fertil. Soils 2019, 55, 149–169. [Google Scholar] [CrossRef]
- Li, H.Y.; Qiu, Y.Z.; Yao, T.; Ma, Y.C.; Zhang, H.R.; Yang, X.L. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Zhang, X.L.; Ma, L.; Gilliam, F.S.; Wang, Q.; Li, C.H. Effects of raised-bed planting for enhanced summer maize yield on rhizosphere soil microbial functional groups and enzyme activity in Henan Province, China. Field Crops Res. 2012, 130, 28–37. [Google Scholar] [CrossRef]
- Kuzmina, N.P.; Ermolaeva, S.V.; Chevychelov, A.P. Microbiological analysis of agropal soil with potato plantings. IOP Conf. Ser. Earth Environ. Sci. 2022, 1045, 012043. [Google Scholar] [CrossRef]
- Ma, Y.J.; Lan, Z.Q.; Zhang, K.G.; Li, B.Y.; Zheng, W.D.; Gao, Y.M.; Li, J.S.; Zhang, X.Y. Effects of Plough Layer Thickness on Soil Nutrients and Cucumber Root Development. Sci. Hortic. 2021, 290, 110498. [Google Scholar] [CrossRef]
- Nerdy. Determination of sodium, potassium, magnesium, and calcium minerals level in fresh and boiled broccoli and cauliflower by atomic absorption spectrometry. IOP Conf. Ser. Earth Environ. Sci. Eng. 2018, 288, 012113. [Google Scholar] [CrossRef]
- Zhu, B.; Gu, H.Z.; He, J.X.; Li, F.C.; Yu, J.; Liu, W.J. The impact of smash-ridge tillage on agronomic traits of tobacco plants, soil enzymatic activity, microbial community structure, and functional diversity. Plant Signal. Behav. 2023, 18, 2260640. [Google Scholar] [CrossRef]
