Abstract
Wheat is one of the most important crops to ensure food production globally. Understanding the mechanism of leaf senescence in wheat plays a crucial role in improving its productivity and resilience under various stress scenarios. In this study, we investigated biochemical, functional, and ultrastructural changes during leaf senescence in wheat genotypes with contrasting drought tolerance. For this, key parameters such as chlorophyll and total protein content, membrane stability, malondialdehyde level, and the activity of antioxidant enzymes (superoxide dismutase, ascorbate peroxidase, guaiacol peroxidase, benzidine peroxidase, and catalase) were comparatively analyzed during both natural and drought-induced senescence. Additionally, the expression of superoxide dismutase isoform genes functioning in different cellular compartments was studied, alongside ultrastructural changes in flag leaves. The experiments involved genotypes of bread wheat (Triticum aestivum L.) and durum (Triticum durum Desf.) wheat. The plants were grown in controlled environment chambers under control and drought conditions using a completely randomized design. After the booting stage, irrigation was discontinued for drought-treated plants. Flag leaves were sampled at 7, 14, 21, 28, and 35 days after anthesis. Drought-tolerant genotypes exhibited slower chlorophyll degradation, lower lipid peroxidation, enhanced membrane stability, and stronger antioxidant responses, allowing them to maintain cellular function longer, whereas sensitive genotypes showed accelerated leaf senescence. Transcript levels of FeSOD increased significantly post-flowering but declined as senescence progressed, while MnSOD expression exhibited a rise towards the later stages of ontogenesis across all studied genotypes. Ultrastructural analysis revealed progressive damage to chloroplast membranes, thylakoid structures, and mesophyll cell walls under stress conditions, particularly in sensitive genotypes. These findings contribute to a deeper understanding of the physiological and molecular responses of wheat to drought stress, offering potential targets for improving crop performance in water-limited environments.
1. Introduction
Wheat is a globally significant crop and a vital source of human nutrition [1]. The senescence of wheat leaves is a key physiological process that directly impacts grain yield and quality. Understanding the mechanisms governing leaf senescence is crucial for developing strategies to enhance wheat productivity and resilience [2,3,4].
Leaf senescence is a developmental process characterized by a series of programmed disassembly and degenerative events, such as the accumulation of reactive oxygen species (ROS), the degradation of macromolecules, the breakdown of intracellular organelles, and the transition from nutrient assimilation to remobilization [5,6]. Natural leaf senescence is typically associated with age and accelerates as the plant transitions from vegetative to reproductive growth [7]. During physiological leaf senescence, stored nutrients accumulated in the leaves are translocated to actively growing organs, and in cereals, to the grain [8]. Premature senescence, induced by unfavorable environmental conditions, can reduce crop yields. Various abiotic stressors, including drought, extreme temperatures, salinity, nutrient deficiencies, and light intensity, as well as biotic factors such as pathogen infections and insect infestations, can hasten the leaf senescence process [9]. Furthermore, genetic factors and hormonal regulation play a pivotal role in controlling the timing and progression of leaf senescence [10,11].
In the context of global climate change, water scarcity is expected to have the most significant negative impact on crop yields worldwide. The complex stress caused by drought can trigger premature leaf senescence and lead to early maturation of the entire plant, resulting in reduced productivity [9]. Drought-induced leaf senescence contributes to plant survival under drought stress in certain species by (i) facilitating the early redirection of resources from vegetative to reproductive development, thereby ensuring the completion of the life cycle in monocarpic species; (ii) enabling the remobilization of nutrients from senescing leaves to younger leaves, which supports the survival of perennial plants; and (iii) reducing water loss at the whole-plant level [12]. It has been observed that under drought conditions, nitrogen availability in the soil decreases, which initiates and accelerates the leaf senescence process [13]. Research on drought-induced leaf senescence is critical not only for understanding how plants survive under adverse climatic conditions but also for improving agricultural productivity, as crops with delayed leaf senescence can be used to enhance crop yields [14,15].
The leaf senescence process is characterized by alterations in the expression of thousands of senescence-associated genes (SAGs) [10,16]. Studies have shown that numerous transcriptional regulators govern senescence by modulating SAG expression [17,18]. In addition to genetic and environmental factors, epigenetic regulation adds a new layer of complexity to our understanding of the control of leaf senescence. Epigenetic modifications, such as DNA methylation and histone modifications, influence the expression of genes associated with aging, offering additional avenues for research and potential targets for intervention [6,19]. Multi-omics approaches have revealed that leaf senescence is governed by multiple layers of regulation, including chromatin remodeling, transcriptional and post-transcriptional processes, and translational and post-translational mechanisms [20,21].
The antioxidant system plays a crucial role in mitigating the effects of aging and environmental stress [22,23]. Antioxidants are vital for maintaining cellular homeostasis and protecting plant tissues from oxidative damage during aging. The equilibrium between the production of ROS and the activity of the antioxidant system is a pivotal factor in determining leaf longevity and overall plant health [24]. In plant cells, the primary sources of ROS are associated with three main pathways: electron transport chains in chloroplasts and mitochondria, various oxidases and peroxidases, and photosensitizing compounds such as chlorophyll. Light-driven photosynthetic activity is the major contributor to ROS generation in chloroplasts, while carboxylase and oxygenase reactions catalyzed by Rubisco in these organelles facilitate both the production and scavenging of molecular oxygen [25]. During leaf senescence, as chlorophyll degrades and photosynthetic activity diminishes, the generation of ROS in chloroplasts increases, leading to oxidative stress [26,27]. This prompts the activation of antioxidant enzymes, such as superoxide dismutase, catalase, and peroxidases, to neutralize ROS and protect cellular components from oxidative damage. The dynamic regulation of antioxidant enzyme activity during leaf senescence is a complex process influenced by a range of intracellular and environmental factors [28,29]. Understanding the intricate relationship between these factors and antioxidant enzyme activity is crucial for elucidating the underlying mechanisms of leaf senescence [30].
Cellular organelles are known to respond rapidly to environmental stress through various adaptive and avoidance mechanisms. Leaf cell organelles across different plant species exhibit distinct changes, reflecting their survival strategies under stress [31]. Cell death in senescent leaves is predominantly associated with the deterioration of intracellular organelles [32]. To date, most research has focused on physiological and biochemical parameters [33], with relatively few studies examining changes at the ultrastructural level. This indicates a gap in the scientific literature regarding the ultrastructural aspects of the aging process [34]. As plants age, the cell membrane’s structure is initially compromised with the subsequent transition of the functional proteins of the membrane through the liquid crystal phase. The accumulation of malondialdehyde, resulting from membrane lipid peroxidation, triggers a series of physiological and biochemical changes. Chloroplasts are reported to undergo degeneration earlier in the aging process compared to mitochondrial and nuclear fragmentation [35].
Our current understanding of leaf senescence is primarily derived from studies using model systems such as Arabidopsis [21,36,37,38]. Research indicates that the molecular and biochemical pathways involved in leaf senescence are highly conserved across various plant species [6,8]. Investigating these pathways in wheat could yield valuable insights into the genetic and regulatory networks that govern this complex process in cereals. Recently, there has been a growing body of research on the aging process in wheat [3,11,14,18,39]. Transcriptomic analysis has revealed key insights into the genetic regulation underlying leaf senescence in wheat [11,18].
Overall, managing senescence in crops such as wheat requires a thorough understanding of complex regulatory networks at various levels, including molecular, biochemical, and physiological processes. In this study, we aim to investigate the mechanisms of drought-induced senescence in contrasting genotypes of durum and bread wheat, explore the role of antioxidant enzymes during flag leaf senescence, and identify ultrastructural changes in leaves during senescence dynamics. Gaining deeper insights into different aspects of leaf senescence may allow us to identify potential targets for manipulating these processes.
2. Materials and Methods
2.1. Cultivation of Plant Material
Seeds of local bread wheat (Triticum aestivum L.) genotypes, including the tolerant Gyrmyzy gul 1 and sensitive Tale 38, as well as durum wheat (Triticum durum Desf.) genotypes, the tolerant Vugar and sensitive Tartar, were obtained from the gene bank of the Research Institute of Crop Husbandry (Baku, Azerbaijan) [40]. The plants were grown in controlled environment chambers under control and drought conditions using a completely randomized design. Five seeds of each genotype were planted in 5 L plastic pots filled with soil mixture consisting of 40% topsoil and 60% natural sand. Throughout the experiment, the ambient temperature was maintained between 19 and 29 °C, and the relative humidity was between 50% and 65%. The plants were grown in controlled environment chambers with a 16 h light/8 h dark cycle. The plants were watered 2–3 times weekly to make sure the soil stayed moist. All plants were provided with equal irrigation until the booting stage. Afterward, watering was stopped for the drought-treated plants, while the control plants continued to be watered regularly. Flag leaves were sampled 7, 14, 21, 28, and 35 days after anthesis (DAAs). Flag leaves were sampled 7 (early grain filling), 14 (mid grain filling), 21 (late grain filling), 28 (milk ripeness), and 35 (dough ripeness) days after anthesis. The leaf samples were immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
2.2. Chlorophyll Content
Total chlorophyll content and chlorophyll a/b ratio were determined spectrophotometrically in the 80% acetone extract according to Porra [41].
