The Use of Integrated Crop–Livestock Systems as a Strategy to Improve Soil Organic Matter in the Brazilian Cerrado
Abstract
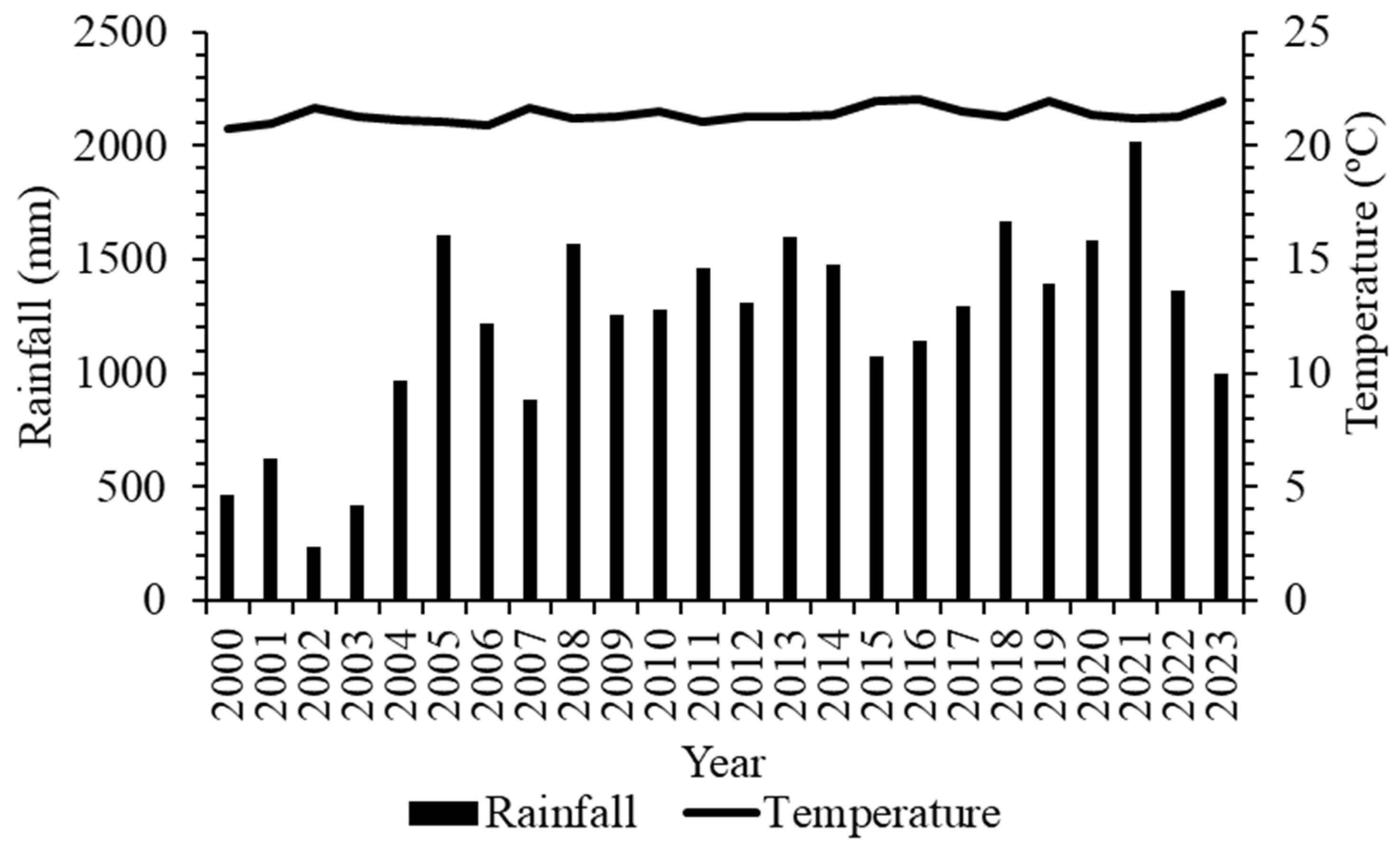
1. Introduction
2. Materials and Methods
2.1. Location and Experimental Design
2.2. Soil Sampling and Processing
2.3. Calculations
2.4. SOM Particle Size Fractionation
2.5. Statistical Analysis
3. Results
3.1. Soil C and N Concentration
3.2. Soil C and N Stocks and C/N Ratio
3.3. Physical Fractionation of Soil
3.4. Principal Components Analysis
4. Discussion
4.1. Soil C and N Dynamics
4.2. Soil Physical Fractionation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, C.M.; Shimbo, J.Z.; Rosa, M.R.; Parente, L.L.; Alencar, A.A.; Rudorff, B.F.T.; Hasenack, H.; Matsumoto, M.; Ferreira, L.G.; Souza-Filho, P.W.M.; et al. Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- Fernandes, M.M.; de Moura Fernandes, M.R.; Garcia, J.R.; Matricardi, E.A.T.; de Souza Lima, A.H.; de Araújo Filho, R.N.; Gomes Filho, R.R.; Piscoya, V.C.; Piscoya, T.O.F.; Cunha Filho, M. Land Use and Land Cover Changes and Carbon Stock Valuation in the São Francisco River Basin, Brazil. Environ. Chall. 2021, 5, 100247. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2015; ISBN 9781107058217. [Google Scholar]
- da Conceição, M.C.; Matos, E.D.S.; Bidone, E.D.; Rodrigues, R.D.A.R.; Cordeiro, R.C. Changes in Soil Carbon Stocks under Integrated Crop-Livestock-Forest System in the Brazilian Amazon Region. Agric. Sci. 2017, 8, 904–913. [Google Scholar] [CrossRef]
- Lal, R. Managing Soils for Negative Feedback to Climate Change and Positive Impact on Food and Nutritional Security. Soil Sci. Plant Nutr. 2020, 66, 1–9. [Google Scholar] [CrossRef]
- dos Santos, C.A.; Rezende, C.D.P.; Pinheiro, É.F.M.; Pereira, J.M.; Alves, B.J.; Urquiaga, S.; Boddey, R.M. Changes in Soil Carbon Stocks after Land-Use Change from Native Vegetation to Pastures in the Atlantic Forest Region of Brazil. Geoderma 2019, 337, 394–401. [Google Scholar] [CrossRef]
- Morais, V.A.; Ferreira, G.W.D.; de Mello, J.M.; Silva, C.A.; de Mello, C.R.; Araújo, E.J.G.; David, H.C.; da Silva, A.C.; Scolforo, J.R.S. Spatial Distribution of Soil Carbon Stocks in the Cerrado Biome of Minas Gerais, Brazil. Catena 2020, 185, 104285. [Google Scholar] [CrossRef]
- Lal, R.; Monger, C.; Nave, L.; Smith, P. The Role of Soil in Regulation of Climate. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20210084. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The Role of Soil Microbes in the Global Carbon Cycle: Tracking the Below-ground Microbial Processing of Plant-derived Carbon for Manipulating Carbon Dynamics in Agricultural Systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Primieri, S.; Muniz, A.W.; Lisboa, H.D.M. Dinâmica Do Carbono No Solo Em Ecossistemas Nativos e Plantações Florestais Em Santa Catarina. Floresta E Ambiente 2017, 24, e00110314. [Google Scholar] [CrossRef]
- Penman, J.; Gytarsky, M.; Hiraishi, T.; Irving, W.; Krug, T. 2006 IPCC—Guidelines for National Greenhouse Gas Inventories; Directrices para los Inventarios Nacionales GEI: Geneva, Switzerland, 2006; Volume 12. [Google Scholar]
