Chemotactic Responses of Oscheius myriophilus to Mollusk Mucus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mollusk Collection and Maintenance
2.2. Nematode Suspensions
2.3. Chemotaxis Assay and Mollusk Mucus Collection
2.4. Statistical Analysis
3. Results
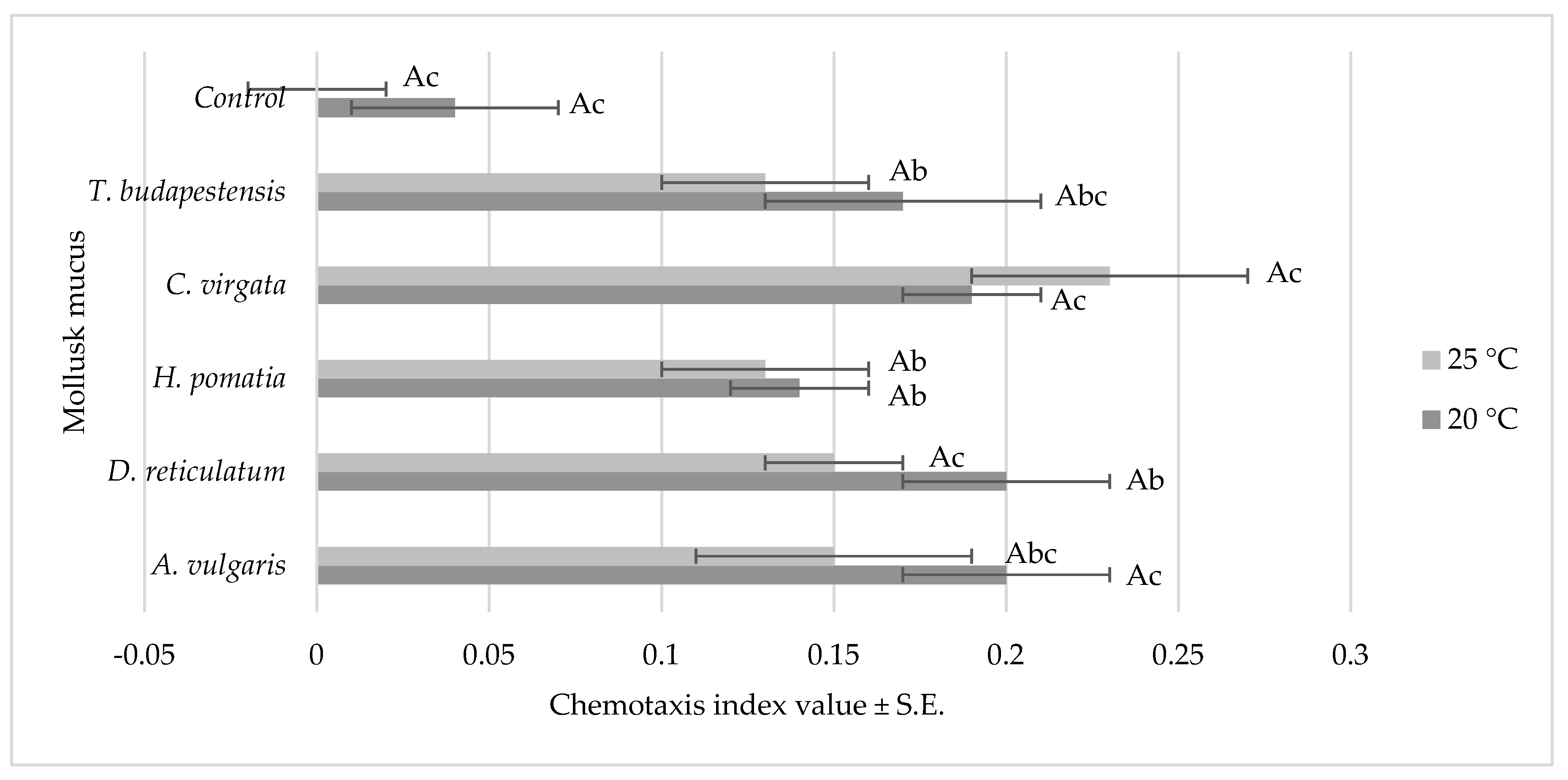
3.1. Nematode Chemoattraction Towards Mollusk Mucus Versus Water
3.1.1. Nematode Motility
3.1.2. Chemotaxis Index
3.2. Comparison of Nematode Chemoattraction Towards Mollusk Mucus in a Choice Test
3.2.1. The Spanish Slug (Arion vulgaris) vs. Other Mollusk Species
3.2.2. The Gray Field Slug (Deroceras reticulatum) vs. Other Mollusk Species
3.2.3. The Roman Snail (Helix pomatia) vs. Other Mollusk Species
3.2.4. The Common White Snail (Cernuella virgata) vs. Other Mollusk Species
3.2.5. The Budapest Keeled Slug (Tandonia budapestensis) vs. Other Mollusk Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glen, D.M.; Moens, R. Agriolimacidae, Arionidae and Milacidae as Pests in West European Cereals. In Molluscs as Crop Pests; Barker, G.M., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 271–300. [Google Scholar]
- Kozłowski, J. Host Plants and Harmfulness of the Arion lusitanicus Mabille, 1868 Slug. J. Plant Prot. Res 2014, 45, 221–233. [Google Scholar]
- Slotsbo, S.; Damgaard, C.; Hansen, L.M.; Holmstrup, M. The Influence of Temperature on Life History Traits in the Iberian Slug, Arion lusitanicus. Ann. Appl. Biol. 2013, 162, 80–88. [Google Scholar] [CrossRef]
- Rowson, B.; Turner, J.; Anderson, R.; Symondson, B. Slugs of Britain and Ireland; FSC Publications: Telford, UK, 2014. [Google Scholar]
- Henderson, I.; Triebskorn, R. Chemical Control of Terrestrial Gastropods. In Molluscs as Crop Pests; Barker, G.M., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 1–31. [Google Scholar]
- Iglesias, J.; Castillejo, J.; Castro, R. The Effects of Repeated Applications of the Molluscicide Metaldehyde and the Biocontrol Nematode Phasmarhabditis hermaphrodita on Molluscs, Earthworms, Nematodes, Acarids and Collembolans: A Two-Year Study in North-West Spain. Pest Manag. Sci. 2003, 59, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Rae, R.G.; Robertson, J.F.; Wilson, M.J. Chemoattraction and Host Preference of the Gastropod Parasitic Nematode Phasmarhabditis hermaphrodita. J. Parasitol. 2009, 95, 517–526. [Google Scholar] [CrossRef]
- Mancini, F.; Woodcock, B.A.; Isaac, N.J.B. Agrochemicals in the Wild: Identifying Links between Pesticide Use and Declines of Nontarget Organisms. Curr. Opin. Environ. Sci. Health 2019, 11, 53–58. [Google Scholar] [CrossRef]
- Castle, G.D.; Mills, G.A.; Gravell, A.; Jones, L.; Townsend, I.; Cameron, D.G.; Fones, G.R. Review of the Molluscicide Metaldehyde in the Environment. Environ. Sci. Water Res. 2017, 3, 415–428. [Google Scholar] [CrossRef]