2.3. Membrane Stability Index (MSI)
Flag leaf samples (2 cm from the middle sections, with three replicates for each sample) were washed three times in deionized water to remove surface electrolytes for measuring MSI. The samples were placed in 10 mL of double-distilled water at 40 °C for 30 min [42]. Electrical conductivity (C1) was measured using a conductivity meter. Subsequently, the same samples were placed in a boiling water bath (100 °C) for 10 min, and their electrical conductivity (C2) was re-recorded. The membrane stability index (MSI) was calculated as MSI = [1 − (C1/C2)] × 100.
2.4. Lipid Peroxidation Assay
The intensity of lipid peroxidation in plants was assessed by measuring the quantity of malondialdehyde (MDA) in flag leaf samples under both normal watering and drought stress conditions [43]. The MDA content was determined spectrophotometrically using a Thermo Scientific Evolution 350 UV/Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) through its reaction with 0.5% thiobarbituric acid at wavelengths 532 nm and 600 nm. The formula used for calculation was A = (D532 − D600)/(E × m), where A represents MDA concentration, D is the optical density, m denotes plant wet biomass, and E is a constant coefficient.
2.5. Total Protein Content
Soluble protein content was determined using the Bradford method (1976) at 595 nm, with bovine serum albumin used to construct the calibration curve [44].
2.6. Enzyme Activity Measurement
2.6.1. Preparation of Enzyme Extract
Flag leaf samples (0.5 g) were crushed in liquid nitrogen and dissolved in a buffer solution containing 100 mM Na-phosphate (pH 7.8), 1 mM EDTA, 2 mM phenylmethylsulfonyl fluoride (PMSF), 1% polyvinylpyrrolidone (PVP), and 0.1% Triton X-100. The homogenized samples were then centrifuged at 15,000× g for 20 min at 4 °C. The resulting supernatant was used to determine the activity of antioxidant enzymes.
2.6.2. Ascorbate Peroxidase (APX, EC 1.11.1.11)
APX activity was measured by monitoring the decrease in optical density at a wavelength of 290 nm using a spectrophotometer Ultrospec 3300 Pro (Amersham Biosciences, Piscataway, NJ, USA). following the method described by Nakano and Asada [45]. The reaction mixture contained 0.1 mM EDTA (pH 8.0), 0.05 mM ascorbate, 0.1 mM H2O2, 50 mM sodium phosphate buffer (pH 7.6), and 100 µL of enzyme extract. The molar extinction coefficient used was 2.8 mM−1 cm−1, with enzyme activity expressed in units of µmol ascorbate/(mg protein·min).
2.6.3. Guaiacol Peroxidase (GPX, EC 1.11.1.7)
The activity of GPX was determined spectrophotometrically by measuring the change in the optical density of the reaction solution at 470 nm over 2 min, as described by Zelinova [46]. The reaction medium consisted of 50 mM sodium phosphate buffer (pH 7.0), 25 mM guaiacol, 25 mM H2O2, and 20 μL of enzyme extract. The molar extinction coefficient (ε) of 26.6 mM−1 cm−1 was used, with enzyme activity expressed in units of µmol guaiacol/(mg protein·min).
2.6.4. Benzidine Peroxidase (BPX, EC 1.11.1.7)
BPX activity was determined spectrophotometrically by monitoring the increase in the optical density of the reaction solution at 590 nm for 1 min, as described by Gechev [47]. The activity of the enzyme was calculated in units of µmol benzidine/(mg protein·min), using an extinction coefficient (ε) of 39 mM−1 cm−1.
2.6.5. Catalase (CAT, E.C. 1.11.1.6)
For the determination of CAT activity, 1 g of flag leaf tissue was homogenized in 10 mL of 50 mM potassium phosphate buffer (pH 7.0). The homogenate was filtered and centrifuged at 8000× g for 10 min to obtain a clear supernatant. For the assay, 2.9 mL of phosphate buffer (pH 7.0) was combined with 25 μL of the enzyme extract. To initiate the reaction, 90 μL of 3% hydrogen peroxide (H2O2) was added to the solution. The decrease in optical density at 240 nm was measured per minute using a spectrophotometer. The enzyme activity was calculated in µmol/(mg protein·min) using a molar extinction coefficient (ε) of 39.4 mM−1cm−1 [48].
2.6.6. Superoxide Dismutase (SOD, EC 1.15.1.1)
To determine the activity of SOD enzyme, SOD Assay Kit-WST (Sigma-Aldrich, St. Louis, MO, USA)) was employed. In this study, the cytosolic isoform of SOD from plant cells was analyzed. Leaves were homogenized in 50 mM potassium phosphate buffer (pH 7.8), and the homogenate was centrifuged. The supernatant, which contained the cytosolic form of SOD, was used for further analysis. The optical density (OD) of the reaction was measured at a wavelength of 450 nm to assess SOD activity.
2.7. Gene Expression Analysis
2.7.1. RNA Extraction and cDNA Synthesis
Total RNA was extracted from leaf material using a Monarch Total RNA Miniprep Kit (New England Biolabs, Ipswich, MA, USA) following the manufacturer’s instructions. Genomic DNA contamination was removed using RNase-free DNase I. The quality and quantity of the extracted RNA were assessed by agarose gel electrophoresis. RNA concentration was measured spectrophotometrically using a NanoDrop 2000C Spectrophotometer (Thermo Scientific, Waltham, MA, USA). Single-stranded cDNA synthesis was performed from the total RNA using a LunaScript RT SuperMix Kit (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s instructions, in a final volume of 20 µL.
2.7.2. Quantitative Real-Time PCR
PCR was conducted using a Mic Real-Time PCR system in a total reaction volume of 20 µL. Each reaction mixture contained 10 µL of Luna Universal qPCR Mix (New England Biolabs, Ipswich, MA, USA), 1 µL of 1:5 diluted cDNA, 0.5 µL each of forward and reverse primers (10 µM), and 7 µL of nuclease-free water. The PCR protocol included an initial denaturation step at 94 °C for 60 s, followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s. No-template controls (NTCs) were included for each primer pair. Each reaction was performed in triplicate (technical replicates) for each of the three biological replicates. Elongation factor 1 alpha (Elf1-α) was chosen as a housekeeping gene due to its stable and consistent expression across different experimental conditions. The primer sequences used for expression analysis are listed in Table 1. Primer efficiency for each pair was determined by the standard curve method using serial dilutions of cDNA, calculated using the formula Efficiency (%) = (10(−1/slope) − 1) × 100. Dissociation curves for each amplicon were analyzed to confirm the specificity of the amplification reactions. Fold change in gene expression (stressed versus control) was calculated using the 2−ΔΔCt method [49].

Table 1.
Sequences of primers used for qRT-PCR.
2.8. Analysis of Leaf Mesophyll Cells by Light and Transmission Electron Microscopy (TEM)
Flag leaves were used for ultrastructural examination. The specimens were fixed in a solution containing 2.5% glutaraldehyde, 2% paraformaldehyde, 4% sucrose, and 0.1% picric acid, prepared in 0.1 M phosphate buffer (pH 7.4). The fixed material was then transported to the Electron Microscopy Laboratory at Azerbaijan Medical University (Baku) for further processing. After fixation for one day, the samples were postfixed in 1% osmium tetroxide solution prepared in phosphate buffer (pH 7.4) for two hours. Araldite-Epon blocks were prepared from the material using standard methods in electron microscopy [50]. Semi-thin (1–2 μm) sections were cut from the blocks using a Leica EM UC7 Ultramicrotome (Leica Microsystems, Buffalo Grove, IL, USA), stained with methylene blue, azure II, and basic fuchsin [51], and viewed under a Primo Star microscope (Carl Zeiss, Oberkochen, Germany). Images of the relevant sections were captured with a digital camera, EOS D650 (Canon, Inc., Tokyo, Japan). Ultrathin sections (50–70 nm) of the blocks, double-stained with uranyl acetate and lead citrate, were examined using a Transmission Electron Microscope, JEM-1400 (JEOL Ltd., Tokyo, Japan), at a voltage of 80–120 kV. Morphometric analysis of the electron micrographs was performed in TIF format using the TEM Imaging Platform software version number 5.2 (Build 3554) developed by Olympus Soft Imaging Solutions GmbH (Münster, Germany).
2.9. Statistical Analysis
The statistical analysis was carried out using SAS software ver9.2 (SAS Institute, 2008). Standard deviation (SD) values are from at least three biological replicates.
3. Results
3.1. Analysis of Variance
The analysis of variance revealed significant differences for all studied parameters across the factors of genotype, treatment, and their interaction (Table 2), highlighting the role of genotypic and physiological differences in the response to stress.

Table 2.
Parameter variations under control and drought treatments.
3.2. Chlorophyll Content
The content of photosynthetic pigments—chlorophyll a and chlorophyll b—was measured in flag leaves at five time points (7, 14, 21, 28, and 35 DAAs) during natural (age-induced) and drought-induced senescence in wheat genotypes with contrasting stress tolerance (Table 3). In all plants, the quantities of both chlorophyll a and chlorophyll b progressively decreased as senescence advanced. At 7 DAAs, although there was a slight reduction in pigment levels in stress-sensitive genotypes, no significant differences were observed in tolerant plants. In contrast to naturally senescing plants, a sharp decline in pigment levels was observed in the flag leaves of drought-stressed wheat at 21 DAAs. This decline was particularly pronounced in sensitive genotypes, with the amount of both chlorophyll a and chlorophyll b being more than twofold lower in drought-exposed tetraploid Tartar and hexaploid Tale 38 compared to 14 DAAs. A significant decrease in chlorophyll a content was observed in naturally senescing plants at 35 DAAs, with reductions of 39%, 40%, 34%, and 40% in Vugar, Tartar, Gyrmyzy gul 1, and Tale 38, respectively. Notably, the reduction in chlorophyll b content in drought-stressed plants was detected earlier: at 14 DAAs in the sensitive genotypes (29% in Tartar and 34% in Tale 38) and at 21 DAAs in the tolerant genotypes (21% in Vugar and 44% in Gyrmyzy gul 1) compared to the previous stage.