- Mao, G.F.; Lu, L.M.; Yang, X.H.; Chen, Y.P.; Zhu, P. Study on the Basic Characteristics and Control Technique of Secondary Salted Soil under Protected Culture of Watermelon and Melon in Shanghai Suburbs. Acta Agric. Shanghai 2005, 01, 58–66. [Google Scholar] [CrossRef]
- Wu, F.Z.; Bao, J.; Liu, S.Q. Effects of Salt Stress on Rhizospheric Soil Bacterial Community Structure and Cucumber Yield. Acta Hortic. Sin. 2010, 37, 741–748. [Google Scholar] [CrossRef]
- Chu, Y.H.; Cui, S.M.; Fu, C.Y.; Yu, C.H.; Liu, W.; Gao, X.Y.; Sun, Q. Effects of different microbial fertilisers on the growth and quality of lettuce. Vegetables 2014, 03, 20–24. [Google Scholar] [CrossRef]
- Mari, G.R.; Ji, C.Y.; Zhou, J. Effects of soil compaction on soil physical properties and nitrogen, phosphorus, potassium uptake in wheat plants. Trans. Chin. Soc. Agric. Eng. 2008, 01, 74–79. [Google Scholar] [CrossRef]
- Li, D. Effect of organic microbial fertilisers on the physico-chemical properties of soil in cucumber fields. Xiandai Hortic. 2017, 7, 15. [Google Scholar] [CrossRef]
- Zhu, J.Y. Study on the Application of Microbial Agents in Cucumber and Tomato Cultivated in Greenhouse. Master’s Thesis, Shandong Agricultural University, Taian, China, 2014. [Google Scholar] [CrossRef]
- Zhao, H.H. Study on Microbial Fertilizer Function and Its Application in Vegetable Production. Heilongjiang Agric. Sci. 2011, 1, 51–53. [Google Scholar] [CrossRef]
