Abstract
Determination of the microbial and enzymatic properties in soil is primarily concentrated on the surface layers of the soil profiles; however, it is well known that the transformation of soil organic matter also occurs in the deeper horizons of the soil profile. The aim of this study was to assess any changes in specific sets of enzyme activities and their associated physicochemical properties as affected by two different agricultural land-use systems and soil depth. Changes in the studied properties were determined across four Luvisol profiles in two agricultural land uses (arable land and vineyards). The enzyme activities associated with the transformation of C, N and P were analyzed. Additionally, the activity of some oxidoreductases and the fluorescein diacetate hydrolysis (FDAH) rate were also determined. Moreover, the content of the various forms of soil carbon, nitrogen, phosphorus (including microbial biomass C, N and P) and some other properties (pH, clay and silt content) were assessed. Agricultural land use significantly affected the microbial biomass content and as well as the studied enzyme activities. Most of the studied enzymes exhibited a higher activity in the grapevine (GV) profiles, which was followed by the winter wheat (WW) profiles; however, the largest variability occurred for the urease activity. There was no clear differentiation between the two studied land uses for the activity of nitrate reductase, dehydrogenases, acid phosphatase, or endo- and exo-cellulase. Irrespective of the plant being cultivated, the soil variables decreased significantly with increasing soil depth, wherein the greatest changes were observed between the surface and sub-surface soil horizons (I–II). The activity of some enzymes (e.g., the urease activity in WW profiles) decreased gradually across the soil profiles, while others were located almost solely within the surface layers (e.g., the nitrate reductase activity in the GV profiles as well as invertase in the WW profiles). The α-glucosidase activity did not exhibit any statistically significant changes along the analyzed profiles. The activity of phenol oxidase and peroxidase also revealed different trends along the studied profiles compared to the other enzymes and did not decrease gradually with depth. The microbial biomass of the C, N and P content was generally the highest in the upper horizons and gradually decreased with depth, wherein the largest decrease was observed between the surface and sub-surface horizon. The studied enzyme activities were more dependent on the soil carbon content compared to the other soil properties. And thus, in the C-rich horizons (C > 4 g kg) for the surface and subsurface layers the enzyme activities were highly correlated with TOC, DOC and MBC content as compared to the deeper, C-low horizons (C < 4 g kg). By examining how the microbial and enzymatic properties change across the soil profiles, it is possible to gain valuable insight into the long-term biogeochemical processes that are involved in soil fertility and in the health of agricultural ecosystems.
1. Introduction
Soil enzymes, which mainly originate from microorganisms and plant roots, participate in many of the biochemical reactions that are associated with the different stages of soil organic matter (SOM) decomposition and as well as in the turnover of soil nutrients prior to their being made available for microorganisms and plants [1,2]. Because enzymes respond to changes in both natural and anthropogenic factors, such as soil use and management practices more rapidly than the other soil properties, they can be used as early indicators of soil health and quality [3]. Enzymes which are active in the soil are for the most part extracellular oxidoreductases and hydrolases. One of the main groups of enzymes taking part in the soil oxidative processes is the group featuring the oxidases such as phenol oxidase and peroxidase. These enzymes are involved in the transformation of the polyphenolic compounds and also in the degradation of the lignin and humic substances [4]. Soil dehydrogenases (EC 1.1.), which are the most frequently studied oxidoreductases, are often considered to be an index of the general soil microbial activity. They play a significant role in the oxidation of soil organic matter by transferring protons and electrons from the organic substrates to the inorganic acceptors [5].
A large number of enzymes, which play fundamental roles in soil organic matter (SOM) transformation, belong to the class of hydrolases. Soil hydrolases catalyze the biochemical reactions of the soil organic matter transformation and play crucial roles in the soil C, N, P and S cycling, which meet the nutrient requirements of soil microbes and plants [6]. Among the specific C-transforming enzymes, which are crucial to the hydrolysis of the most abundant, complex plant-derived polysaccharides (cellulose, starch, xylan, lignin, pectin, chitin, inulin) into small available compounds, the complexes of the cellulases and xylanases are most often studied [6]. A wide range of N-transforming enzymes has been found and they are most often studied in the soil. Thus, the proteolytic enzymes (proteases) hydrolyze proteins into amino acids, which are further converted into the mineral N forms (ammonium and nitrate) [7,8]. Additionally, the soil urease activity is widely studied because of its significance in agriculture due to the decomposition of urea, a popular mineral fertilizer [7,9]. Although several enzymes are involved in the mineralization of the organic P compounds, acid (EC 3.1.3.1) and alkaline (EC 3.1.3.2), phosphomonoesterases (phosphatases), are most often studied because of the role they play in the hydrolysis of the esters and anhydrides of orthophosphoric acid to inorganic P (phosphates), which are then available to soil microorganisms and plants [10,11].
The estimation of microbial biomass by determination of C, N and P content is a very popular technique used in soil microbiology. Although the microbial biomass constitutes at most a slight pool of soil organic matter (less than 5%), it is considered to be its most labile constituent and plays a significant role in the decomposition of plant and animal residues and nutrient cycling [12,13].
Different land uses that are associated with various cultivated plants and management practices such as mineral and organic fertilization, tillage and crop rotations are widely known to affect the microbial and enzymatic properties of soil [14,15,16]. Plants stimulate the soil microbial activity through the production of root exudates, and they also produce and liberate their own extracellular enzymes [17]. A major impact on soil microbial content and enzymatic activity is caused by the differences in the root mass and structure, which occurs mainly in relation to number of small roots that are present and is related to a high rate of metabolic activity [18]. Many studies have determined the influence of land use and management practices on enzyme activities and microbial properties, but most have been restricted to the surface soil horizons [19]. In fact, topsoils are more exposed to the various management practices that are associated with agricultural soil use compared to the deeper soils. Because the growth in plant species and the related management practices, which could also significantly affect the microbial biomass and activity throughout the soil profile, it is essential to assess the influence of plant species diversity, e.g., to determine how plants with a contrasting morphology of the root system affect the soil enzymes down the soil profile [20,21,22].
In this study, the effects of two distinct agricultural land-use systems (vineyards and arable land) on soil properties throughout the soil profiles were compared. Among the cultivated cereals, the cultivation of which has a long history in Poland, wheat is the most important cereal and occupies about 22% of the arable fields [23]. Grapevines (Vitis vinifera L.) are a woody perennial crop that has been cultivated in Poland since the beginning of the 1980s and are mainly located in the south of the country. Currently (2022), there are 483 vineyards covering 769 hectares [24], while in the Cuiavia–Pomerania Voivodeship there are 11 vineyards, which occupy an area of 18.2 hectares [25]. The average age of a vineyard in Poland is 16 years, while in the Cuiavia–Pomerania Voivodeship it is 6 years. Previously, it was noted that grapevines are best suited to land that has lack of water and recurrent drought as well as to soils with a relatively low fertility, and thus, they play a role in the adaptation to climate change via agriculture [26,27]. On the other hand, viticulture can have a profound impact on the soil properties due to the intensive tillage, the removal of crop residues, and the chemical control of weeds and pests, wherein the negative changes increase with prolonged winegrape cultivation [28].
Most studies concerning the soil microbial and enzymatic properties of agricultural soils have been restricted to the upper horizons (up to 30 cm) of the soil, while their status and functions in the sub-surface layers are less understood, even though it has been established that soil microbes and enzymes impact the SOM transformation across the entire soil profile [29,30]. It was previously noted that the microbial properties and enzymatic activities generally decrease with soil depth [20,21,30] but that they are still substantially high and active in the deep soils, and what is more, occasionally these activities are even higher than in the topsoil [8,31,32]. That is why it is particularly important to determine the variations in the microbial variables with depth, since to limit the research to topsoil not only restricts our knowledge concerning changes in the soil microbial and enzymatic activity but may also limit our insight into the distinctive and possibly important processes of the SOM transformation, as these have a substantial effect on soil ecosystems. Although the principal agent that affects the microbial and enzymatic variables with soil depth is the content of total soil organic C, the concentrations of the labile, easily degradable substrates that are suitable for microbial and enzymatic activity are also important. In general terms, over 50% of the organic C in soils is deposited in the deeper soil layers (below 30 cm), where C cycling is slower compared to the surface soils because the structure and availability of the organic matter as well as variations in the microbial and enzyme properties [33,34]. Moreover, other abiotic factors such as the characteristics of the parent material, including the soil texture (proportion of sand, silt and clay), bulk density, soil pH, air–water conditions, have all been considered to be important factors that can affect the soil microbial biomass content and enzymatic activity [21].