Table 3.
Dynamics of changes in photosynthetic pigments during the post-anthesis period.
3.3. Membrane Stability
During the post-anthesis period, due to the aging process, membrane stability in the flag leaves exhibited a declining trend (Figure 1). At 7 DAAs, this parameter was at a similar level across all genotypes. However, in the subsequent periods, membrane stability in stressed plants was lower compared to those under normal conditions. Significant decreases in the MSI were observed at 21 DAAs compared to the previous stage, with reductions of 15% and 12% in the stress-tolerant Vugar and Gyrmyzy gul 1 genotypes, respectively, and 31% and 37% in the stress-sensitive genotypes Tartar and Tale 38, respectively.
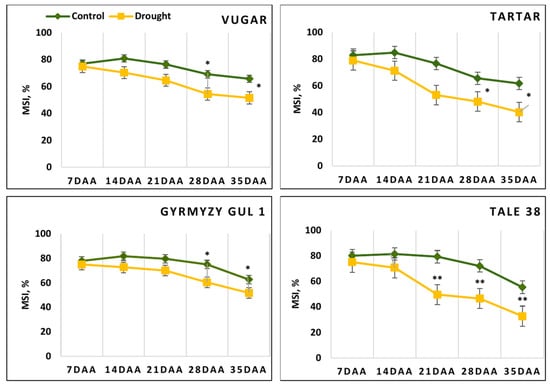
Figure 1.
Dynamics of membrane stability index (MSI) in wheat flag leaves during natural and drought-induced senescence. DAAs—days after anthesis. Data points represent mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01) using pairwise t tests.
3.4. Malondialdehyde Content
An increase in the content of MDA, an indicator of lipid peroxidation, was observed in wheat genotypes during natural senescence (Figure 2). The level of MDA during drought-induced senescence was higher than the level of normal irrigation in all genotypes. This difference was more pronounced and occurred earlier in stress-sensitive genotypes. At 21 DAAs, no significant difference in MDA levels was observed between control and stressed variants of the drought-tolerant durum wheat genotype Vugar, while a 2.3-fold increase was noted in the stress-sensitive bread wheat genotype Tale 38. Towards the end of senescence, the difference in MDA content between control and drought-stressed variants became more pronounced, showing increases of 1.55-, 1.73-, 2.25-, and 2.4-fold in Vugar, Tartar, Gyrmyzy gul 1, and Tale 38, respectively.
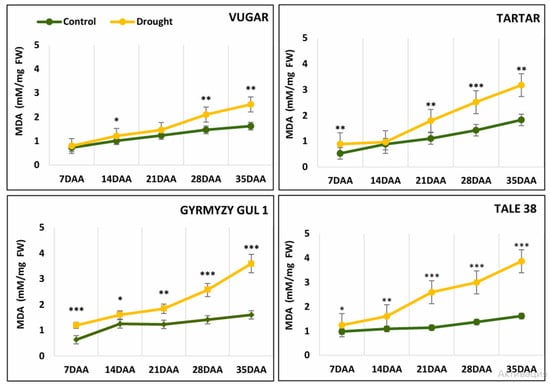
Figure 2.
Changes in malondialdehyde (MDA) content in wheat flag leaves during senescence. DAAs—days after anthesis; FW—fresh weight. Data points represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01; *** p < 0.001) using pairwise t tests.
3.5. Soluble Protein Content
The total soluble protein content is shown in Figure 3. At 7 DAAs, no significant differences were observed between genotypes for this parameter. In naturally senescing plants, the protein content increased until 28 DAAs and then sharply decreased at 35 DAAs; in the drought-tolerant durum wheat genotype Vugar the content decreased by 12%, in the tolerant bread wheat genotype Gyrmyzy gul 1 it decreased by 25%, and in the susceptible genotypes Tartar and Tale 38 it decreased by 24% and 30%, respectively. In stressed plants, the curve reflecting soluble protein content showed changes earlier, at 21 DAAs, similar to other parameters. The only exception was Vugar, which, like the control plants, exhibited a decrease in protein content after 28 DAAs.
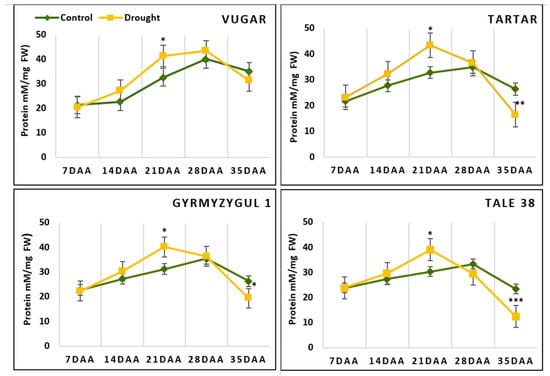
Figure 3.
Total soluble protein content in wheat flag leaves during senescence. DAAs—days after anthesis; FW—fresh weight. Data points represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01; *** p < 0.001) using pairwise t tests.
3.6. The Activity of SOD
At 7 DAAs, the activity of SOD was similar across all variants (Figure 4). By 14 DAAs, enzyme activity increased, with a more significant rise observed in stress-exposed genotypes. In naturally aging wheat, the maximum SOD activity was recorded at 28 DAAs. In contrast, during stress-induced senescence, the maximum activity occurred earlier, at 21 DAAs. Towards the end of ontogenesis (35 DAAs), SOD activity declined. A sharp decrease in enzyme activity was observed in stress-exposed bread wheat (65% and 73% in Gyrmyzy gul 1 and Tale 38, respectively) compared to durum wheat (32% and 50% in Vugar and Tartar, respectively).
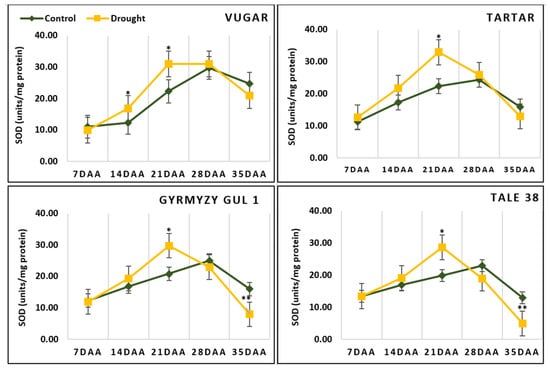
Figure 4.
The activity of SOD during natural and stress-induced flag leaf senescence in wheat genotypes. DAAs—days after anthesis. Data points represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01;) using pairwise t tests.
3.7. Gene Expression Analysis of SOD Isoforms
To determine which SOD isoforms are closely involved in the senescence process, gene expression analysis was performed. Transcript levels of CuZnSOD, FeSOD, and MnSOD were examined (Figure 5). The transcript level of CuZnSOD was similar across all variants at 7 DAAs. In naturally aging plants, the expression of this isoform increased progressively, reached a peak at 28 DAAs, and then decreased at 35 DAAs. Under stress conditions, the maximum expression of this gene was observed at 21 DAAs, after which the expression decreased, reaching a minimum level at 35 DAAs. The expression pattern of FeSOD during flag leaf senescence differed. In all genotypes, the transcription of this isoform significantly increased after flowering, peaked at 21 DAAs, and then declined sharply. At 35 DAAs, compared to control plants, the transcript level of FeSOD decreased by 29%, 21%, 30%, and 48% in the genotypes Vugar, Tartar, Gyrmyzy gul 1, and Tale 38, respectively. MnSOD expression was higher towards the end of ontogenesis, with transcript levels reaching a maximum at 35 DAAs. In the tolerant genotypes Vugar and Gyrmyzy gul 1, the transcript level of this isoform increased by 12%, while in the sensitive durum wheat genotype Tartar, it increased by around 39%, and in bread wheat Gyrmyzy gul 1 it increased by nearly 20%.
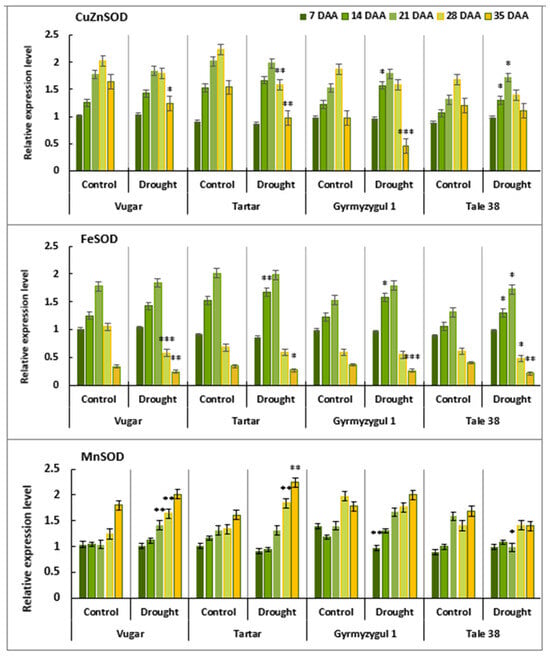
Figure 5.
Transcript levels of CuZnSOD, FeSOD, and MnSOD in flag leaves of wheat plants during natural and stress-induced senescence. DAAs—days after anthesis. The fold change in expression was calculated using the 2−ΔΔCt method. Bars represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01; *** p < 0.001) using pairwise t tests.