- Wang, W.; Akhtar, K.; Ren, G.; Yang, G.; Feng, Y.; Yuan, L. Impact of Straw Management on Seasonal Soil Carbon Dioxide Emissions, Soil Water Content, and Temperature in a Semi-Arid Region of China. Sci. Total Environ. 2019, 652, 471–482. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-associated Forms to Address Global Change in the 21st Century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Congreves, K.A.; Hooker, D.C.; Hayes, A.; Verhallen, E.A.; Van Eerd, L.L. Interaction of Long-Term Nitrogen Fertilizer Application, Crop Rotation, and Tillage System on Soil Carbon and Nitrogen Dynamics. Plant Soil 2017, 410, 113–127. [Google Scholar] [CrossRef]
- Rigon, J.P.G.; Calonego, J.C. Soil Carbon Fluxes and Balances of Crop Rotations under Long-Term No-Till. Carbon Balance Manag. 2020, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Lavallee, J.M. Soil Organic Matter Formation, Persistence, and Functioning: A Synthesis of Current Understanding to Inform Its Conservation and Regeneration. Adv. Agron. 2022, 172, 1–66. [Google Scholar]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of Organic Matter in Temperate Soils: Mechanisms and Their Relevance under Different Soil Conditions—A Review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of Soil Organic Matter Dynamics to Physical Protection and Tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Grandy, A.S.; Robertson, G.P. Aggregation and Organic Matter Protection Following Tillage of a Previously Uncultivated Soil. Soil Sci. Soc. Am. J. 2006, 70, 1398–1406. [Google Scholar] [CrossRef]
- Denef, K.; Six, J. Contributions of Incorporated Residue and Living Roots to Aggregate-associated and Microbial Carbon in Two Soils with Different Clay Mineralogy. Eur. J. Soil Sci. 2006, 57, 774–786. [Google Scholar] [CrossRef]
- Haddix, M.L.; Gregorich, E.G.; Helgason, B.L.; Janzen, H.; Ellert, B.H.; Francesca Cotrufo, M. Climate, Carbon Content, and Soil Texture Control the Independent Formation and Persistence of Particulate and Mineral-Associated Organic Matter in Soil. Geoderma 2020, 363, 114160. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Bayer, C.; Martin-Neto, L.; Mielniczuk, J.; Pavinato, A. Armazenamento de Carbono Em Frações Lábeis Da Matéria Orgânica de Um Latossolo Vermelho Sob Plantio Direto. Pesqui. Agropecuária Bras. 2004, 39, 677–683. [Google Scholar] [CrossRef]
- Frazão, L.A.; Santana, I.K.D.S.; Campos, D.V.B.D.; Feigl, B.J.; Cerri, C.C. Estoques de Carbono e Nitrogênio e Fração Leve Da Matéria Orgânica Em Neossolo Quartzarênico Sob Uso Agrícola. Pesqui. Agropecuária Bras. 2010, 45, 1198–1204. [Google Scholar] [CrossRef]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different Climate Sensitivity of Particulate and Mineral-Associated Soil Organic Matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- Ayarza, M.; Rao, I.; Vilela, L.; Lascano, C.; Vera-Infanzón, R. Soil Carbon Accumulation in Crop-Livestock Systems in Acid Soil Savannas of South America: A Review. Adv. Agron. 2022, 173, 163–226. [Google Scholar]
- Gmach, M.-R.; Dias, B.O.; Silva, C.A.; Nóbrega, J.C.A.; Lustosa-Filho, J.F.; Siqueira-Neto, M. Soil Organic Matter Dynamics and Land-Use Change on Oxisols in the Cerrado, Brazil. Geoderma Reg. 2018, 14, e00178. [Google Scholar] [CrossRef]
- Loss, A.; Pereira, M.G.; Perin, A.; Coutinho, F.S.; Cunha dos Anjos, L.H. Particulate Organic Matter in Soil under Different Management Systems in the Brazilian Cerrado. Soil Res. 2012, 50, 685. [Google Scholar] [CrossRef]
