Abstract
In response to the challenge of resource recycling, this review investigates the removal of phytotoxicity from agricultural waste for its application as a growing media component. Agricultural waste typically exhibits high phytotoxicity due to the presence of substances such as phenols, organic acids, ammonia, nitrogen, and heavy metals. These substances hinder seed germination and plant growth, posing a significant barrier to the use of agricultural waste as a growing media component. Thus, it is imperative to mitigate or eliminate phytotoxicity before effectively utilizing agricultural waste. This review rigorously analyzes an extensive array of recent studies, scrutinizing diverse technologies for the mitigation of phytotoxicity in agricultural wastes. The methods investigated include the four most common methods—composting, heat treatment, washing, and aging, and a recently introduced method, ammonium incubation. Each method was assessed considering its underlying principles, effects in application, and respective advantages and disadvantages. This review suggests that successful phytotoxicity mitigation in agricultural waste hinges on reducing the content or activity of phytotoxic substances. Moreover, this study emphasizes that future phytotoxicity mitigation efforts should aim for economic efficiency while maximizing the preservation of the original material volume and weight. This study offers insightful guidance for technical professionals aiming to mitigate the phytotoxicity of agricultural waste, thereby fostering sustainable agricultural practices.
Keywords:
agricultural waste; growing media; phytotoxicity; composting; biochar; ammonium incubation 1. Introduction
Soilless cultivation refers to a planting method that does not use natural soil but uses other substances as the growing media for plants [1,2]. In recent years, the demand for soilless cultivation has been growing due to the continuous decrease in arable land, bringing soilless cultivation substrates into the spotlight [3,4]. Owing to its good water retention, fertility retention, and breathability, peat has become the most widely used soilless cultivation substrate worldwide [5,6]. Soilless cultivation with peat substrates can save water resources, improve plant yield and quality, and reduce the occurrence of pests and diseases, and is suitable for the growth of various vegetables, flowers, and ornamental plants [7]. However, excessive peat extraction also brings serious environmental problems such as wetland degradation, biodiversity reduction, and greenhouse gas emissions [5,8]. In the long run, this could potentially result in alterations to the regional climate, which may negatively impact crop growth [9]. In order to protect the environment and resources, it is necessary to explore sustainable peat substrate alternatives [10,11].
Agricultural waste refers to various residues produced in agricultural production and processing, such as poultry and livestock manure, straw, sawdust, fallen leaves, fruit peels, mushroom residue, etc. [12]. These agricultural wastes are mostly plant-derived and contain rich organic matter as well as plant nutrients such as nitrogen, phosphorus, potassium, and trace elements. They have the potential to be used as growing media components [8,11,13,14,15]. Many studies have shown the feasibility of using agricultural waste as a growing media component to replace peat [11,16,17,18,19].
While agricultural waste may serve as an alternative to peat in growing media, numerous studies have observed a notable decrease in biological yield when using this waste as a growing media component. This reduction is typically ascribed to phytotoxicity [20,21]. Phytotoxicity refers to any adverse effect on plants caused by specific substances or growth conditions [21]. The germination index (GI) is usually used to measure the phytotoxicity level of materials. It is generally considered that the material has reached the harmless standard when the GI ≥ 80% [22,23]. Phenolic substances are generally considered to be the main phytotoxic substances in woody waste [24,25,26]. In addition, organic acids, NH4+, heavy metals, and other substances can also cause phytotoxicity in agricultural waste composting [27,28]. Besides phytotoxic substances, certain physicochemical properties of substrate material, including pH and EC levels beyond the optimal range and inadequate aeration, can also cause phytotoxicity [14,29]. Agricultural waste is usually mainly composed of various plant residues and poultry and livestock manure, so it is usually rich in phenolic substances, organic acids, ammonia nitrogen, and other phytotoxic substances [30,31,32]. In addition, agricultural waste also usually contains high levels of pollutants, such as high concentrations of heavy metals and polycyclic aromatic hydrocarbons, which often lead to phytotoxicity [22]. Before being used as a growing media component, the phytotoxicity of agricultural waste must be reduced or eliminated.
Throughout the annals of history, humanity has devised numerous methods to harness agricultural waste for the purpose of bolstering agricultural productivity. Composting stands as the earliest method humans employed to harness the potential of agricultural waste resources [33]. Up to now, comprehensive research has been conducted on composting technologies for diverse types of agricultural waste [31,34]. In recent years, biochar research in agriculture has gained momentum, and considerable attention has been paid to the realm of biomass heat treatment [15,35]. Currently, composting and thermally treated products of agricultural waste are mainly used as soil additives to improve soil fertility, but increasing efforts are tapping into their potential as growing media components [36,37]. When used as soil amendments or fertilizer components, agricultural waste composting or thermally treated products only form a minor fraction of the plant root environment. Thus, the effect of material phytotoxicity may be negligible [38,39]. However, when they are used as growing media, they form the majority of the plant root environment. Consequently, phytotoxicity emerges as an inescapable issue [40,41]. For this reason, increasing research on agricultural waste compost and heat treatment has focused on the issue of phytotoxicity in recent years [42,43,44,45]. In addition, many scholars have explored various new ways to remove phytotoxicity and proposed many valuable insights for the utilization of agricultural waste substrates. Regrettably, to our knowledge, there is currently no systematic elaboration of these valuable studies from the perspective of phytotoxicity removal.
To systematically elucidate research on phytotoxicity removal technologies for agricultural waste as a growing media component, this review undertook a comprehensive literature search across multiple databases. The literature selection and review process adhered to strict standards, ensuring coverage of as many relevant documents as possible. During this process, the study focused on the methods, results, and conclusions of the research, ensuring their scientific validity and accuracy. It also assessed the working principles, application effects, and advantages and disadvantages of each technology. All referenced literature was meticulously recorded and cited, ensuring transparency and traceability.
This review synthesizes several decades of work aimed at mitigating the phytotoxicity of agricultural waste and delves into four common methods—composting, heat treatment, aging, and washing—along with a newly reported rapid detoxification technique called ammonium incubation (incubation treatment with ammonium salt). These technologies generally aim to mitigate phytotoxicity by diminishing the content or activity of phytotoxic substances. This study provides a systematic summary of the principles, application effects, and pros and cons of these technologies, with an emphasis on advancements made to enhance these methods. Considering the substantial volume of agricultural waste, its use as growing media represents an innovative approach to resource utilization. Consequently, this study primarily aims to enrich the comprehension of phytotoxicity and serve as a reference for related research and applications in phytotoxicity mitigation for agricultural waste. This review aspires to draw more scholars into conducting research on the utilization of agricultural waste as growing media, thus contributing to the resolution of resource recycling challenges.
2. Removal of Phytotoxicity by Composting
Composting is a process of decomposing organic matter into stable, humus-like products through microorganisms under controlled conditions [31]. It is essentially the mineralization and humification of organic matter under the action of microorganisms [34]. Composting is generally carried out under aerobic conditions, usually including the stages of heating, high temperature, and maturation [31]. The practical agricultural operation process involves the careful adjustment of the physicochemical properties of the materials, including the C/N ratio and moisture content, with the objective of facilitating the rapid proliferation of microorganisms. This microbial activity, in turn, accelerates the decomposition of readily biodegradable organic matter, ultimately yielding compost products that exhibit a higher level of stability [46,47]. During aerobic composting, microbial metabolic activity releases a large amount of heat, raising the temperature of the pile to above 50 °C. In certain cases, composters may introduce thermophilic bacteria, pushing the temperature even higher, sometimes surpassing 100 °C. This elevated temperature serves to effectively eliminate pathogens, pests, and weed seeds, thereby meeting the necessary criteria for plant growing [48,49,50]. Owing to its simplicity and efficiency, composting has been the most widely used method for harnessing the resource potential of agricultural waste [51,52].