3.8. The Activity of Peroxidases
The activity of peroxidase enzymes, including APX, BPX, and GPX, was measured in flag leaves at five stages after anthesis (Figure 6). In naturally senescing plants, APX activity increased after anthesis, reaching a maximal level at 28 DAAs, and then decreased at 35 DAAs. In stress-exposed plants, the enzyme activity in the drought-tolerant durum wheat genotype Vugar increased two-fold, reaching its maximum level at 28 DAAs. In the drought-sensitive durum wheat genotype Tartar, the maximum APX activity was observed at 21 DAAs, while in both bread wheat genotypes, it was observed at 14 DAAs. By 35 DAAs, compared to the control, the enzyme activity in stressed plants decreased by 24%, 64%, 59%, and 54% in the Vugar, Tartar, Gyrmyzy gul 1, and Tale 38 genotypes, respectively.
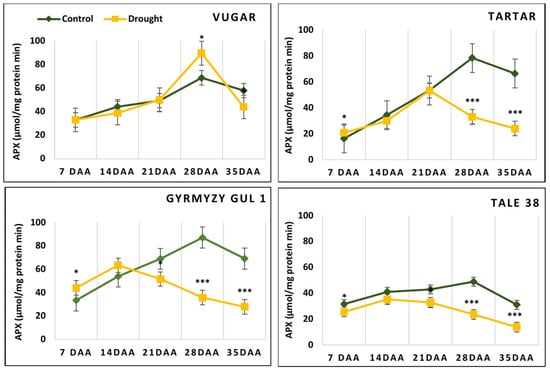
Figure 6.
Dynamics of ascorbate peroxidase (APX) activity during natural and stress-induced flag leaf senescence in wheat genotypes. DAAs—days after anthesis. Data points represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; *** p < 0.001) using pairwise t tests.
BPX activity reached a maximum in all genotypes at 28 DAAs and sharply decreased at 35 DAAs. In this stage under stressed conditions, BPX activity was higher in stressed plants compared to control plants, reaching 92%, 83%, 72%, and 17% in the Vugar, Tartar, Gyrmyzy gul 1, and Tale 38 genotypes, respectively (Figure 7).
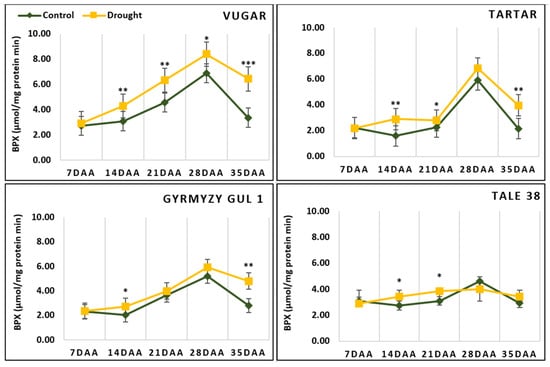
Figure 7.
Dynamics of benzidine peroxidase (BPX) activity during natural and stress-induced flag leaf senescence in wheat genotypes. DAAs—days after anthesis. Data points represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01; *** p < 0.001) using pairwise t tests.
The activity of GPX in flag leaves of both naturally senescing and drought-stressed wheat genotypes also increased after anthesis, peaking at 28 DAAs. Similar to other peroxidases, GPX activity decreased again at 35 DAAs, with a significant reduction observed, particularly in bread wheat genotypes (Figure 8). In naturally senescing durum wheat under control conditions, this decrease was 23% in Vugar and 22% in Tartar, while in bread wheat it was 60% and 47% in Gyrmyzy gul 1 and Tale 38, respectively. Under stress conditions, GPX activity in durum wheat decreased by 20% in Vugar and 36% in Tartar, while in Gyrmyzy gul 1 and Tale 38 it decreased by 61% and 57%, respectively.
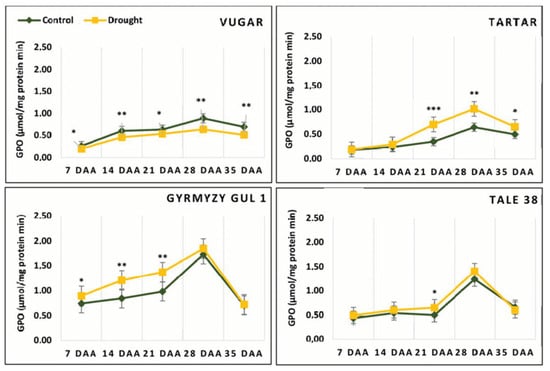
Figure 8.
Dynamics of guaiacol peroxidase (GPX) activity during natural and stress-induced flag leaf senescence in wheat genotypes. DAAs—days after anthesis. Data points represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01; *** p < 0.001) using pairwise t tests.
3.9. The Activity of CAT
In contrast to peroxidases, CAT activity reached its maximum earlier (14 DAAs). As senescence progressed, CAT activity decreased in both control and drought-exposed plants. In plants that senesced more rapidly due to stress, with the exception of the drought-tolerant genotype Vugar, which showed a reduction in CAT activity by 9% compared to the control, the other genotypes showed an increase of 32%, 6%, and 26% in Tartar, Gyrmyzy gul 1, and Tale 38, respectively (Figure 9).
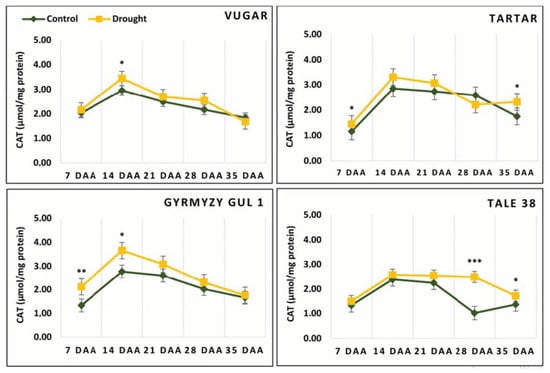
Figure 9.
The activity of CAT during natural and stress-induced flag leaf senescence in wheat genotypes. DAAs—days after anthesis. Data points represent the mean ± se (n = 5). Asterisks indicate significance difference (* p < 0.05; ** p < 0.01; *** p < 0.001) using pairwise t tests.
3.10. Light Microscopy
Changes occurring in the flag leaves of bread wheat genotypes were examined by light microscopy at 7, 21, and 35 days after anthesis (DAAs) (Figure 10 and Figure 11). Figure 10A shows the anatomical structure of the flag leaf of the Gyrmyzy gul 1 genotype under normal watering conditions at 7 DAAs.
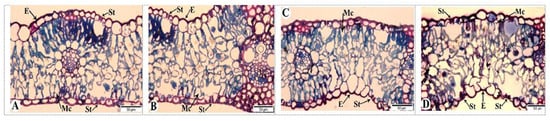
Figure 10.
Light microscopy images of the flag leaves of the Gyrmyzy gul 1 genotype. DAAs—days after anthesis. (A)—7 DAAs under normal watering conditions; (B–D)—7, 21, and 35 DAAs under drought stress, respectively. Designations: E—epidermis; Mc—mesophyll cell; St—stomata. Semi-thin section (1 µm), one-step staining.
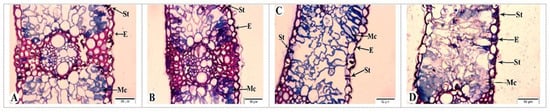
Figure 11.
Light microscopy images of the flag leaves of the Tale 38 genotype. DAAs—days after anthesis. (A)—7 DAAs under normal watering conditions; (B–D)—7, 21, and 35 DAAs under drought stress, respectively. Designations: E—epidermis; Mc—mesophyll cell; St—stomata. Semi-thin section (1 µm), one-step staining.
The anatomy of the flag leaf exhibits distinct features: the epidermis (E), stomata (St), and mesophyll cells (Mcs) are visible. The stomata are open, and the mesophyll cells are rich in chloroplasts. At 7 DAAs under drought stress (Figure 10B), no significant anatomical differences are observed. By 21 DAAs, both open and closed stomata are present, and some areas of the epidermis show signs of thickening (Figure 10C). At 35 DAAs, all stomata are observed to be closed. In addition to the thickening of various epidermal layers, some mesophyll cell walls exhibit damage (Figure 10D).
In contrast to the tolerant genotype, the Tale 38 genotype exhibits more pronounced changes in anatomical structure during the examination of its flag leaves (Figure 11). At 7 DAAs under drought stress, the structural elements of the leaves are similar to those in the control group (Figure 11A,B). However, at 21 DAAs, some stomata are closed, and thickening of the epidermal cell layers and damage to the walls of some mesophyll cells are observed (Figure 11C). By 35 DAAs, pathological changes in the flag leaves of the Tale 38 genotype have intensified. Specifically, the epidermal layer shows increased thickening in some areas, with structural integrity compromised; most stomata remain open, and the walls of mesophyll cells are disrupted. Additionally, there is a reduction in the number of chloroplasts in the mesophyll cell cytoplasm (Figure 11D).
3.11. Transmission Electron Microscopy
Changes in the ultrastructure of flag leaves in the Gyrmyzy gul 1 and Tale 38 genotypes during drought-induced leaf senescence were also examined using TEM (Figure 12 and Figure 13). In the control leaves of the Gyrmyzy gul 1 genotype at 7DAAs, cultivated under normal watering conditions, the ultrastructure clearly shows cell walls (Cws), chloroplasts (Chs), the central nucleus (N), lipid droplets (Lds), mitochondria (M), internal thylakoid membranes of chloroplasts (Ths), and plastoglobuli (Pl) (Figure 12A).
