Impact of Drying–Wetting Cycles on Nitrification Inhibitors (DMPP and DMPSA) in a Greenhouse Experiment with Two Contrasting Mediterranean Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation and Soil Selection
2.2. Experimental Procedure and Conditions
2.3. Soil N-min Sampling and Analysis
2.4. GHG Sampling and Analysis
2.5. Soil Inhibitor Concentration Sampling and Analysis
2.6. Sampling and Analysis of Soil Nitrification Microorganisms
2.7. Calculations and Statistical Analysis
3. Results
3.1. Temperature and Soil Moisture Conditions
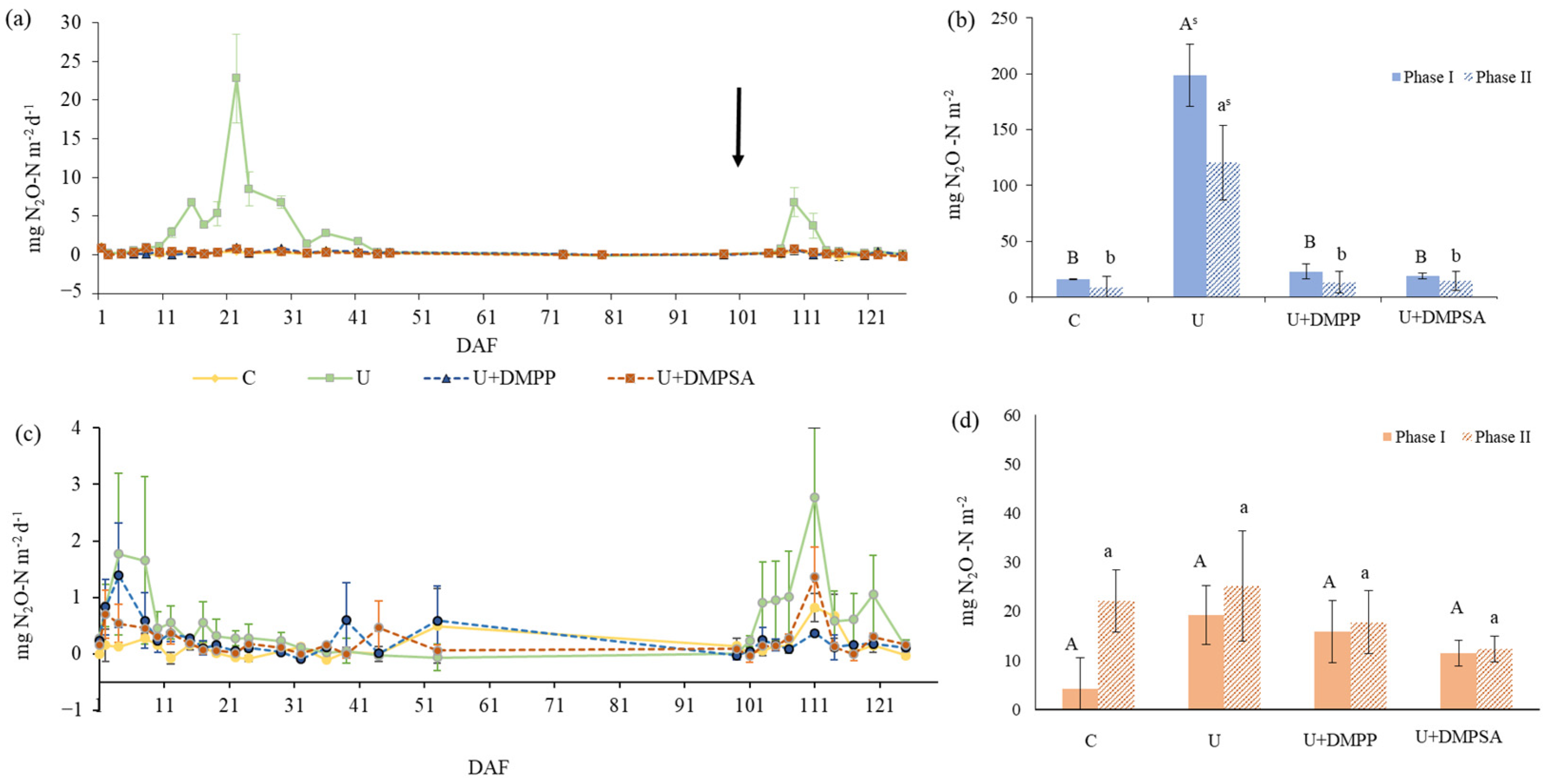
3.2. Soil GHG Emissions (N2O and CO2)
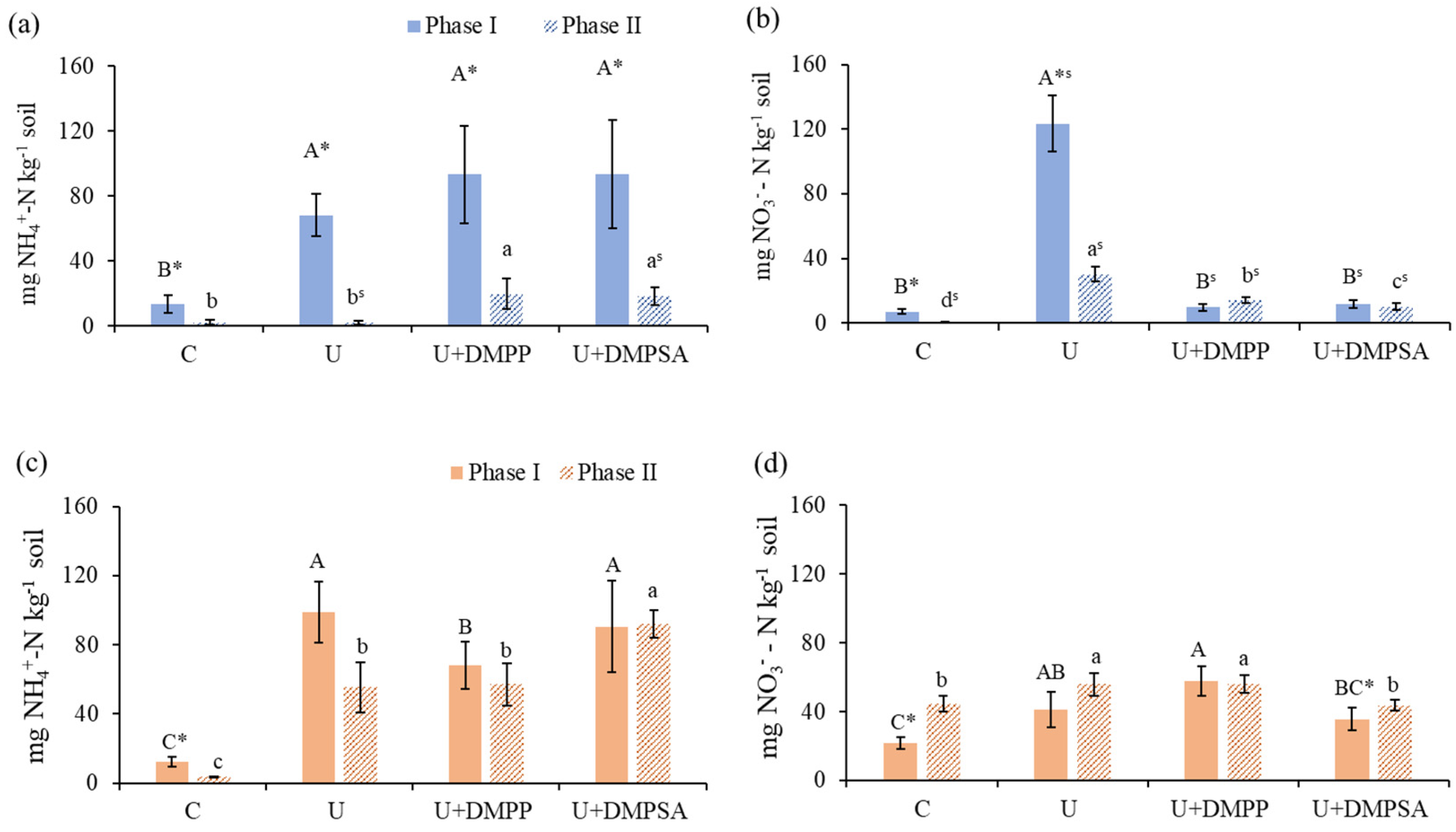
3.3. Soil N-min
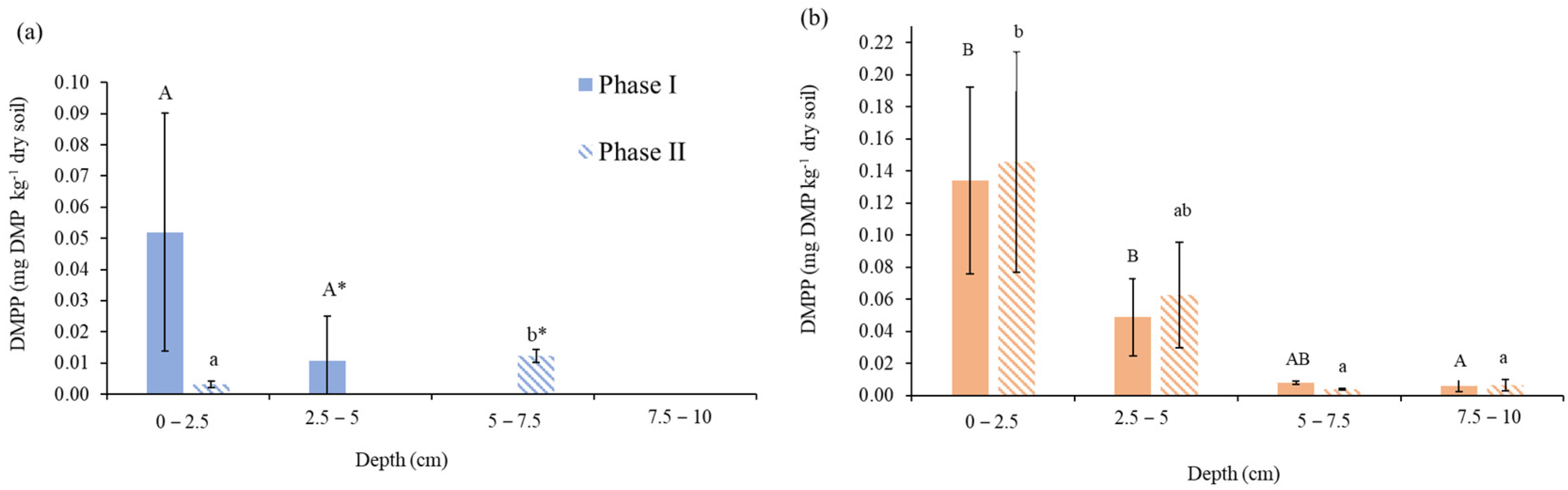
3.4. Persistence of DMPP in the Soil Profile
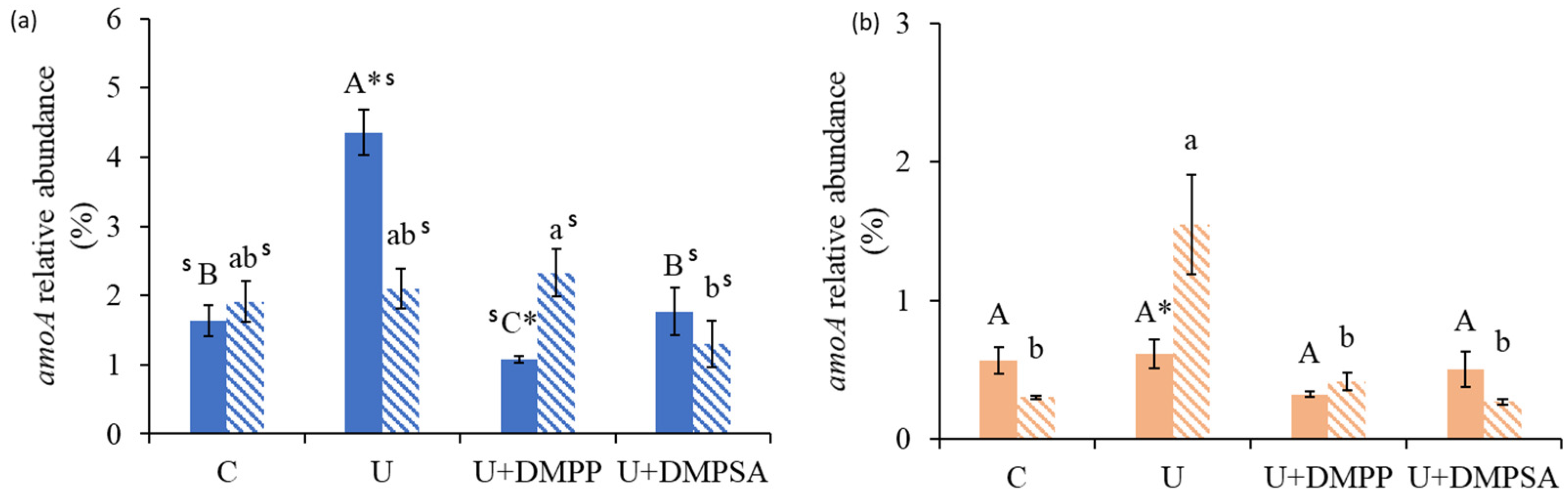
3.5. Abundances of Nitrifying Genes at Different Stages of the Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Meisner, A.; Snoek, B.L.; Nesme, J.; Dent, E.; Jacquiod, S.; Classen, A.T.; Priemé, A. Soil microbial legacies differ following drying-rewetting and freezing-thawing cycles. ISME J. 2020, 14, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Barrat, H.A.; Evans, J.; Chadwick, D.R.; Clark, I.M.; Le Cocq, K.; Cardenas, L. The impact of drought and rewetting on N2O emissions from soil in temperate and Mediterranean climates. Eur. J. Soil Sci. 2020, 72, 2504–2516. [Google Scholar] [CrossRef]
- Guardia, G.; García-Gutiérrez, S.; Rodríguez-Pérez, R.; Recio, J.; Vallejo, A. Increasing N use efficiency while decreasing gaseous N losses in a non-tilled wheat (Triticum aestivum L.) crop using a double inhibitor. Agric. Ecosyst. Environ. 2021, 319, 107546. [Google Scholar] [CrossRef]