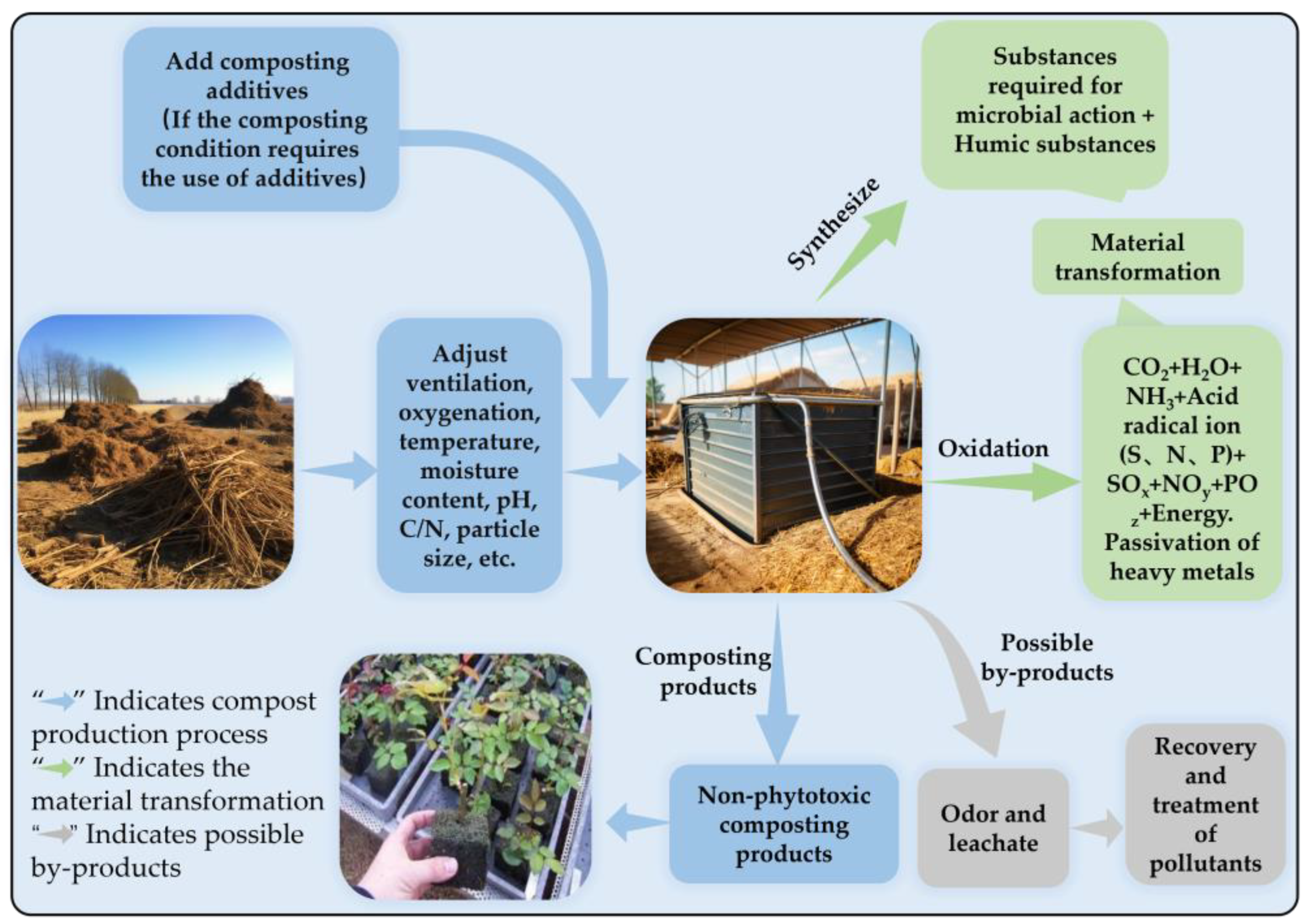
Composting is also the most commonly used method to reduce or eliminate the phytotoxicity of agricultural waste. It can reduce phytotoxicity by decomposing, transforming, and aggregating phytotoxic substances and reducing the bioavailability of toxins (Figure 1). Specifically, composting typically reduces the phytotoxicity of materials by reducing the concentration of organic acids and ammonium ions and the bioavailability of heavy metals in the material. For instance, a study conducted by Wang et al. [28] highlighted that the initial concentrations of acetic acid and butyric acid in raw composting materials exceeded 400 mg/L and 200 mg/L, respectively. However, after the composting process, these organic acids were nearly undetectable in the materials, resulting in a substantial reduction in phytotoxicity. Kong et al. [53] employed farm animal manure for composting. Their findings revealed a negative correlation between the NH4+-N concentration and the GI. Following the composting process, the NH4+-N concentration dropped from an initial level of 300 mg/L to near 0 mg/L, thereby ensuring that the compost material met the harmless criteria. Zhao et al. [54] analyzed the correlation between heavy metal ions and phytotoxicity, confirming that the phytotoxicity of compost products was positively correlated with the content of As, Cd, Hg, Cr, Fe, Mn, and Pb. Tiquia et al. [23] also revealed that the main contributors to the phytotoxicity of pig manure compost were extractable copper, extractable zinc, and NH4+-N. Importantly, these substances gradually decreased during the composting process.
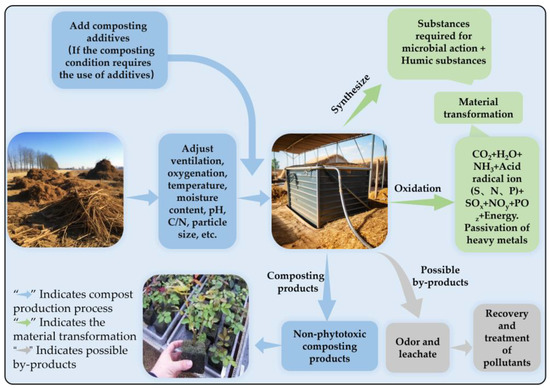
Figure 1.
Diagram of composting process and its phytotoxicity removal mechanism.
Although composting can notably mitigate the phytotoxicity of agricultural waste, it is important to note that this process is relatively time-consuming. The entire composting procedure required to attain full maturation typically spans several months [30]. In addition, some studies have also shown that even after undergoing thorough composting treatment, compost products may still retain phytotoxicity; the variability inherent in the materials, coupled with the differences in composting methods and equipment, can result in diverse composting outcomes [55]. For example, Siles-Castellano et al. [56] analyzed the evolution of phytotoxicity in five compost materials from fifteen industrial composting facilities. The results showed that compost products derived from municipal solid waste and plant residues, even after undergoing composting treatment, always exhibited phytotoxicity. They also revealed that inappropriate EC, pH values, and heavy metal content were the primary factors leading to phytotoxicity in these compost products. To address this issue, there is a growing focus on optimizing composting measures. This involves improving the composting environment in various ways, such as regulating temperature and enhancing ventilation, with the aim of accelerating the composting process, reducing the duration required for compost maturation, and diminishing the phytotoxicity of final compost products. As an example, Tong et al. [57] conducted a comparative study examining the distinctions between static treatment, flipping treatment, forced ventilation treatment, and acidified forced ventilation. Their findings suggested that forced ventilation and acidification could enhance composting efficiency, shorten treatment times, mitigate ammonia volatilization, and reduce greenhouse gas emissions. Rosimara Zittel et al. [58] prepared mixtures using different reactors and different types of waste to carry out reactor compression mixed composting at different stages, thereby generating mature and non-toxic compost faster. Furthermore, the inherent characteristics of compost raw materials, such as C/N and moisture content, may also cause variations in compost outcomes. Rosimara Zittel et al. [59] used tobacco, garden waste, sawdust, and sludge to form substrates with different C/N contents for composting. The experimental group with a C/N of 20.1 exhibited superior detoxification, yielding compost products with the highest GI. Generally speaking, maintaining the C/N of the compost pile within the range of 18–30 and the water content within 60–75% promotes nutrient utilization by microorganisms. This, in turn, facilitates the conversion of phytotoxic organic substances and reduces the bioavailability of heavy metals [60,61].
While adjusting the pile composition and optimizing the process flow can improve compost quality and reduce outcome phytotoxicity, it is worth noting that in practical composting operations, there may be limitations on these measures. Consequently, the application of additives has often been regarded as an efficient and easy-to-master strategy to accelerate composting processes [34]. Some representative cases are shown in Table 1.
Many additives can effectively enhance the humification of compost and reduce phytotoxicity, but their effects on the composting process are different. As an illustration, Wang et al. [62] introduced superphosphate and biological additives into chicken manure compost. Their findings revealed that both additives effectively accelerated the composting process and reduced the phytotoxicity of the material. Superphosphate was found to promote the humification process and reduce phytotoxicity, while biological additives facilitated the formation of precursor substances and the humification process. In Wang et al.’s study [28], it was highlighted that carbon-rich additives, such as mushroom substrates, corn straw, and waste branches, can diminish the levels of total soluble nitrogen, ammonium nitrogen, and low molecular weight organic acids in the compost, thereby contributing to a decrease in phytotoxicity. Sun’s team, as reported in [63], found that adding bean dregs and crab shell powder for composting led to various improvements in compost conditions such as compost temperature, specific surface area, average pore size, pH value (the concentration of hydrogen ions, used to measure the acidity or alkalinity of a solution), and EC value (electrical conductivity, used to measure the concentration of soluble ions in the growing media). These improvements further facilitated microbial growth and enhanced enzyme activity. Moderate use of these two additives accelerated the decomposition of the composting process, shortened the composting duration, and improved the maturity and stability of compost products. Yin et al. [64] conducted composting with the addition of bean dregs and flue gas gypsum. This measure accelerated the degradation of lignocellulose and improved the water retention, nutrient content, pore distribution, and soil structure of the final compost products, and reduced their phytotoxicity. Pei et al. [65] used fruit residue, biochar, and manganese dioxide as additives for agricultural waste composting. Their results proved that these additives could significantly promote metabolic product transformation, optimize bacterial community structure, and effectively remove phytotoxic substances in agricultural waste, thereby improving the GI.
Biochar has gained significant popularity as a compost additive in recent years. Its incorporation into compost can effectively mitigate phytotoxicity by improving the physicochemical properties of the compost mixture. This includes boosting microbial activity, facilitating the decomposition of organic matter, promoting nitrogen conversion processes, diminishing the bioavailability of heavy metals, and enhancing overall compost maturity [66,67]. For example, Chen et al. [46] reported that biochar improved the pore structure of compost materials, leading to enhanced oxygen availability, the prevention of anaerobic environments, and the overall enhancement of compost quality. These effects collectively contributed to a reduction in phytotoxicity. A study by Sánchez-García et al. [68] also showed that biochar could prevent the formation of large chunks of material, thus promoting gas exchange and reducing phytotoxicity caused by poor material structure and gas exchange. In addition to improving the pore structure, Xiao et al. [67] pointed out that biochar could serve as a habitat for microorganisms, providing energy and nutrients for microbial activity, thereby promoting the microbial conversion of phytotoxic substances. In addition, since ammonia nitrogen is a substrate for nitrification, the addition of biochar to compost can accelerate the conversion of ammonia to nitrate and nitrite nitrogen, thus reducing the phytotoxicity caused by high concentrations of ammonia nitrogen [69,70]. A study by Chen et al. [46] proved that biochar can significantly reduce the extractable heavy metal content during the composting process of river bottom sediment and agricultural waste mixtures. Arshad et al. [71] also demonstrated that biochar reduced the bioavailability and migration of certain metal elements during the composting process, thereby reducing the phytotoxicity arising from excessive concentrations of heavy metals in compost materials. Additionally, it is worth noting that biochar contains high levels of FA-like and HA-like substances. These substances can expedite the formation of humus-like substances, thereby promoting a faster maturation of the compost and ensuring it meets harmless standards [72].
In summary, the incorporation of additives has commonly been considered a practical and efficient method to expedite the reduction of phytotoxicity in compost products. Inorganic additives such as metal oxides, minerals, and phosphate fertilizers offer cost-effective and environmentally friendly options; however, their efficacy in phytotoxicity removal is limited. Organic additives, particularly biochar, can address phytotoxicity from multiple aspects but come with a slightly higher cost. Martínez-Gallardo and colleagues [73] demonstrated that the use of bioadditives enhances the maturity of compost. Nevertheless, the applicability of bioadditives in composting requires validation through extensive research. Therefore, the simultaneous use of multiple additives has been proposed as a strategy that can potentially reduce costs, enhance phytotoxicity removal effects, and stabilize outcomes [34]. For example, Qu and colleagues [74] proved that composite additives not only improve compost maturity and phytotoxicity removal effects but also reduce the number and cost of additives. Duan et al. [75] found that because biochar has a larger specific surface area and stability, it provides a suitable habitat for microorganisms. The synergistic effect of biochar with biological additives could significantly accelerate the humification process of cow dung composting. Research by Li and colleagues [76] also confirmed that using 2% peanut shell biochar and 0.5% microbial inoculant as composite additives significantly improved compost maturity and reduced phytotoxicity. However, it is important to note that research on the detoxification of compost using composite additives remains relatively limited. Future studies on the promotion of phytotoxicity reduction in compost may benefit from exploring the combined use of multiple additives.

