Growing Media pH and Nutrient Concentrations for Fostering the Propagation and Production of Lingonberry (Vaccinium vitis-idaea L.)
Abstract
1. Introduction
2. Materials and Methods
3. Results
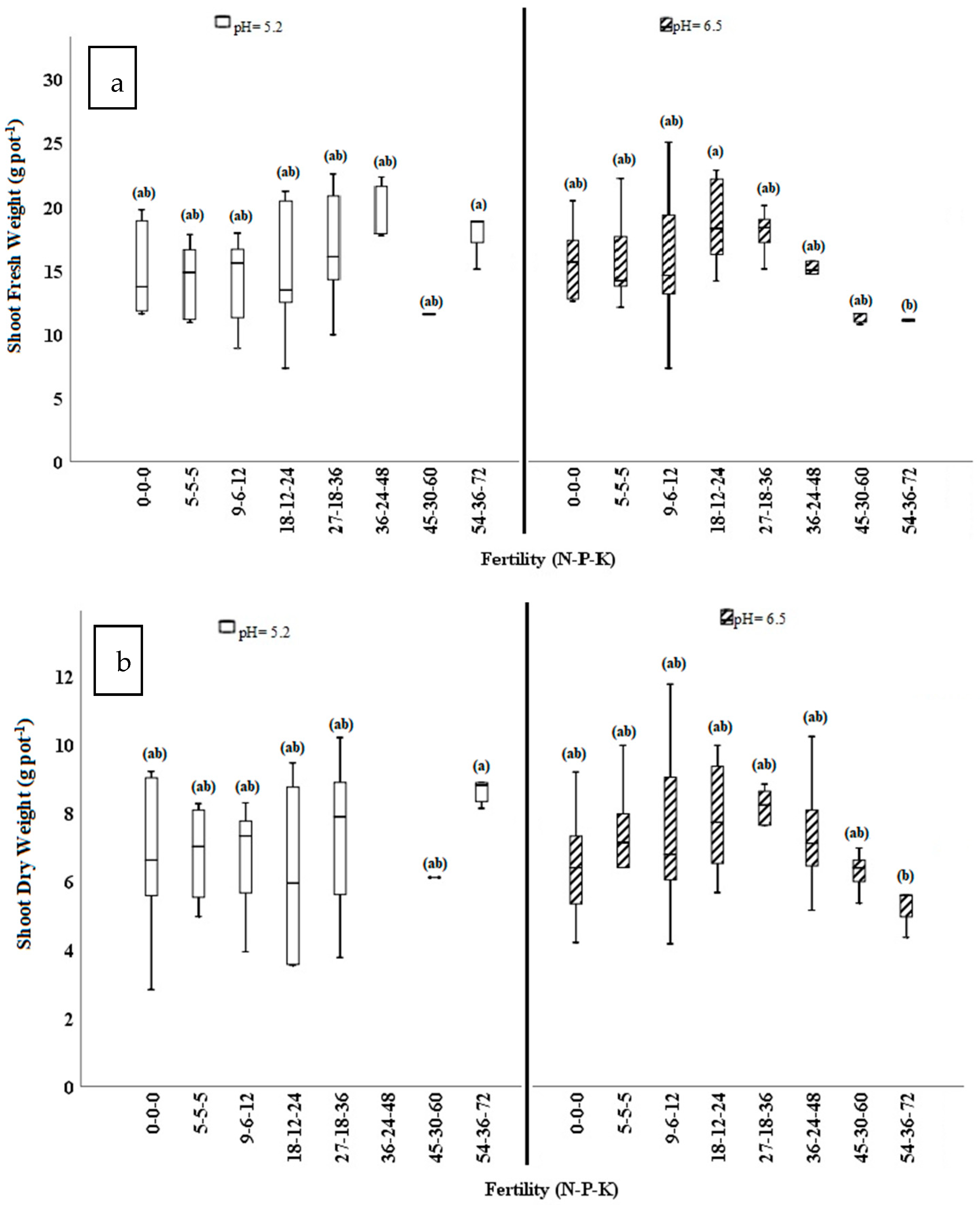
3.1. Plant Parameters
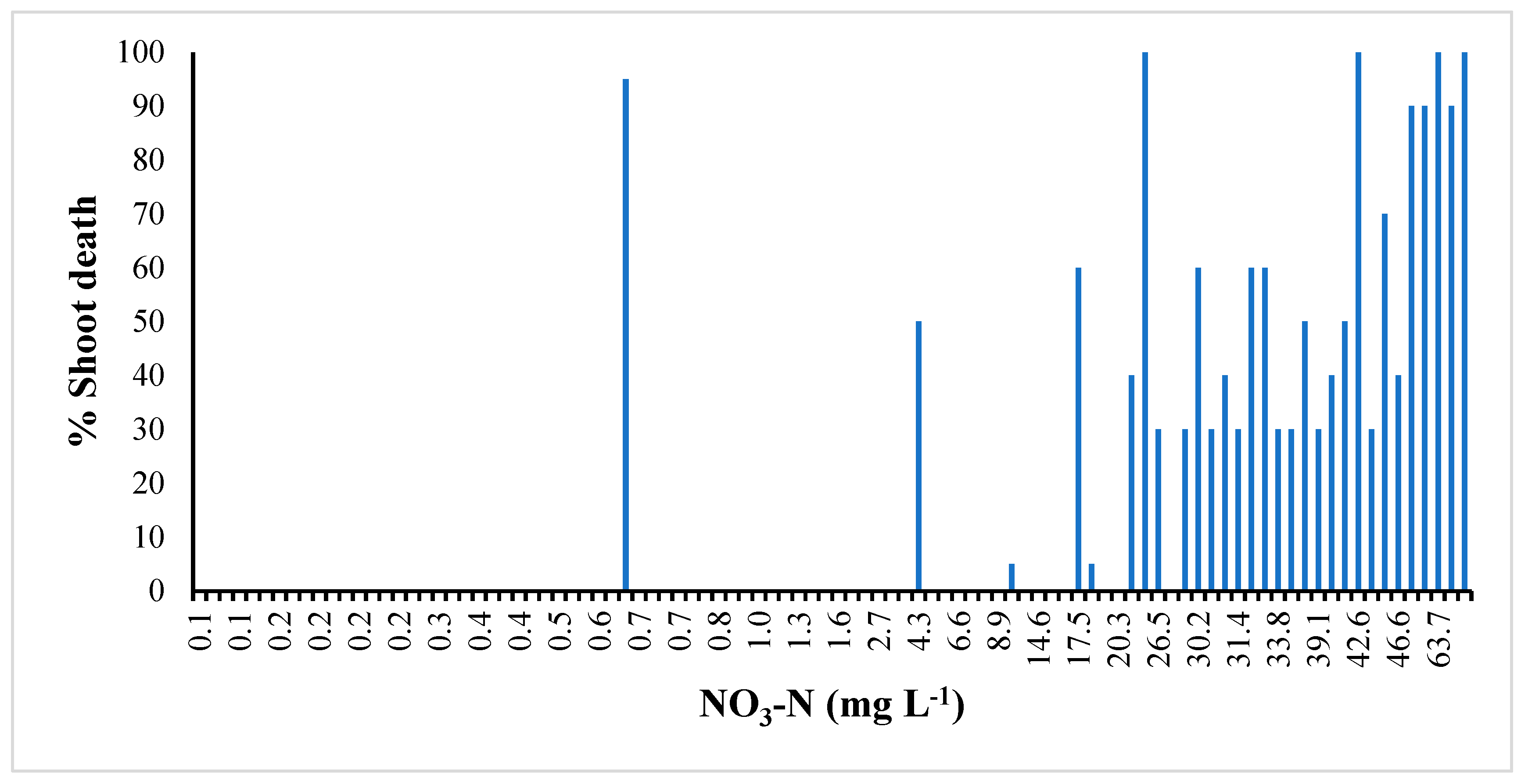
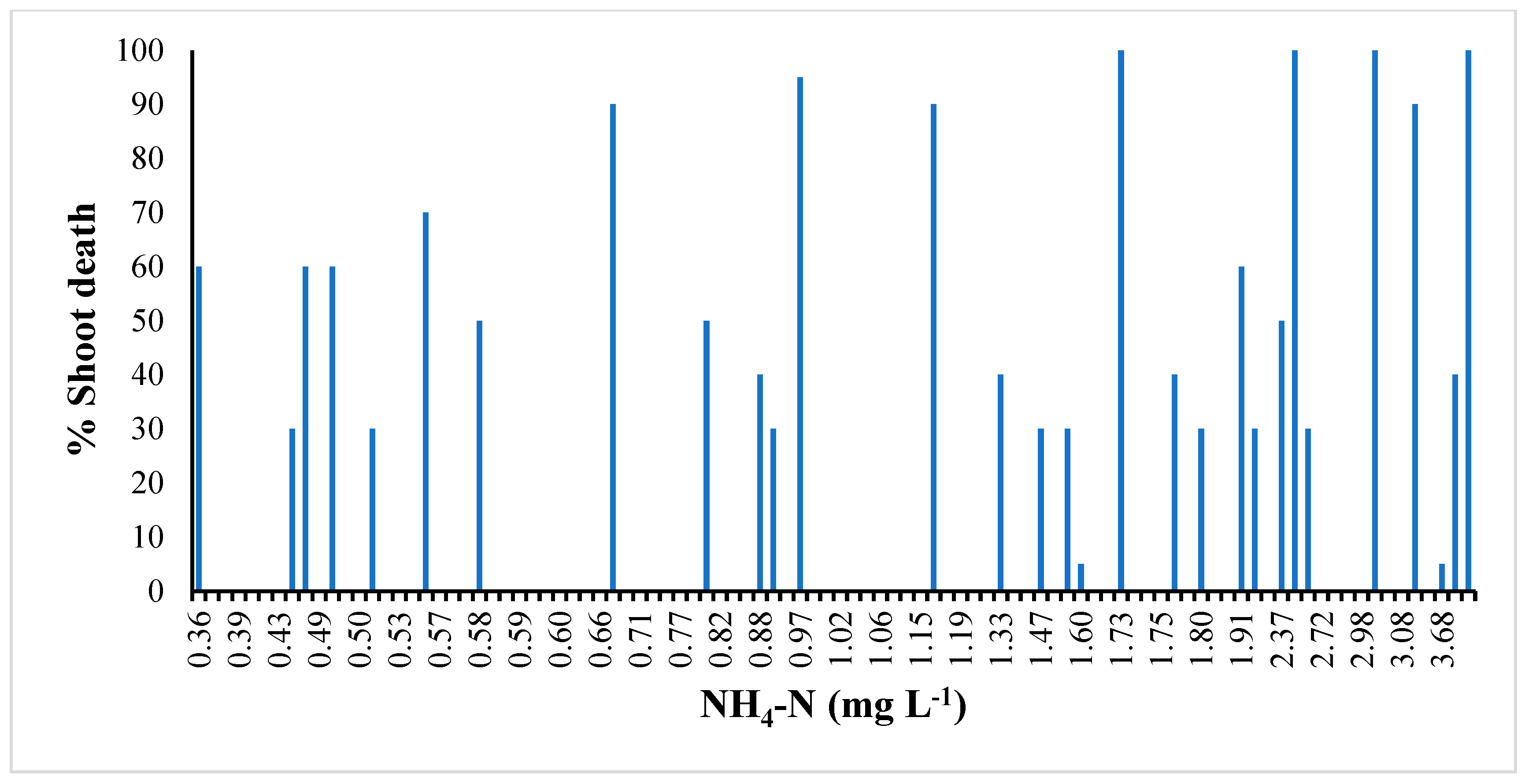
3.2. Growing Media Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Olsen, C. Studies on the hydrogen ion concentration of the soil and its significance to the vegetation, especially to the natural distribution of plants. CR Lab Carlsberg. 1921, 15, 1–160. [Google Scholar]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) fruit as a source of bioactive compounds with health-promoting effects—A review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef] [PubMed]
- Opal Consulting Inc. Labrador Wild Berry Strategic Development Plan. A Strategy to Expand the Capacity of the Wild Berry Industry in Southern Labrador: Opal Consulting Inc. and P & G Management Services; Sep. 2010. p. 86. Available online: http://nakn.ca/data/4/wildberry_study-zone_4_and_5.pdf (accessed on 22 March 2024).