- Montoya, M.; Guardia, G.; Recio, J.; Castellano-Hinojosa, A.; Gines, C.; Bedmar, E.J.; Álvarez, J.M.; Vallejo, A. Zinc-nitrogen co-fertilization influences N2O emissions and microbial communities in an irrigated maize field. Geoderma 2021, 383, 114735. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Influence of drying–rewetting frequency on soil bacterial community structure. Microb. Ecol. 2003, 45, 63–71. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Chang. Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Harris, E.; Diaz-Pines, E.; Stoll, E.; Schloter, M.; Schulz, S.; Duffner, C.; Li, K.; Moore, K.L.; Ingrisch, J.; Reinthaler, D.; et al. Denitrifying pathways dominate nitrous oxide emissions from managed grassland during drought and rewetting. Sci. Adv. 2021, 7, eabb7118. [Google Scholar] [CrossRef]
- Leitner, S.; Homyak, P.M.; Blankinship, J.C.; Eberwein, J.; Jenerette, G.D.; Zechmeister-Boltenstern, S. Linking NO and N2O emission pulses with the mobilization of mineral and organic N upon rewetting dry soils. Soil Biol. Biochem. 2017, 115, 461–466. [Google Scholar] [CrossRef]
- Liu, R.; Hayden, H.; Suter, H.; He, J.; Chen, D. The effect of nitrification inhibitors in reducing nitrification and the ammonia oxidizer population in three contrasting soils. J. Soils Sediments 2015, 15, 1113–1118. [Google Scholar] [CrossRef]
- Bozal-Leorri, A.; Subbarao, G.V.; Kishii, M.; Urmeneta, L.; Kommerell, V.; Karwat, H.; Braun, H.-J.; Aparicio-Tejo, P.M.; Ortiz-Monasterio, I.; González-Murua, C.; et al. Biological nitrification inhibitor-trait enhances nitrogen uptake by suppressing nitrifier activity and improves ammonium assimilation in two elite wheat varieties. Front. Plant Sci. 2022, 13, 1034219. [Google Scholar] [CrossRef]