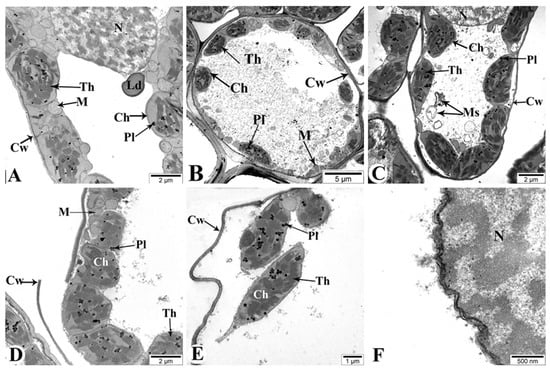
Figure 12.
Transmission electron microscopy (TEM) images of the flag leaves of the Gyrmyzy gul 1 genotype. DAAs—days after anthesis. (A)—7 DAAs under normal watering conditions; (B–F)—7, 21, and 35 DAAs under drought stress, respectively. Designations: N—nucleus; Cw—cell wall; Th—thylakoid; Ld—lipid droplet; M—mitochondria; Ch—chloroplast; Pl—plastoglobuli; Ms—myelin-like structure. Ultrathin sections (50–70 nm), stained with uranyl acetate and lead citrate.
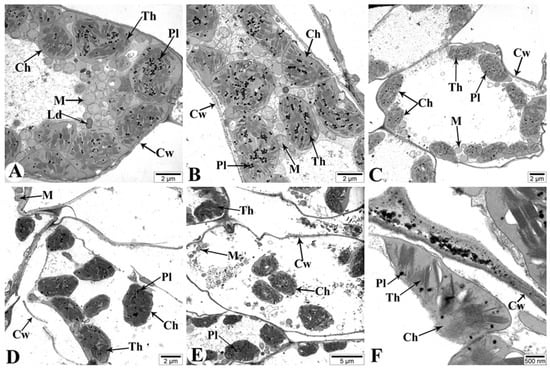
Figure 13.
Transmission electron microscopy (TEM) images of the flag leaves of the Tale 38. (A)—7 DAAs under normal watering conditions; (B–F)—7, 21, and 35 DAAs under drought stress, respectively. Designations: Cw—cell wall; Th—thylakoid; Ld—lipid droplet; M—mitochondria; Ch—chloroplast; Pl—plastoglobuli. Ultrathin sections (50–70 nm), stained with uranyl acetate and lead citrate.
Figure 12B shows the ultrastructural appearance of flag leaves at 7 DAAs under drought conditions. No significant pathology is observed. At 21 DAAs, although the cell walls maintain a normal structure, some organelles with membranous structures, such as mitochondria, exhibit structural damage and transform into myelin-like structures (Ms) (Figure 12C). More severe changes compared to the control are observed at 35 DAAs (Figure 12D–F). Specifically, some mesophyll cell walls are damaged (Figure 12D), chloroplast membranes are compromised, and connections between chloroplasts are severed, with alterations in thylakoid membrane structures (Figure 12E). The nuclear envelope of mesophyll cells is thickened and structurally disrupted in some areas (Figure 12F).
Figure 13 depicts the changes occurring in the mesophyll cells of the Tale 38 genotype during leaf senescence, as observed using TEM.
Figure 13A illustrates the structural elements of mesophyll cells in flag leaves of plants grown under normal watering conditions at 7 DAAs. At this stage under drought conditions, no significant pathology is observed in the flag leaves (Figure 13B). At 21 DAAs, chloroplast membranes are damaged and their connections are severed. The crystals in the mitochondria are not clearly visible, and some mitochondria are severely damaged and completely disintegrated (Figure 13C). At 35 DAAs, most mesophyll cell walls are severely damaged and fragmented in several places (Figure 13D). The majority of chloroplast membranes are damaged and fragmented, causing the chloroplasts to disassociate from the cell walls and migrate toward the center of the cytoplasm (Figure 13E). Additionally, most membranous structures in the cytoplasm are also damaged. Ultrastructural analysis of the chloroplasts reveals that the thylakoid membranes are damaged and disintegrated, with vacuoles forming in the intermembrane spaces (Figure 13F).
4. Discussion
Leaf senescence is characterized by specific changes at the cellular, biochemical, and molecular levels. Observing these changes collectively provides a comprehensive understanding of the mechanisms behind leaf aging in plants. These changes occur in a coordinated manner to facilitate the remobilization of nutrients during the progression of leaf senescence, especially under drought stress. Our study identified a sequence of events occurring during flag leaf senescence induced by drought in contrasting wheat genotypes. Significant chlorophyll degradation occurred concurrently with increased lipid peroxidation and decreased membrane stability. This was further accompanied by enhanced activation of antioxidant system components and a reduction in total soluble protein levels, ultimately affecting the leaf cell ultrastructure. Our findings indicate that chlorophyll loss during natural senescence occurs gradually. Under drought stress, chlorophyll a and b levels in tolerant genotypes sharply declined by 21 DAAs, while in sensitive genotypes, chlorophyll b decreased earlier, by 14 DAAs. Accelerated leaf senescence in response to drought is considered an important adaptive survival mechanism, as it reduces the plant’s overall water requirements [9]. Chlorophyll degradation is regarded as a precursor to leaf senescence, as it is associated with nutrient remobilization [8]. Chlorophyll loss may play a role in photoprotection during the early stages of drought-induced leaf senescence when chloroplasts still retain some photosynthetic activity. The loss of chlorophyll reduces the controlled effects of singlet oxygen formation in thylakoids [52]. This protection may be particularly important in the initial phase of leaf senescence under drought, as it could prevent the rapid dismantling of chloroplasts, which, in the absence of protective mechanisms, could threaten nutrient remobilization [53]. Once chlorophyll degradation occurs, the pigment–protein complex dissociates, and enzymes can break down proteins that may constitute a significant amount of the nitrogen present in chloroplasts [15]. Additionally, some studies suggest that the onset of leaf senescence may occur before chlorophyll degradation becomes apparent. Pic et al. [54] demonstrated that a gene homologous to SAG2 from Arabidopsis, which codes for a cysteine protease and is considered a specific marker of senescence, is expressed in water-stressed potted pea plants before a decline in photosynthesis, chlorophylls, or protein levels. In our study, we observed a gradual increase in MDA levels during natural leaf senescence, with a noticeable sharp rise in stress-sensitive genotypes starting from 28 DAAs and in drought-stressed genotypes from 21 DAAs. Previous research also reported an increase in MDA with elevated O2• levels during light-induced leaf senescence in wheat [55]. A premature senescence mutant from a rice mutant library exhibited increased MDA levels [56]. MDA is a product of lipid peroxidation in cell membranes, indicating damage to the ROS cellular membrane system. Accordingly, our experiments showed a decrease in the membrane stability index with advancing senescence, which was especially pronounced in drought-stressed plants. Interestingly, our study found that the number of soluble proteins in drought-stressed plants was higher initially compared to those under control conditions. This increase appears to be due to the effect of drought stress rather than the natural senescence process. Similarly, Maqsood et al. reported that water deficit stress resulted in a significant increase in soluble proteins in wheat genotypes [57].
In our research, a sharp decrease in protein content was observed starting from 21 DAAs. Protein degradation is a crucial catabolic process during leaf senescence and plays a significant role in nutrient recycling, particularly of nitrogen. Chloroplastic proteases, which increase in synthesis during drought-induced senescence, can mobilize up to 75% of the total cellular nitrogen in leaves [13]. The increased expression and activity of serine, cysteine, aspartic, and metalloproteases has been detected in senescing leaves [27,58,59,60,61]. Although many proteins degrade during senescence, some remain intact. The decrease in protein amount was more rapid in early aging lines compared to normal aging lines in wheat [62]. Protein content also decreased in Tropaeolum majus leaves during senescence [63] and in French bean leaves during both natural and dark-induced senescence [64].