- Debnath, S.C. Genetic diversity and erosion in berries. In Sustainable Development and Biodiversity; Ahuja, M.R., Jain, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 8. [Google Scholar]
- Dróżdż, P.; Šėžienė, V.; Wójcik, J.; Pyrzyńska, K. Evaluation of bioactive compounds, minerals and antioxidant activity of lingonberry (Vaccinium vitis-idaea L.) fruits. Molecules 2017, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Zielińska-Wasielica, J.; Olkowicz, M. Inhibitory effects of lingonberry (Vaccinium vitis-idaea L.) fruit extract on obesity-induced inflammation in 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Funct. Foods 2019, 54, 371–380. [Google Scholar] [CrossRef]
- Debnath, S.C.; Arigundam, U. In vitro propagation strategies of medicinally important berry crop, lingonberry (Vaccinium vitis-idaea L.). Agronomy 2020, 10, 744. [Google Scholar] [CrossRef]
- Foley, S.L.; Debnath, S.C. Influence of in vitro and ex vitro propagation on anthocyanin content and anti-oxidant activity of lingonberries. J. Hortic. Sci. Biotechnol. 2007, 82, 114–118. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Reich, L. Lingonberry: Dainty Looks, Sturdy Disposition, and Tasty Berries. Arnoldia 2004, 63, 18–25. [Google Scholar] [CrossRef]
- Penhallegon, R.H. Lingonberry Production Guide for the Pacific Northwest: Oregon State University Extension Bulletin Number PNW 583- E; Oregon State University: Corvallis, OR, USA, 2006. [Google Scholar]
- Gustavsson, B. Lingonberry breeding and cultivation (Vaccinium vitis-idaea L.). Acta Hortic. 1993, 346, 311–313. [Google Scholar] [CrossRef]
- Eeva, T.; Holmström, H.; Espín, S.; Sánchez-Virosta, P.; Klemola, T. Leaves, berries and herbivorous larvae of bilberry Vaccinium myrtillus as sources of metals in food chains at a Cu-Ni smelter site. Chemosphere 2018, 210, 859–866. [Google Scholar] [CrossRef]
- Grelet, G.A.; Alexander, I.J.; Proe, M.F.; Frossard, J.S.; Millard, P. Leaf habit influences nitrogen remobilization in Vaccinium species. J. Exp. Bot. 2001, 52, 993–1002. [Google Scholar] [CrossRef]
- Karlsons, A.; Tomsone, S.; Lazdāne, M.; Osvalde, A. Effect of fertilization on growth of lingonberry (Vaccinium vitis-idaea L.). Agron. Res. 2021, 19, 1039–1051. [Google Scholar] [CrossRef]
- Nestby, R.; Krogstad, T.; Joner, E.; Vohnik, M. The effect of NP fertilization on European blueberry (Vaccinium myrtillus L.) development on cultivated land in mid-Norway. J. Berry Res. 2014, 4, 147–157. [Google Scholar] [CrossRef]
- Bujor, O.C.; Ginies, C.; Popa, V.I.; Dufour, C. Phenolic compounds and antioxidant activity of lingonberry (Vaccinium vitis-idaea L.) leaf, stem and fruit at different harvest periods. Food Chem. 2018, 252, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Havas, P.; Kubin, E. Structure, growth and organic matter content in the vegetation cover of an old spruce forest in northern Finland. Ann. Bot. Fenn. 1983, 20, 115–149. [Google Scholar]
- Lähdesmäki, P.; Pakonen, T.; Saari, E.; Laine, K.; Tasanen, L.; Havas, P. Changes in total nitrogen, protein, amino acids and NH4+ in tissues of bilberry, Vaccinium myrtillus, during the growing season. Ecography 1990, 13, 31–38. [Google Scholar] [CrossRef]