- de Sant-Anna, S.A.C.; Jantalia, C.P.; Sá, J.M.; Vilela, L.; Marchão, R.L.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Changes in Soil Organic Carbon during 22 Years of Pastures, Cropping or Integrated Crop/Livestock Systems in the Brazilian Cerrado. Nutr. Cycl. Agroecosystems 2017, 108, 101–120. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Filho, J.C.A.; Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System; Embrapa: Brasília, Brasil, 2018; Volume 5. [Google Scholar]
- Chapuis Lardy, L.; Brossard, M.; Lopes Assad, M.; Laurent, J.-Y. Carbon and Phosphorus Stocks of Clayey Ferralsols in Cerrado Native and Agroecosystems, Brazil. Agric. Ecosyst. Environ. 2002, 92, 147–158. [Google Scholar] [CrossRef]
- Arnold, S.L.; Schepers, J.S. A Simple Roller-Mill Grinding Procedure for Plant and Soil Samples. Commun. Soil Sci. Plant Anal. 2004, 35, 537–545. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo; Embrapa: Brasília, Brasil, 2017. [Google Scholar]
- Sisti, C.P.J.; dos Santos, H.P.; Kohhann, R.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Change in Carbon and Nitrogen Stocks in Soil under 13 Years of Conventional or Zero Tillage in Southern Brazil. Soil Tillage Res. 2004, 76, 39–58. [Google Scholar] [CrossRef]
- Cerri, C.C.; Feller, C.; Balesdent, J.; Victoria, R.L.; Plenecassagne, A. Application Du Traçage Isotopique Naturel En 13C, à l’étude de La Dynamique de La Matière Organique Dans Les Sols. Comptes Rendus L’académie Des Sci. Paris 1985, 300, 423–428. [Google Scholar]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Computer Statistical Analysis System. Ciência E Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Team, R.D.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Dray, S.; Dufour, A.-B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Boddey, R.M.; Macedo, R.; Tarré, R.M.; Ferreira, E.; de Oliveira, O.C.; de PRezende, C.; Cantarutti, R.B.; Pereira, J.M.; Alves, B.J.R.; Urquiaga, S. Nitrogen Cycling in Brachiaria Pastures: The Key to Understanding the Process of Pasture Decline. Agric. Ecosyst. Environ. 2004, 103, 389–403. [Google Scholar] [CrossRef]
- Groppo, J.D.; Lins, S.R.M.; Camargo, P.B.; Assad, E.D.; Pinto, H.S.; Martins, S.C.; Salgado, P.R.; Evangelista, B.; Vasconcellos, E.; Sano, E.E.; et al. Changes in Soil Carbon, Nitrogen, and Phosphorus Due to Land-Use Changes in Brazil. Biogeosciences 2015, 12, 4765–4780. [Google Scholar] [CrossRef]
- Xu, S.; Silveira, M.L.; Inglett, K.S.; Sollenberger, L.E.; Gerber, S. Soil Microbial Community Responses to Long-Term Land Use Intensification in Subtropical Grazing Lands. Geoderma 2017, 293, 73–81. [Google Scholar] [CrossRef]
- Damian, J.M.; da Silva Matos, E.; e Pedreira, B.C.; de Faccio Carvalho, P.C.; de Souza, A.J.; Andreote, F.D.; Premazzi, L.M.; Cerri, C.E.P. Pastureland Intensification and Diversification in Brazil Mediate Soil Bacterial Community Structure Changes and Soil C Accumulation. Appl. Soil Ecol. 2021, 160, 103858. [Google Scholar] [CrossRef]
- Frazão, L.A.; Cardoso, P.H.S.; Neta, M.N.A.; Mota, M.F.C.; de Souza Almeida, L.L.; Ribeiro, J.M.; Bicalho, T.F.; Feigl, B.J. Carbon and Nitrogen Stocks and Organic Matter Fractions in the Topsoil of Traditional and Agrisilvicultural Systems in the Southeast of Brazil. Soil Res. 2021, 59, 794–805. [Google Scholar] [CrossRef]
- Freitas IC, d.e.; Alves, M.A.; Pena, A.N.L.; Ferreira, E.A.; Frazão, L.A. Changing the Land Use from Degraded Pasture into Integrated Farming Systems Enhance Soil Carbon Stocks in the Cerrado Biome. Acta Sci. Agron. 2023, 46, e63601. [Google Scholar] [CrossRef]