Table 1.
Changes in physicochemical properties and phytotoxicity using different composting techniques.
Table 1.
Changes in physicochemical properties and phytotoxicity using different composting techniques.
| Raw Material | Composting | Changes in Phytotoxicity-Related Factors (Star → Finish, Conventional Composting vs. Improved Composting) | Changes in Phytotoxicity (GI, Conventional Composting vs. Improved Composting) * | Composting Days Required to Reach Safety Standards (GI > 80%, Conventional Composting vs. Improved Composting) * | References | |||
|---|---|---|---|---|---|---|---|---|
| Conventional | Improved | Phytotoxicity-Related Substances * | pH * | EC *(mS‧cm−1) | ||||
| Urban Green Waste | C/N = 25, water content = 60–70% | Composting with 35% Soybean Residue and 25% Crab Shell Powder Added | / | 6.1 vs. 7.3 | 1.8 → 1.2 vs. 1.5 → 1.8 | 75% vs. 163% | 30 Days vs. Earlier than the Conventional Group | [63] |
| Urban Green Waste | C/N = 25, water content = 60–70% | Composting with the Addition of Flue Gas Desulfurization Gypsum and Silage | Ammonia Nitrogen (mg/kg): 354.44 vs. 73.24–120.24 | 8.3 → 8.2 vs. (7.3–7.4) → (7.5–7.8) | 1.19 → 1.26 vs. (1.1–1.4) → (1.2–1.5) | 76% vs. 115–130% | 42 Days vs. Earlier than the Conventional Group | [64] |
| Chicken Manure + Sawdust | C/N = 18, water content = 65%, | Composting with the Addition of Biochar, Biofungicide, and Superphosphate | Ammonia Nitrogen (mg/L): 600 → 180 vs. (500 -530) → (120 -200) | 7.7 → 8.5 vs. (7.0–7.7) → (8.0–8.5) | 4.15 → 3.8 vs. 4.15 → (3.4–6.0) | 68.9% vs. 89.7–95.2% | Unable to Reach Safety Standards vs. 14 Days | [62] |
| Beer Spent Grain | water content = 60–70% | Composting with the Addition of Biochar at Levels of 0%, 5%, 10%, and 15% | Ammonia Nitrogen (mg/g): 0.06 → 0.30 vs. (0.05–0.06) → (0.10–0.33) | 6.28 → 8.35 vs. (7.01–7.12) → (8.03–8.33) | / | 115% vs. 120–140% | 21 Days vs. 14 Days | [77] |
| Pig Manure + Sawdust | / | Composting with the Addition of Biochar at Levels of 0%, 3%, 5%, and 10% | / | 7.4 vs. 8.27–8.4 | All Treatments Showed No Difference: Around 0.85 mS/cm | 58% vs. 119–171% | / | [78] |
| Urban Green Waste | C/N = 25–30, water content = 60–70% | Composting with 35% Kitchen Waste and 15.5% Montmorillonite Added | Ammonia Nitrogen (mg/kg): 800 → 400 vs. 200 → 0 | 6.2 vs. 7.44 | 1.87 vs. 3.53 | 70% vs. 145% | Unable to Reach Safety Standards vs. Earlier than 21 Days | [79] |
| Chicken Manure + Sawdust | C/N = 25, water content = 65% | Composting with the Addition of Biochar at Levels of 0%, 3%, 5%, and 10% | Ammonia Nitrogen (mg/kg): 300 → 250 vs. 300 → (150–200) | All Treatments Showed Similar Trends: (7.4–7.6) → (8.0–8.1) | 2.25 → 2.8 vs. (2.35–2.45) → (1.9–2.2) | 70% vs. 90–120% | Unable to Reach Safety Standards vs. Earlier than 50 Days | [80] |
| Sheep Manure | water content = 65% | Composting with the Addition of 15% Wet-Weight Mushroom Substrate, Corn Straw, and Garden Waste | Ammonia Nitrogen (mg/L): 300 → 120 vs. (200–240) → (20–60) Small Molecule Acids (No Difference in All Treatments): Formic Acid (mg/L): (10–50) → 10; Propionic Acid (mg/L): (10–20) → (2–10); Acetic Acid (mg/L): (400–800) → 0; Butyric Acid (mg/L): (200–350) → 0 | 8.4 → 8.8 vs. (7.7–7.9) → (8.1–8.4) | 4.7 → 4.7 vs. (4.1–4.3) → (3.5–4.3) | 50% vs. 120–150% | Unable to Reach Safety Standards vs. 14 Days | [28] |
| Sludge, Cigarette Tobacco, Sawdust, Garden Waste, | / | Composting Substrate Mixed with Tobacco, Sludge, Garden Pruning Waste, and Sawdust at C/N = 18.6, 15.5, and 20.1 | / | C/N: 18.6:8.0 → 8.2 C/N: 15.5:7.2 → 8.1 C/N: 20.1:6.6 → 8.1 | C/N: 18.6: 2.2 → 4.1 C/N: 15.5: 2.3 → 4.9 C/N: 20.1: 1.6 → 1.9 | (C/N: 18.6: 30%; C/N: 15.5: 35%; C/N: 20.1: 40%) → (C/N: 18.6: 70%; C/N: 15.5: 92%; C/N: 20.1: 100%) | C/N: 15.5, C/N: 20.1 60–120 Days; C/N: 18.6 Unable to Reach Safety Standards | [58] |
| Plant Straw, and Chicken Manure | C/N = 30, water content = 60% | Composting with the Addition of 5% Biochar, 5% Gypsum, and 5% Biochar + 5% Gypsum, | Ammonia Nitrogen (No Difference in All Treatments, g/kg): 0.3 → 0.1 | 7.5 → 7.3 vs. (7.1–7.5) → (7.0–7.3) | 0.8 → 0.4 vs. (0.8–2.3) → (0.4–1.8) | 90% vs. 120% | 49 days vs. 45–49 days | [74] |
* These data were extracted using Web Plot Digitizer from figures in the original reference.
3. Removing Phytotoxicity by Heat Treatment
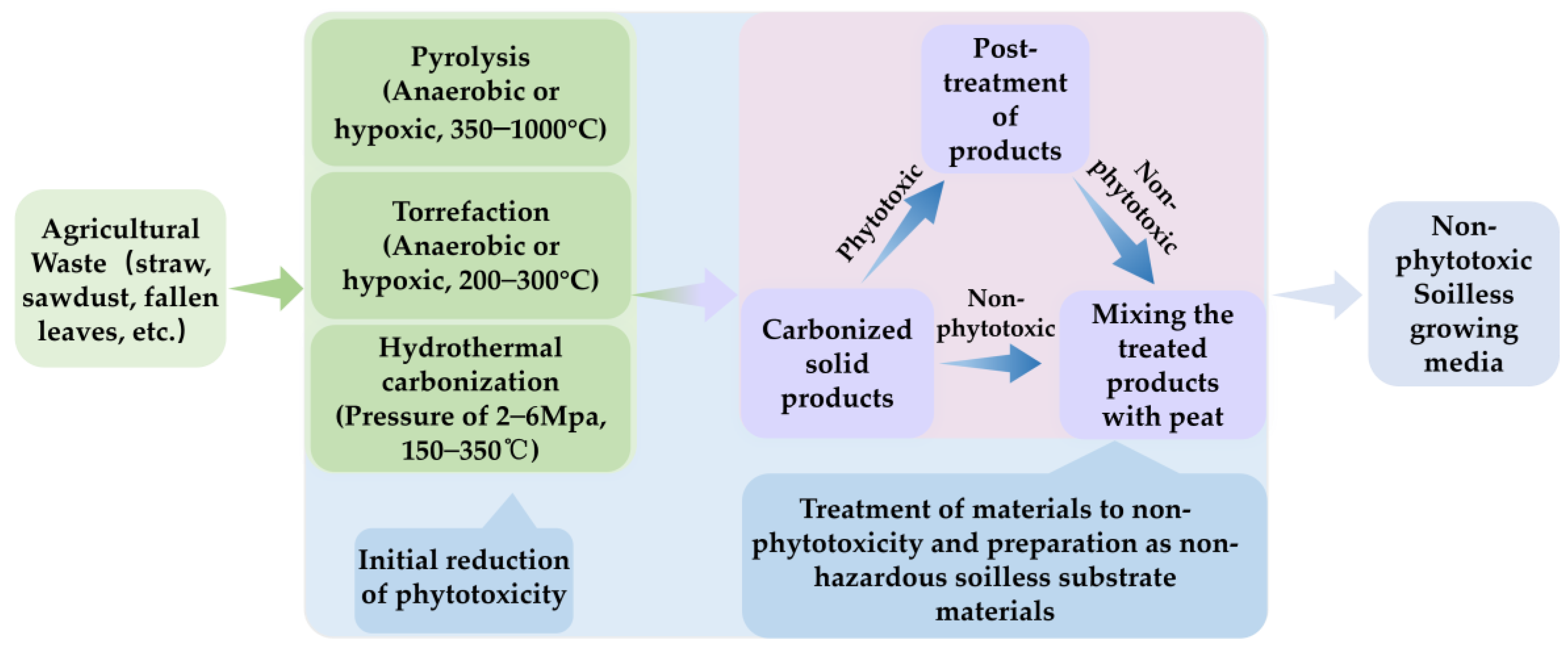
Heat treatments, including pyrolysis, torrefaction, and hydrothermal carbonization (HTC), are common methods for biomass treatment [81,82,83]. The representative product of heat-treated biomass for agricultural applications is biochar. Biochar refers to the solid, carbon-rich part obtained through the thermochemical conversion of biomass in a limited oxygen environment [69]. Pyrolysis, typically conducted under anaerobic or anoxic conditions within a temperature range of 350–1000 °C, results in the gradual decomposition of cellulose and lignin under thermal cracking conditions [84]. Torrefaction is an incomplete pyrolysis process that occurs under anaerobic or low-oxygen conditions at 200–300 °C [85]. Compared with pyrolysis, torrefaction has lower energy consumption. However, due to the lower degree of carbonization, it is generally considered that biomass torrefaction products cannot provide the same carbon sequestration capacity as biochar [86]. As a result, compared with biochar, less attention has been paid to biomass torrefaction products [82]. The HTC process requires a moderate temperature of 150–350 °C and a certain pressure (2–6 Mpa); thus, HTC is particularly suitable for raw materials with a high water content, such as crop straw and sludge [87,88]. Hydrothermal carbonization, with a carbon efficiency as high as 80–100%, significantly outperforms pyrolysis, which has a carbon efficiency of about 50%. Given its similar physicochemical properties to peat, HTC is regarded as a promising technology for using biomass as growing media [89]. Biomass heat treatment products are generally quite stable and have a high porosity and specific surface area. They can significantly improve soil physicochemical properties and improve nutrient utilization efficiency. Therefore, they are widely used in agricultural production. Some common agricultural applications of biomass heat treatment products, such as soil amendments, compost additives, slow-release fertilizer carriers, etc., have been extensively studied [39,67,90,91]. In contrast, there are much fewer studies on biomass heat treatment products as growing media components. However, several scholars have underscored the significant potential of biomass heat treatment products as components of growing media [24,41,92]. Here, this review focuses on reviewing the research on heat treatment to eliminate the phytotoxicity of agricultural waste materials, concludes how production conditions affect the phytotoxicity of the products, and elaborates on their application as growing media components (Figure 2).