- Huérfano, X.; Estavillo, J.M.; Torralbo, F.; Vega-Mas, I.; González-Murua, C.; Fuertes-Mendizábal, T. Dimethylpyrazole-based nitrification inhibitors have a dual role in N2O emissions mitigation in forage systems under Atlantic climate conditions. Sci. Total Environ. 2022, 807, 150670. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Li, Y.; Ran, Y.; Liu, Y.; Zhang, Q.; Li, Z.; He, Y.; Xu, J.; Di, H. High temperatures inhibited the growth of soil bacteria and archaea but not that of fungi and altered nitrous oxide production mechanisms from different nitrogen sources in an acidic Soil. Soil Biol. Biochem. 2017, 107, 168–179. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, L.; Hu, S.; Compton, J.E.; Greaver, T.L.; Li, Q. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob. Chang. Biol. 2015, 21, 1249–1257. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethylpyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Sci. Rep. 2016, 6, 22075. [Google Scholar] [CrossRef]
- Rodrigues, J.; Lasa, M.B.; Aparicio-Tejo, P.M.; González-Murua, C.; Marino, D. 3,4-Dimethylpyrazole phosphate and 2-(N-3,4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: Quantification in plant tissues and toxicity assays. Sci. Total Environ. 2018, 624, 1180–1186. [Google Scholar] [CrossRef]
- Bozal-Leorri, A.; Corrochano-Monsalve, M.; Vega-Mas, I.; Aparicio-Tejo, P.M.; González-Murua, C.; Marino, D. Evidences towards deciphering the mode of action of dimethylpyrazole-based nitrification inhibitors in soil and pure cultures of Nitrosomonas europaea. Chem. Biol. Technol. Agric. 2022, 9, 56. [Google Scholar] [CrossRef]
- Torralbo, F.; Menéndez, S.; Barrena, I.; Estabillo, J.M.; Marino, D.; Gonzalez-Murua, C. Dimethyl pyrazol-based nitrification inhibitors effect on nitrifying and denitrifying bacteria to mitigate N2O emission. Sci. Rep. 2017, 7, 13810. [Google Scholar] [CrossRef]