- Diekow, J.; Mielniczuk, J.; Knicker, H.; Bayer, C.; Dick, D.P.; Kögel-Knabner, I. Carbon and Nitrogen Stocks in Physical Fractions of a Subtropical Acrisol as Influenced by Long-Term No-till Cropping Systems and N Fertilisation. Plant Soil 2005, 268, 319–328. [Google Scholar] [CrossRef]
- Batlle-Bayer, L.; Batjes, N.H.; Bindraban, P.S. Changes in Organic Carbon Stocks upon Land Use Conversion in the Brazilian Cerrado: A Review. Agric. Ecosyst. Environ. 2010, 137, 47–58. [Google Scholar] [CrossRef]
- de Souza Almeida, L.L.; Frazão, L.A.; Lessa, T.A.M.; Fernandes, L.A.; de Carvalho Veloso, Á.L.; Lana, A.M.Q.; de Souza, I.A.; Pegoraro, R.F.; Ferreira, E.A. Soil Carbon and Nitrogen Stocks and the Quality of Soil Organic Matter under Silvopastoral Systems in the Brazilian Cerrado. Soil Tillage Res. 2021, 205, 104785. [Google Scholar] [CrossRef]
- Tracy, B.F.; Zhang, Y. Soil Compaction, Corn Yield Response, and Soil Nutrient Pool Dynamics within an Integrated Crop-Livestock System in Illinois. Crop Sci. 2008, 48, 1211–1218. [Google Scholar] [CrossRef]
- Gentry, L.E.; Below, F.E.; David, M.B.; Bergerou, J. Source of the Soybean N Credit in Maize Production. Plant Soil 2001, 236, 175–184. [Google Scholar] [CrossRef]
- Lal, R. Carbon Management in Agricultural Soils. Mitig. Adapt. Strateg. Glob. Change 2007, 12, 303–322. [Google Scholar] [CrossRef]
- Liu, X.-J.A.; Sun, J.; Mau, R.L.; Finley, B.K.; Compson, Z.G.; van Gestel, N.; Brown, J.R.; Schwartz, E.; Dijkstra, P.; Hungate, B.A. Labile Carbon Input Determines the Direction and Magnitude of the Priming Effect. Appl. Soil Ecol. 2017, 109, 7–13. [Google Scholar] [CrossRef]
- Gazolla, P.R.; Guareschi, R.F.; Perin, A.; Pereira, M.G.; Rossi, C.Q. Frações Da Matéria Orgânica Do Solo Sob Pastagem, Sistema Plantio Direto e Integração Lavoura-Pecuária. Semin. Ciências Agrárias 2015, 36, 693. [Google Scholar] [CrossRef][Green Version]
- Ojima, D.S.; Schimel, D.S.; Parton, W.J.; Owensby, C.E. Long- and Short-Term Effects of Fire on Nitrogen Cycling in Tallgrass Prairie. Biogeochemistry 1994, 24, 67–84. [Google Scholar] [CrossRef]
- Boakye, M.K.; Little, I.T.; Panagos, M.D.; Jansen, R. Effects of Burning and Grazing on Plant Species Percentage Coverand Habitat Condition in the Highland Grassland of Mpumalanga Province, South Africa. J. Anim. Plant Sci. 2013, 23, 603–610. [Google Scholar]
- Abdalla, K.; Chivenge, P.; Everson, C.; Mathieu, O.; Thevenot, M.; Chaplot, V. Long-Term Annual Burning of Grassland Increases CO2 Emissions from Soils. Geoderma 2016, 282, 80–86. [Google Scholar] [CrossRef]
- Manson, A.; Jewitt, D.; Short, A. Effects of Season and Frequency of Burning on Soils and Landscape Functioning in a Moist Montane Grassland. Afr. J. Range Forage Sci. 2007, 24, 9–18. [Google Scholar] [CrossRef]
- Roscoe, R.; Buurman, P.; Velthorst, E.; Vasconcellos, C. Soil Organic Matter Dynamics in Density and Particle Size Fractions as Revealed by the 13C/12C Isotopic Ratio in a Cerrado’s Oxisol. Geoderma 2001, 104, 185–202. [Google Scholar] [CrossRef]
- Corbeels, M.; Scopel, E.; Cardoso, A.; Bernoux, M.; Douzet, J.; Neto, M.S. Soil Carbon Storage Potential of Direct Seeding Mulch-based Cropping Systems in the Cerrados of Brazil. Glob. Change Biol. 2006, 12, 1773–1787. [Google Scholar] [CrossRef]