- Lehmushovi, A. Trials with the cowberry in Finland. Acta Hortic. 1976, 61, 301–308. [Google Scholar] [CrossRef]
- Trajkovski, V. Facts about lingonberries (cowberries, partridgeberries). Fruit Var. J. 1987, 41, 39. [Google Scholar]
- Holloway, P.S. Studies on Vegetative and Reproductive Growth of Lingonberry, Vaccinium vitis-idaea L. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 1983. [Google Scholar]
- Gustavsson, B.A. Genetic variation in horticulturally important traits of fifteen wild lingonberry Vaccinium vitis-idaea L. populations. Euphytica 2001, 120, 173–182. [Google Scholar] [CrossRef]
- Debnath, S.C.; Keske, C. Technological advances in the propagation and improvement of Newfoundland and Labrador berries. In Food Futures: Growing a Sustainable Food System for Newfoundland and Labrador; Memorial University Press: St. John's, NL, Canada, 2018; pp. 381–409. [Google Scholar]
- Ross, K.A.; Godfrey, D.; Fukumoto, L. The chemical composition, antioxidant activity and α-glucosidase inhibitory activity of water-extractable polysaccharide conjugates from northern Manitoba lingonberry. Cogent Food Agric. 2015, 1, 1109781. [Google Scholar] [CrossRef]
- Chester, A.L.; McGraw, J.B. Effects of nitrogen addition on the growth of Vaccinium uliginosum and Vaccinium vitis-idaea. Can. J. Bot. 1983, 61, 2316–2322. [Google Scholar] [CrossRef]
- Sharifi, M.; Salimi, K.; Rosa, D.; Hart, M. Screening Cover Crops for Utilization in Irrigated Vineyards: A Greenhouse Study on Species’ Nitrogen Uptake and Carbon Sequestration Potential. Plants 2024, 13, 1959. [Google Scholar] [CrossRef] [PubMed]
- Ingestad, T. Mineral nutrient requirements of Vaccinium vitis idaea and V. myrtillus. Physiol. Plant. 1973, 29, 239–246. [Google Scholar] [CrossRef]
- Teär, J.I. Vegetativ och Fruktifikativ Utveckling hos Vildväxande och Odlade Lingon; Alfa-Laval: Tumba, Sweden, 1972. [Google Scholar]
- Davenport, J.R. The effect of nitrogen fertilizer rates and timing on cranberry yield and fruit quality. J. Am. Soc. Hortic. Sci. 1996, 121, 1089–1094. [Google Scholar] [CrossRef]
- Read, D.J. The structure and function of the ericoid mycorrhizal root. Ann. Bot. 1996, 77, 365–374. [Google Scholar] [CrossRef]
- Mäkipää, R. Response patterns of Vaccinium myrtillus and V. vitis-idaea along nutrient gradients in boreal forest. J. Veg. Sci. 1999, 10, 17–26. [Google Scholar] [CrossRef]
- Mäkipää, R. Effects of nitrogen fertilization on the humus layer and ground vegetation under closed canopy in boreal coniferous stands. Silva Fenn. 1994, 28, 81–94. [Google Scholar] [CrossRef][Green Version]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Assosiates, Inc.: Sunderland, MA, USA, 2015. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Hendershot, W.; Lalande, H.; Duquette, M. Soil reaction and exchangeable acidity. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; Canadian Society of Soil Science, CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 8th ed.; Pearson Education India: Hoboken, NJ, USA, 2014. [Google Scholar]