- Guardia, G.; Cangani, M.T.; Andreu, G.; Sanz-Cobena, A.; García-Marco, S.; Álvarez, J.M.; Recio-Huetos, J.; Vallejo, A. Effect of inhibitors and fertigation strategies on GHG emissions, NO fluxes and yield in irrigated maize. Field Crops Res. 2017, 204, 135–145. [Google Scholar] [CrossRef]
- Corrochano-Monsalve, M.; Huérfano, X.; Menéndez, S.; Torralbo, F.; Fuertes-Mendizábal, T.; Estavillo, J.M.; González-Murua, C. Relationship between tillage management and DMPSA nitrification inhibitor efficiency. Sci. Total Environ. 2020, 718, 134748. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Zhang, L.; Song, Y.; Li, Y.; Zheng, Y.; Zhang, J.; et al. Effects of Nitrification Inhibitors on Soil Nitrification and Ammonia Volatilization in Three Soils with Different pH. Agronomy 2021, 11, 1674. [Google Scholar] [CrossRef]
- Guan, T.; Lei, J.; Fan, Q.; Liu, R. Soil Factors Key to 3,4-Dimethylpyrazole Phosphate (DMPP) Efficacy: EC and SOC Dominate over Biotic Influences. Microorganisms 2024, 12, 1787. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Danielson, R.E.; Sutherland, P.L. Porosity. In Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 443–460. [Google Scholar]
- Sanchez-Martín, L.; Beccaccia, A.; De Blas, C.; Sanz-Cobena, A.; García-Rebollar, P.; Estellés, F.; Marsden, K.; Chadwick, D.; Vallejo, A. Diet management to effectively abate N2O emissions from surface-applied pig slurry. Agric. Ecosyst. Environ. 2007, 239, 1–11. [Google Scholar] [CrossRef]
- Pavelka, M.; Acosta, M.; Kiese, R.; Altimir, N.; Brümmer, C.; Crill, P.; Darenova, E.; Fuß, R.; Gielen, B.; Graf, A.; et al. Standardisation of chamber technique for CO2, N2O and CH4 fluxes measurements from terrestrial ecosystems. Int. Agrophys. 2018, 32, 569–587. [Google Scholar] [CrossRef]