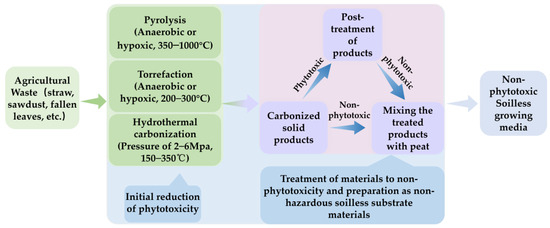
Figure 2.
Schematic diagram of the production process of heat-treated soilless culture substrates.
During the heat treatment process, the phytotoxic components in the material are volatilized/condensed and degraded/generated at high temperatures, resulting in a significant alteration in the phytotoxicity. The alteration is typically manifested as a substantial decrease in the material’s phytotoxicity [93,94,95]. For instance, organic substances like polyphenols were eliminated from a garden waste mixture through the process of slow pyrolysis, enabling the treated materials to meet harmless standards [96]. Steam explosion treatment notably diminished the phenolic content in oak chips. Consequently, the treated oak chips ceased to impede the growth of Chinese cabbage, marking a significant contrast to the pre-treatment phase [97]. Furthermore, after the pyrolysis and hydrothermal carbonization processes of urban sewage sludge, the bioavailability of heavy metals was reduced, and there was a decrease in phytotoxicity [98]. In addition, all hydrochars exhibited superior germination compared to untreated raw materials such as sludge, coffee grounds, and grape pomace [99]. Moreover, after using eucalyptus globulus bark treated by a low-temperature hydrothermal treatment and then mixed with peat as a substrate for cultivating Chinese cabbage, the growth statistics of Chinese cabbage were better than those of commercial substrates, indicating that it is non-phytotoxic [24].
However, materials may still retain phytotoxicity after heat treatment [100]. Mumme et al. [101] measured the phytotoxicity of various biochars. Some heat treatment products still inhibited plant growth, and different biochars showed great variations in phytotoxicity levels. A study by Busch et al. [102] compared the phytotoxicity of biochar and hydrochar products under various processes and found that some hydrochars still had a high level of phytotoxicity, while the overall phytotoxicity of biochar was lower than that of hydrochar. Furthermore, the research results of Bargmann et al. [81] also showed that biochar usually has no effect on seed germination or even has a slight positive effect, while hydrochar usually has a significant negative impact on seed germination. The phytotoxicity of these hydrochars was mainly caused by the presence of water-soluble or volatile organic compounds [81,103,104]. Buss et al. [105] also pointed out that volatile organic compounds (VOCs) with the highest potential to cause phytotoxicity include low-molecular-weight organic acids and phenols, which is attributed to their high mobility. Furthermore, polycyclic aromatic hydrocarbons (PAHs), which are byproducts of the pyrolysis process, can also contribute to phytotoxicity [106,107]. Furthermore, the formation of phytotoxic compounds, such as furans and polycyclic aromatic hydrocarbons, was generated during the pyrolysis process, and these phytotoxic substances then dissolved into the bio-oil. This bio-oil had the potential to be adsorbed onto the biochar following recondensation during the carbonization process, thereby transforming the biochar into a pollutant carrier and leading to high levels of phytotoxicity [103,108,109]. Besides volatile substances, high concentrations of heavy metals are also a main reason for the phytotoxicity of biochar [110,111,112]. In addition, the adsorption of ammonia nitrogen and organic and inorganic composite pollutants by biochar and high pH and EC levels can also have negative effects on plant growth [102,103].
The properties of biochar vary due to different raw materials and heat treatment conditions [39]. The type of raw material, heat treatment conditions (temperature, time, and oxidation conditions), and variations in pre- and post-treatment steps significantly influence the composition of elemental and surface functional groups as well as the pore structure and quantity of biochar [69,113,114]. These alterations result in substantial variations in the properties of biochar, thereby affecting the final outcome of phytotoxicity removal [115,116,117]. Considering the variations in raw materials and heat treatment processes, the effects of heat treatments on phytotoxicity removal as observed in representative studies are listed in Table 2 [81,86,118].

Table 2.
Phytotoxicity and pH of products from different raw materials and heat treatment processes.
Compared with low-temperature biochar, high-temperature biochar often has lower phytotoxicity. This can be attributed to the fact that high temperatures are more conducive to reducing the content of organic pollutants in biochar and reducing the bioavailability of heavy metals [104,119]. However, higher temperatures often mean a higher pH of the product, and high pH is generally not conducive to plant growth. Furthermore, higher temperatures result in increased energy consumption, which, in turn, significantly raises production costs. Compared with high-temperature pyrolysis, hydrothermal carbonization is more efficient and consumes less energy, and largely retains physicochemical properties similar to peat. However, due to the low processing temperature, it cannot effectively eliminate volatile and water-soluble organic phytotoxic substances, nor can it passivate harmful heavy metals, so it is difficult to directly use for growing media components [120,121].
To create high-quality, heat-treated growing media components at a low cost and with minimal energy consumption, numerous studies are concentrating on the post-treatment process of HTC; the aim is to entirely eliminate the phytotoxicity of HTC products (Table 3). Choosing suitable raw materials and optimizing preparation and post-treatment processes have been proven to be feasible methods to reduce the phytotoxicity of HTC. For example, research by Martin Hitzl et al. [90] proposed that secondary heat treatment of prepared HTC at 275 °C can remove more than 99% of volatile organic phytotoxic substances. Research by Intani et al. [122] pointed out that fresh biochar and biochar water extract both have severe phytotoxicity, but the washing treatment of biochar can effectively reduce its phytotoxicity. Similarly, research by Islam [87] showed that untreated biochar, fresh HTC, and aging hydrothermal carbon all have strong phytotoxicity, but washed hydrothermal carbon shows lower phytotoxicity.

Table 3.
Effectiveness of post-treatment strategies for phytotoxicity removal.
4. Removing Phytotoxicity by Washing
Washing is the technique of using water or other solvents to diminish the pollutant content of materials, thereby enhancing their quality and applicability [127]. Initially, research on washing methods was primarily aimed at solving the problem of heavy metal soil pollution, with a focus on reducing the excessive concentrations of these metals in the soil [128]. In recent years, washing has also been used to treat agricultural waste to remove its phytotoxicity [122].
Washing can be categorized into two types based on the type of washing agent used: water washing and chemical washing [129]. Water washing is the simplest and most common method. It uses deionized water to wash contaminated media to dilute and eliminate pollutants. The advantage of water washing is that it is simple to operate and low in cost. The disadvantage is that it is inefficient, requires a large amount of water resources, and may lead to wastewater discharge [130,131]. Chemical washing refers to the use of chemical reagents to treat contaminated materials in order to increase the solubility of harmful substances or alter their chemical properties, thereby accelerating their removal process. Chemical washing is efficient and can choose reagents suitable for specific pollutants, but it is complicated to operate, high in cost, and may produce some phytotoxic or difficult-to-degrade chemicals, causing secondary pollution [132]. As shown in Table 4, different washing agents have their limitations and the properties of commonly used agents can vary significantly [133].

Table 4.
Advantages, scope of application, and disadvantages of detergent types.