- Jantalia, C.P.; Resck, D.V.S.; Alves, B.J.R.; Zotarelli, L.; Urquiaga, S.; Boddey, R.M. Tillage Effect on C Stocks of a Clayey Oxisol under a Soybean-Based Crop Rotation in the Brazilian Cerrado Region. Soil Tillage Res. 2007, 95, 97–109. [Google Scholar] [CrossRef]
- Liu, M.; Ussiri, D.A.N.; Lal, R. Soil Organic Carbon and Nitrogen Fractions under Different Land Uses and Tillage Practices. Commun. Soil Sci. Plant Anal. 2016, 47, 1528–1541. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Leifeld, J.; Heiling, M.; Hajdas, I. Age and Thermal Stability of Particulate Organic Matter Fractions Indicate the Presence of Black Carbon in Soil. Radiocarbon 2015, 57, 99–107. [Google Scholar] [CrossRef]
- Cusack, D.F.; Torn, M.S.; McDowell, W.H.; Silver, W.L. The Response of Heterotrophic Activity and Carbon Cycling to Nitrogen Additions and Warming in Two Tropical Soils. Glob. Change Biol. 2010, 16, 2555–2572. [Google Scholar] [CrossRef]
- Knicker, H.; Nikolova, R.; Dick, D.P.; Dalmolin, R.S.D. Alteration of Quality and Stability of Organic Matter in Grassland Soils of Southern Brazil Highlands after Ceasing Biannual Burning. Geoderma 2012, 181, 11–21. [Google Scholar] [CrossRef]
- Hilscher, A.; Knicker, H. Degradation of Grass-Derived Pyrogenic Organic Material, Transport of the Residues within a Soil Column and Distribution in Soil Organic Matter Fractions during a 28month Microcosm Experiment. Org. Geochem. 2011, 42, 42–54. [Google Scholar] [CrossRef]
- Corazza, E.J.; Silva, J.E.; Resck, D.V.S.; Gomes, A.C. Comportamento de Diferentes Sistemas de Manejo Como Fonte Ou Depósito de Carbono Em Relação à Vegetação de Cerrado. Rev. Bras. Ciência do Solo 1999, 23, 425–432. [Google Scholar] [CrossRef]
- Braz, S.P.; Urquiaga, S.; Alves, B.J.; Jantalia, C.P.; Guimarães, A.P.; dos Santos, C.A.; dos Santos, S.C.; Machado Pinheiro, É.F.; Boddey, R.M. Soil Carbon Stocks under Productive and Degraded Brachiaria Pastures in the Brazilian Cerrado. Soil Sci. Soc. Am. J. 2013, 77, 914–928. [Google Scholar] [CrossRef]
- Man, M.; Deen, B.; Dunfield, K.E.; Wagner-Riddle, C.; Simpson, M.J. Altered Soil Organic Matter Composition and Degradation after a Decade of Nitrogen Fertilization in a Temperate Agroecosystem. Agric. Ecosyst. Environ. 2021, 310, 107305. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Kirkegaard, J.A.; Richardson, A.E.; Wade, L.J.; Blanchard, C.; Batten, G. Stable Soil Organic Matter: A Comparison of C:N:P:S Ratios in Australian and Other World Soils. Geoderma 2011, 163, 197–208. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-Nutrient Stoichiometry to Increase Soil Carbon Sequestration. Soil Biol. Biochem. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Soil Carbon Sequestration in Prairie Grasslands Increased by Chronic Nitrogen Addition. Ecology 2012, 93, 2030–2036. [Google Scholar] [CrossRef]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.M.; de Moraes, J.C.; Albrecht, A. Soil Organic Matter, Biota and Aggregation in Temperate and Tropical Soils—Effects of No-Tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- Awoonor, J.K.; Adiyah, F.; Dogbey, B.F. Land-Use Change on Soil C and N Stocks in the Humid Savannah Agro-Ecological Zone of Ghana. J. Environ. Prot. 2022, 13, 32–68. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Clim. 2019, 1, 482133. [Google Scholar] [CrossRef]