- Benckiser, G.; Christ, E.; Herbert, T.; Weiske, A.; Blome, J.; Hardt, M. The nitrification inhibitor 3,4-dimethylpyrazole-phosphate (DMPP)—Quantification and effects on soil metabolism. Plant Soil 2013, 371, 257–266. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Behrens, S.; Azizian, M.F.; McMurdie, P.J.; Sabalowsky, A.; Dolan, M.E.; Semprini, L.; Spormann, A.M. Monitoring abundance and expression of “Dehalococcoides” species chloroethene-reductive dehalogenases in a tetrachloroethene-dechlorinating flow column. Appl. Environ. Microbiol. 2008, 74, 5695–5703. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural Soil. Soil Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef]
- Guo, J.; Ling, N.; Chen, H.; Zhu, C.; Kong, Y.; Wang, M.; Shen, Q.; Guo, S. Distinct drivers of activity, abundance, diversity and composition of ammonia-oxidizers: Evidence from a long-term field experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Taylor, A.E.; Vajrala, N.; Giguere, A.T.; Gitelman, A.I.; Arp, D.J.; Myrold, D.D.; Sayavedra-Soto, L.; Bottomley, P.J. Use of aliphatic n-alkynes to discriminate soil nitrification activities of ammonia-oxidizing thaumarchaea and bacteria. Appl. Environ. Microbiol. 2013, 79, 6544–6551. [Google Scholar] [CrossRef]
- Davidson, E.A.; Hart, S.C.; Shanks, C.A.; Firestone, M.K. Measuring gross nitrogen mineralization, and nitrification by ¹⁵N isotopic pool dilution in intact soil cores. Eur. J. Soil Sci. 1991, 42, 335–349. [Google Scholar] [CrossRef]
- Montoya, M.; Juhanson, J.; Hallin, S.; García-Gutiérrez, S.; García-Marco, S.; Vallejo, A.; Recio, J.; Guardia, G. Nitrous oxide emissions and microbial communities during the transition to conservation agriculture using N-enhanced efficiency fertilisers in a semiarid climate. Soil Biol. Biochem. 2022, 170, 108687. [Google Scholar] [CrossRef]
- Menéndez, S.; Barrena, I.; Setien, I.; González-Murua, C.; Estavillo, J.M. Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol. Biochem. 2012, 53, 82–89. [Google Scholar] [CrossRef]