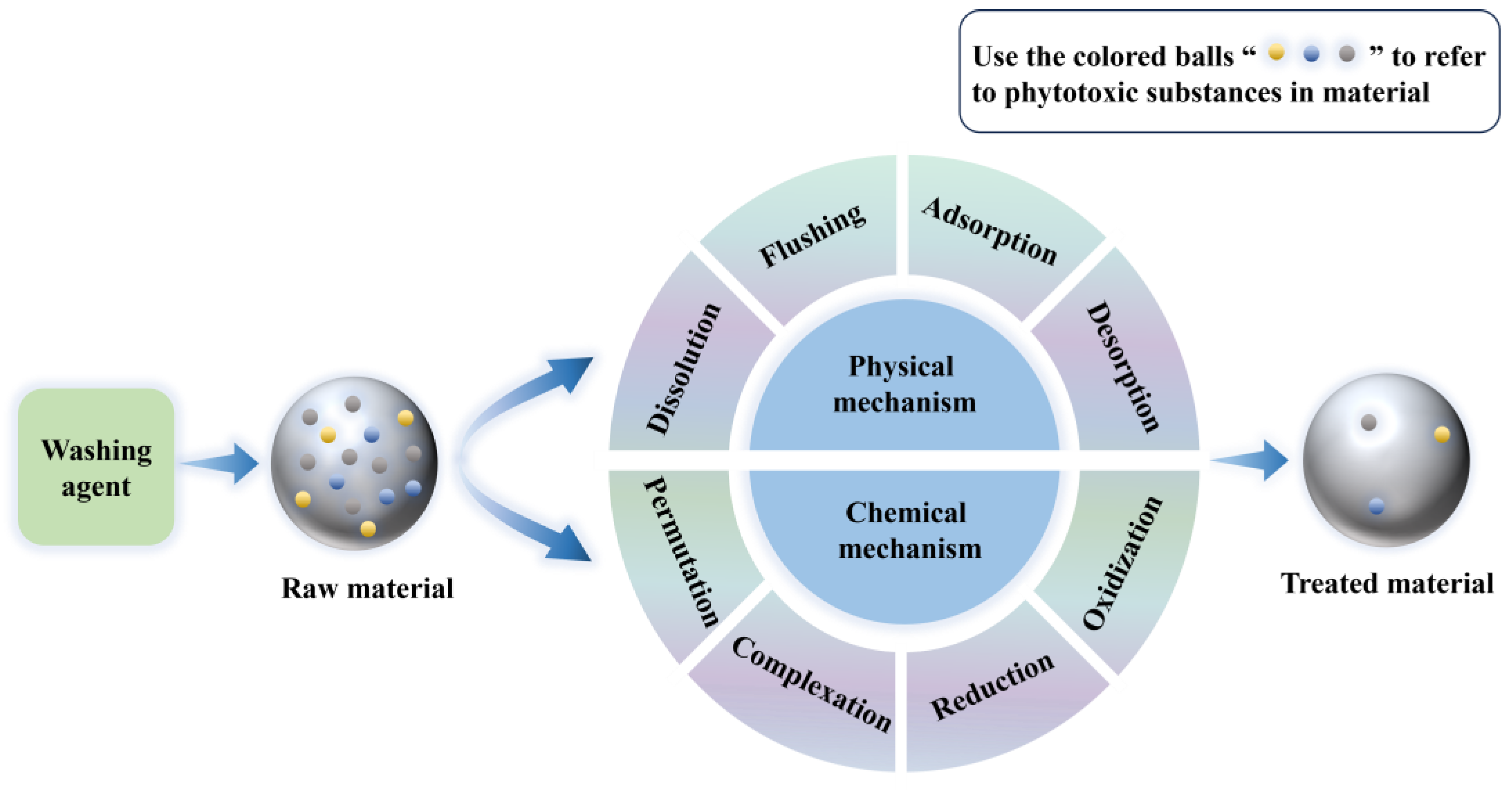
Phytotoxicity removal mechanisms for washing methods can be divided into two categories (Figure 3): physical mechanism—by the flushing, dissolution, adsorption, and desorption of pollutants in the material with water or other washing agents, whereby pollutants are transferred from the solid phase to the liquid phase or gas phase, thereby reducing their content and bioavailability in the material—and chemical mechanism—through oxidation, reduction, complexation, permutation, and other reactions between water or other washing agents and the pollutants in the material, which are transformed into more soluble, less phytotoxic, or more easily degradable forms, thereby reducing their content and bioavailability in the material [133,134]. It is important to note that washing is only suitable for treating some lightly or moderately polluted soils and soilless substrates, especially materials containing soluble or adsorbable harmful substances. For some difficult-to-remove pollutants, such as organochlorine pesticides and polycyclic aromatic hydrocarbons, washing may not achieve the expected effect and may need to be combined with other methods for comprehensive treatment [135,136].

Figure 3.
Mechanism of phytotoxicity removal by washing.
Due to significant differences in the physicochemical properties of different phytotoxic substances and washing agents, the removal effects of washing methods on material phytotoxicity vary [137]. Generally speaking, organic pollutants are more difficult to remove than inorganic pollutants, and high-concentration pollutants are more difficult to remove than low-concentration pollutants [138]. Furthermore, the washing time and frequency affect the contact time and number of washes between pollutants and washing agents. The longer the washing time and the higher the frequency, the better the removal effect, but it may also cause the material to be overly moist. Therefore, it is necessary to determine the appropriate washing time and frequency based on the migration speed of pollutants and the water demand of substrates or plants [130]. Furthermore, different washing agents have different solubilities and affinities for pollutants. Usually, the higher the concentration of the washing agent, the better the removal effect, but a too-high concentration of the washing agent may cause damage to the material. Therefore, it is necessary to choose a suitable washing agent and dosage based on the characteristics of pollutants and the tolerance of substrates or plants [129]. Moreover, the washing temperature and pH will affect the chemical reaction rate and equilibrium state between pollutants and washing agents [139]. The higher the temperature and the farther away from neutral pH, the better the removal effect usually is [140]. But, it is necessary to consider whether too high a temperature and too acidic or alkaline an environment will cause thermal damage or acid-base damage to materials, and pH will greatly affect nutrient element availability [11]. Therefore, it is necessary to control washing temperature and pH value according to pollutant reaction kinetics and use a suitable temperature and pH range for the material [141,142].
Although washing methods can efficiently mitigate phytotoxicity through straightforward procedures and observable outcomes, they also pose challenges, including the consumption and contamination of water resources. Consequently, it is essential to devise more eco-friendly washing technologies; investigate novel, safe washing agents; and establish scientifically sound washing standards and regulations.
5. Removing Phytotoxicity by Aging
Composting, heat treatment, and washing are all technologies that require human intervention to remove phytotoxicity from agricultural waste. Aging treatment refers to the open-air storage of materials and natural weathering for 2–18 months, without adding fertilizers or additives and without adjusting the physicochemical properties of the materials in order to achieve the purpose of removing phytotoxicity [1,143]. Organic carbon, ammonia nitrogen, sulfur, and other elements in plant waste are converted into harmless states such as carbon dioxide, ammonia, nitrate nitrogen, nitrite nitrogen, sulfate, etc. by microorganisms and enzymes [25,144]. Moreover, phytotoxic substances such as phenols, aldehydes, and ketones in plant waste can be removed by aging, while the suitability of the waste as a growing media component can be improved [145].
The efficiency of phytotoxicity removal through natural aging is determined by various factors, primarily environmental temperature, humidity, and the material’s pH value and C/N ratio [146,147,148]. Temperature, humidity, and pH are important factors affecting the activity of microorganisms and enzymes. Too-high or too-low temperatures, humidity, and pH will inhibit the activity and metabolism of microorganisms and enzymes [149,150,151]. The C/N ratio is an important factor affecting the conversion rate of organic carbon and nitrogen [152]. Generally, the more suitable the C/N ratio of the material, the faster the natural aging speed is. Too-high or too-low C/N ratios will lead to the accumulation or deficiency of organic carbon or nitrogen [153]. The most used aging waste is coir and bark [1,80,143,146]. In this review, several cases are used to illustrate aging methods for agricultural waste as a growing media component. Research by Ma and Nichols [154] pointed out that fresh coconut shells have high phytotoxicity and are used as a cultivation substrate to significantly inhibit lettuce growth. After the aging treatment of coconut shells, the phenol content is reduced and the inhibition of lettuce growth is also weakened. Witcher’s team [155] also conducted a phytotoxicity test on fresh and aged pine bark. Although the growth of plants grown in aged pine bark growing media is not as good as that of plants grown in peat substrate, the phytotoxicity is significantly reduced compared to fresh pine bark growing media. Furthermore, Tuckeldoe et al. [156] planted peppers with soil and aged coir growing media, respectively. The planting effect of aged coir growing media was better than soil planting. Furthermore, Buamscha et al. [144] used fresh and aged cedar bark as growing media, respectively. The geraniums planted in aged cedar bark growing media grew faster and had higher leaf nitrogen contents. Similarly, Chemetova et al. [146] found that fresh Acacia melanoxylon bark also has a strong phytotoxic effect, but, after the aging treatment of Acacia melanoxylon bark, phenolic substances were completely removed and no longer inhibited celery germination. And Altland et al. [143] compared the physical and hydraulic properties of fresh pine bark, short-time-aged pine bark, and long-time-aged pine bark. The aged pine bark had a finer particle size and stronger water storage capacity, so it was more suitable for use in soilless cultivation.
Generally, the benefits of natural aging are clear: cost-effectiveness, ease of implementation, and no requirement for chemical additives. However, natural aging is time-consuming, yields inconsistent results, and is limited by the availability of raw materials. These factors make it challenging to industrialize for the production of high-quality growing media [157].