Oxidative stress, resulting from an imbalance between ROS and antioxidants, intensifies during plant senescence [57]. Under optimal conditions, constitutive antioxidant levels manage basal ROS production [65]. The induction of antioxidant synthesis counters increased ROS production at low concentrations during moderate stress [28]. This is also characteristic of the early stages of leaf senescence in plants subjected to drought stress, where enhanced photo- and antioxidant protection is observed [66]. Senescence may progress further due to higher ROS production, potentially triggered by an oxidative burst [30]. Cell death can occur when antioxidant defenses are overwhelmed by excessively large, rapid, and uncontrolled ROS accumulation [22]. In plants, the antioxidant defense is primarily constituted by the enzymatic actions of superoxide dismutase (SOD, EC 1.15.1.1), which catalyzes the disproportionation of O2− to H2O2 and O2. SOD is considered one of the first lines of defense [67,68]. Three main types of SOD are described in plants based on their metal cofactors: Cu/Zn-SOD (found in the cytosol, chloroplast stroma, peroxisomes, and apoplast), Mn-SOD (present in mitochondria, peroxisomes, apoplast, and cell walls), and Fe-SOD (restricted to the chloroplast stroma of certain plant species [69]. In our study, we investigated the expression levels of Cu/Zn-SOD, Mn-SOD, and Fe-SOD genes to determine which isoform plays a major role during senescence. Cu/Zn-SOD expression increased during the senescence process, peaked at 28 DAAs, and then significantly decreased in all naturally senescing plants. In plants that senesced rapidly under stress, it reached its maximum activity at 21 DAAs and then sharply declined. In contrast, Fe-SOD expression significantly differ between the control and stress variants, remaining high until late grain filling (21 DAAs) and then sharply declining as senescence progressed. Mn-SOD transcript levels increased proportionally with the senescence process in almost all variants, reaching a maximum at 35 DAAs. A comparison of expression levels between tolerant and sensitive genotypes in the control and treatment variants revealed that only Fe-SOD showed a strict association with tolerance, as transcript levels significantly increased in tolerant genotypes of both durum and bread wheat. The transcription level of Cu/Zn-SOD was elevated in the sensitive genotype among durum wheat varieties and in the tolerant genotype among bread wheat varieties. Mn-SOD transcript levels did not differ significantly between tolerant and sensitive genotypes under drought. These findings align with some studies but also contradict others. For instance, Cu/Zn-SOD and Mn-SOD activity increased at the beginning of senescence in bean cotyledons [70] but decreased as senescence progressed. Mn-SOD activity continually decreased in pea leaves [71] from the onset of senescence. In wheat, Cu/Zn-SOD gene expression remained consistent in early and late aging lines immediately after the flag leaf expansion [62]. Aliyeva et al. demonstrated that SOD activity in wheat leaves under drought stress increased in tolerant genotypes but decreased in sensitive ones [72]. Saed-Moucheshi et al. [73] found that a tolerant triticale genotype had significantly higher expression levels of Mn-SOD and Cu/Zn-SOD compared to a susceptible genotype, while Fe-SOD levels did not differ. Mn-SOD showed higher expression in shoots, Fe-SOD was more expressed in roots, and Cu/Zn-SOD was higher in the roots of the tolerant genotype but lower in the roots of the susceptible one, indicating genotype specificity. The authors suggested that Mn-SOD is likely a general isozyme responding to stress, while Cu/Zn-SOD is more genotype-specific, with higher expression in certain genotypes like the tolerant triticale in their study. Zhao et al. found that SOD expression levels and malondialdehyde content in rice were significantly correlated, with SOD levels being much lower in heat-sensitive cultivars compared to tolerant ones [74]. Similarly, Mohammadi et al. [75] reported that Cu/Zn-SOD was significantly expressed in common bean plants under drought stress, with a higher induction rate in tolerant genotypes. Xie et al. [76] also noted that Cu/Zn-SOD likely played a crucial role in regulating total SOD activity and ROS detoxification under stress conditions. Based on our findings, Fe-SOD appears to be responsible for responding to drought stress, while Mn-SOD was mainly induced by aging, showing minimal differences between drought and stress conditions. In our opinion, the observed discrepancies depend on the genomic differences between durum and bread wheat genotypes and the developmental stage of the plant. It is shown that the effectiveness of plant antioxidant systems in detoxifying ROS and protecting against oxidative damage depends on plant species and genus, genetic background, stress intensity, and the growth stage of plants exposed to stress [77]. Some researchers, such as Awan et al. [78], Sheoran et al. [79], and Wang et al. [80], have suggested that Mn-SOD is crucial for drought tolerance. Sheoran et al. [79] found that Mn-SOD gene expression significantly increased under drought stress, showing a strong correlation with overall SOD activity, which contradicts our results. A genome-wide analysis by Jiang et al. [81] identified 26 SOD genes in wheat, including 17 Cu/Zn-SODs, 6 Fe-SODs, and 3 Mn-SODs.
Hydrogen peroxide is more efficiently scavenged by ascorbate peroxidase (APX), which has a better affinity for H2O2 than CAT and is widely distributed in various cellular compartments, including chloroplasts, where CAT is absent, as well as in the cytosol, mitochondria, and peroxisomes [82]. APX6, in particular, is associated with senescence, being induced in aging leaves and in response to senescence-promoting stimuli like abscisic acid (ABA), extended darkness, and osmotic stress. APX6 is thought to modulate ROS/redox homeostasis and signaling in aging leaves, playing a crucial role in both developmental and stress-induced senescence programs [30]. In our research, we compared the activity of peroxidases, specifically APX, BPX, and GPX. APX exhibited the highest activity among these enzymes and its activity increased during the senescence process. In drought-induced senescence, APX activity peaked earlier compared to naturally senescing plants, where maximum APX activity was observed at 28 DAAs. In the sensitive durum wheat genotype Tartar at 21 DAAs and in bread wheat genotypes at 14 DAAs, the enzyme showed maximum activity. The only exception was the tolerant durum wheat genotype Vugar, which peaked at 28 DAAs, similar to the control variant. Both GPX and BPX reached maximum activity at 28 DAAs. GPX activity was comparatively lower in durum wheat genotypes than in bread wheat and showed less fluctuation in activity dynamics. There were no significant differences in GPX activity between stress and control conditions. Conversely, BPX activity was higher in drought-stressed plants. The activity of guaiacol peroxidase was examined in durum wheat genotypes under drought stress during the flowering, milk ripeness, and wax ripeness stages, showing increased activity toward the end of ontogenesis. Similarly, benzidine peroxidase activity also reached a maximum during the wax ripeness phase [83,84,85]. Similar trends for guaiacol peroxidase were observed in bread wheat seedlings acclimated to low temperatures [86]. Multiple forms of peroxidases operate under different growth and development stages of plants, oxidizing various substrates and functioning under diverse environmental conditions.
CAT is a heme-containing enzyme that catalyzes the rapid decomposition of H2O2 to H2O and O2. It is predominantly found in peroxisomes, where it scavenges H2O2 generated during photorespiration and β-oxidation of fatty acids. CAT has also been identified in mitochondria and cytosol, but not in chloroplasts [82]. In our study, CAT activity peaked at 14 DAAs and subsequently decreased. This pattern is consistent with reports of increased CAT activity at the beginning of senescence, followed by a decline as senescence progresses [62]. Studies across various plant species have shown a continual decrease in CAT enzyme activity from the onset of senescence in cucumber and bean cotyledons [87]. In maize, CAT activity increased until 25 days after tasseling but decreased thereafter [88]. In wheat, CAT activity was lower in early aging lines compared to normal aging lines across different sampling times [62].
Drought-induced leaf senescence is accompanied by a range of structural changes within cells [89]. We investigated the ultrastructural changes associated with senescence induced by age and drought stress in flag leaves using light and electron microscopy at three time points after anthesis. The observed structural damage intensified over time. By the end of senescence, mesophyll cell walls in the sensitive genotype were significantly damaged and disintegrated compared to the tolerant genotype. In both genotypes, chloroplast membranes were damaged and fragmented, and due to the loss of connections between them, chloroplasts migrated toward the center of the cytoplasm. Additionally, in the sensitive genotype Tale 38, most membrane-bound structures within the cytoplasm were also significantly disintegrated. Thylakoid membranes inside chloroplasts were damaged in both genotypes, and vacuoles formed in the intermembrane spaces. Chloroplasts are among the first organelles to undergo destruction during senescence, while nuclei and mitochondria maintain their integrity until the later stages of leaf senescence [90]. This is because oxidative stress plays a critical role in the progression of leaf senescence, and chloroplasts are among the most potent intracellular generators of reactive oxygen species. The transformation of chloroplasts into gerontoplasts characterizes the senescence reorganization phase of leaves. Chloroplasts lose volume and density due to extensive loss of stromal components and thylakoids, and the number and size of plastoglobuli increase with aging. Ultrastructural studies of plastoglobuli confirm their significant role in protecting thylakoid membranes from oxidative damage through the intensive synthesis and storage of lipids (e.g., carotenoids). Plastoglobuli are found in both chloroplasts and chromoplasts. Under various stress conditions, the amount of plastoglobuli increases as thylakoids disintegrate [91]. During drought, cultivar-specific differences in the ultrastructure and functions of mitochondria in autumn wheat have been observed [92]. Leaf mitochondria in drought-tolerant cultivars are better preserved compared to sensitive ones, which can be considered a trait contributing to drought tolerance in plants. These changes occur in drought-induced leaf senescence in field-grown plants [12] and are also considered good indicators of leaf senescence in response to other stresses [93]. Thus, both light and electron microscopy studies have revealed that as the duration of leaf senescence increases, more severe changes are observed in the ultrastructure of flag leaves.
5. Conclusions
This study highlights the critical role of antioxidant enzymes and cellular integrity in mitigating the effects of drought-induced senescence in wheat genotypes. The contrasting responses between drought-tolerant and drought-sensitive genotypes underline the complexity of the senescence process under stress. The increase in MDA levels indicates more pronounced oxidative damage to membranes in drought-sensitive genotypes. In contrast, drought-tolerant genotypes exhibited higher antioxidant enzyme activity, which helps mitigate oxidative stress and slow down the senescence process. Additionally, they showed greater expression of antioxidant enzyme genes, enhancing their ability to cope with drought. Sensitive genotypes experienced more severe damage to chloroplasts and cell walls under drought conditions compared to the tolerant ones, which led to rapid leaf senescence. These findings provide valuable insights for breeding programs aimed at developing drought-resilient wheat varieties, emphasizing the importance of maintaining antioxidant balance and cellular ultrastructure in prolonging leaf vitality under drought conditions.