- Scagel, C.F.; Schreiner, R.P. Changes in the Concentration of Nutrients and Irrigation Solution Electrical Conductivity during Container Production of Rhododendron and Blueberry. HortScience 2006, 41, 1793–1801. [Google Scholar]
- Rosen, C.J.; Bierman, P.M. Nutrient Management for Fruit and Vegetable Crop Production: Using Manure and Compost as Nutrient Sources for Vegetable Crops; University of Minnesota Extension: St Paul, MN, USA, 2005. [Google Scholar]
- Cain, J. Blueberry chlorosis in relation to leaf pH and mineral composition. Proc. Amer. Soc. Hort. Sci. 1954, 64, 61–70. [Google Scholar]
- Colgrove, M.S.; Roberts, A.N. Growth of the azalea as influenced by ammonium and nitrate nitrogen. Am. Soc. Hortic. Sci. 1956, 68, 522–536. [Google Scholar]
| Fertilizer Application Rate (15-Weeks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | N | P | K | Urea | H3PO3 1 | KCl | Urea | H3PO3 | KCl |
| kg ha−1 | kg ha−1 | L ha−1 | kg ha−1 | mg pot−1 | µL pot−1 | mg pot−1 | |||
| 0–0–0 (Control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5–5–5 | 5 | 5 | 5 | 11 | 18.6 | 8 | 8.9 | 14.9 | 6.7 |
| 9–6–12 | 9 | 6 | 12 | 20 | 22.3 | 20 | 16.0 | 17.9 | 16.0 |
| 18–12–24 | 18 | 12 | 24 | 40 | 44.7 | 40 | 32.0 | 35.7 | 32.0 |
| 27–18–36 | 27 | 18 | 36 | 60 | 67.0 | 60 | 48.0 | 53.6 | 48.0 |
| 36–24–48 (Standard) | 36 | 24 | 48 | 80 | 89.3 | 80 | 64.0 | 71.5 | 64.0 |
| 45–30–60 | 45 | 30 | 60 | 100 | 112 | 100 | 80.0 | 89.3 | 80.0 |
| 54–36–72 | 54 | 36 | 72 | 120 | 134 | 120 | 96.0 | 107 | 96.0 |
| Treatment | Plant Height | Growth Volume | Shoot Fresh Biomass | Shoot Dry Biomass | Root Fresh Biomass | Root Dry Biomass | Root-to-Shoot Ratio | Total Fresh Biomass | Total Dry Biomass | Growing Media EC1:20 1 Post-Harvest | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | cm3 | g | g | g | g | g | g | g | dS m−1 | ||
| pH | |||||||||||
| 5.2 | 4.46 | 370 | 15.4 | 7.16 | 102 | 11.3 | 1.73 | 118 | 18.5 | 0.567 | |
| 6.5 | 6.09 | 501 | 15.3 | 6.95 | 97.7 | 11.5 | 1.65 | 114 | 18.2 | 0.673 | |
| Fertility (kg ha−1) | |||||||||||
| 0–0–0 | 4.68 | 425 ab 2 | 15.3 ab | 6.54 | 131 a | 15.3 a | 2.56 a | 146 a | 21.8 | 0.318 c | |
| 5–5–5 | 4.79 | 431 ab | 15.01 ab | 6.90 | 95.3 ab | 11.1 ab | 1.62 ab | 110 ab | 18.0 | 0.341 c | |
| 9–6–12 | 6.07 | 438 ab | 15 ab | 7.05 | 92.0 ab | 10.7 ab | 1.52 b | 107 ab | 17.7 | 0.345 c | |
| 18–12–24 | 5.68 | 622 a | 16.7 a | 6.99 | 109 ab | 12.2 ab | 1.88 ab | 128 ab | 18.9 | 0.294 c | |
| 27–18–36 | 6.28 | 468 ab | 17.3 a | 7.48 | 108 ab | 12.1 ab | 1.65 ab | 123 ab | 19.1 | 0.417 c | |
| 36–24–48 | 5.43 | 479 ab | 17.0 a | 7.99 | 111 ab | 10.8 ab | 1.43 b | 129 ab | 18.9 | 0.719 bc | |
| 45–30–60 | 3.71 | 245 b | 11.6 b | 6.23 | 79.8 b | 8.79 b | 1.38 b | 91.5 b | 15.0 | 1.11 ab | |
| 54–36–72 | 5.43 | 394 ab | 14.9 ab | 7.21 | 74.5 b | 9.79 ab | 1.44 b | 97.3 ab | 16.9 | 1.41 a | |
| SEM 3 | 0.271 | 28.2 | 0.416 | 0.189 | 4.06 | 0.498 | 0.081 | 4.17 | 0.617 | 0.058 | |
| Source of variation | df | p Value | |||||||||
| pH | 1 | 0.003 | 0.011 | 0.920 | 0.535 | 0.713 | 0.771 | 0.668 | 0.757 | 0.945 | 0.203 |
| Fertility | 7 | 0.271 | 0.055 | 0.007 | 0.268 | 0.008 | 0.085 | 0.003 | 0.015 | 0.285 | 0.000 |