- Wang, J.Z. Study on the Application Effect of Microbial Agents in Cucumber Seedling and Substrate Culture. Master’s Thesis, Northwest A&F University, Yangling, China, 2021. [Google Scholar] [CrossRef]
- Han, D.W.; Zhang, D.; Han, D.Z.; Ren, H.L.; Wang, Z.; Zhu, Z.J.; Sun, H.Y.; Wang, L.X.; Qu, Z.C.; Yuan, M. Effects of salt stress on soil enzyme activities and rhizosphere microbial structure in salt-tolerant and -sensitive soybean. Sci. Rep. 2023, 13, 17057. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.L.; Zhang, X.M.; Hong, Y.; Liu, D.F. Effect of long-term fertilization and tillage system on soil enzyme activities. Soils Fertil. Sci. China 2008, 2, 27–30, 60. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhang, Q.; Gao, Z.X.; Ma, X.Q.; Qu, F.; Hu, X.H. Effects of Two Microbial Agents on Yield, Quality and Rhizosphere Environment of Autumn Cucumber Cultured in Organic Substrate. Sci. Agric. Sin. 2021, 54, 3077–3087. [Google Scholar] [CrossRef]
- Liu, S.Y. Effects of EM fertilizer on rhizosphere soil enzyme activity in cucumber. China Cucurbits Veg. 2016, 29, 11–13. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; Lei, S.N.; Li, Y.; Huang, W.; Ma, R.Q. Revealing the variation and stability of bacterial communities in tomato rhizosphere micro-biota. Microorganisms 2020, 8, 170. [Google Scholar] [CrossRef]
- Liu, P.J.; Xiao, J.; Sun, B.H.; Gao, M.X.; Zhang, S.L.; Yang, X.Y.; Feng, H. Variation of bacterial community structure and the main influencing factors in Eum-orthic Anthrosols under different fertilization regimes. J. Plant Nutr. Fertil. 2020, 26, 307–315. [Google Scholar] [CrossRef]