- Wilson, M.J.; Rae, R. Phasmarhabditis hermaphrodita as a Control Agent for Slugs. In Nematode Pathogenesis of Insects and Other Pests; Campos-Herrera, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 509–521. [Google Scholar]
- Morand, S.; Wilson, M.J.; Glen, D.M. Nematodes (Nematoda) Parasitic in Terrestrial Gastropods. In Natural Enemies of Terrestrial Molluscs; Barker, G.M., Ed.; CABI Publishing: Wallingford, UK, 2004; pp. 525–557. [Google Scholar]
- Mc Donnell, R.; Howe, D.; Denver, D. First Report of the Gastropod-Killing Nematode, Phasmarhabditis californica, in Washington, U.S.A. J. Nematol. 2023, 55, 20230013. [Google Scholar] [CrossRef] [PubMed]
- Rae, R.G.; Robertson, J.F.; Wilson, M.J. The Chemotactic Response of Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) to Cues of Deroceras reticulatum (Mollusca: Gastropoda). Nematology 2006, 8, 197–200. [Google Scholar]
- Sheehy, L.; Cutler, J.; Weedall, G.D.; Rae, R. Microbiome Analysis of Malacopathogenic Nematodes Suggests No Evidence of a Single Bacterial Symbiont Responsible for Gastropod Mortality. Front. Immunol. 2022, 13, 878783. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, J.; Melby, K.K.; Magnusson, C.; Nielsen, A.; Rydning, J.; Wendell, P.H.M.; Alsanius, B.; Krokene, P.; Thomsen, I.M.; Wright, S.A.; et al. Risk Assessment of the Biocontrol Product Nemaslug 2.0 with the Active Organisms Phasmarhabditis californica (Strain P19D) and Moraxella osloensis. Sci. Opin. Panel Plant Health Norw. Sci. Comm. Food Environ. 2021, 16, 1–37. [Google Scholar]
- Pieterse, A.; Tiedt, L.R.; Malan, A.P.; Ross, J.L. First Record of Phasmarhabditis papillosa (Nematoda: Rhabditidae) in South Africa, and Its Virulence Against the Invasive Slug, Deroceras panormitanum. Nematology 2017, 19, 1035–1050. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S.; Tóth, T.; Ádám, S.; Lakatos, T.; Majić, I. Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata. Agronomy 2023, 13, 1386. [Google Scholar] [CrossRef]
- Andrássy, I. A Taxonomic Review of the Suborder Rhabditina (Nematoda: Secernentia); ORSTOM: Paris, France, 1983; p. 241. [Google Scholar]
- Sudhaus, W.; Hooper, D.J. Rhabditis (Oscheius) guentheri sp. n., an Unusual Species with Reduced Posterior Ovary, with Observations on the Dolichura and Insectivora Groups (Nematoda: Rhabditidae). Nematologica 1994, 40, 508–533. [Google Scholar] [CrossRef]
- Kumar, P.; Jamal, W.; Somvanshi, V.S.; Chauhan, K.; Mumtaz, S. Description of Oscheius indicus n. sp. (Rhabditidae: Nematoda) from India. J. Nematol. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Půža, V.; Jaffuel, G.; Blanco-Pérez, R.; Čepulyte Rakauskiene, R.; Turlings, T. Unraveling the Intraguild Competition between Oscheius spp. Nematodes and Entomopathogenic Nematodes: Implications for Their Natural Distribution in Swiss Agricultural Soils. J. Invertebr. Pathol. 2015, 132, 216–227. [Google Scholar] [CrossRef]