- Mattila, T.J.; Vihanto, N. Agricultural Limitations to Soil Carbon Sequestration: Plant Growth, Microbial Activity, and Carbon Stabilization. Agric. Ecosyst. Environ. 2024, 367, 108986. [Google Scholar] [CrossRef]
- Kroschewski, B.; Richter, C.; Baumecker, M.; Kautz, T. Effect of Crop Rotation and Straw Application in Combination with Mineral Nitrogen Fertilization on Soil Carbon Sequestration in the Thyrow Long-Term Experiment Thy_D5. Plant Soil 2023, 488, 121–136. [Google Scholar] [CrossRef]
- Freixo, A.A.; Canellas, L.P.; Machado, P.L.O.A. Propriedades Espectrais Da Matéria Orgânica Leve-Livre e Leve Intra-Agregado de Dois Latossolos Sob Plantio Direto e Preparo Convencional. Rev. Bras. Ciência do Solo 2002, 26, 445–453. [Google Scholar] [CrossRef]
- Cerli, C.; Celi, L.; Kalbitz, K.; Guggenberger, G.; Kaiser, K. Separation of Light and Heavy Organic Matter Fractions in Soil—Testing for Proper Density Cut-off and Dispersion Level. Geoderma 2012, 170, 403–416. [Google Scholar] [CrossRef]
- Bieluczyk, W.; Pereira, M.G.; Guareschi, R.F.; Bonetti, J.A.; Freó, V.A.; Silva Neto, E.C. Granulometric and Oxidizable Carbon Fractions of Soil Organic Matter in Crop-Livestock Integration Systems. Semin. Ciências Agrárias 2017, 38, 607. [Google Scholar] [CrossRef]
- Loss, A.C.; Pereira, M.G.; Beutler, S.J.; Perin, A.; Piccolo, M.D.C.; Assunção, S.A.; Zonta, E. The Impact of Agricultural Systems in the Soil Organic Matter Content in Brazilian Cerrado. Int. J. Res. Granthaalayah 2019, 7, 220–244. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of Soil Organic Matter via Biochemical and Physical Pathways of Litter Mass Loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating Plant Litter Quality, Soil Organic Matter Stabilization, and the Carbon Saturation Concept. Glob. Change Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [PubMed]






| LUM | Depth | pH | Al | Ca | Mg | K | P |
|---|---|---|---|---|---|---|---|
| H2O | cmolc dm3 | mg dm−3 | |||||
| PM | 0–5 | 6.35 | 0.00 | 4.10 | 2.17 | 54.54 | 1.40 |
| 5–10 | 6.21 | 0.00 | 3.67 | 1.32 | 35.00 | 0.40 | |
| 10–20 | 6.12 | 0.00 | 2.60 | 0.96 | 21.88 | 0.17 | |
| 20–30 | 5.85 | 0.00 | 1.44 | 0.66 | 17.38 | 0.11 | |
| ICL | 0–5 | 5.80 | 0.01 | 6.11 | 2.13 | 67.04 | 30.04 |
| 5–10 | 5.81 | 0.01 | 4.42 | 1.62 | 37.51 | 19.20 | |
| 10–20 | 5.52 | 0.03 | 2.69 | 0.83 | 22.74 | 3.17 | |
| 20–30 | 4.96 | 0.14 | 1.48 | 0.57 | 20.46 | 0.30 | |
| MT | 0–5 | 5.64 | 0.00 | 4.08 | 1.14 | 41.95 | 31.83 |
| 5–10 | 5.63 | 0.02 | 3.50 | 1.16 | 25.40 | 25.43 | |
| 10–20 | 5.17 | 0.11 | 2.21 | 0.54 | 28.58 | 9.44 | |
| 20–30 | 4.96 | 0.17 | 1.31 | 0.37 | 29.21 | 2.30 | |
| NT | 0–5 | 6.08 | 0.00 | 5.21 | 1.68 | 66.23 | 33.34 |
| 5–10 | 6.26 | 0.00 | 4.62 | 1.60 | 52.12 | 38.20 | |
| 10–20 | 5.76 | 0.00 | 2.92 | 0.92 | 39.30 | 9.54 | |
| 20–30 | 5.06 | 0.18 | 1.29 | 0.67 | 32.96 | 3.24 | |
| NC | 0–5 | 4.51 | 0.84 | 0.29 | 0.23 | 39.79 | 0.01 |
| 5–10 | 4.52 | 0.69 | 0.06 | 0.09 | 24.79 | 0.01 | |
| 10–20 | 4.65 | 0.49 | 0.02 | 0.05 | 20.34 | 0.01 | |
| 20–30 | 4.48 | 0.63 | 0.03 | 0.06 | 33.30 | 0.04 | |
| Depth (cm) | PM | ICL | MT | NT | NC |
|---|---|---|---|---|---|
| 0–5 | 1.01 ± 0.026 | 0.97 ± 0.016 | 1.14 ± 0.025 | 1.08 ± 0.038 | 0.76 ± 0.035 |
| 5–10 | 1.11 ± 0.022 | 1.17 ± 0.041 | 1.11 ± 0.021 | 1.03 ± 0.039 | 1.00 ± 0.046 |
| 10–20 | 1.07 ± 0.016 | 1.11 ± 0.028 | 1.12 ± 0.019 | 1.10 ± 0.014 | 0.95 ± 0.035 |