- Yuan, H.C.; Wu, H.; Ge, T.D.; Li, K.L.; Wu, J.S.; Wang, J.R. Effects of long-term fertilization on bacterial and archaeal diversity and community structure within subtropical red paddy soils. Chin. J. Appl. Ecol. 2015, 26, 1807–1813. [Google Scholar] [CrossRef]


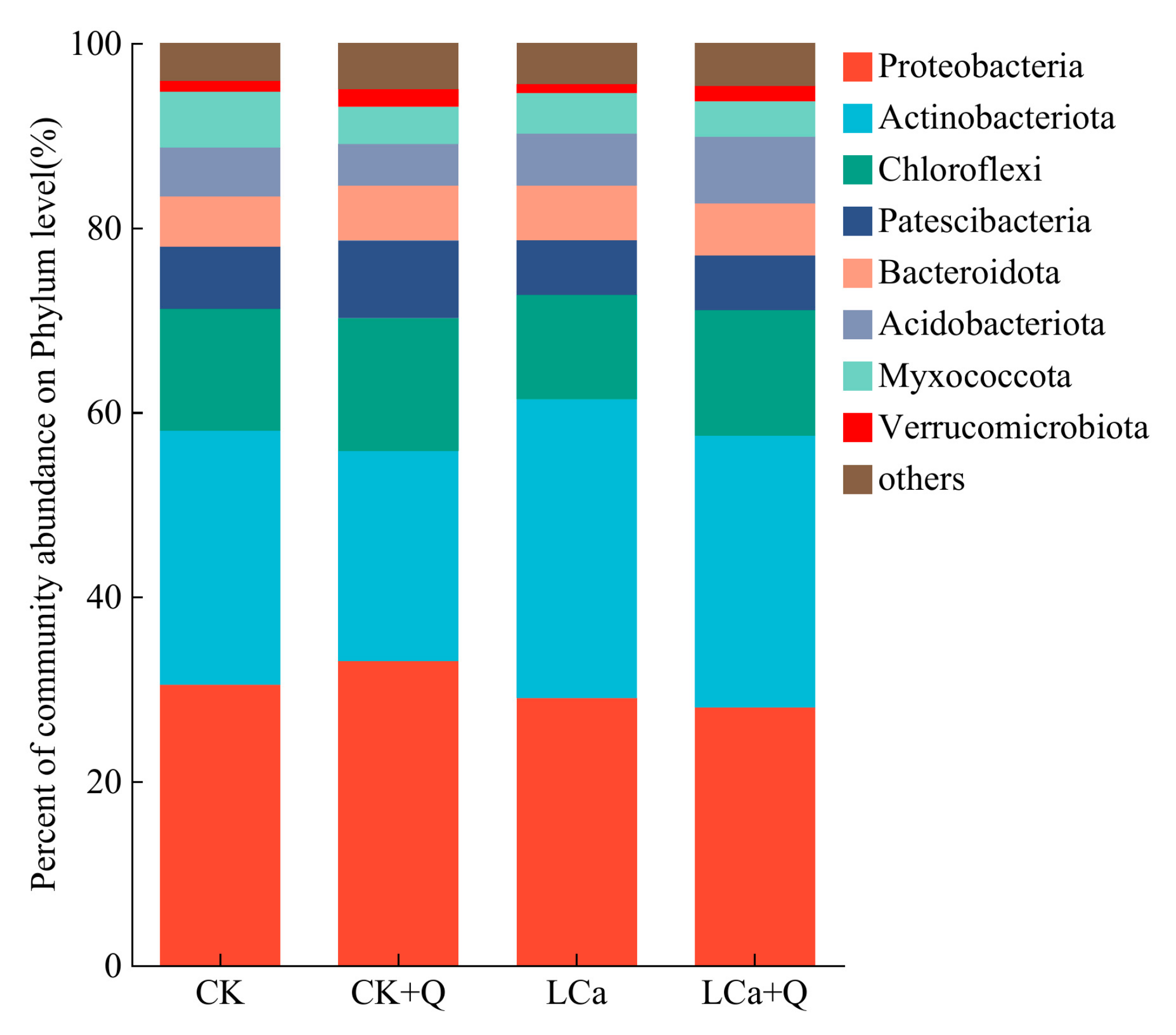
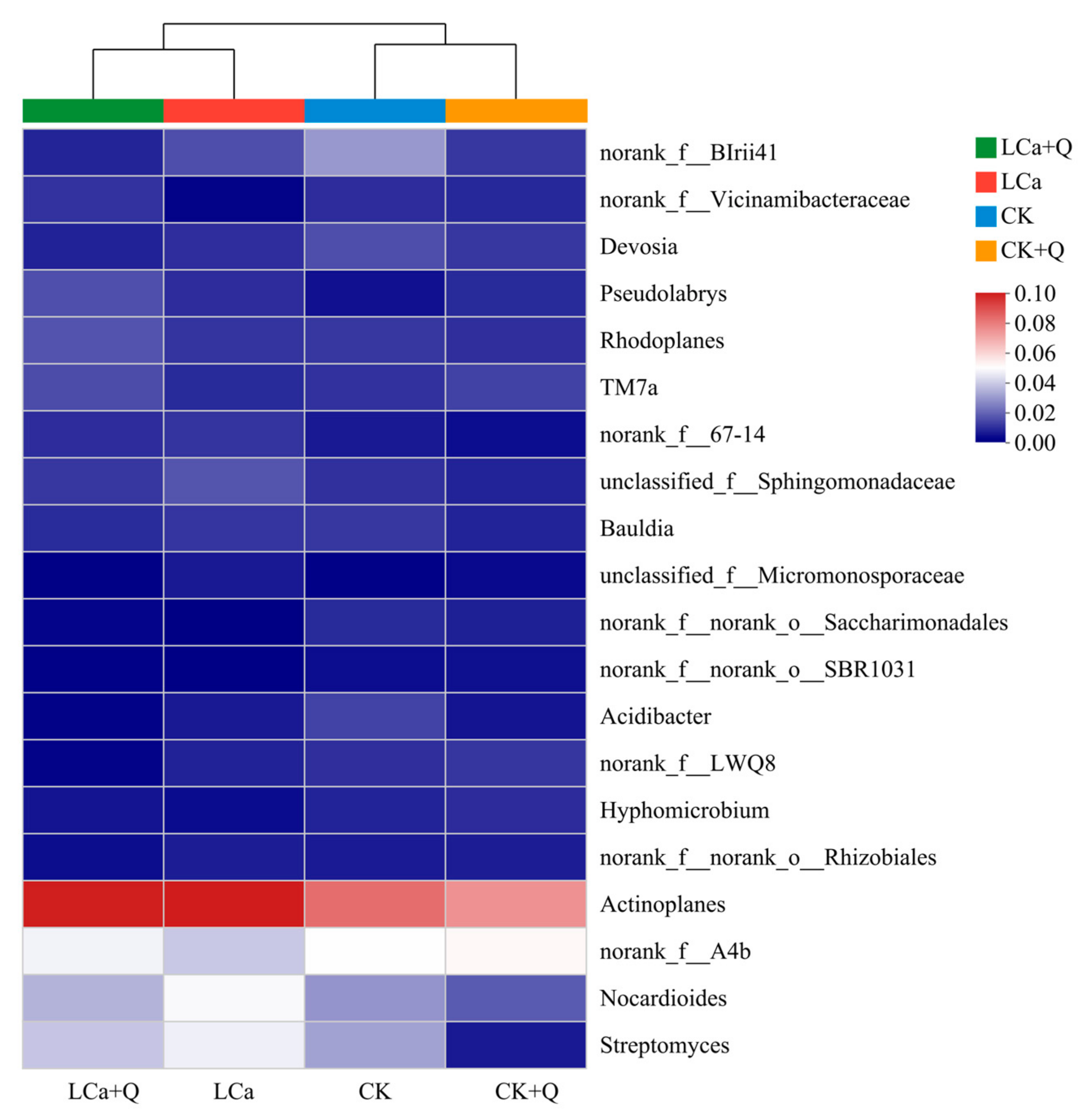
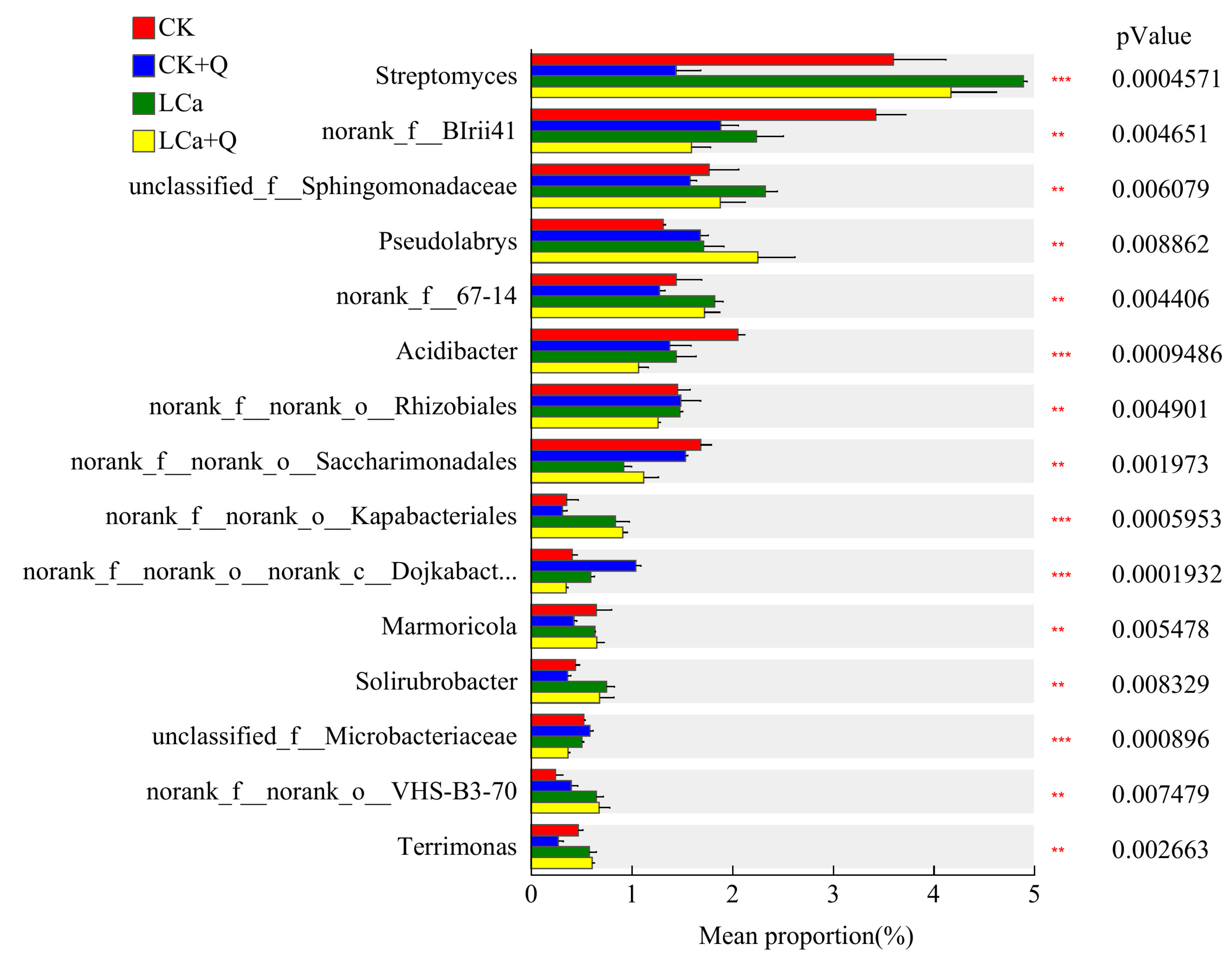
| Treatment | Shoot Fresh Weight g | Shoot Dry Weight g | Root Fresh Weight g | Root Dry Weight g | Total Fresh Weight g | Total Dry Weight g |
|---|---|---|---|---|---|---|
| CK | 555.31 ± 5.43 b | 52.32 ± 1.06 b | 67.93 ± 4.03 b | 5.69 ± 0.25 b | 623.24 ± 9.40 b | 58.01 ± 1.29 b |
| CK+Q | 604.80 ± 4.02 a | 58.72 ± 1.86 a | 78.09 ± 0.99 a | 6.82 ± 0.18 a | 682.89 ± 4.99 a | 65.54 ± 2.03 a |
| LCa | 457.63 ± 5.58 d | 43.33 ± 1.23 c | 56.69 ± 2.73 c | 4.76 ± 0.04 c | 514.33 ± 8.16 d | 48.09 ± 1.19 c |
| LCa+Q | 504.72 ± 0.44 c | 50.06 ± 0.60 b | 66.52 ± 1.38 b | 5.36 ± 0.02 b | 571.23 ± 1.64 c | 55.42 ± 0.60 b |
| Treatment | pH | EC (us·cm−1) | Bulk Density (g·cm−3) | Porosity (%) |
|---|---|---|---|---|
| CK | 6.34 ± 0.12 b | 140.00 ± 2.14 d | 0.15 ± 0.00 b | 88.29 ± 0.87 b |
| CK+Q | 6.05 ± 0.05 c | 133.00 ± 2.52 c | 0.12 ± 0.00 d | 92.79 ± 0.58 a |
| LCa | 6.64 ± 0.04 a | 163.13 ± 1.42 a | 0.16 ± 0.00 a | 81.57 ± 1.94 c |