- Castro-Ortega, I.R.; Caspeta-Mandujano, J.M.; Suárez-Rodríguez, R.; Peña-Chora, G.; Ramírez Trujillo, J.A.; Cruz-Pérez, K.; Sosa, I.A.; Hernández-Velázquez, V.M. Oscheius myriophilus (Nematoda: Rhabditida) Isolated in Sugar Cane Soils in Mexico with Potential to Be Used as Entomopathogenic Nematode. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Poinar, G.O. Rhabditis myriophila sp. n., (Rhabditidae: Rhabditida), Associated with the Millipede, Oxidis gracilis (Polydesmida: Diplepoda). Proc. Helm. Soc. Wash. 1986, 53, 232–236. [Google Scholar]
- Erbaş, Z.; Demir, İ.; Demirbağ, Z. Isolation and Characterization of a Parasitic Nematode, Oscheius myriophila (Nematoda: Rhabditida), Associated with European Mole Cricket, Gryllotalpa gryllotalpa (Orthoptera: Gryllotalpidae). Hacet. J. Biol. Chem. 2017, 45, 197–203. [Google Scholar] [CrossRef]
- Hapca, S.; Crawford, J.; Rae, R.; Wilson, M.; Young, I. Movement of the Parasitic Nematode Phasmarhabditis hermaphrodita in the Presence of the Slug Deroceras reticulatum. Biol. Control 2007, 41, 223–229. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of Entomopathogenic Nematodes by Insect-Damaged Maize Roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. An Investigation on the Chemotactic Responses of Different Entomopathogenic Nematode Strains to Mechanically Damaged Maize Root Volatile Compounds. Exp. Parasitol. 2013, 134, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Jagodič, A.; Ipavec, N.; Trdan, S.; Laznik, Ž. Attraction Behaviors: Are Synthetic Volatiles, Typically Emitted by Insect-Damaged Brassica nigra Roots, Navigation Signals for Entomopathogenic Nematodes (Steinernema and Heterorhabditis)? BioControl 2017, 62, 515–524. [Google Scholar] [CrossRef]
- Andrus, P.; Rae, R. Natural Variation in Chemoattraction of Phasmarhabditis hermaphrodita, Phasmarhabditis neopapillosa, and Phasmarhabditis californica Exposed to Slug Mucus. Nematology 2019, 21, 479–488. [Google Scholar] [CrossRef]
- Small, R.W.; Bradford, C. Behavioral Responses of Phasmarhabditis hermaphrodita (Nematoda: Rhabditida) to Mucus from Potential Hosts. Nematology 2008, 19, 591–598. [Google Scholar] [CrossRef]
- Nermut’, J.; Půža, V.; Mráček, Z. The Response of Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) and Steinernema feltiae (Nematoda: Steinernematidae) to Different Host-Associated Cues. Biol. Control 2012, 61, 201–206. [Google Scholar] [CrossRef]
- Andrus, P.; Ingle, O.; Coleman, T.; Rae, R. Gastropod Parasitic Nematodes (Phasmarhabditis sp.) Are Attracted to Hyaluronic Acid in Snail Mucus by cGMP Signalling. J. Helminthol. 2018, 94, e9. [Google Scholar] [CrossRef] [PubMed]