Author Contributions
Conceptualization, I.M.H.; methodology, D.R.A., S.M.R., F.H.R. and E.K.G.; investigation, T.Y.I., S.M.R., D.R.A., F.H.R. and E.K.G.; validation, D.R.A. and S.M.R.; formal analysis, S.M.R.; writing—original draft preparation, S.M.R., T.Y.I., F.H.R. and E.K.G.; writing—review and editing, I.M.H., D.R.A. and S.M.R.; visualization, S.M.R., T.Y.I., F.H.R. and E.K.G.; supervision, I.M.H.; project administration, I.M.H.; funding acquisition, I.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Education of the Republic of Azerbaijan under the project titled "Creation of infrastructure for the development of new directions of genetic engineering in Azerbaijan, application of genome editing methods in biomedical research and genome selection" (2023–2024).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ROS, reactive oxygen species; SAGs, senescence-associated genes; DAAs, days after anthesis, MSI, membrane stability index; MDA, malondialdehyde; FW, fresh weight; SOD, superoxide dismutase; APX, ascorbate-peroxidase; GPX, guaiacol-peroxidase; BPX, benzidine-peroxidase; CAT, catalase, TEM, transmission electron microscopy
References
- Cheng, S.; Feng, C.; Wingen, L.U.; Cheng, H.; Riche, A.B.; Jiang, M.; Leverington-Waite, M.; Huang, Z.; Collier, S.; Orford, S.; et al. Harnessing landrace diversity empowers wheat breeding. Nature 2024, 632, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, H.; Wang, H.; He, Y. Research progress on the relationship between leaf senescence and quality, yield and stress resistance in horticultural plants. Front. Plant Sci. 2022, 13, 1044500. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.A.; Orford, S.; Lage, J.; Griffiths, S. Capturing and selecting senescence variation in wheat. Front. Plant Sci. 2021, 12, 638738. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Tian, X.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. Quantifying senescence in bread wheat using multispectral imaging from an unmanned aerial vehicle and QTL mapping. Plant Physiol. 2021, 187, 2623–2636. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Wang, P.; Wang, H.; Wang, L.; Sun, F.; Jing, H.C. Genome-wide association study of image-based trait reveals the genetic architecture of dark-induced leaf senescence in rice. J. Exp. Bot. 2024, erae391. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Lim, P.O.; Nam, H.G. Leaf senescence: Systems and dynamics aspects. Annu. Rev. Plant Biol. 2019, 70, 347–376. [Google Scholar] [CrossRef]
- Aloryi, K.D.; Jing, H.C.; Dijkwel, P.P. Comparison of leaf senescence regulation between distantly related plant species uncovers knowledge gaps and opportunities for plant improvement strategies. Environ. Exp. Bot. 2023, 214, 105474. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Tan, S.; Sha, Y.; Sun, L.; Li, Z. Abiotic stress-induced leaf senescence: Regulatory mechanisms and application. Int. J. Mol. Sci. 2023, 24, 11996. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Tan, S.; Yang, Q.; Wang, H.-L.; Xia, X.; Luo, J.; Guo, H.; Zhang, Z.; Li, Z. LSD 4.0: An improved database for comparative studies of leaf senescence. Mol. Hortic. 2022, 2, 24. [Google Scholar] [CrossRef]
- Sultana, N.; Islam, S.; Juhász, A.; Ma, W. Wheat leaf senescence and its regulatory gene network. Crop J. 2021, 9, 703–717. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Hajibarat, Z.; Saidi, A. Senescence-associated proteins and nitrogen remobilization in grain filling under drought stress condition. J. Genet. Eng. Biotechnol. 2022, 20, 101. [Google Scholar] [CrossRef]
- Joshi, S.; Choukimath, A.; Isenegger, D.; Panozzo, J.; Spangenberg, G.; Kant, S. Improved wheat growth and yield by delayed leaf senescence using developmentally regulated expression of a cytokinin biosynthesis gene. Front. Plant Sci. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 81, 603–622. [Google Scholar] [CrossRef]
- Camargo Rodriguez, A.V. Integrative modelling of gene expression and digital phenotypes to describe senescence in wheat. Genes 2021, 12, 909. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.; Tan, S.; Li, Z. Transcription Factors-Regulated Leaf Senescence: Current Knowledge, Challenges and Approaches. Int. J. Mol. Sci. 2023, 24, 9245. [Google Scholar] [CrossRef]
- Borrill, P.; Harrington, S.A.; Simmonds, J.; Uauy, C. Identification of transcription factors regulating senescence in wheat through gene regulatory network modelling. Plant Physiol. 2019, 180, 1740–1755. [Google Scholar] [CrossRef]
- Yolcu, S.; Li, X.; Li, S.; Kim, Y.J. Beyond the genetic code in leaf senescence. J. Exp. Bot. 2018, 69, 801–810. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Guo, P.; Xia, X.; Guo, H.; Li, Z. Multiple layers of regulation on leaf senescence: New advances and perspectives. Front. Plant Sci. 2021, 12, 788996. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Lyu, J.I.; Woo, H.R.; Lim, P.O. New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wang, J. Mitochondrial functions in leaf senescence: Insights from metabolic and cellular dynamics. J. Plant Physiol. 2023, 271, 153681. [Google Scholar]
- Nousis, L.; Kanavaros, P.; Barbouti, A. Oxidative Stress-Induced Cellular Senescence: Is Labile Iron the Connecting Link? Antioxidants 2023, 12, 1250. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, W.; Pang, J.; Zhou, M.; Liu, J.; Zhao, J.; Yang, M. Integrated physiological and metabolomic analyses reveal changes during the natural senescence of Quercus mongolica leaves. PLoS ONE 2023, 18, e0289272. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive Oxygen Species (ROS) generation and detoxifying in plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Yu, L. The role of chloroplasts in leaf senescence and related signaling pathways. Int. J. Mol. Sci. 2023, 24, 1147. [Google Scholar]
- Buet, A.; Costa, M.L.; Martínez, D.E.; Guiamet, J.J. Chloroplast protein degradation in senescing leaves: Proteases and lytic compartments. Front. Plant Sci. 2019, 10, 747. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Chen, C.; Galon, Y.; Rahmati Ishka, M.; Malihi, S.; Shimanovsky, V.; Twito, S.; Miller, G. Ascorbate peroxidase 6 delays the onset of age-dependent leaf senescence. Plant Physiol. 2021, 185, 441–456. [Google Scholar]
- Ďúranová, H.; Šimora, V.; Ďurišová, Ľ.; Olexiková, L.; Kovár, M.; Požgajová, M. Modifications in ultrastructural characteristics and redox status of plants under environmental stress: A review. Plants 2023, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Tefera, A.; Kebede, M.; Tadesse, K.; Getahun, T. Morphological, physiological, and biochemical characterization of drought-tolerant wheat (Triticum spp.) varieties. Int. J. Agron. 2021, 2021, 8811749. [Google Scholar] [CrossRef]
- Gan, S.S. Hypothesis: The subcellular senescence sequence of a mesophyll cell mirrors the cell origin and evolution. Mol. Hortic. 2022, 2, 27. [Google Scholar] [CrossRef]
- Tamary, E.; Nevo, R.; Naveh, L.; Levin-Zaidman, S.; Kiss, V.; Savidor, A.; Adam, Z. Chlorophyll catabolism precedes changes in chloroplast structure and proteome during leaf senescence. Plant Direct 2019, 3, e00127. [Google Scholar] [CrossRef]
- Yumoto, G.; Nishio, H.; Muranaka, T.; Sugisaka, J.; Honjo, M.N.; Kudoh, H. Seasonal switching of integrated leaf senescence controls in an evergreen perennial Arabidopsis. Nat. Commun. 2024, 15, 4719. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, M.; Liu, Y.; Wang, B.; Wei, H.; Kong, D.; Wang, H. Arabidopsis FHY3 and FAR1 function in age gating of leaf senescence. Front. Plant Sci. 2021, 12, 770060. [Google Scholar] [CrossRef]
- Zhao, X.; Zhuang, W.; Zhang, Y. The relationship between leaf senescence and stress tolerance in plants. J. Plant Growth Regul. 2023, 42, 1–15. [Google Scholar]
- Yan, F.; Yu, Z.; Shi, Y. Optimized border irrigation delays winter wheat flag leaf senescence and promotes grain filling. Front. Plant Sci. 2023, 14, 1051323. [Google Scholar] [CrossRef]
- Allahverdiyev, T.I. Physiological traits of durum wheat (Triticum durum Desf.) and bread wheat (Triticum aestivum L.) genotypes under drought stress. Agric. Sci. 2015, 6, 848–859. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Deshmukh, P.; Sairam, R.; Shukla, D. Measurement of ion leakage as a screening technique for drought resistance in wheat genotypes. Indian J. Plant Physiol. 1991, 35, 89–91. [Google Scholar]
- Wang, H.F.; Zhong, X.H.; Shi, W.Y.; Guo, B. Study of malondialdehyde (MDA) content, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in chickens infected with avian infectious bronchitis virus. Afr. J. Biotechnol. 2011, 10, 9213–9217. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Zelinova, V.; Mistrik, I.; Palove-Balang, P.; Tamas, L. Peroxidase activity against guaiacol, NADH, chlorogenic acid, ferulic acid and coniferyl alcohol in root tips of Lotus japonicus and L. corniculatus grown under low pH and aluminium stress. Biologia 2010, 65, 279–283. [Google Scholar] [CrossRef]
- Gechev, T.; Gadjiev, I.; Van Breusagem, F.; Inze, D.; Dukiandjiev, S.; Toneva, V.; Minkov, I. Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol. Life Sci. 2002, 59, 708–714. [Google Scholar] [CrossRef]