6. Removing Phytotoxicity by Ammonium Incubation
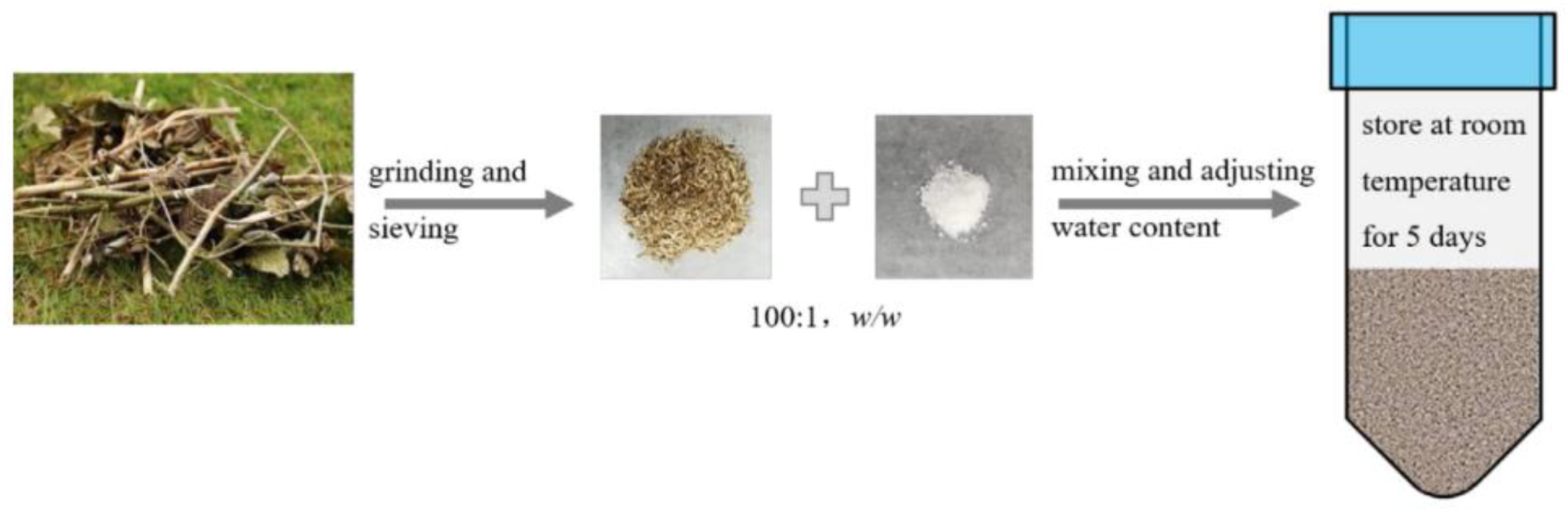
This review provides a comprehensive overview of four common phytotoxicity removal technologies. These four methods are relatively mature and can often achieve the non-phytotoxic standard (GI ≥ 80%). However, each method has its drawbacks. Composting requires an extended processing period and specific equipment investments. It also results in nitrogen loss, inconsistent outcomes, and the emission of waste liquids and gases. Heat treatment demands significant equipment input and energy consumption while emitting substances like bio-oil and biogas. Washing necessitates substantial water and washing agents, leading to considerable chemical wastewater discharge, which is not environmentally friendly. Aging involves an extended processing period along with waste liquid discharge. These limitations restrict their application in the industrial utilization of agricultural waste as growing media. For the industrial utilization of agricultural waste as growing media, prerequisites include shorter processing times, ease of operation, reproducibility, and stable yield and quality. Additionally, consideration must be given to cost reduction and minimizing environmental pollution. Recently, Zhou and colleagues [158] conducted an evaluation of the phytotoxicity of six common plant wastes in southwestern China. They highlighted a strong correlation between the intensity of phytotoxicity and the content of organic acids in these wastes. Drawing on these findings, they suggested a novel approach for mitigating the phytotoxicity of green waste called ammonium incubation. The basic idea of ammonium incubation is to have a specific detoxifying agent react chemically with the phytotoxic substances in the material and reduce the activity or concentration of the phytotoxic substances, thereby eliminating or reducing the phytotoxicity. Specifically, they mixed green waste and ammonium salts (ammonium carbonate, ammonium bicarbonate, etc.) at a mass ratio of 1–2%, adjusted the moisture content to 60–70%, and then placed them at room temperature for 3–7 days (Figure 4). The experiment showed that this technology could significantly reduce the content of organic acids and their derivatives in green waste within 5 days, increasing the seed germination index (GI) of representative green waste mixtures from less than 5% to more than 100%. During the ammonium incubation process of green waste, complex biological and non-biological reactions involving ammonium salts, organic acids, and other phytotoxic substances may occur, thereby reducing the phytotoxicity of green waste. This treatment process had low energy consumption and low pollution and very good prospects for industrial application.
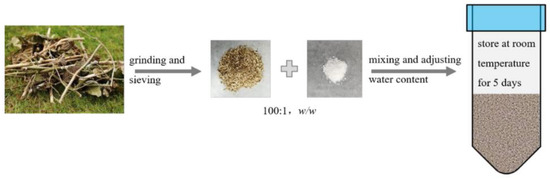
Figure 4.
The rapid detoxification process (quoted from the original reference).
7. Conclusions and Future Research Expected
Recent research on phytotoxicity removal technologies for agricultural waste as a growing media component has been examined in this review. Four common methods (composting, heat treatment, washing, aging) and a new technology called ammonium incubation have been identified as effective in significantly reducing the content or bioavailability of phytotoxic substances. The main findings indicate that while these methods can transform agricultural waste into nearly harmless growing media materials, they also present several disadvantages such as inconsistent product quality, high energy consumption, loss of volume and weight, lengthy processing times, and secondary pollution. The advantages and disadvantages of the various methods are compared in Table 5.

Table 5.
The advantages and disadvantages of phytotoxicity removal technologies.
Given these findings, future efforts should focus on optimizing these phytotoxicity removal technologies to minimize material loss and processing costs while avoiding secondary pollution. Furthermore, maintaining the original volume of agricultural waste and removing its phytotoxicity should be prioritized when it is utilized as a growing media component as soilless cultivation requires a large volume and weight of the substrate [159]. The implications of this study suggest that future research could explore economical and environmentally friendly washing agents and efficient washing technologies. Additionally, the chemical detoxification route based on detoxifying agents warrants further attention. This method can achieve rapid and nearly zero-energy-consumption removal of phytotoxicity while preserving the volume, weight, and physicochemical properties of the raw materials. This approach could particularly benefit the industrial use of agricultural waste as a growing media component. In conclusion, this review underscores the importance of thoroughly investigating the sources of phytotoxicity in different agricultural wastes, identifying the composition of their phytotoxic substances, and developing efficient removal methods. The findings are hoped to inspire further research in this area, contributing to the resolution of resource recycling challenges.
Author Contributions
Conceptualization, W.C. and W.Z.; software, Q.B.; validation, J.L.; formal analysis, W.C. and J.C.; resources, Q.B.; data curation, W.C. and J.L.; writing—original draft preparation, W.C.; writing—review and editing, W.C., Q.B., J.C. and W.Z.; supervision, W.Z.; project administration, Z.Q. and W.Z.; funding acquisition, Z.Q. and W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Science and Technology Program, grant numbers 2021JDRC0025 and 2022YFN0028; Hunan Provincial Natural Science Foundation, grant number 2021JJ30296; Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences, grant number ASTIP2023-34-IUA-04; and Local Financial Project of the National Agricultural Science and Technology Center, grant number NASC2023ST09.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the School of Mechanical Engineering, Chengdu University; Institute of Urban Agriculture, Chinese Academy of Agricultural Sciences; and Chengdu National Agricultural Science and Technology Center for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Türkten, H.; Ceyhan, V. Environmental Efficiency in Greenhouse Tomato Production Using Soilless Farming Technology. J. Clean. Prod. 2023, 398, 136482. [Google Scholar] [CrossRef]
- Caputo, S.; Rumble, H.; Schaefer, M. “I like to Get My Hands Stuck in the Soil”: A Pilot Study in the Acceptance of Soil-Less Methods of Cultivation in Community Gardens. J. Clean. Prod. 2020, 258, 120585. [Google Scholar] [CrossRef]
- Li, H.; Song, W. Spatial Transformation of Changes in Global Cultivated Land. Sci. Total Environ. 2023, 859, 160194. [Google Scholar] [CrossRef]
- Amesbury, M.J.; Gallego-Sala, A.; Loisel, J. Peatlands as Prolific Carbon Sinks. Nat. Geosci. 2019, 12, 880–881. [Google Scholar] [CrossRef]
- Gruda, N. Sustainable peat alternative growing media. Acta Hortic. 2012, 927, 973–979. [Google Scholar] [CrossRef]
- Jayasinghe, G.Y.; Arachchi, I.D.L.; Tokashiki, Y. Evaluation of Containerized Substrates Developed from Cattle Manure Compost and Synthetic Aggregates for Ornamental Plant Production as a Peat Alternative. Resour. Conserv. Recycl. 2010, 54, 1412–1418. [Google Scholar] [CrossRef]
- Maher, M.; Prasad, M.; Raviv, M. Organic Soilless Media Components. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2008; pp. 459–504. ISBN 978-0-444-52975-6. [Google Scholar]
- Xue, Y.; Bai, X.; Zhao, C.; Tan, Q.; Li, Y.; Luo, G.; Wu, L.; Chen, F.; Li, C.; Ran, C.; et al. Spring Photosynthetic Phenology of Chinese Vegetation in Response to Climate Change and Its Impact on Net Primary Productivity. Agric. For. Meteorol. 2023, 342, 109734. [Google Scholar] [CrossRef]
- García-Mier, L.; Guevara-González, R.; Mondragón-Olguín, V.; Del Rocío Verduzco-Cuellar, B.; Torres-Pacheco, I. Agriculture and Bioactives: Achieving Both Crop Yield and Phytochemicals. Int. J. Mol. Sci. 2013, 14, 4203–4222. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Muylle, H.; De Windt, I.; Van Acker, J.; Ameloot, N.; Moreaux, K.; Coucke, P.; Debode, J. Plant Fibers for Renewable Growing Media: Potential of Defibration, Acidification or Inoculation with Biocontrol Fungi to Reduce the N Drawdown and Plant Pathogens. J. Clean. Prod. 2018, 203, 1143–1154. [Google Scholar] [CrossRef]
- Li, Z. Resource Utilization of Agricultural Solid Waste. J. Integr. Agric. 2021, 20, 1119–1120. [Google Scholar] [CrossRef]
- Chemetova, C.; Fabião, A.; Gominho, J.; Ribeiro, H. Range Analysis of Eucalyptus Globulus Bark Low-Temperature Hydrothermal Treatment to Produce a New Component for Growing Media Industry. Waste Manag. 2018, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naasz, R.; Caron, J.; Legault, J.; Pichette, A. Efficiency Factors for Bark Substrates: Biostability, Aeration, or Phytotoxicity. Soil Sci. Soc. Am. J. 2009, 73, 780–791. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, Y.; Zhao, J.; Ouyang, X.; Zhao, Y.; Weng, L.; Md Yasir, A.; Chen, Y.; Li, Y. Effect of Agricultural Organic Inputs on Nanoplastics Transport in Saturated Goethite-Coated Porous Media: Particle Size Selectivity and Role of Dissolved Organic Matter. Environ. Sci. Technol. 2022, 56, 3524–3534. [Google Scholar] [CrossRef]
- Agarwal, P.; Saha, S.; Hariprasad, P. Agro-Industrial-Residues as Potting Media: Physicochemical and Biological Characters and Their Influence on Plant Growth. Biomass Convers. Biorefin. 2023, 13, 9601–9624. [Google Scholar] [CrossRef]