The aim of this study was to assess the reaction of a set of soil microbial, enzymatic and physico-chemical variables to diverse land-use systems and also to the depth in the soil profiles. For the purpose of the study, four Luvisol profiles were compared. They differed in terms of the agricultural soil use and management (arable field—winter wheat, WW; vineyard—grapevine, GV). It was hypothesized that (1) the enzymatic and microbial properties would change depending on the land use/vegetation cover and that although such changes are expected to be most predominant in the upper horizons, the appropriate soil management and the cultivation of a plant species with a contrasting root biomass and structure would also change the studied properties across the soil profiles; (2) the studied properties would be the highest in the surface horizons and are expected to decrease with depth to varying degrees. They would have a different pattern of activity throughout the soil profiles because of the different functions they play in the organic matter transformation, e.g., the presence of the various amounts of available substrates for their reactions; and (3) the enzyme activities in the surface and sub-surface horizons of the soil profiles would be primarily determined by the content of soil C and N (including the microbial biomass C and N), while the enzymatic activity below the surface horizons would also be expected to be affected by other soil properties such as soil pH and soil texture, e.g., the clay and silt content. The hypotheses outlined above were tested based on a determination of the 17 enzymatic properties that characterize the total microbial activity and that are involved in the soil C, N and P cycles as well as other soil variables across four profiles of Luvisols that were excavated from two distinct land uses (an arable field and a vineyard).
2. Materials and Methods
2.1. Research Sites and Soil Sampling
The studied areas were located in the South Baltic Lake District, Cuiavia–Pomerania Province, Krajna Lake District (NW Poland), that have a long history of agricultural use. Four separate profiles were selected and excavated to represent a distinct land-use system and soils were sampled in each genetic horizon to a depth of at least 150 cm (up to the bedrock). Profiles 1 and 2 were excavated in the field with the winter wheat (WW) (Triticum aestivum L.), while profiles 3 and 4 were located in the vineyard sampling area, which was founded in 2012 (Table 1).

Table 1.
Characteristic of the study sites.
Description of the soil profiles is given in Table 2. In the autumn, the WW was fertilized with mineral phosphorus and potassium as Lubofoska (P-12%, K-24%) at doses of 50 kg and 80 kg (pure ingredients), respectively. Nitrogen fertilization (as NH4NO3; N-34% and urea; N-46%) was added at the following rates: 40 kg ha−1 was used before the sowing of WW (together with Lubofoska), 60 kg ha−1 was applied in spring and the last addition (40 kg ha−1) was made during the WW shooting. The vineyard soil was not fertilized. The black mechanical fallow (mechanical weed regulation) was treated between the grapevine rows, while the weeds in the rows (between the plants) were eliminated by hand. The residues of the weeds were left and then incorporated into the soil.

Table 2.
Description of the soil profiles.
Soil samples were collected according to the individual genetic horizons in the soil profiles. We selected five blocks in each horizon, wherein the dimensions of the blocks were dependent on the horizon thickness. We collected three individual samples from the middle part of each block, which were mixed to make one bulk sample. The five pooled samples (from each block) were analyzed individually for the chosen variables and the average value for the five examined blocks was presented for each horizon. The soil samples that had a mass of 250 g were taken in the field. The soil samples collected from the surface horizons were placed in plastic boxes, which allow for gas exchange, while the samples that were taken from the deeper horizons were placed in sealed containers which generated an atmosphere with a reduced oxygen content.
All of the samples were placed in a portable refrigerator (4 °C) (Dometic CoolFreeze CFX 35 Professional, 32 L, Dometic Group, Solna, Sweeden). Upon arrival at the laboratory, subsamples (250 g) for physical and chemical property analysis were air-dried, ground and sieved through a 2 mm mesh. Subsamples for the determination of microbial biomass and enzymatic activity (250 g) were stored at 4 °C for 2 weeks in order to complete the required analysis.
2.2. Soil Enzyme Assays
The potential activity (i.e., activity which is not limited by the appropriate substrate concentrations) of the 17 different enzymes was measured. One gram of soil was incubated with an appropriate substrate and buffer. After incubation, the reaction was stopped, and the concentration of the released product (in the filtrate or supernatant) was measured using a spectrophotometric assay technique.
2.2.1. Indicators of the Overall Soil Microbiological and Hydrolytic Activity
A method developed by Schinner and von Mersi [35] was adopted in order to assess the dehydrogenase (DHA) activity by monitoring the release of red-colored formazan (INTF) from soil treated with a 0.2% INT solution (2-(4-iodophenyl)-3-(4-nitrophenyl(-5-phenyl-2H-tetrazolium chloride) as a substrate and incubated for 24 h with THAM buffer (1 M, pH 7.0). The general hydrolytic activity of the studied soils was determined by the assessment of the rate of hydrolysis of fluorescein diacetate sodium salt (FDSS) in a phosphate buffer (60 mM, pH 7.6) during a 60 min long incubation period at 37 °C [36]. The reaction was terminated by addition of the mixture of methanol and chloroform (1:2). Finally, the soil reaction mixture was centrifuged, and the concentration of the product was measured at 490 nm.
2.2.2. C- and P-Cycling Hydrolases
The activity of α-glucosidase (αGlu), β-glucosidase (βGlu), cellobiohydrolase (exoCel), β-xylosidase (exoXyl), acid and alkaline phosphatase (AcP, AlP) and phosphodiesterases (PDE) was determined using a method based on p-nitrophenol as the end product released after the incubation of soil samples with 1 mL of an appropriate substrate (25 mM p-nitrophenyl-α-D-glucopyranoside, 25 mM p-nitrophenyl-β-D-glucopyranoside, 1.2 mM p-nitrophenyl-β-D-cellobiopyronoside, 10 mM p-nitrophenyl β-D-xylopyranoside, 15 mM p-nitrophenyl phosphate disodium and 5 mM bisnitrophenyl-p-nitrophenyl phosphate and 4 mL of an appropriate buffer (pH 6.0, 5.5 and 5.0) during 1 h at 37 °C [37,38,39,40]. The activity of endo-cellulase (EC 3.2.1.4), endo-xylanase (EC 3.2.1.8) and invertase (EC 3.2.1.26) was determined based on reducing sugars as the end product [41]. The soil samples were incubated for 24 h at 50 °C (except for invertase—3 h) with the acetate buffer (2 M, pH 5.5) and an appropriate substrate (0.7% carboxymethyl cellulose sodium salt, CMC; 1.2% xylan and 10% sucrose, respectively). The determination of the reducing sugars (glucose, xylose as well as a mixture of glucose and fructose, respectively) was carried out using the Prussian blue method which involves the reduction in ferricyanide ions in alkaline solution followed by the formation of Prussian blue (ferric ferrocyanide). This was measured quantitatively at 690 nm.
2.2.3. N-Transforming Enzymes
In order to determine the urease activity (UR), the soil samples were incubated (2 h, 37 °C) with urea as a substrate and borate buffer (pH 10.0) [42]. The content of released ammonium was extracted using a 1 M KCl solution and determined using a modified Berthelot reaction. A method developed by Kandeler [43] was adopted to assess nitrate reductase activity (NR) by monitoring the release of nitrite (NO2) from soil treated with KNO3 (25 mM) as a substrate (24 h at 25 °C). The reaction was terminated by the addition of 4 M KCl solution, and the reaction mixture was filtered. The concentration of liberated NO2 was determined in the filtrate with a color reagent and ammonium chloride buffer (0.2 M, pH 8.5). The protocol of Ladd and Butler [44] was used to determine the casein-protease activity. Soil samples were incubated (2 h in a water bath at 50 °C) with 2.5 mL of an Na-caseinate solution (2%) and 2.5 mL of Tris buffer (0.2 M, pH 8.0). After incubation, 5 mL of a 10% trichloroacetic acid (TCA) was added to precipitate the remaining casein and the reaction mixture was filtered. In order to assess the content of tyrosine, 0.5 mL of the filtrate was mixed with sodium carbonate (1.4 M) and Folin–Ciocalteu reagent (0.2 M).
2.2.4. Soil Oxidase Activity
The activity of the phenol oxidase (POX, EC 1.10.3.2) was assessed by using 3,4-dihydroxy-L-phenylalanine (L-DOPA) as a substrate according to the method of Bach et al. [45]. The soil samples were mixed with 3 mL of an acetate buffer (pH 5.0) and 2 mL of L-DOPA (10 mM) in centrifuge tubes. The samples were incubated in a shaking water bath (100 rev min−1) 25 °C for 30 min. In order to stop the reaction, the suspensions were centrifuged, and the concentration of the product was measured spectrophotometrically in each sample (at 475 nm). The peroxidase (PER, EC 1.11.1.7) activity was assessed as well as the POX, but 8 replications were carried out. Four replicated were prepared to determine the POX activity (as above) and four were prepared with the addition of H2O2 (0.3%), as the second substrate used to determine the total oxidative activity as was proposed by Sinsabaugh et al. [46].