- Barth, G.; von Tucher, S.; Schmidhalter, U. Influence of soil parameters on the effect of 3,4-dimethylpyrazole-phosphate as a nitrification inhibitor. Biol. Fertil. Soils 2001, 34, 98–102. [Google Scholar] [CrossRef]
- Barth, G.; von Tucher, S.; Schmidhalter, U. Effectiveness of 3,4-dimethylpyrazole phosphate as nitrification inhibitor in soil as influenced by inhibitor concentration, application form, and soil matric potential. Pedosphere 2018, 18, 378–385. [Google Scholar] [CrossRef]
- Sidhu, P.K.; Taggert, B.I.; Chen, D.; Wille, U. Degradation of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate in soils: Indication of chemical pathways. ACS Agric. Sci. Technol. 2021, 1, 540–549. [Google Scholar] [CrossRef]
- Doran, G.S.; Condon, J.R.; Kaveney, B.F. Rapid analysis of the nitrification inhibitor 3,4-dimethylpyrazole phosphate in soil using LC-MS/MS. J. Environ. Anal. Chem. 2018, 98, 606–621. [Google Scholar] [CrossRef]
- Bond, W. Soil Physical Methods for Estimating Recharge-Part 3; CSIRO Publishing: Melbourne, Australia, 1998. [Google Scholar]
- Congreves, K.A.; Phan, T.; Farrell, R.E. Revisiting the relationship between soil moisture and N2O production pathways by measuring 15N2O isotopomers. Soil 2019. in review. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Forge, T.A.; Goyer, C.; Brin, L.D. Effect of soil acidification on nitrification in Soil. Can. J. Soil Sci. 2015, 95, 359–363. [Google Scholar] [CrossRef]
- Li, Z.; Xu, P.; Bo, X.; Wu, J.; Han, Z.; Guo, S.; Li, K.; Shen, M.; Wang, J.; Zou, J. Soil pH-dependent efficacy of DMPP in mitigating nitrous oxide under different land uses. Geoderma 2024, 449, 117018. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, Y.; Lindsey, S.; Shi, Y.; Wei, Z.; Wang, L.; Zhang, L. Effects of soil properties on urea-N transformation and efficacy of nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP). Soil Sci. Plant Nutr. 2021, 68, 228–237. [Google Scholar] [CrossRef]