- Kumar, G.M.; Knowles, N.R. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kuo, J. Processing plant tissues for ultrastructural study. Electron Microsc. Methods Protoc. 2014, 1117, 39–55. [Google Scholar]
- Morikawa, S.; Sato, A.; Ezaki, T. A simple, one-step polychromatic staining method for epoxy-embedded semithin tissue sections. Microscopy 2018, 67, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Španić, V.; Šunić, K.; Duvnjak, J.; Hu, Y.G.; Katanić, Z. Chlorophyll a fluorescence during flag leaf senescence of field-grown winter wheat plants under drought conditions. Ann. Appl. Biol. 2023, 183, 80–92. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Krupinska, K.; Shimakawa, G. The impact of photosynthesis on initiation of leaf senescence. Physiol. Plant. 2019, 166, 148–164. [Google Scholar] [CrossRef]
- Pic, E.; de La Serve, B.T.; Tardieu, F.; Turc, O. Leaf senescence induced by mild water deficit follows the same sequence of macroscopic, biochemical, and molecular events as monocarpic senescence in pea. Plant Physiol. 2002, 128, 236–246. [Google Scholar] [CrossRef]
- Liu, Y.N.; Xu, Q.Z.; Li, W.C.; Yang, X.H.; Zheng, Q.; Li, B.; Li, H.W. Long-term high light stress induces leaf senescence in wheat (Triticum aestivum L.). Photosynthetica 2019, 57, 3. [Google Scholar] [CrossRef]
- Huang, Q.N.; Shi, Y.F.; Zhang, X.B.; Song, L.X.; Feng, B.H.; Wang, H.M.; Xu, X.; Li, X.H.; Guo, D.; Wu, J.L. Single base substitution in OsCDC48 is responsible for premature senescence and death phenotype in rice. J. Integr. Plant Biol. 2016, 58, 12–28. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Shahbaz, M.; Kanwal, S.; Kaleem, M.; Shah, S.M.R.; Luqman, M.; Farhat, F. Methionine promotes the growth and yield of wheat under water deficit conditions by regulating the antioxidant enzymes, reactive oxygen species, and ions. Life 2022, 12, 969. [Google Scholar] [CrossRef]
- Díaz-Mendoza, M.; Velasco-Arroyo, B.; Santamaría, M.E.; González-Melendi, P.; Martínez, M.; Díaz, I. Plant senescence and proteolysis: Two processes with one destiny. Genet. Mol. Biol. 2016, 39, 329–338. [Google Scholar] [CrossRef]
- Christiansen, M.W.; Gregersen, P.L. Members of the barley NAC transcription factor gene family show differential co-regulation with senescence-associated genes during senescence of flag leaves. J. Exp. Bot. 2014, 65, 4009–4022. [Google Scholar] [CrossRef]
- Hollmann, J.; Gregersen, P.L.; Krupinska, K. Identification of predominant genes involved in regulation and execution of senescence-associated nitrogen remobilization in wheat. J. Exp. Bot. 2014, 65, 4101–4113. [Google Scholar] [CrossRef]
- Roberts, I.N.; Caputo, C.; Criado, M.V.; Funk, C. Senescence-associated proteases in plants. Physiol. Plant. 2012, 145, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Hongwei, L.; Gui, W.; Shudong, L.; Qiang, A.; Zheng, Q.; Bin, L.; Zhensheng, L. Comparative changes in the antioxidant system in the flag leaf of early and normally senescing near-isogenic lines of wheat (Triticum aestivum L.). Plant Cell Rep. 2014, 33, 1109–1120. [Google Scholar]
- Karatas, I.; Ozturk, L.; Ersahin, Y.; Okatan, Y. Effects of auxin on photosynthetic pigments and some enzyme activities during dark-induced senescence of Tropaeolum leaves. Pak. J. Bot. 2010, 42, 1881–1888. [Google Scholar]
- Lambert, R.; Quiles, F.A.; Gálvez-Valdivieso, G.; Piedras, P. Nucleases activities during French bean leaf aging and dark-induced senescence. J. Plant Physiol. 2017, 218, 235–242. [Google Scholar] [CrossRef]
- Laus, M.N.; De Santis, M.A.; Flagella, Z.; Soccio, M. Changes in Antioxidant Defence System in Durum Wheat under Hyperosmotic Stress: A Concise Overview. Plants 2022, 11, 98. [Google Scholar] [CrossRef]
- Huseynova, I.M. Photosynthetic characteristics and enzymatic antioxidant capacity of leaves from wheat cultivars exposed to drought. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1516–1523. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Mao, H.; Chen, M.; Su, Y.; Wu, N.; Yuan, M.; Yuan, S.; Brestic, M.; Zivcak, M.; Zhang, H.; Chen, Y. Comparison on photosynthesis and antioxidant defense Systems in Wheat with different Ploidy levels and Octoploid Triticale. Int. J. Mol. Sci. 2018, 19, 3006. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Prochazkova, D.; Wilhelmova, N. Leaf senescence and activities of the antioxidant enzymes. Biol. Plant. 2007, 51, 401–406. [Google Scholar] [CrossRef]
- Jiménez, A.; Hernández, J.A.; Pastori, G.; del Río, L.A.; Sevilla, F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998, 118, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Aliyeva, D.R.; Gurbanova, U.A.; Rzayev, F.H.; Gasimov, E.K.; Huseynova, I.M. Biochemical and ultrastructural changes in wheat plants during drought stress. Biochemistry 2023, 88, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Saed-Moucheshi, A.; Sohrabi, F.; Fasihfar, E.; Baniasadi, F.; Riasat, M.; Mozafari, A.A. Superoxide dismutase (SOD) as a selection criterion for triticale grain yield under drought stress: A comprehensive study on genomics and expression profiling, bioinformatics, heritability, and phenotypic variability. BMC Plant Biol. 2021, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, L.; Liu, J.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol. Biochem. 2018, 122, 90–101. [Google Scholar] [CrossRef]
- Mohammadi, M.; Tavakoli, A.; Pouryousef, M.; Fard, E.M. Study the effect of 24-epibrassinolide application on the Cu/Zn-SOD expression and tolerance to drought stress in common bean. Physiol. Mol. Biol. Plants 2020, 23, 459–474. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res. Int. 2019, 2019, 435–455. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Pakniyat, H.; Pirasteh-Anosheh, H.; Azooz, M.M. Role of ROS as signaling molecules in plants. In Oxidative Damage to Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 585–620. [Google Scholar]
- Awan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant. 2020, 147, 125–136. [Google Scholar] [CrossRef]
- Sheoran, S.; Thakur, V.; Narwal, S.; Turan, R.; Mamrutha, H.; Singh, V.; Tiwari, V.; Sharma, I. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl. Biochem. Biotechnol. 2015, 177, 1282–1298. [Google Scholar] [CrossRef]
- Wang, W.; Xia, M.; Chen, J.; Yuan, R.; Deng, F.; Shen, F. Gene expression characteristics and regulation mechanisms of superoxide dismutase and its physiological roles in plants under stress. Biochemistry 2018, 81, 465–480. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, L.; He, Y.; Zhang, H.; Li, W.; Chen, H.; Yin, J. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). PeerJ 2019, 7, e8062. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Huseynova, I.; Aliyeva, D.; Aliyev, J. Subcellular localization and responses of superoxide dismutase isoforms in local wheat varieties subjected to continuous soil drought. Plant Physiol. Biochem. 2014, 81, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Huseynova, I.; Aliyeva, D.; Mammadov, A.; Aliyev, J. Hydrogen peroxide generation and antioxidant enzyme activities in the leaves and roots of wheat cultivars subjected to long-term soil drought stress. Photosynth. Res. 2015, 1125, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Huseynova, I.; Rustamova, S.; Suleymanov, S.; Aliyeva, D.; Mammadov, A.; Aliyev, J. Drought-induced changes in photosynthetic apparatus and antioxidant components of wheat (Triticum durum Desf.) varieties. Photosynth. Res. 2016, 130, 215–223. [Google Scholar] [CrossRef]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Changes in activity of antioxidative enzymes in wheat (Triticum aestivum) seedlings under cold acclimation. Physiol. Plant. 1998, 104, 747–752. [Google Scholar] [CrossRef]
- Manoharan, K.; Karuppanapandian, T.; Sinha, P.B.; Prasad, R. Membrane degradation, accumulation of phosphatidic acid, stimulation of catalase activity and nuclear DNA fragmentation during 2,4-D-induced leaf senescence in mustard. J. Plant Biol. 2005, 48, 394–403. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Paluch-Lubawa, E.; Stolarska, E.; Sobieszczuk-Nowicka, E. Dark-induced barley leaf senescence—A crop system for studying senescence and autophagy mechanisms. Front. Plant Sci. 2021, 12, 635619. [Google Scholar] [CrossRef]
- Smart, C.M. Gene expression during leaf senescence. New Phytol. 1994, 126, 419–448. [Google Scholar] [CrossRef]
- Austin, J.R.; Frost, E.; Vidi, P.A.; Kessler, F.; Staehelin, L.A. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef]
- Challabathula, D.; Analin, B.; Mohanan, A.; Bakka, K. Differential modulation of photosynthesis, ROS and antioxidant enzyme activities in stress-sensitive and -tolerant rice cultivars during salinity and drought upon restriction of COX and AOX pathways of mitochondrial oxidative electron transport. J. Plant Physiol. 2022, 268, 153583. [Google Scholar] [CrossRef]
- Vollenweider, P.; Ottiger, M.; Günthardt-Goerg, M.S. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environ. Pollut. 2003, 124, 101–118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).