2.3. Microbial Biomass Carbon, Nitrogen and Phosphorus Assays
In order to assess microbial biomass carbon (MBC), nitrogen (MBN) and phosphorus (MBP), the chloroform fumigation–extraction procedure was used as proposed by Vance et al. [47] and Brookes et al. [48]. The method is based on the difference between the C or N content extracted from the fumigated and non-fumigated soil samples (25 g, 50% WHC) using 0.5 M K2SO4 at a ratio of 5:1. After extraction, the soil suspension was centrifuged at 200 rev min−1 for 30 min and filtered if required. Sub-samples of the supernatants or filtrates from both the fumigated and unfumigated soils were analyzed for the extractable C and total N. To account for the incomplete recovery of microbial C and N content, the microbial biomass values were calculated by dividing the difference between the fumigated and unfumigated samples by a correction factor (=kEN) of 0.45 and 0.54, respectively [49,50]. Both the fumigated and non-fumigated soil samples were subjected to extraction with 0.5 M NaHCO3 (pH 8.5) to evaluate the content of microbial biomass P (MBP). The suspensions were filtered and the P content in the filtrate was assessed colorimetrically as proposed by Olsen and Sommers [51] by using a UV Vis Evolution 220 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The following formula: Pmic = Ep/Kp was used to evaluate the microbial biomass P. In this formula, Ep indicates the increase in extractable P in fumigated soil as compared to the control and Kp is the fraction of MBP which is extracted after fumigation.
2.4. Analysis of Physico-Chemical Properties
The particle size distribution of the soil samples was determined using the Casagrande method (modified by Prószyński). The content of the sand fraction was determined according to the sieving method [52]. In order to determine the gravimetric moisture level, which was essential for performing an enzymatic activity calculation, the moist soil samples were dried at 105 °C until the samples reached a constant weight. The soil samples were placed in a solution of 0.01 M CaCl2 and analyzed for pH by using an S210 Seven Compact™ pH meter (Mettler-Toledo, Greifensee, Switzerland). Total organic carbon (TOC) was analyzed using a Vario Max CNS analyzer (Elementar, Jena, Germany). The content of soil dissolved organic carbon (DOC) was determined using a 0.004 M CaCl2 solution after a 1 h long extraction with a soil sample to extractant ratio of 1/10. The DOC contents of the extract solutions were assessed using a TOCN Multi N/C 3100 analyzer (Analityk, Jena, Germany). The available P content was assessed according to the method of Egner– Riehm and 0.1 M ammonium lactate with pH = 3.7 was used as an extracting solution. After the extraction, P was determined using spectrophotometry after color development with ammonium molybdate and SnCl2 [53]. Soil mineral nitrogen (N-NO3− and N-NH4+) was determined in the field-moist soil samples using 2 M KCl and 1% K2SO4, respectively. The N-NO3− concentration was assessed using the phenol-2,4-disulfonic acid method. Using this method, the nitration of phenol disulfonic acid yields an orange–brown solution, which was measured using spectrophotometric assay technique (435 nm) [54]. The N-NH4+ content was evaluated using the standard indophenol blue method, which is based on the reaction of alkaline phenol and hypochlorite with ammonia to form an indophenol blue complex which is proportional to the content of ammonia in the studied sample [54].
2.5. Assessment of Root Biomass and Structure
After the initial preparation (manual sorting and wet-sieving), the fresh weight of the plant roots, including all roots and not only those of the cultivated plants, were considered. Firstly, the morphological characteristics of the roots systems were assessed. To accomplish this, each root sample was scanned with an Epson expression 10,000 XL root scanner with WinRhizo V3.10 software (Régent Instruments Inc., Québec, QC, Canada). The root length (mm dm−3) was calculated using WinRhizo software. Finally, the roots were dried at 70 °C until a constant weight was obtained.
2.6. Statistical Analyses
Since most of the obtained results did not show a normal distribution according to the Shapiro–Wilk test (e.g., the activity of all enzymes beside the FDAH, urease, microbial biomass C and N, dissolved organic C and N), they were log-transformed in order to reduce their variability. Since the transformation improved the normality of the data, all further analyses were carried out using the corrected data. Two experimental factors were considered. The soil depths (or soil genetic horizons) were the first experimental factor, which were compared along the soil profile (within the same soil use scenario) and a one-way analysis of variance (ANOVA) was performed to determine the effect of this factor on the studied properties. Additionally, a one-way analysis of variance was performed to assess the changes in the studied variables as they are affected by land uses/plant cover, wherein the genetic horizon lying at the same/similar depth in soil profiles with different uses were compared. An analysis of variance was performed based on the mean values for profiles 1 and 2, as well as 3 and 4). A Tukey’s Honest Significant Difference (HSD) test was performed with a 95% confidence interval in order to assess the significance of differences between pairs group means. To estimate the linear correlation between all of the determined properties a Pearson’s correlation coefficient (p < 0.05 significance level) was applied. In order to reduce the dimensionality of a dataset the Principal Component Analysis (PCA) based on the average data set of the measured variables was applied. The first two principal components (PC1 and PC2) were chosen for the ordination of the cases. All of the statistical analyses were performed using Statistica 13.1 for Windows 10 software.
3. Results
3.1. Physico-Chemical Properties across the Soil Profiles
In general terms, the soil depth significantly affected most of the studied properties. They were the highest in the surface layers and decreased to a varying degree down the soil profiles (Table 3, Table 4, Table 5 and Table 6).

Table 3.
Total organic carbon and total nitrogen as dependent on cultivated plant and soil depth, mean ± SE, n = 5.

Table 4.
Dissolved organic carbon and nitrogen as dependent on cultivated plant and soil depth, mean ± SE, n = 5.

Table 5.
Some physico-chemical properties as dependent on cultivated plant and soil depth; mean ± SE, n = 5.

Table 6.
Mineral nitrogen forms and available phosphorus as dependent on cultivated plant and soil depth; mean ± SE, n = 5.
The concentration of total organic C and total N (TOC, TN) as well as their dissolved forms (DOC, DNt) were the highest in the Ap horizon and generally deceased with depth, while both the highest and statistically significant differences were mainly noted in the I and II layers (Table 3 and Table 4).
In the deeper layers (III–V) the results were lower and the differences between them were statistically insignificant. The soil pH in CaCl2 ranged from moderately acidic to alkaline (6.37 to 7.09) and was the highest in the deeper horizons, while it was lower and statistically insignificant in all four of the horizons that were located above the deeper layer (Table 5). The clay content ranged from 8.5% to 19.0%, but its vertical distribution had different patterns in the winter wheat (WW) and grapevine (GV) profiles. In the WW profiles, the clay content was the highest in layers III and IV (E2, Bt, EB horizons) and the lowest in the surface layer (Table 5). In the GV profiles the clay content was the highest in layer IV (CK1, Bt horizons), while it was lowest in the surface and sub-surface layers. The silt fraction ranged between 33.1 and 47.5%, and in the WW profiles it was highest in the deepest layers, while it was significantly lower in layers I–IV. In the GV profiles, the silt content was similar and did not differentiate significantly in layers I, II, IV and V, while it was the lowest in the middle layer (III) (Table 5).
No clear trends were observed for in the content of the mineral forms and the available P content throughout the soil profiles (Table 6). In the WW profiles, the content of NO3− and NH4+ decreased significantly with depth, while no clear trends were observed in these variables as they were determined in the GV profiles. The only common observation was the lowest content of these variables in the deepest horizons of both WW and GV profiles.
3.2. Vertical Distributions of the Soil Enzymatic Activities and Microbial Biomass Content
The soil variables decreased significantly with increasing soil depth, wherein the changes were most pronounced between the surface soil horizons (I–II) (Table 7, Table 8 and Table 9, Figure 1, Figure 2 and Figure 3). The degree of the vertical changes in the soil properties with depth differed across land uses and were dependent on the studied variables themselves.

Table 7.
The N-transforming soil enzyme activities as dependent on cultivated plant and soil depth; mean ± SE, n = 5.

Table 8.
The C-cycling soil enzyme activities as dependent on cultivated plant and soil depth; mean ± SE, n = 5.

Table 9.
The P-cycling soil enzyme activities as dependent on cultivated plant and soil depth; mean ± SE, n = 5.
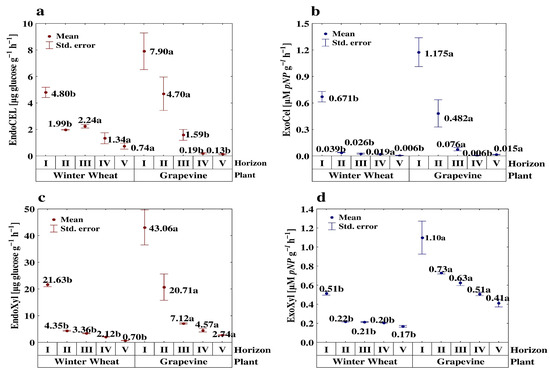
Figure 1.