- Nocentini, M.; Panettieri, M.; García De Castro Barragán, J.M.; Mastrolonardo, G.; Knicker, H. Recycling Pyrolyzed Organic Waste from Plant Nurseries, Rice Production and Shrimp Industry as Peat Substitute in Potting Substrates. J. Environ. Manag. 2021, 277, 111436. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Deppe, N.A.; Palmquist, D.E.; Berhow, M.A. Extracted Sweet Corn Tassels as a Renewable Alternative to Peat in Greenhouse Substrates. Ind. Crops Prod. 2011, 33, 514–517. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of Biochars Derived from Wood Pellets and Pelletized Wheat Straw as Replacements for Peat in Potting Substrates. Ind. Crops Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Machrafi, Y.; Prévost, D.; Beauchamp, C.J. Toxicity of Phenolic Compounds Extracted from Bark Residues of Different Ages. J. Chem. Ecol. 2006, 32, 2595–2615. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of Composting on Phytotoxicity of Spent Pig-Manure Sawdust Litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tiquia, S.M. Reduction of Compost Phytotoxicity during the Process of Decomposition. Chemosphere 2010, 79, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Chemetova, C.; Mota, D.; Fabião, A.; Gominho, J.; Ribeiro, H. Low-Temperature Hydrothermally Treated Eucalyptus Globulus Bark: From by-Product to Horticultural Fiber-Based Growing Media Viability. J. Clean. Prod. 2021, 319, 128805. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, I.S.; Park, E.; Cho, B.-K.; Lee, C.W.; Choi, J.M. Changes in the Chemical Properties of Coir Dust with Increasing Aging Time and Development of a Method for Determining Moderate Aging Degree. Hortic. Environ. Biotechnol. 2021, 62, 547–557. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, X.; Qi, Z.; Wang, H.; Yang, R.; Lin, W.; Li, J.; Zhou, W.; Ronsse, F. Superheated Steam as Carrier Gas and the Sole Heat Source to Enhance Biomass Torrefaction. Bioresour. Technol. 2021, 331, 124955. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, P.; Khwairakpam, M.; Kalamdhad, A.S. Bio-Inherent Attributes of Water Hyacinth Procured from Contaminated Water Body–Effect of Its Compost on Seed Germination and Radicle Growth. J. Environ. Manag. 2020, 257, 109990. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, Y.; Kong, Y.; Ma, R.; Yuan, J.; Li, G. Key Factors Affecting Seed Germination in Phytotoxicity Tests during Sheep Manure Composting with Carbon Additives. J. Hazard. Mater. 2022, 421, 126809. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent Mushroom Substrates as Component of Growing Media for Germination and Growth of Horticultural Plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef]
- Fourti, O. The Maturity Tests during the Composting of Municipal Solid Wastes. Resour. Conserv. Recycl. 2013, 72, 43–49. [Google Scholar] [CrossRef]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive Review on Agricultural Waste Utilization and High-Temperature Fermentation and Composting. Biomass Convers. Biorefin. 2023, 13, 5445–5468. [Google Scholar] [CrossRef]
- Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Olive Mill Waste Composting: A Review. Int. Biodeterior. Biodegrad. 2013, 85, 108–119. [Google Scholar] [CrossRef]
- Engledow, F.L. The Waste Products of Agriculture: Their Utilization as Humus. Nature 1931, 128, 854–855. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Li, Y.; Liu, Y.; Jiang, H.; Li, H.; Yuan, Y.; Chen, Y.; Zou, B. Improving the Humification by Additives during Composting: A Review. Waste Manag. 2023, 158, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Suarez, E.; Tobajas, M.; Mohedano, A.F.; Reguera, M.; Esteban, E.; De La Rubia, A. Effect of Garden and Park Waste Hydrochar and Biochar in Soil Application: A Comparative Study. Biomass Convers. Biorefin. 2023, 13, 16479–16493. [Google Scholar] [CrossRef]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Panainte-Lehadus, M.; Mosnegutu, E.; Tomozei, C.; Chitimus, D.; Irimia, O. Assessment of Manure Compost Used as Soil Amendment—A Review. Processes 2023, 11, 1167. [Google Scholar] [CrossRef]
- Islam, M.A.; Paul, J.; Akter, J.; Islam, M.A.; Limon, S.H. Conversion of Chicken Feather Waste via Hydrothermal Carbonization: Process Optimization and the Effect of Hydrochar on Seed Germination of Acacia auriculiformis. J. Mater. Cycles Waste Manag. 2021, 23, 1177–1188. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.; Grünhage, L.; Müller, C. Simple Biotoxicity Tests for Evaluation of Carbonaceous Soil Additives: Establishment and Reproducibility of Four Test Procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef]
- Gell, K.; Van Groenigen, J.; Cayuela, M.L. Residues of Bioenergy Production Chains as Soil Amendments: Immediate and Temporal Phytotoxicity. J. Hazard. Mater. 2011, 186, 2017–2025. [Google Scholar] [CrossRef]
- Basirat, M.; Mousavi, S.M.; Dehghani, F.; Davoudi, M.H. Exploratory Research on the Adoption of New Organic Wastes for Production of Greenhouse Cucumber in Soilless Culture. Waste Biomass Valor 2023, 14, 2367–2374. [Google Scholar] [CrossRef]
- Roehrdanz, M.; Greve, T.; De Jager, M.; Buchwald, R.; Wark, M. Co-Composted Hydrochar Substrates as Growing Media for Horticultural Crops. Sci. Hortic. 2019, 252, 96–103. [Google Scholar] [CrossRef]
- Batista-Barwinski, M.J.; Venturieri, G.A.; Miller, P.R.M.; Testolin, R.C.; Niero, G.; Somensi, C.A.; Almerindo, G.I.; Ariente-Neto, R.; Radetski, C.M.; Cotelle, S. Swine Slaughterhouse Biowaste: An Environmental Sustainability Assessment of Composting, Amended Soil Quality, and Phytotoxicity. Environ. Technol. 2022, 1–8. [Google Scholar] [CrossRef]
- Carballo, T.; Gil, M.V.; Calvo, L.F.; Morán, A. The Influence of Aeration System, Temperature and Compost Origin on the Phytotoxicity of Compost Tea. Compos. Sci. Util. 2009, 17, 127–139. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Cheng, H.; Luo, Y.; Oh, K.; Meng, X.; Zhang, H.; Liu, N.; Chang, M. Seedling Establishment Test for the Comprehensive Evaluation of Compost Phytotoxicity. Sustainability 2022, 14, 11920. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, Y.; Wu, Y.; Chen, Y.; Zeng, G.; Zhang, J.; Li, H. Influence of Biochar on Heavy Metals and Microbial Community during Composting of River Sediment with Agricultural Wastes. Bioresour. Technol. 2017, 243, 347–355. [Google Scholar] [CrossRef]
- Moreno, J.; López, M.J.; Vargas-García, M.C.; Suárez-Estrella, F. Recent advances in microbial aspects of compost production and use. Acta Hortic. 2013, 1013, 443–457. [Google Scholar] [CrossRef]
- Koyama, M.; Nagao, N.; Syukri, F.; Rahim, A.A.; Kamarudin, M.S.; Toda, T.; Mitsuhashi, T.; Nakasaki, K. Effect of Temperature on Thermophilic Composting of Aquaculture Sludge: NH3 Recovery, Nitrogen Mass Balance, and Microbial Community Dynamics. Bioresour. Technol. 2018, 265, 207–213. [Google Scholar] [CrossRef]
- Nakasaki, K.; Araya, S.; Mimoto, H. Inoculation of Pichia Kudriavzevii RB1 Degrades the Organic Acids Present in Raw Compost Material and Accelerates Composting. Bioresour. Technol. 2013, 144, 521–528. [Google Scholar] [CrossRef]
- Vinnerås, B. Sanitation and Hygiene in Manure Management. In Animal Manure Recycling; Sommer, S.G., Christensen, M.L., Schmidt, T., Jensen, L.S., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 91–104. ISBN 978-1-118-48853-9. [Google Scholar]
- Yu, Z.; Liu, X.; Zhao, M.; Zhao, W.; Liu, J.; Tang, J.; Liao, H.; Chen, Z.; Zhou, S. Hyperthermophilic Composting Accelerates the Humification Process of Sewage Sludge: Molecular Characterization of Dissolved Organic Matter Using EEM–PARAFAC and Two-Dimensional Correlation Spectroscopy. Bioresour. Technol. 2019, 274, 198–206. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Yang, T.; Wei, Z.; Li, Y.; Wei, Y.; Chen, X.; Wang, L. Effects of Exogenous Protein-like Precursors on Humification Process during Lignocellulose-like Biomass Composting: Amino Acids as the Key Linker to Promote Humification Process. Bioresour. Technol. 2019, 291, 121882. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, G.; Chen, W.; Yang, Y.; Ma, R.; Li, D.; Shen, Y.; Li, G.; Yuan, J. Phytotoxicity of Farm Livestock Manures in Facultative Heap Composting Using the Seed Germination Index as Indicator. Ecotoxicol. Environ. Saf. 2022, 247, 114251. [Google Scholar] [CrossRef]
- Zhao, X.; Xi, B.; He, X.; Li, D.; Tan, W.; Zhang, H.; Wang, X.; Yang, C. The Impacts of Metal Ions on Phytotoxicity Mediate by Microbial Community during Municipal Solid Waste Composting. J. Environ. Manag. 2019, 242, 153–161. [Google Scholar] [CrossRef]
- Siles-Castellano, A.B.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Estrella-González, M.J.; Moreno, J. Industrial Composting of Low Carbon/Nitrogen Ratio Mixtures of Agri-Food Waste and Impact on Compost Quality. Bioresour. Technol. 2020, 316, 123946. [Google Scholar] [CrossRef]
- Siles-Castellano, A.B.; López, M.J.; López-González, J.A.; Suárez-Estrella, F.; Jurado, M.M.; Estrella-González, M.J.; Moreno, J. Comparative Analysis of Phytotoxicity and Compost Quality in Industrial Composting Facilities Processing Different Organic Wastes. J. Clean. Prod. 2020, 252, 119820. [Google Scholar] [CrossRef]
- Tong, B.; Wang, X.; Wang, S.; Ma, L.; Ma, W. Transformation of Nitrogen and Carbon during Composting of Manure Litter with Different Methods. Bioresour. Technol. 2019, 293, 122046. [Google Scholar] [CrossRef]