- Laznik, Ž.; Franin, K.; Trdan, S.; Vidrih, M.; Majić, I. Chemotactic Response and Motility of the Mollusc Parasitic Nematode Phasmarhabditis papillosa towards Mucus from Different Mollusc Species. BioControl 2022, 67, 345–356. [Google Scholar] [CrossRef]
- Laznik, Ž.; Majić, I.; Trdan, S.; Malan, A.; Pieterse, A.; Ross, J.L. Is Phasmarhabditis papillosa (Nematoda: Rhabditidae) a Possible Biological Control Agent against the Spanish Slug, Arion vulgaris (Gastropoda: Arionidae)? Nematology 2020, 23, 577–585. [Google Scholar] [CrossRef]
- Kaya, H.K.; Stock, S.P. Techniques in Insect Nematology. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 281–324. [Google Scholar]
- O’Halloran, D.M.; Burnell, A.M. An Investigation of Chemotaxis in the Insect Parasitic Nematode Heterorhabditis bacteriophora. Parasitology 2003, 127, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I.; Horvitz, H.R. Chemosensory Neurons with Overlapping Functions Direct Chemotaxis to Multiple Chemicals in C. elegans. Neuron 1991, 7, 729–742. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Thomas, J.H.; Horvitz, H.R. Chemosensory Cell Function in the Behavior and Development of Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Glen, D.M.; George, S.K.; Butler, R.C. The Rhabditid Nematode Phasmarhabditis hermaphrodita as a Potential Biocontrol Agent for Slugs. Biocontrol Sci. Technol. 1993, 3, 503–511. [Google Scholar] [CrossRef]

| Factor | Df | F | p |
|---|---|---|---|
| Mollusk mucus | 5 | 10.61 | <0.01 |
| Temperature (T) | 1 | 10.43 | 0.02 |
| Temporal replication | 9 | 1.73 | 0.52 |
| Spatial replication | 2 | 0.87 | 0.51 |
| Mollusk mucus × T | 5 | 1.27 | 0.65 |
| Residual | 79 | ||
| Total (Corrected) | 101 |
| Factor | Df | F | p |
|---|---|---|---|
| Mollusk mucus | 5 | 7.63 | <0.01 |
| Temperature (T) | 1 | 1.78 | 0.43 |
| Temporal replication | 9 | 0.69 | 0.63 |
| Spatial replication | 2 | 0.44 | 0.60 |
| Mollusk mucus × T | 5 | 2.31 | 0.63 |
| Residual | 79 | ||
| Total (Corrected) | 101 |
| Spanish Slug (A. vulgaris) | |||
|---|---|---|---|
| Factor | Df | F | p |
| Mollusk mucus | 3 | 6.22 | <0.01 |
| Temperature (T) | 1 | 3.73 | 0.06 |
| Temporal replication | 9 | 0.60 | 0.79 |
| Spatial replication | 2 | 0.41 | 0.66 |
| Mollusk mucus × T | 3 | 5.53 | <0.01 |
| Residual | 61 | ||
| Total (Corrected) | 79 | ||
| Gray Field Slug (D. reticulatum) | |||
| Mollusk mucus | 3 | 55.62 | <0.01 |
| Temperature (T) | 1 | 1.21 | 0.28 |
| Temporal replication | 9 | 0.70 | 0.71 |
| Spatial replication | 2 | 0.38 | 0.69 |
| Mollusk mucus × T | 3 | 28.59 | <0.01 |
| Residual | 61 | ||
| Total (Corrected) | 79 | ||
| Roman Snail (H. pomatia) | |||