| 20–30 | 1.19 ± 0.024 | 1.13 ± 0.039 | 1.04 ± 0.016 | 1.14 ± 0.052 | 1.10 ± 0.032 |
| Depth (cm) | PM | ICL | MT | NT | NC |
|---|---|---|---|---|---|
| 2001 | |||||
| 0–5 | 24.09 ± 0.66 | 28.13 ± 1.11 | 25.22 ± 0.59 | 25.47 ± 0.80 | 29.67 ± 0.45 |
| 5–10 | 22.61 ± 0.63 ab | 24.89 ± 0.46 ab | 24.12 ± 0.29 ab | 22.39 ± 0.40 b | 26.62 ± 0.51a |
| 10–20 | 20.30 ± 0.71 | 22.00 ± 0.14 | 22.33 ± 0.58 | 21.84 ± 0.21 | 20.41 ± 0.39 |
| 20–30 | 14.44 ± 0.31 d | 18.67 ± 0.31 ab | 15.90 ± 0.52 cd | 20.41 ± 0.22 a | 17.68 ± 0.34 abc |
| 2009 | |||||
| 0–5 | 30.48 ± 0.93 b | 36.88 ± 0.24 a | 24.13 ± 0.32 c | 36.88 ± 0.24 a | 29.01 ± 0.51 b |
| 5–10 | 24.25 ± 0.88 | 25.53 ± 0.46 | 24.45 ± 0.24 | 25.53 ± 0.46 | 25.18 ± 0.58 |
| 10–20 | 21.31 ± 0.75 | 21.90 ± 0.61 | 22.05 ± 0.14 | 21.13 ± 0.65 | 21.43 ± 0.33 |
| 20–30 | 17.10 ± 0.53 | 20.45 ± 1.24 | 17.45 ± 0.34 | 21.20 ± 1.17 | 16.47 ± 0.19 |
| 2013 | |||||
| 0–5 | 28.05 ± 0.78 bc | 37.78 ± 0.52 a | 23.30 ± 1.18 c | 31.15 ± 0.75 b | 37.58 ± 0.46 a |
| 5–10 | 23.86 ± 0.39 ab | 26.17 ± 0.45 ab | 23.97 ± 0.49 ab | 22.65 ± 0.24 b | 30.03 ± 1.61 a |
| 10–20 | 21.65 ± 1.32 | 22.83 ± 0.20 | 21.29 ± 0.37 | 20.95 ± 0.25 | 21.39 ± 0.36 |
| 20–30 | 14.65 ± 1.33 | 19.73 0.44 | 17.80 ± 0.35 | 18.19 ± 0.82 | 18.04 ± 0.43 |
| 2023 | |||||
| 0–5 | 35.53 ± 0.25 ab | 39.53 ± 0.51 a | 24.83 ± 0.04 c | 36.02 ± 0.96 ab | 32.54 ± 0.64 b |
| 5–10 | 22.82 ± 0.58 | 24.80 ± 0.75 | 22.69 ± 0.25 | 23.32 ± 0.42 | 27.00 ± 0.17 |
| 10–20 | 17.22 ± 0.60 b | 23.57 ± 0.77 a | 18.91 ± 0.40 ab | 20.60 ± 0.20 ab | 22.24 ± 0.28 a |
| 20–30 | 14.32 ± 0.73 b | 18.39 ± 0.30 ab | 15.96 ± 0.35 ab | 17.63 ± 0.44 ab | 20.18 ± 0.21 a |
| Depth (cm) | PM | ICL | MT | NT | NC |
|---|---|---|---|---|---|
| 2001 | |||||
| 0–5 | 1.33 ± 0.04 | 1.68 ± 0.06 | 1.37 ± 0.04 | 1.39 ± 0.05 | 1.61 ± 0.04 |
| 5–10 | 1.25 ± 0.02 | 1.41 ± 0.03 | 1.27 ± 0.01 | 1.20 ± 0.02 | 1.39 ± 0.05 |
| 10–20 | 1.05 ± 0.03 | 1.11 ± 0.01 | 1.18 ± 0.03 | 1.19 ± 0.02 | 1.05 ± 0.03 |
| 20–30 | 0.84 ± 0.02 c | 1.00 ± 0.01 ab | 0.84 ± 0.03 c | 1.07 ± 0.01 a | 0.92 ± 0.01 bc |
| 2009 | |||||
| 0–5 | 1.36 ± 0.07 c | 2.27 ± 0.02 a | 1.48 ± 0.02 c | 1.97 ± 0.06 ab | 1.68 ± 0.03 bc |
| 5–10 | 1.44 ± 0.03 | 1.52 ± 0.01 | 1.30 ± 0.01 | 1.37 ± 0.03 | 1.42 ± 0.03 |
| 10–20 | 1.25 ±0.04 | 1.29 ± 0.02 | 1.14 ± 0.01 | 1.20 ± 0.01 | 1.20 ± 0.02 |
| 20–30 | 0.95 ± 0.03 ab | 1.12 ± 0.03 a | 0.92 ± 0.02 b | 1.09 ± 0.02 ab | 0.99 ±0.01 ab |
| 2013 | |||||
| 0–5 | 1.66 ± 0.05 b | 2.71 ± 0.04 a | 2.45 ± 0.01 b | 2.25 ± 0.08 a | 2.25 ± 0.07 a |
| 5–10 | 1.43 ± 0.02 | 1.58 ± 0.02 | 1.38 ± 0.01 | 1.36 ± 0.07 | 1.75 ± 0.06 |
| 10–20 | 1.24 ± 0.07 | 1.35 ± 0.02 | 1.14 ± 0.02 | 1.20 ± 0.08 | 1.16 ± 0.06 |
| 20–30 | 0.91 ± 0.03 | 1.15 ± 0.02 | 0.93 ± 0.01 | 1.00 ± 0.04 | 0.95 ± 0.04 |
| 2023 | |||||
| 0–5 | 1.98 ± 0.06 | 2.97 ± 0.05 | 2.08 ± 0.06 | 2.67 ± 0.10 | 2.01 ± 0.10 |
| 5–10 | 1.52 ± 0.05 b | 1.74 ± 0.04 a | 1.46 ± 0.05 b | 1.75 ± 0.08 b | 1.94 ± 0.09 ab |
| 10–20 | 1.10 ± 0.04 | 1.63 ± 0.11 | 1.24 ± 0.05 | 1.15 ± 0.05 | 1.35 ± 0.03 |
| 20–30 | 0.81 ± 0.03 | 1.69 ± 0.07 | 0.90 ± 0.06 | 0.88 ± 0.05 | 1.02 ± 0.04 |
| Depth (cm) | PM | ICL | MT | NT | NC | CV |
|---|---|---|---|---|---|---|
| C-POM (g kg−1 of soil) | % | |||||
| 0–5 | 2.31 b | 3.12 a | 1.26 b | 1.19 b | 1.74 b | 31.16 |
| 5–10 | 1.83 | 1.82 | 2.14 | 2.02 | 1.30 | 30.40 |
| 10–20 | 1.49 | 1.38 | 1.46 | 1.31 | 1.15 | 27.39 |
| 20–30 | 0.90 | 1.13 | 0.94 | 0.82 | 0.79 | 27.86 |