| LCa+Q | 6.42 ± 0.03 ab | 147.87 ± 1.25 b | 0.13 ± 0.00 c | 86.06 ± 0.31 b |
| Treatment | Organic Matter (g·kg−1) | Nitrogen (%) | Phosphorus (μg·g−1 DW) | Potassium (mg·g−1 DW) |
|---|---|---|---|---|
| CK | 305.93 ± 5.17 b | 2.63 ± 0.02 b | 144.95 ± 0.30 b | 15.45 ± 0.21 c |
| CK+Q | 258.96 ± 7.47 c | 2.05 ± 0.05 d | 122.61 ± 2.73 c | 13.24 ± 0.28 d |
| LCa | 356.04 ± 12.11 a | 2.96 ± 0.04 a | 173.46 ± 2.85 a | 18.89 ± 0.06 a |
| LCa+Q | 309.81 ± 4.50 b | 2.45 ± 0.03 c | 150.05 ± 3.65 b | 16.52 ± 0.17 b |
| Treatment | Seq Num. | Base Num. | Mean Length | Min Length | Max Length | Coverage/% |
|---|---|---|---|---|---|---|
| CK-1 | 67,503 | 27,816,132 | 412.07 | 203 | 490 | 98.49% |
| CK-2 | 62,622 | 25,829,676 | 412.47 | 217 | 495 | 98.37% |
| CK-3 | 65,159 | 26,901,175 | 412.85 | 249 | 486 | 98.59% |
| CK+Q-1 | 60,164 | 24,797,631 | 412.17 | 232 | 498 | 98.40% |
| CK+Q-2 | 61,575 | 25,388,341 | 412.32 | 246 | 492 | 98.35% |
| CK+Q-3 | 63,455 | 26,174,644 | 412.49 | 203 | 486 | 98.36% |
| LCa-1 | 60,819 | 25,084,857 | 412.45 | 201 | 507 | 98.56% |
| LCa-2 | 56,338 | 23,242,520 | 412.55 | 240 | 468 | 98.53% |
| LCa-3 | 61,609 | 25,428,312 | 412.74 | 203 | 482 | 98.50% |
| LCa+Q-1 | 60,386 | 24,884,589 | 412.09 | 204 | 535 | 98.35% |
| LCa+Q-2 | 56,917 | 23,477,478 | 412.49 | 246 | 507 | 98.49% |
| LCa+Q-3 | 67,447 | 27,784,810 | 411.95 | 245 | 467 | 98.45% |
| Mean | 62,000 | 25,567,514 | 412.39 | 224 | 493 | 98.45% |