- Benckiser, G.; Schartel, T.; Weiske, A. Control of NO3− and N2O emissions in agroecosystems: A review. Agron. Sustain. Dev. 2015, 35, 1059–1074. [Google Scholar] [CrossRef]
- McCarty, G.W. Modes of action of nitrification inhibitors. Biol. Fertil. Soils 1999, 29, 1–9. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Martin, L.; Bozal-Leorri, A.; Rodrigues, J.M.; González-Murua, C.; Aparicio, P.; García-Marco, S.; Vallejo, A. Impact of Drying–Wetting Cycles on Nitrification Inhibitors (DMPP and DMPSA) in a Greenhouse Experiment with Two Contrasting Mediterranean Soils. Agronomy 2024, 14, 2620. https://doi.org/10.3390/agronomy14112620
Sánchez-Martin L, Bozal-Leorri A, Rodrigues JM, González-Murua C, Aparicio P, García-Marco S, Vallejo A. Impact of Drying–Wetting Cycles on Nitrification Inhibitors (DMPP and DMPSA) in a Greenhouse Experiment with Two Contrasting Mediterranean Soils. Agronomy. 2024; 14(11):2620. https://doi.org/10.3390/agronomy14112620
Chicago/Turabian StyleSánchez-Martin, Laura, Adrián Bozal-Leorri, Janaina M. Rodrigues, Carmen González-Murua, Pedro Aparicio, Sonia García-Marco, and Antonio Vallejo. 2024. "Impact of Drying–Wetting Cycles on Nitrification Inhibitors (DMPP and DMPSA) in a Greenhouse Experiment with Two Contrasting Mediterranean Soils" Agronomy 14, no. 11: 2620. https://doi.org/10.3390/agronomy14112620
APA StyleSánchez-Martin, L., Bozal-Leorri, A., Rodrigues, J. M., González-Murua, C., Aparicio, P., García-Marco, S., & Vallejo, A. (2024). Impact of Drying–Wetting Cycles on Nitrification Inhibitors (DMPP and DMPSA) in a Greenhouse Experiment with Two Contrasting Mediterranean Soils. Agronomy, 14(11), 2620. https://doi.org/10.3390/agronomy14112620