Effect of the cultivated plant and soil depth on the endo-cellulase (endoCel) (a), exo-cellulase (exoCel) (b), endo-xylanase (endoXyl) (c) and exo-xylanase (exoXyl) (d) activity. Different small letters indicate significant differences (p < 0.05) between cultivated plants (in the same soil depth/layer).
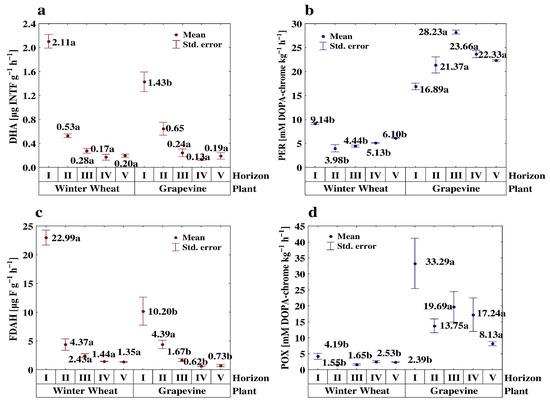
Figure 2.
Effect of the cultivated plant and soil depth on dehydrogenases (DHA) (a), peroxidase (PER) (b), of hydrolysis of fluorescein diacetate sodium salt (FDAH) (c) and phenol oxidase (POX) (d) activity. Different small letters indicate significant differences (p < 0.05) between cultivated plants (in the same soil depth/layer).
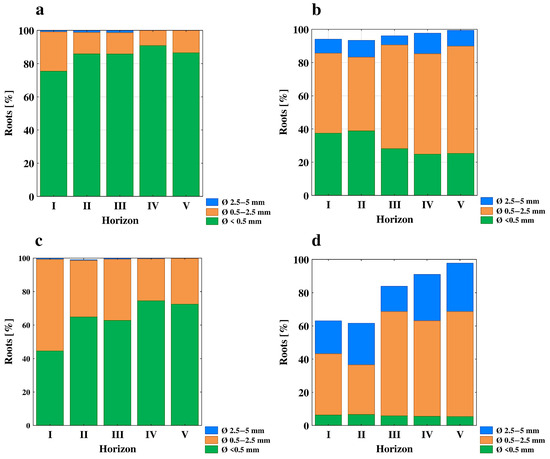
Figure 3.
Contribution of different root diameters (mm) in the total length of the root system; winter wheat profiles (a,c) and grapevine profiles (b,d).
The activity of some enzymes (e.g., the UR activity in the WW profiles) decreased gradually across the soil profiles, while those of the others were located almost solely in the surface layers (e.g., the NR activity in vineyard profiles as well as the INV activity in the WW profiles). Although the αGlu activity did not show any statistically significant changes along the analyzed profiles, a slightly higher activity of this enzyme occurred in the sub-surface horizons (II) than in the upper ones (I) (Table 8). Most of the studied enzyme activities (except for the αGlu activity) declined from the surface layers to the deeper horizons by 84–99.7%. The exception was the activity of exoXyl and the FDAH ratio across the winter wheat profiles (Figure 1d and Figure 2c).
There was a different trend in the POX and PER activities along the studied profiles compared to the other enzymes (Figure 2b,d).
In the winter wheat profiles, these enzymes exhibited the highest activity in the surface horizons, and their activity was significantly higher in the two deepest layers than in the II and III horizons. With regard to the activity of these enzymes along the vineyard profiles, the highest level of the PER activity occurred in the third (III) horizon, while the lowest was found in the surface layers. Although the activity of POX was highest in the upper layers, no clear trends were observed in the sub-surface horizons of the GV profiles. There was a significantly higher level of POX activity in the middle layer (III) compared to the sub-surface layers (II) and both of the deepest layers.
The content of microbial biomass C, N and P was generally the highest in the upper horizons and significantly but gradually decreased with depth (Table 10).

Table 10.
Microbial biomass C, N and P as dependent on cultivated plant and soil depth; mean ± SE, n = 5.
The greatest decrease in the values of this variable was found between the surface and sub-surface horizons (42% for the MBC and MBN content and 31% for the MBP concentration). The TOC/TN ratio ranged between 4.45 and 10.5 and irrespective of the cultivated plant the highest ratio was calculated for the upper layer (I) and the deepest horizon (V) (Table 11). With regards to the MBC/MBN ratio, there were no significant changes in this ratio between the WW and GV profiles in the surface and sub-surface horizons, while in the deeper layers this ratio was found to be significantly higher in the vineyard profiles than in the WW profiles (Table 11).

Table 11.
The TOC/TN and MBC/MBN ratios as dependent on cultivated plant and soil depth; mean ± SE, n = 5.
The contribution of MBC to TOC, which ranged from 0.67 to 4.69, was significantly higher in the vineyard profiles than in the WW profiles, with the exception of the second horizon (II) where the opposite trend was noted. No clear trends were found when the vertical changes of this ratio was evaluated (Table 12). The contribution of MBN to TN ranged between 1.20 and 6.54 and it was significantly higher in the vineyard profiles than in the WW profiles (Table 12).

Table 12.
The contribution of microbial biomass C and N in TOC and TN content as dependent on cultivated plant and soil depth; mean ± SE, n = 5.
The only exception, with regard to this ratio, was the lack of any significant difference in the third horizon. The highest values of this ratio were found in the middle horizons of both profiles, while the lowest results were assessed in the deepest horizons.
3.3. The Studied Variable as Affected by the Land-Use System
While the TOC content was significantly higher in the GV profiles compared to the WW profiles in the I and II soil layers, there were no clear changes in this property in the deeper soil horizons (Table 3). Irrespective of the plants being cultivated, the TN concentration was similar in the surface and the two deepest layers of all of the studied profiles, although no clear trend was observed for II and III horizons (Table 3). There was a lack of clear changes for the DOC content, while the DNt content was significantly higher throughout the WW profiles compared to the GV profiles (Table 4). No significant changes were observed in the pH values and silt content as regards the influence of the agricultural soil-use systems. The clay content was significantly higher in the surface horizons across the GV profiles compared to the WW profiles, while the opposite trend was noted for this variable in the sub-surface (II) layer (Table 5). The nitrate N content was significantly higher across the WW profiles compared to the GV profiles, while for ammonium N content, this was true only for the surface layers and the opposite trend was found in the deeper layers (II–V) (Table 6). The agricultural land use had a significant effect on most of the studied enzymes at all of the tested depths/horizons. Most of them (UR, PRO, βGlu, αGlu, INV, AlP, PDE, FDAH, PER, POX, endoXyl, exoXyl) exhibited a higher activity in the vineyard profiles followed by the winter wheat profiles (Table 7, Table 8 and Table 9, Figure 1 and Figure 2). The greatest variability was found for the UR activity, which was determined to be nine times higher in the surface and sub-surface horizons of the vineyard profile (55.5 and 22.6 mg NH4+ kg−1 h−1), compared to the winter wheat profile (5.90 and 2.39 mg N–NH4+ kg−1 h−1) (Table 7). There was no clear differentiation between both of the land uses for the NR, DHA, AcP, endoCel and exoCel activities. Generally, a significantly higher content of microbial biomass as expressed as MBC, MBN and MBP was found across the GV profiles compared to the WW profiles (Table 10).
3.4. Root Biomass and Morphology
The root biomass was found to be significantly higher in all of the horizons of the vineyard profiles as compared to the WW profiles and decreased with an increasing soil depth in the WW profiles. In the GV profiles, the root biomass was the highest in both the surface and sub-surface horizons (22.4 and 24.5 g dm−3), while in the deeper layers of these profiles, the values of the root biomass were markedly lower and there were no clear trends in its variability (Table 13). Irrespective of the plant being cultivated, the length of the roots (the total length of the root system and roots below 0.5 mm) were greatest in the surface horizons (I) of the studied profiles, while there were no clear changes in this variable in the deeper horizons (Table 13). In the WW profiles, the root lengths were similar in layers II, III and IV, while they were found to be significantly lower in the deeper horizon. In turn, in the sub-surface horizons of vineyard profiles there was no clear direction of this property. For the WW profiles, both the length and the surface area were the highest in the case of roots with the diameter of less than 0.5 mm (Figure 3a,c).

Table 13.
Root biomass and length in the studied profiles (the average values and selected intervals) dependent on cultivated plant and soil depth.