| pH × Fertility | 7 | 0.798 | 0.621 | 0.013 | 0.026 | 0.629 | 0.931 | 0.818 | 0.724 | 0.768 | 0.155 |
| Treatment | NH4+-N | NO3−-N | Plant Death Tissue | Shoots N | Shoots Carbon | Roots-N | Roots Carbon | |
|---|---|---|---|---|---|---|---|---|
| mg N L−1 | % | % | ||||||
| pH | ||||||||
| 5.2 | 1.33 | 13.7 | 11.8 | 1.29 | 49.2 | 1.02 | 41.6 | |
| 6.5 | 1.34 | 13.4 | 11.6 | 1.40 | 49.0 | 1.06 | 41.8 | |
| Fertility (kg ha−1) | ||||||||
| 0–0–0 | 0.91 b 1 | 2.00 c | 0.00 c | 1.05 d | 49.7 a | 0.862 c | 41.4 | |
| 5–5–5 | 1.31 ab | 1.26 c | 0.00 c | 1.11 d | 49.6 a | 0.941 abc | 41.6 | |
| 9–6–12 | 0.99 ab | 2.24 c | 7.50 abc | 1.17 d | 49.7 a | 1.03 abc | 42.6 | |
| 18–12–24 | 1.25 ab | 0.86 c | 0.00 c | 1.26 cd | 49.4 ab | 0.951 abc | 40.0 | |
| 27–18–36 | 1.39 ab | 5.38 c | 3.33 bc | 1.32 cd | 49.3 ab | 1.11 ab | 42.2 | |
| 36–24–48 | 1.20 ab | 21.7 b | 12.5 abc | 1.47 abc | 49.1 ab | 1.17 a | 41.7 | |
| 45–30–60 | 1.44 ab | 42.6 a | 34.6 ab | 1.74 a | 48.4 bc | 1.18 a | 42.1 | |
| 54–36–72 | 2.18 a | 32.3 ab | 35.4 a | 1.60 ab | 47.9 c | 1.09 ab | 41.9 | |
| SEM 2 | 1.103 | 1.97 | 2.82 | 0.032 | 0.108 | 0.018 | 0.285 | |
| Source of variation | df | p Value | ||||||
| pH | 1 | 0.955 | 0.926 | 0.968 | 0.023 | 0.270 | 0.145 | 0.721 |
| Fertility | 7 | 0.084 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.534 |
| pH × Fertility | 7 | 0.538 | 0.733 | 0.594 | 0.589 | 0.878 | 0.943 | 0.773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© His Majesty the King in Right of Canada, as represented by the Minister of Agriculture and Agri-Food, 2024. This material is licensed under the terms of the Canada Open Government Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided you acknowledge the source of the information or link to any attribution statement specified in the license. To view this license, visit https://open.canada.ca/en/open-government-licence-canada
Share and Cite
Sharifi, M.; Debnath, S.C.; Hajiaghaei-Kamrani, M.; Rabie, B.; Forsyth, J. Growing Media pH and Nutrient Concentrations for Fostering the Propagation and Production of Lingonberry (Vaccinium vitis-idaea L.). Agronomy 2024, 14, 2533. https://doi.org/10.3390/agronomy14112533
Sharifi M, Debnath SC, Hajiaghaei-Kamrani M, Rabie B, Forsyth J. Growing Media pH and Nutrient Concentrations for Fostering the Propagation and Production of Lingonberry (Vaccinium vitis-idaea L.). Agronomy. 2024; 14(11):2533. https://doi.org/10.3390/agronomy14112533
Chicago/Turabian StyleSharifi, Mehdi, Samir C. Debnath, Monireh Hajiaghaei-Kamrani, Bill Rabie, and Jillian Forsyth. 2024. "Growing Media pH and Nutrient Concentrations for Fostering the Propagation and Production of Lingonberry (Vaccinium vitis-idaea L.)" Agronomy 14, no. 11: 2533. https://doi.org/10.3390/agronomy14112533
APA StyleSharifi, M., Debnath, S. C., Hajiaghaei-Kamrani, M., Rabie, B., & Forsyth, J. (2024). Growing Media pH and Nutrient Concentrations for Fostering the Propagation and Production of Lingonberry (Vaccinium vitis-idaea L.). Agronomy, 14(11), 2533. https://doi.org/10.3390/agronomy14112533