| Mollusk mucus | 3 | 16.62 | <0.01 |
| Temperature (T) | 1 | 7.43 | <0.01 |
| Temporal replication | 9 | 1.18 | 0.33 |
| Spatial replication | 2 | 0.53 | 0.59 |
| Mollusk mucus × T | 3 | 5.48 | <0.01 |
| Residual | 61 | ||
| Total (Corrected) | 79 | ||
| Common White Snail (C. virgata) | |||
| Mollusk mucus | 3 | 4.27 | <0.01 |
| Temperature (T) | 1 | 58.95 | <0.01 |
| Temporal replication | 9 | 1.42 | 0.20 |
| Spatial replication | 2 | 0.77 | 0.47 |
| Mollusk mucus × T | 3 | 1.20 | 0.32 |
| Residual | 61 | ||
| Total (Corrected) | 79 | ||
| Budapest Keeled Slug (T. budapestensis) | |||
| Mollusk mucus | 3 | 11.92 | <0.01 |
| Temperature (T) | 1 | 0.92 | 0.34 |
| Temporal replication | 9 | 1.02 | 0.44 |
| Spatial replication | 2 | 0.46 | 0.63 |
| Mollusk mucus × T | 3 | 8.87 | <0.01 |
| Residual | 61 | ||
| Total (Corrected) | 79 | ||
| Temperature (°C) | Chemotaxis Index Between Mucus Treatments | |||
|---|---|---|---|---|
| A. v.–C. v. | A. v.–D. r. | A. v.–H. p. | A. v.–T. b. | |
| 20 | −0.21 ± 0.07 Aa | −0.04 ± 0.05 Ab | 0.16 ± 0.04 Bc | −0.13 ± 0.04 Aab |
| 25 | 0.00 ± 0.04 Bab | −0.06 ± 0.03 Aa | 0.04 ± 0.05 Abc | 0.11 ± 0.05 Bc |
| C. v.–A. v. | C. v.–D. r. | C. v.–H. p. | C. v.–T. b. | |
| 20 | 0.21 ± 0.07 Ba | 0.42 ± 0.03 Bc | 0.30 ± 0.05 Bab | 0.34 ± 0.05 Bbc |
| 25 | 0.00 ± 0.04 Aa | 0.08 ± 0.04 Aab | 0.07 ± 0.04 Aab | 0.12 ± 0.04 Ab |
| T. b.–A. v. | T. b.–C. v. | T. b.–D. r. | T. b.–H. p. | |
| 20 | 0.13 ± 0.04 Bd | −0.34 ± 0.05 Aa | 0.00 ± 0.03 Ac | −0.12 ± 0.04 Ab |
| 25 | −0.11 ± 0.05 Aa | −0.12 ± 0.04 Ba | 0.03 ± 0.05 Ab | −0.01 ± 0.05 Bab |
| D. r.–A. v. | D. r.–C. v. | D. r.–H. p. | D. r.–T. b. | |
| 20 | 0.04 ± 0.05 Ab | −0.42 ± 0.03 Ba | −0.02 ± 0.04 Ab | 0.00 ± 0.03 Ab |
| 25 | 0.06 ± 0.03 Ab | −0.08 ± 0.04 Aa | −0.10 ± 0.05 Aa | −0.03 ± 0.05 Aa |
| H. p.–A. v. | H. p.–C. v. | H. p.–D. r. | H. p.–T. b. | |
| 20 | −0.16 ± 0.04 Ab | −0.30 ± 0.05 Aa | 0.02 ± 0.04 Ac | 0.12 ± 0.04 Bd |
| 25 | −0.04 ± 0.05 Ba | −0.07 ± 0.04 Ba | 0.10 ± 0.05 Ab | 0.01 ± 0.05 Aab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laznik, Ž.; Trdan, S.; Šavli, K. Chemotactic Responses of Oscheius myriophilus to Mollusk Mucus. Agronomy 2024, 14, 3049. https://doi.org/10.3390/agronomy14123049
Laznik Ž, Trdan S, Šavli K. Chemotactic Responses of Oscheius myriophilus to Mollusk Mucus. Agronomy. 2024; 14(12):3049. https://doi.org/10.3390/agronomy14123049
Chicago/Turabian StyleLaznik, Žiga, Stanislav Trdan, and Klara Šavli. 2024. "Chemotactic Responses of Oscheius myriophilus to Mollusk Mucus" Agronomy 14, no. 12: 3049. https://doi.org/10.3390/agronomy14123049
APA StyleLaznik, Ž., Trdan, S., & Šavli, K. (2024). Chemotactic Responses of Oscheius myriophilus to Mollusk Mucus. Agronomy, 14(12), 3049. https://doi.org/10.3390/agronomy14123049