These roots made up most of the root system (more than 80% of the total root system length, with the exception of the surface horizon and on average 65% of the total root system surface). The highest contribution of these roots was observed in the two deepest layers, while the lowest was found for the surface horizon. Since almost no roots between 2.5 and 5 mm thick were found, especially in two the deepest layers, the rest of the root systems were roots with a diameter of 0.5–2.5 mm. In contrast to the WW profiles, in the vineyard profiles, both the length and the surface area were the highest for roots with a diameter of 0.5–2.5 mm (Figure 3b,d). They made up most of the root system (between 40% and almost 60% of the total root system length and on average 60% of the total root system surface). The vineyard profile roots of <0.5 mm made up less than 10% of the total root system surface and ranged from 30% (III, IV and V layer) to almost 40% (I and II layer) of the total root system length (Figure 3b,d). In addition, the contribution of the roots between 2.5 and 5 mm thick was also significant as they comprised more than 20% of the total root system surface and between 5 and 12% of the total root system length.
3.5. Relationship between the Studied Properties—Analysis of Correlation and PCA
An analysis of correlation was performed separately for the winter wheat and grapevine profiles (Tables S1 and S2). Additionally, the studied properties were correlated separately for C-rich surface horizons (the TOC content > 4 g kg−1) and for the C-low horizons (the TOC content < 4 g kg−1) (Tables S3 and S4). All of the studied enzyme activities were significantly and positively correlated with TOC, TN, DOC, DNt and microbial biomass content (MBC, MBN, MBP) both in the WW profile and in the GV profiles. Only the PER activity in the GV profiles was correlated significantly but negatively with these properties. Most of the studied enzymes were significantly correlated with the mineral N forms in the WW profiles, while in the GV profiles, the lack of the significant correlations were observed. The clay content in the GV profiles was not significantly correlated with the set of the studied enzyme activities, while no consistent trends were observed in the case of the silt content in these profiles. In turn, the clay content was significantly but negatively correlated with the studied enzyme activities (except for the αGlu activity), while the silt content did not reveal any significant relationship with enzymatic activity. Across the GV profiles only αGlu was significantly correlated with pH in CaCl2, while throughout the WW profiles only four enzymes (end-Cel, UR, AlP) were significantly but negatively correlated with the values of this property. Considering a separate correlation analysis made due to different contents of carbon, it was observed that the studied enzyme activities revealed higher correlation coefficients in the C-rich and lower layers, as well as the lack of a significant correlation in the C-low horizons. The exception was the PER activity, which revealed either a negative or the lack of a significant correlation with C and N forms in both of the considered land uses.
The PCA analysis identified four components which accounted for 86.3% of the total variance, most of which (78.8%) could be explained by PC1 and PC2 (Figure 4a,b).
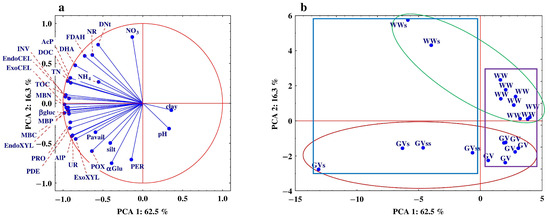
Figure 4.
Principal component analysis derived from the studied soil properties. (a) Plot of the first two principal components (PC) for the measured soil properties. Abbreviations are explained under Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11 and Table 12 and Figure 1 and Figure 2; (b) principal component analysis of the variables in the soil horizons in all 4 profiles: WW—winter wheat profiles, GV—grapevine profiles, WWs and GVs—surface horizons (Ap) of WW and GV profiles, GVss—sub-surface (II) horizons of GV profiles, WW and GV—deeper horizons (III–V) of WW and GV profiles.
Most of the studied properties were negatively correlated with PC1, because all of them had high negative loading scores concerning this component which ranged from −0.638 to −0.980 (Figure 4a). This proves that PCA1 explains the very high percentage of variance (e.g., 96% endoXyl). In turn, PCA2 clearly separated the studied variables into three groups. One group of variables explained the high positive correlation (DHA, FDAH, NR), while some of them (POX, aGlu, PER) were more closely but negatively correlated with this component. However, most of the determined variables did not show any significant correlation with PCA2. A PCA analysis showed that PCA1 separated layers (surface and sub-surface) with higher and lower organic matter content. Cases with a higher OM content showed a negative correlation with PCA1, while cases with a lower OM content demonstrated a positive correlation with this component. It was also noted that the increase in correlation coefficients was related to the increase in organic carbon content (Figure 4b). However, PCA2 divided the samples into 2 groups: the samples collected from winter wheat profiles (positive relationship) and the samples taken from profiles located in vineyards (the negative relationship).
4. Discussion
4.1. Land Use and Plant Cover as the Main Factors That Affect the Soil Microbial and Enzymatic Variables
Land use and cultivation, especially in the long-term, is well known to affect enzymatic activity in several ways (1) through direct variations in the soil physico-chemical variables, (2) through changes in the abundance and structure of the microbial communities and (3) through changes in the content and quality of soil organic matter [55,56,57,58]. Most of the studies concerning the effect of agricultural land use and plant cover on the soil microbial and enzymatic activity are focused on the upper soil layers, while fewer authors have assessed the effect of land use and the plants being grown on these variables in the deep soils [8,30,58,59]. In our study, as was expected, there were significant differences in the enzymatic activities and microbial biomass among the compared soil profiles that represented different land uses. The 8-year-long viticulture significantly improved the microbial biomass content and enzymatic activity levels compared to the winter wheat cultivation, while the TOC content was significantly higher in only three horizons of the vineyard profiles (surface, sub-surface and the third layer). Additionally, other physico-chemical properties such as DOC, DNt, pH, clay and silt were quite similar in the compared soil profiles; however, this does not explain the differences observed in the microbial and enzymatic properties. Previous studies have shown that the input of residues that consist of leaves and stems resulted in increases in the overall SOM and nutrient availability. However, it should be pointed out that this positive effect occurred in the first few years of cultivation and that a decrease in these positive effects was observed with prolonged winegrape cultivation [60]. In our study, after 8 years of viticulture, the activities of most of the studied enzymes were significantly higher throughout the GV profiles than across the WW profiles. This was associated with the content of microbial biomass, which was definitely higher in the GV profiles compared to the WW profiles. It is commonly known that soil microorganisms are the main source of enzymes in the soil [61]. In turn, the higher microbial biomass content was probably associated with a higher nutrient status (P content—Table 6) as a result of the previous soil management regime.
A substantially higher activity of POX and PER was observed in the soil samples from GV profiles compared to the soil that had been collected from the WW profiles (1.8–6.4 times in the case of the PER and 3.4–11.9 times for the POX activity). This was probably associated with the accumulation of different phenolic compounds in the grapevine roots which were further secreted into the soil. Weidner et al. [62] found that this might indicate that these compounds play an important role in the adaptation of roots to growth under different stress conditions such as a water deficit or extreme temperatures. The increased activity of some enzymes such as catalase, peroxidase or phenol oxidase in such conditions was considered to be one of the possible plant defense mechanisms [63]. In addition to the POX and PER activity, there was a significantly higher degree in the activity of the C-transforming enzymes in the GV profiles than in the WW profiles. This might have been associated with a high content of grapevine residues such as lignin and cellulose derivatives that are difficult to decompose, which are possible enzyme substrates. It is possible that this resulted in the higher and more stable activity of xylanases, cellulases and β-glucosidase [60].
Although the microbial biomass C and N content was significantly higher in the GV than in the WW surface soil (almost 100%), the opposite trend was observed for the DHA. Such differences might be due to the fact that these variables reflect the various metabolic statuses of the soil microbial communities. DHA activity is only an indicator of a metabolically active microorganism, while the microbial biomass content, which is expressed in terms of MBC, MBN and MBP in this study, includes both the active (proliferating) and inactive (dormant, dead) pool of the microbial population [64].
The lower activity of most of the studied enzymes in the WW profiles as opposed to the GV profiles might be explained by the different farming practices that were used in the two agricultural systems that were compared. The conventional tillage that was used in winter wheat cultivation included the removal of plant residues (straw), the use of agrochemicals (pesticides, fertilizers) as well as various mechanical treatments, e.g., plowing or harrowing, which can cause structural soil damage. All of these management practices accelerate the decomposition of organic carbon and decrease the level of enzyme activities and the content of the microbial biomass. The higher microbial biomass content (expressed in terms of C, N and P) that was observed in GV soils than in the WW soils was not consistent with the literature results [65,66] and reflected the higher supply of labile C and other nutrients, which were provided by litter and crop residues.