| Treatment | Alpha Diversity Index | ||||
|---|---|---|---|---|---|
| Sobs | ACE | Chao1 | Shannon | Simpson | |
| CK | 2039 bc | 2649.04 bc | 2641.54 b | 6.04 ab | 0.008 a |
| CK+Q | 2186 a | 2833.40 a | 2843.38 a | 6.22 a | 0.006 a |
| LCa | 1960 c | 2545.82 c | 2573.43 b | 5.90 b | 0.011 a |
| LCa+Q | 2119 ab | 2733.28 ab | 2748.50 ab | 6.15 a | 0.007 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhang, L.; Wei, L.; Yang, Y.; Wang, Z.; Qiao, B.; Han, L. Effect of Bacillus amyloliquefaciens QST713 on Inter-Root Substrate Environment of Cucumber under Low-Calcium Stress. Agronomy 2024, 14, 542. https://doi.org/10.3390/agronomy14030542
Li B, Zhang L, Wei L, Yang Y, Wang Z, Qiao B, Han L. Effect of Bacillus amyloliquefaciens QST713 on Inter-Root Substrate Environment of Cucumber under Low-Calcium Stress. Agronomy. 2024; 14(3):542. https://doi.org/10.3390/agronomy14030542
Chicago/Turabian StyleLi, Bin, Li Zhang, Lincao Wei, Yujie Yang, Zhexuan Wang, Bo Qiao, and Lingjuan Han. 2024. "Effect of Bacillus amyloliquefaciens QST713 on Inter-Root Substrate Environment of Cucumber under Low-Calcium Stress" Agronomy 14, no. 3: 542. https://doi.org/10.3390/agronomy14030542
APA StyleLi, B., Zhang, L., Wei, L., Yang, Y., Wang, Z., Qiao, B., & Han, L. (2024). Effect of Bacillus amyloliquefaciens QST713 on Inter-Root Substrate Environment of Cucumber under Low-Calcium Stress. Agronomy, 14(3), 542. https://doi.org/10.3390/agronomy14030542