| N-POM (g kg−1 of soil) | ||||||
| 0–5 | 0.06 b | 0.17 a | 0.06 b | 0.04 b | 0.07 b | 41.74 |
| 5–10 | 0.04 | 0.08 | 0.12 | 0.10 | 0.05 | 41.98 |
| 10–20 | 0.03 ab | 0.06 a | 0.06 ab | 0.05 ab | 0.04 b | 21.21 |
| 20–30 | 0.02 | 0.04 | 0.03 | 0.03 | 0.03 | 39.17 |
| C:N ratio-POM | ||||||
| 0–5 | 39.64 a | 18.12 b | 23.85 b | 32.59 a | 23.65 b | 13.88 |
| 5–10 | 40.99 a | 21.81 c | 18.22 c | 20.27 c | 27.37 b | 9.08 |
| 10–20 | 38.69 a | 22.15 c | 23.93 c | 25.54 bc | 31.09 b | 10.72 |
| 20–30 | 40.18 a | 26.53 b | 27.46 b | 32.04 ab | 30.75 ab | 15.01 |
| C-MOAM (g kg−1 of soil) | ||||||
| 0–5 | 33.21 a | 36.41 a | 23.57 b | 34.82 a | 30.80 a | 7.94 |
| 5–10 | 20.99 b | 22.98 ab | 20.55 b | 21.28 b | 25.70 a | 8.42 |
| 10–20 | 15.72 c | 22.19 a | 17.44 bc | 19.28 abc | 21.09 ab | 10.66 |
| 20–30 | 13.41 b | 17.26 ab | 15.02 b | 16.80 ab | 19.38 a | 11.70 |
| N-MOAM (g kg−1 of soil) | ||||||
| 0–5 | 1.92 b | 2.79 a | 2.02 b | 2.63 ab | 1.92 b | 14.30 |
| 5–10 | 1.48 ab | 1.65 ab | 1.34 b | 1.64 ab | 1.89 a | 14.13 |
| 10–20 | 1.06 | 1.57 | 1.17 | 1.10 | 1.31 | 21.85 |
| 20–30 | 0.79 | 1.64 | 0.86 | 0.86 | 0.99 | 23.39 |
| C:N ratio in MOAM | ||||||
| 0–5 | 17.52 a | 13.06 bc | 11.79 c | 13.31 abc | 16.23 ab | 12.98 |
| 5–10 | 14.32 | 13.88 | 15.47 | 13.27 | 13.94 | 12.13 |
| 10–20 | 14.86 | 14.52 | 15.04 | 17.98 | 16.09 | 14.96 |
| 20–30 | 16.92 | 13.43 | 18.06 | 20.81 | 19.68 | 22.72 |
| 13C abundance of carbon in POM (‰) | ||||||
| 0–5 | −16.09 a | −17.09 b | −21.98 c | −22.43 c | −25.86 d | 2.98 |
| 5–10 | −17.65 a | −19.39 b | −20.35 b | −20.14 b | −25.05 c | 3.38 |
| 10–20 | −18.72 a | −20.30 ab | −21.95 b | −21.71 b | −24.89 c | 3.41 |
| 20–30 | −19.17 a | −21.21 b | −22.56 b | −21.97 b | −24.35 c | 3.09 |
| Proportion of C4 carbon in POM (%) | ||||||
| 0–5 | 72.73 a | 60.26 a | 30.95 b | 25.21 b | 0 | 38.37 |
| 5–10 | 58.47 | 45.60 | 37.38 | 41.09 | 0 | 44.07 |
| 10–20 | 48.32 a | 35.51 b | 23.29 b | 25.19 b | 0 | 25.47 |
| 20–30 | 43.33 a | 25.66 b | 15.96 bc | 20.73 bc | 0 | 40.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, S.; Souza, W.; Homem, B.; Ramalho, I.; Borré, J.; Pereira, M.; Pinheiro, É.; Marchao, R.; Alves, B.; Boddey, R.; et al. The Use of Integrated Crop–Livestock Systems as a Strategy to Improve Soil Organic Matter in the Brazilian Cerrado. Agronomy 2024, 14, 2547. https://doi.org/10.3390/agronomy14112547
Soares S, Souza W, Homem B, Ramalho I, Borré J, Pereira M, Pinheiro É, Marchao R, Alves B, Boddey R, et al. The Use of Integrated Crop–Livestock Systems as a Strategy to Improve Soil Organic Matter in the Brazilian Cerrado. Agronomy. 2024; 14(11):2547. https://doi.org/10.3390/agronomy14112547
Chicago/Turabian StyleSoares, Stallone, Wesley Souza, Bruno Homem, Israel Ramalho, João Borré, Marcos Pereira, Érika Pinheiro, Robelio Marchao, Bruno Alves, Robert Boddey, and et al. 2024. "The Use of Integrated Crop–Livestock Systems as a Strategy to Improve Soil Organic Matter in the Brazilian Cerrado" Agronomy 14, no. 11: 2547. https://doi.org/10.3390/agronomy14112547
APA StyleSoares, S., Souza, W., Homem, B., Ramalho, I., Borré, J., Pereira, M., Pinheiro, É., Marchao, R., Alves, B., Boddey, R., & Urquiaga, S. (2024). The Use of Integrated Crop–Livestock Systems as a Strategy to Improve Soil Organic Matter in the Brazilian Cerrado. Agronomy, 14(11), 2547. https://doi.org/10.3390/agronomy14112547