Previously, it was observed that the activities of the soil enzymes that participate in the transformation of the main nutrients respond negatively to applied mineral fertilization and are frequently inversely correlated with the available forms of these nutrients in the soil [67]. The patterns of the UR and NR activity that were observed in the WW profiles might be explained by the opposite effects of the NH4NO3 application. NR is an adaptative enzyme and is only induced in the presence of a substrate that has nitrate ions, while in the soil environment, it is inhibited by ammonium ions [68,69]. In fact, in this study, a significantly higher nitrate-N content and NR activity were observed in the WW profiles (mainly in the surface horizon). Additionally, these two variables were positively correlated (with r = 0.493). It is important to mention that no such relationship was observed in the soil that had been collected across the GV profiles, where no N mineral fertilization was applied. Previously, it was found that a high amount of N-ammonium decreases urease activity [70]. This explanation may apply in part to the relationship that was observed between these variables; the UR activity was several times higher in the GV profiles samples than in the WW profiles, while the content of ammonium-N was significantly higher in the WW soil as compared to the GV soil. However, such a relationship was only true for the surface horizon.
Variation in the enzymatic activity between the compared land uses might be related to the different mass of the root system and its architecture. Plant roots either produce enzymatic protein themselves, e.g., acid phosphatase, or they improve the living conditions for microorganisms, which are the main source of enzymes in the soil, in both the surface and in the deeper horizons of the soil profile [71]. The greatest effect on the development of soil microbial and enzymatic properties has been attributed to the number of small roots (usually of the length of Ø < 0.5 mm) due to their high rate of metabolic activity [8]. For example, the annual cropping systems such as cereals have a well-developed bundle root system, which is mainly located in the topsoil horizons. This type of root system is known to secrete the root exudates that stimulate the development and activity of the microbial communities in the rhizosphere [72,73]. This is in agreement with previous studies, in which the highest level of enzymatic activity in the surface soil layers was associated with the density of fine roots, which are believed to play a crucial role in the transformation of SOM and nutrient release by stimulating microbial decomposers and via the enzyme activity [8,74]. As opposed to the bundle, shallow-rooted system, plants that have been cultivated in one place, in the long-term such as grapevine, usually develop multi-branching root systems, that include a woody structure of older roots from which other permanent roots arise and grow in different directions and can then produce many small, fine roots. Such a root system has the potential to penetrate the lower soil horizons thereby substantially affecting the soil microbial and enzymatic properties. Additionally, some authors have suggested that the arbuscular mycorrhizal fungi (AMF), which are associated with grape roots enhance water uptake and the absorption of nutrients such as phosphorus and nitrogen, which are important for improving the nutritional status of the grapevine [75]. The results of our study have indicated that in the 8-year-long period of viticulture, a significantly higher root mass (relative to the WW profiles) might account for the increase in the soil microbial content and certain enzyme activities. Even though the highest impact on the soil microbial and enzymatic variables was previously attributed to the number of small roots (usually with a length of Ø < 0.5 mm), our data did not confirm this. The root system of the WW plants had a significantly longer length of the total root system and a greater number of roots with a length of Ø < 0.5 mm relative to the GV plants; however, this fact was not reflected in the level of the enzymatic activity, which was usually higher in the soil samples that had been taken from the GV profiles.
4.2. Changes in the Soil Properties across the Soil Profiles
As was expected, most of the studied enzymes exhibited the highest level of activity in the surface soil horizons, which gradually decreased with depth. The higher values of the studied enzymatic and microbial properties that were observed in the surface layers (and in some cases in the sub-surface soil) were for the most part attributed to a higher content of TOC and TN as well as their dissolved forms (DOC, DNt) that were found in these layers. In fact, it was previously observed that organic and dissolved C and N forms are among the most important agents that control the enzyme activities across the soil profiles [76]. Such a relationship was confirmed by the high values of the correlation coefficients between the microbial biomass, the activity of the studied enzymes and the content of the C and N forms, wherein the greater values of the correlation coefficients were calculated for the C-rich surface horizons (TOC content > 4 g kg−1) as compared to the C-low horizons (TOC content < 4 g kg−1) (Tables S3 and S4). Since the content of C and N is known to decrease significantly with the depth of a soil profile, the values of the microbial and enzymatic properties are also expected to decrease [77]. The decrease in the microbial and enzymatic properties with depth is also caused by the lower content of nutrients, the spatial separation between the microbial communities and enzymes as well as their substrates, and finally, due to some unfavorable conditions, i.e., inadequate pH and redox state, high bulk density and anaerobic conditions that occur in the deeper horizons compared to the surface soil layers [21,78]. Unlike the rapid turnover of the labile, easy-to-mineralize forms of C in topsoil, the turnover time of organic C in the deep soil horizons is longer because the soil C in these locations tends to be absorbed by clay or other minerals and is more protected from mineralization and stabilized as a C sink [31,33,79]. However, recent research has revealed that, despite the characteristic slow turnover of organic C in deep soils, it remains accessible for decomposition. This occurs even as the stability of C compounds increases further town the soil profile [80]. Similar to our data, other researchers have noted a rather high level in the activity of oxidases in the deep soil horizons compared to the topsoil layers, which might be related to the number of irregular polymers and/or the number of toxic substances, which are both irrespective of the soil organic carbon content [34,81].
Previously, the vertical distribution of soil microbial end enzymatic properties was observed to be closely associated with differences in the abiotic factors such as the air–water conditions, parent material, texture, pH, bulk density, soil porosity, redox potential and, finally, with the nutrient distribution across the soil profiles [82]. The differences that were identified were mainly observed in the deeper soil horizons, which have a significantly lower C content than the surface layers [83]. In this study, however, other soil properties such as the soil texture and soil pH were revealed to have relatively little impact on these properties throughout the studied soil profiles, irrespective of the soil-use system and the role that they play in organic matter transformation. This finding was confirmed by the lack of any significant correlation between enzymatic activity and the other studied properties, in both the C-rich surface horizons (TOC content > 4 g kg−1) and in the C-low horizons (TOC content < 4 g kg−1).
5. Conclusions
The significantly higher microbial content and activity of almost all of the studied enzymes in the vineyards compared to winter wheat cultivation was possibly associated with the ecological approach to viticulture in this case (without the use of mineral fertilization and fungicides). Additionally, the root biomass was significantly higher in all of the horizons of the GV profiles compared to the WW profiles, which probably contributed to the higher values of the soil microbial and enzymatic properties in the GV profiles. In turn, contrary to expectations, we did not find any clear effect of the root system architecture, i.e., the length and surface area, roots with a diameter less than 0.5 mm on the assessed soil properties across the studied profiles.
Most of the studied C, N and P-acquiring enzymes exhibited their highest activity in the surface soil horizons, which then decreased significantly with soil depth, irrespective of the soil-use system. However, the studied enzymes differed in their response to the depth of the soil profiles, and thus some of them exhibited the highest degree of activity in the topsoil, which decreased sharply with depth, starting with the sub-surface layers (i.e., the NR and INV activities in the WW profiles), while others (i.e., the αGlu) showed a similar degree of activity across the soil profiles in both agricultural systems. These differences in the enzymatic activity were possibly associated with the number of available substrates which are necessary for the enzymatic reactions to occur, and this might be explained by the different roles that enzymes play in the SOM transformation.
Contrary to expectations, the microbial biomass content and enzyme activities were primarily determined by the organic C and N content and their availability, not only in the surface and sub-surface horizons, but also in the deeper layers. In turn, other soil properties such as soil texture and soil pH were revealed to have relatively little impact on these properties throughout the studied soil profiles, irrespective of the soil-use system and soil depth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14010083/s1, Table S1. Correlation matrix between studied properties throughout the winter wheat profiles; Table S2. Correlation matrix between studied properties throughout the grapevine profiles; Table S3. Correlation matrix between studied properties in the deeper, C-low horizons (C < 4 g kg) (all the studied profiles are considered together); Table S4. Correlation matrix between studied properties in the surface and sub-surface, C-rich horizons (C > 4 g kg) (all the studied profiles are considered together).
Author Contributions
Conceptualization, A.P.-D., J.D., B.K. and M.G.; methodology, A.P.-D., J.D., B.K. and M.G.; software, A.P.-D., J.D., B.K. and M.G.; investigation, A.P.-D., J.D., B.K. and M.G.; resources, A.P.-D. and J.D.; writing—original draft preparation, A.P.-D. and J.D.; writing—review and editing, A.P.-D., B.K. and M.G.; visualization, A.P.-D. and J.D.; project administration, A.P.-D.; funding acquisition, A.P.-D. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland, grant number 2018/29/B/NZ9/00982.
Data Availability Statement
The data used to support the findings of this study are available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Venkatesan, S.; Senthurpandian, V.K. Comparison of enzyme activity with depth under tea plantations and forested sites in south India. Geoderma 2006, 137, 212–216. [Google Scholar]
- Baldrian, P. Distribution of extracellular enzymes in soils: Spatial heterogeneity and determining factors at various scales. Soil Sci. Soc. Am. J. 2014, 78, 11–18. [Google Scholar] [CrossRef]
- Okur, N.; Altindişli, A.; Cengel, M.; Gocmez, S.; Kayikcioğlu, H.H. Microbial biomass and enzyme activity in vineyard soils under organic and conventional farming systems. Turk. J. Agric. For. 2009, 33, 413–423. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Wolińska, A.; Bennicelli, R. Dehydrogenase activity response to soil reoxidation process described as varied condition of water potential, air porosity and oxygen availability. Pol. J. Environ. Stud. 2010, 19, 651–657. [Google Scholar]
- Piotrowska-Długosz, A. Significance of the enzymes associated with soil C and N transformation. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R.S., Pathan, S.I., Ceccherini, M.T., Eds.; Springer Nature: Singapore, 2020; pp. 339–437. [Google Scholar]
- Furtak, K.; Gałązka, A. Enzymatic activity as a popular parameter used to determine the quality of the soil environment. Pol. J. Agron. 2019, 37, 22–30. [Google Scholar]
- Piotrowska-Długosz, A.; Długosz, J.; Gryta, A.; Frac, M. Responses of N-cycling enzyme activities and functional diversity of soil microorganisms to soil depth, pedogenic processes and cultivated plants. Agronomy 2022, 12, 264. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Hu, C.; Liu, B. Long-term urea fertilization alters the composition and increases the abundance of soil ureolytic bacterial communities in an upland soil. FEMS Microb. Ecol. 2019, 95, fiz044. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, A.E., Eds.; Soil Biology Book Series; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 230–243. [Google Scholar]
- Orczewska, A.; Piotrowska, A.; Lemanowicz, J. Soil acid phosphomonoesterase activity and phosphorus forms in ancient and post-agricultural black alder [Alnus glutinosa (L.) Gaertn.] woodlands. Acta Soc. Bot. Pol. 2012, 81, 81–86. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Turner, B.L.; Lambers, H.; Condron, L.M.; Cramer, M.D.; Leake, J.R.; Richardson, A.E.; Smith, S.E. Soil microbial biomass and the fate of phosphorus during long-term ecosystem development. Plant Soil 2013, 367, 225–234. [Google Scholar] [CrossRef]
- Zimmermann, M.; Bird, M.I. Temperature sensitivity of tropical forest soil respiration increase along an altitudinal gradient with ongoing decomposition. Geoderma 2012, 187–188, 8–15. [Google Scholar] [CrossRef]
- van Gestel, N.C.; Reischke, S.; Bååth, E. Temperature sensitivity of bacterial growth in a hot desert soil with large temperature fluctuations. Soil Biol. Biochem. 2013, 65, 180–185. [Google Scholar] [CrossRef]
- Meena, A.; Rao, K.S. Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region, India. Ecol. Process. 2021, 10, 16. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Zhang, B.; Song, K.; Li, X.; Li, J.; Li, F.; Duan, H. Spatial distribution of soil organic carbon and analysis of related factors in croplands of the black soil region, Northeast China. Agric. Ecosyst. Environ. 2006, 113, 73–81. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Bierza, W.; Sierka, E.; Błońska, A.; Besenyei, L.; Woźniak, G. The role of plants and soil properties in the enzyme activities of substrates on hard coal mine spoil heaps. Sci. Rep. 2021, 11, 5155. [Google Scholar] [CrossRef] [PubMed]
- Mganga, K.; Razavi, B.; Kuzyakov, J. Microbial and enzymes response to nutrient additions in soils of Mt. Kilimanjaro region depending on land use. Eur. J. Soil Biol. 2015, 69, 33–40. [Google Scholar] [CrossRef]
- Herold, N.; Schöning, I.; Berner, D.; Haslwimmer, H.; Kandeler, E.; Michalyik, B.; Schrumpf, M. Vertical gradient of potential enzymes activities in soil profiles of European beech, Norwaz spruce and Scots pine dominated forest sites. Pedobiol. J. Soil Ecol. 2014, 57, 181–189. [Google Scholar] [CrossRef]
- Stone, M.M.; De Forest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Maharjan, M.; Sanaullah, M.; Razavi, B.; Kuzyakov, Y. Effect of land use and management practices on microbial biomass and enzyme activities in subtropical top- and sub-soils. Appl. Soil Ecol. 2017, 113, 22–28. [Google Scholar] [CrossRef]
- GUS. Agriculture in 2017, Warsaw. 2018. Available online: http://stat.gov.pl/en/topics/agriculture-forestry/agriculture/agriculture-in-2017,4,14.html (accessed on 15 February 2019).
- Powierzchnia Winnic w Polsce. Available online: https//www.gov.pl/web/kowr/wykazy-rejestry (accessed on 3 December 2023).
- Struktura Winiarstwa w Polsce w 2022 Roku. Available online: https//www.enoloportal.pl/aktualności/struktura-powierzchni-upraw-winorosli-w-polskich-winnicach-2022/ (accessed on 3 December 2023).
- Santillan, D.; Garrote, L.; Iglesias, A.; Sotes, V. Climate change risks and adaptation: New indicators for Mediterranean viticulture. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 881–899. [Google Scholar]
- Lazcano, C.; Decock, C.; Wilson, S.G. Defining and managing for healthy vineyard soils, intersections with the concept of terroir. Front. Environ. Sci. 2020, 8, 68. [Google Scholar] [CrossRef]
- Giagnoni, L.; Maienza, A.; Baronti, S.; Primo Vaccari, F.; Genesio, L.; Taiti, C.; Martellini, T.; Scodellini, R.; Cincinelli, A.; Costa, C.; et al. Long-term soil biological fertility, volatile organic compounds and chemical properties in a vineyard soil after biochar amendment. Geoderma 2019, 344, 127–136. [Google Scholar] [CrossRef]
- Ko, D.; Yoo, G.; Yun, S.T.; Jun, S.C.; Chung, H. Bacterial and fungal community composition across the soil depth profiles in a fallow field. J. Ecol. Environ. 2017, 41, 34. [Google Scholar] [CrossRef]
- Eilers, K.G.; Debenport, S.; Anderson, S.; Fierer, N. Digging deeper to find unique microbial communities: The strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol. Biochem. 2012, 50, 58–65. [Google Scholar] [CrossRef]
- Hsiao, C.J.; Sassenrath, G.F.; Zeglin, L.H.; Hettiarachchi, G.M.; Rice, C.W. Vertical changes of soil microbial properties in claypan soils. Soil Biol. Biochem. 2018, 121, 154–164. [Google Scholar] [CrossRef]
- Marinari, S.; Marabottini, R.; Falsone, G.; Vianello, G.; Antisari, L.V.; Agnelli, A.; Massaccesi, L.; Cocco, S.; Cardelli, V.; Serrani, D.; et al. Mineral weathering and lessivage affect microbial community and enzyme activity in mountain soils. Appl. Soil Ecol. 2021, 167, 104024. [Google Scholar] [CrossRef]
- Rumpel, C.; Kőgel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Schnecker, J.; Wild, B.; Takriti, M.; Alves, R.J.E.; Gentsch, N.; Gittel, A.; Hofer, A.; Klaus, K.; Knoltsch, A.; Lashchinskiy, N.; et al. Microbial community composition shapes enzyme patterns in topsoil and subsoil horizons along a latitudinal transect in Western Siberia. Soil Biol. Biochem. 2015, 83, 106–115. [Google Scholar] [CrossRef]
- Schinner, F.; von Mersi, W. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Browman, M.G.; Tabatabai, M.A. Phosphodiesterase activity of soils. Soil Sci. Soc. Am. J. 1978, 42, 284–290. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Šnajdar, J.; Valášková, V.; Merhautová, V.; Cajthaml, T.; Baldrian, P. Activity and spatial distribution of lignocellulose-degrading enzymes during forest soil colonization by saprotrophic basidiomycetes. Enzyme Microb. Technol. 2008, 43, 186–192. [Google Scholar] [CrossRef]
- Deng, S.; Popova, I. Carbohydrate hydrolases. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; Volume 9, pp. 185–207. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonia. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Kandeler, E. Enzymes Involved in Nitrogen Metabolism. In Methods in Soil Biology; Scinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 163–184. [Google Scholar]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and peptide derivates as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Bach, C.E.; van Horn, D.J.; Warnock, D.D.; Weintraub, M.N. Measuring phenol oxidase and peroxidase activities with pyrogallol, L-DOPA, and ABTS: Effect of assay conditions and soil type. Soil Biol. Biochem. 2013, 76, 183–191. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinsen, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method for measuring microbial biomass in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Olfs, H.W. The variability between different analytical procedures and laboratories for measuring soil microbial biomass C and biomass N by the fumigation extraction method. J. Plant Nutr. Soil Sci. 2011, 161, 51–58. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Polish Norm PN-ISO 11277; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. Polish Committee for Standardization: Warsaw, Poland, 2005.
- Egnér, H.; Riehm, H.; Domingo, W.R. Studies concerning the chemical analysis of soils as background for soil nutrient assessment II: Chemical extracting methods to determinate the phosphorous and potassium content of soil. Kungl. Lantbr. Ann. 1960, 26, 199–215. (In German) [Google Scholar]
- Bashour, I.I.; Sayegh, A.H. Methods of Analysis for Soils of Arid and Semi-Arid Regions; Food and Agriculture Organization of the United States: Rome, Italy, 2007; 128p. [Google Scholar]
- Raiesi, F.; Beheshti, A. Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of northwest Iran. Appl. Soil Ecol. 2014, 75, 63–70. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of soil physico-chemical properties and enzyme activities to long-term reclamation of coastal saline soil, Eastern China. Sci. Total Environ. 2017, 607–608, 1419–1427. [Google Scholar] [CrossRef]
- Silva-Olaya, A.; Mora-Motta, D.A.; Cgerubin, M.R.; Grados, D.; Somenahally, A.; Ortiz-Morea, F.A. Soil enzyme responses to land use change in the tropical rainforest of the Colombian Amazon region. PLoS ONE 2021, 16, e0255669. [Google Scholar] [CrossRef]
- Frąc, M.; Pertile, G.; Panek, J.; Gryta, A.; Oszust, K.; Lipiec, J.; Usowicz, B. Mycobiome Composition and Diversity under the Long-Term Application of Spent Mushroom Substrate and Chicken Manure. Agronomy 2021, 11, 410. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Sas-Paszt, L.; Frąc, M. The status of soil microbiome as affected by the application of phosphorus biofertilizer: Fertilizer enriched with beneficial bacterial strains. Int. J. Mol. Sci. 2020, 21, 8003. [Google Scholar] [CrossRef]
- Xue, T.; Yang, F.; Li, R.; Li, Y.; Xu, G.; Zhang, L. The impact of Viticulture on Soil Characteristics and Microbial Communities in the Ningxia Region of Northwest China. Horticulture 2022, 8, 1097. [Google Scholar] [CrossRef]
- Miguel, D.L.; da Silva, E.R.; da Silva, C.F.; Pereira, M.G.; Leite, L.F.C. Soil microbiological properties and enzyme activity in agroforestry systems compared with monoculture, natural regeneration, and native caatinga. Biosci. J. 2020, 36, 1–16. [Google Scholar] [CrossRef]
- Weinder, S.; Karolak, M.; Karamać, M.; Kosińska, A.; Amarowicz, R. Phenolic compounds and properties of antioxidants in grapevine roots (Vitis vinifera L.) under drought stress followed by recovery. Acta Soc. Bot. Pol. 2009, 78, 97–103. [Google Scholar]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Ma, W.; Guao, Q.; Ahu, X.; Xiang, F. Dominant plant identity determines soil extracellular enzyme activities of its entire community in a semi-arid grassland. Appl. Soil Ecol. 2021, 161, 103872. [Google Scholar] [CrossRef]
- Montecchia, M.; Correa, O.; Soria, M.; Frey, S.; García, A.; Garland, J. Multivariate approach to characterizing soil microbial communities in pristine and agricultural sites in Northwest Argentina. Appl. Soil. Ecol. 2011, 47, 176–183. [Google Scholar] [CrossRef]
- Dick, R.P. A review: Long-term effects of agricultural systems on soil biochemical and microbial parameters. Agric. Ecosys. Environ. 1992, 40, 25–36. [Google Scholar] [CrossRef]
- Mc Carty, G.W.; Bremner, J.M. Regulation of assimilatory nitrate reductase activity in soil by microbial assimilation of ammonium. Proc. Natl Acad. Sci. USA 1992, 89, 453–456. [Google Scholar] [CrossRef]
- Rice, C.W.; Tiedje, J.M. Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol. Biochem. 1989, 21, 597–602. [Google Scholar] [CrossRef]
- Burket, J.Z.; Dick, R.P. Microbial and soil parameters in relation to N mineralization in soils of diverse genesis under differing management systems. Biol. Fertil. Soils 1998, 27, 430–438. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietiö, O.M.; Straková, P.; Prommer, J.; Wild, B.; Hagner, M.; Pihlatie, M.; Fritze, H.; Richter, A.; Heinonsalo, J. Plant roots increase both decomposition and stable organic matter formation in boreal forest soil. Nat. Commun. 2019, 10, 3982. [Google Scholar] [CrossRef] [PubMed]
- Ravenek, J.M.; Bessler, H.; Engels, C.; Scherer-Lorezen, M.; Gesssler, A.; Gockele, A.; De Luca, E.; Temperton, V.M.; Ebeling, A.; Roschner, C.; et al. Long-term study of root biomass in a biodiversity experiment reveals shifts in diversity effects over time. Oikos 2014, 123, 1528–1536. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017, 7, 44641. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.P.; Fuchslueger, L.; Fleischer, K.; Andersen, K.M.; Assis, R.L.; Baccaro, F.B.; Camargo, P.B.; Cordeiro, A.L.; Grandis, A.; Hartley, I.P.; et al. Fine roots stimulate nutrient release during early stages of leaf litter decomposition in a Central Amazon rainforest. Plant Soil 2021, 469, 287–303. [Google Scholar] [CrossRef]
- Aguilera, P.; Ortiz, N.; Becerra, N.; Turrini, A.; Gaínza-Cortés, F.; Silva-Flores, P.; Aguilar-Paredes, A.; Romero, J.K.; Jorquera-Fontena, E.; Mora, M.L.L.; et al. Application of Arbuscular Mycorrhizal Fungi in Vineyards: Water and Biotic Stress Under a Climate Change Scenario: New Challenge for Chilean Grapevine Crop. Front. Microbiol. 2022, 13, 826571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, K.; Luo, Y.; Du, L.; Tian, R.; Wang, S.; Shen, Y.; Zhang, J.; Li, N.; Shao, W.; et al. Responses of soil enzyme activity to long-term nitrogen enrichment and water addition in a typical steppe. Agronomy 2023, 13, 1920. [Google Scholar] [CrossRef]
- Hao, J.; Chai, Y.N.; Lopes, L.D.; Ordóñez, R.A.; Wright, E.; Archontoulis, S.; Schachtman, D.P. The effects of soil depth on the structure of microbial communities in agricultural soils in Iowa (United States). Appl. Environ. Microbiol. 2021, 87, e02673-20. [Google Scholar] [CrossRef]
- Liu, G.; Bai, Z.; Cui, G.; He, W.; Kongling, Z.; Ji, G.; Gong, H.; Li, D. Effects of Land Use on the Soil Microbial Community in the Songnen Grassland of Northeast China. Front. Microbiol. 2022, 13, 865184. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.; Robinson, D.; Yang, Z.; Guo, J.; Xie, J.; Fu, S.; Zhou, L.; Yang, Y. Large amounts of easily decomposable carbon stored in subtropical forest subsoil is associated with r-strategy-dominated soil microbes. Soil Biol. Biochem. 2016, 95, 233–242. [Google Scholar] [CrossRef]
- Dove, N.C.; Arogyaswamy, K.; Billings, S.A.; Botthoff, J.K.; Carey, C.J.; Cisco, C.; De Forest, J.L.; Fairbanks, D.; Fierer, N.; Gallery, R.E.; et al. Continental-scale patterns of extracellular enzyme activity in the subsoil: An overlooked reservoir of microbial activity. Environ. Res. Lett. 2020, 15, 1040a1. [Google Scholar] [CrossRef]
- Eloy Alves, R.J.; Callejas, I.A.; Marschmann, G.L.; Mooshammer, M.; Singh, H.W.; Whitney, B.; Torn, M.S.; Brodie, E.L. Kinetic properties of microbial exoenzymes vary with soil depth but have similar temperature sensitivities through the soil profile. Front. Microbiol. 2021, 12, 735282. [Google Scholar] [CrossRef] [PubMed]
- Kempen, B.; Brus, D.J.; Stoorvogel, J.J. Three-dimensional mapping of soil organic matter content using soil type–specific depth functions. Geoderma 2011, 162, 107–123. [Google Scholar] [CrossRef]
- Długosz, J.; Piotrowska-Długosz, A. Changes in carbon-degrading enzyme activities and microbial biomass content—The effect of soil depth and soil-forming processes. Appl. Soil Ecol. 2022, 180, 104629. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).