- Zittel, R.; Da Silva, C.P.; Domingues, C.E.; Seremeta, D.C.H.; Estrada, R.A.; De Campos, S.X. Composting of Smuggled Cigarettes Tobacco and Industrial Sewage Sludge in Reactors: Physicochemical, Phytotoxic and Spectroscopic Study. Waste Manag. 2018, 79, 537–544. [Google Scholar] [CrossRef]
- Zittel, R.; Pinto Da Silva, C.; Domingues, C.E.; De Oliveira Stremel, T.R.; De Almeida, T.E.; Vieira Damiani, G.; Xavier De Campos, S. Treatment of Smuggled Cigarette Tobacco by Composting Process in Facultative Reactors. Waste Manag. 2018, 71, 115–121. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of Aeration Rate, C/N Ratio and Moisture Content on the Stability and Maturity of Compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Prasher, S.O.; Yan, B.; Ou, Y.; Cui, H.; Cui, Y. Organic Matter, a Critical Factor to Immobilize Phosphorus, Copper, and Zinc during Composting under Various Initial C/N Ratios. Bioresour. Technol. 2019, 289, 121745. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Yang, Y.; Ma, R.; Shen, Y.; Li, G.; Yuan, J. Superphosphate, Biochar, and a Microbial Inoculum Regulate Phytotoxicity and Humification during Chicken Manure Composting. Sci. Total Environ. 2022, 824, 153958. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Effects of Bean Dregs and Crab Shell Powder Additives on the Composting of Green Waste. Bioresour. Technol. 2018, 260, 283–293. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, L.; Li, R. Effects of Additives on Physical, Chemical, and Microbiological Properties during Green Waste Composting. Bioresour. Technol. 2021, 340, 125719. [Google Scholar] [CrossRef]
- Pei, F.; Cao, X.; Sun, Y.; Kang, J.; Ren, Y.; Ge, J. Manganese Dioxide Eliminates the Phytotoxicity of Aerobic Compost Products and Converts Them into a Plant Friendly Organic Fertilizer. Bioresour. Technol. 2023, 373, 128708. [Google Scholar] [CrossRef]
- Li, Y.; Kumar Awasthi, M.; Sindhu, R.; Binod, P.; Zhang, Z.; Taherzadeh, M.J. Biochar Preparation and Evaluation of Its Effect in Composting Mechanism: A Review. Bioresour. Technol. 2023, 384, 129329. [Google Scholar] [CrossRef]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent Developments in Biochar Utilization as an Additive in Organic Solid Waste Composting: A Review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Alburquerque, J.A.; Sánchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar Accelerates Organic Matter Degradation and Enhances N Mineralisation during Composting of Poultry Manure without a Relevant Impact on Gas Emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef]
- Ferraro, G.; Pecori, G.; Rosi, L.; Bettucci, L.; Fratini, E.; Casini, D.; Rizzo, A.M.; Chiaramonti, D. Biochar from Lab-Scale Pyrolysis: Influence of Feedstock and Operational Temperature. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Zhu, Z.; Zhao, Y.; Jia, L.; Lv, P. Humus Formation Driven by Ammonia-Oxidizing Bacteria during Mixed Materials Composting. Bioresour. Technol. 2020, 311, 123500. [Google Scholar] [CrossRef]
- Arshad, M.; Khan, A.H.A.; Hussain, I.; Badar-uz-Zaman; Anees, M.; Iqbal, M.; Soja, G.; Linde, C.; Yousaf, S. The Reduction of Chromium (VI) Phytotoxicity and Phytoavailability to Wheat (Triticum aestivum L.) Using Biochar and Bacteria. Appl. Soil Ecol. 2017, 114, 90–98. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Luo, C.; Shao, L.; He, P. Humification Characterization of Biochar and Its Potential as a Composting Amendment. J. Environ. Sci. 2014, 26, 390–397. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; Estrella-González, M.J.; Suárez-Estrella, F.; López-González, J.A.; Jurado, M.M.; Toribio, A.J.; López, M.J. Effect of Upstream Bioactivation of Plant Residues to Accelerate the Composting Process and Improve Product Quality. Agronomy 2023, 13, 1638. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Zhang, X.; Gao, L.; Tian, Y. Biochar Combined with Gypsum Reduces Both Nitrogen and Carbon Losses during Agricultural Waste Composting and Enhances Overall Compost Quality by Regulating Microbial Activities and Functions. Bioresour. Technol. 2020, 314, 123781. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Verma, S.; Wang, Q.; Chen, H.; Ren, X.; Zhang, Z.; Awasthi, M.K. Positive Impact of Biochar Alone and Combined with Bacterial Consortium Amendment on Improvement of Bacterial Community during Cow Manure Composting. Bioresour. Technol. 2019, 280, 79–87. [Google Scholar] [CrossRef]
- Li, R.; Meng, H.; Zhao, L.; Zhou, H.; Shen, Y.; Zhang, X.; Ding, J.; Cheng, H.; Wang, J. Study of the Morphological Changes of Copper and Zinc during Pig Manure Composting with Addition of Biochar and a Microbial Agent. Bioresour. Technol. 2019, 291, 121752. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, H.; Zhao, X.; Cui, H.; Wei, Z. Reducing Nitrogen Loss and Phytotoxicity during Beer Vinasse Composting with Biochar Addition. Waste Manag. 2017, 61, 150–156. [Google Scholar] [CrossRef]
- Milon, A.R.; Chang, S.W.; Ravindran, B. Biochar Amended Compost Maturity Evaluation Using Commercial Vegetable Crops Seedlings through Phytotoxicity Germination Bioassay. J. King Saud. Univ. Sci. 2022, 34, 101770. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Food Waste and Montmorillonite Contribute to the Enhancement of Green Waste Composting. Process Saf. Environ. Prot. 2023, 170, 983–998. [Google Scholar] [CrossRef]
- Chung, W.J.; Chang, S.W.; Chaudhary, D.K.; Shin, J.; Kim, H.; Karmegam, N.; Govarthanan, M.; Chandrasekaran, M.; Ravindran, B. Effect of Biochar Amendment on Compost Quality, Gaseous Emissions and Pathogen Reduction during in-Vessel Composting of Chicken Manure. Chemosphere 2021, 283, 131129. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass Torrefaction: An Overview on Process Parameters, Economic and Environmental Aspects and Recent Advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Lustosa Filho, J.F.; Melo, L.C.A.; De Assis, I.R.; De Oliveira, T.S. Influence of Pyrolysis Temperature and Feedstock on the Properties of Biochars Produced from Agricultural and Industrial Wastes. J. Anal. Appl. Pyrolysis 2020, 149, 104839. [Google Scholar] [CrossRef]
- Hans-Peter Schmidt European Biochar Certificate (EBC)—Guidelines, version 6.1; European Biochar Foundation (EBC): Arbaz, Switzerland, 2015. [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A State-of-the-Art Review of Biomass Torrefaction, Densification and Applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Backer, R.; Ghidotti, M.; Schwinghamer, T.; Saeed, W.; Grenier, C.; Dion-Laplante, C.; Fabbri, D.; Dutilleul, P.; Seguin, P.; Smith, D.L. Getting to the Root of the Matter: Water-Soluble and Volatile Components in Thermally-Treated Biosolids and Biochar Differentially Regulate Maize (Zea mays) Seedling Growth. PLoS ONE 2018, 13, e0206924. [Google Scholar] [CrossRef]
- Islam, M.A.; Limon, M.S.H.; Romić, M.; Islam, M.A. Hydrochar-Based Soil Amendments for Agriculture: A Review of Recent Progress. Arab. J. Geosci. 2021, 14, 102. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Gruda, N. Current and future perspective of growing media in Europe. Acta Hortic. 2012, 960, 37–43. [Google Scholar] [CrossRef]
- Hitzl, M.; Mendez, A.; Owsianiak, M.; Renz, M. Making Hydrochar Suitable for Agricultural Soil: A Thermal Treatment to Remove Organic Phytotoxic Compounds. J. Environ. Chem. Eng. 2018, 6, 7029–7034. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Zhu, Y. Changes in Abiotic Dissipation Rates and Bound Fractions of Antibiotics in Biochar-Amended Soil. J. Clean. Prod. 2020, 256, 120314. [Google Scholar] [CrossRef]
- Carlile, W.R.; Cattivello, C.; Zaccheo, P. Organic Growing Media: Constituents and Properties. Vadose Zone J. 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Adhikari, S.; Timms, W.; Mahmud, M.A.P. Optimising Water Holding Capacity and Hydrophobicity of Biochar for Soil Amendment—A Review. Sci. Total Environ. 2022, 851, 158043. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Does Biochar Application Alleviate Soil Compaction? Review and Data Synthesis. Geoderma 2021, 404, 115317. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of Different Feedstocks-Based Biochar on Soil Remediation: A Review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Gascó, G.; Paz-Ferreiro, J.; Fernández, J.M.; Plaza, C.; Méndez, A. The Effect of Pruning Waste and Biochar Addition on Brown Peat Based Growing Media Properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, J.S.; Ha, S.Y.; Choi, J.H.; Yang, J.-K. Suitability of Thermal Treated Sawdust as Replacements for Peat Moss in Horticultural Media. J. Agric. Life Sci. 2015, 49, 105–115. [Google Scholar] [CrossRef]
- Alipour, M.; Asadi, H.; Chen, C.; Rashti, M.R. Bioavailability and Eco-Toxicity of Heavy Metals in Chars Produced from Municipal Sewage Sludge Decreased during Pyrolysis and Hydrothermal Carbonization. Ecol. Eng. 2021, 162, 106173. [Google Scholar] [CrossRef]
- Farru, G.; Dang, C.H.; Schultze, M.; Kern, J.; Cappai, G.; Libra, J.A. Benefits and Limitations of Using Hydrochars from Organic Residues as Replacement for Peat on Growing Media. Horticulturae 2022, 8, 325. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A Critical Review of the Possible Adverse Effects of Biochar in the Soil Environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Mumme, J.; Getz, J.; Prasad, M.; Lüder, U.; Kern, J.; Mašek, O.; Buss, W. Toxicity Screening of Biochar-Mineral Composites Using Germination Tests. Chemosphere 2018, 207, 91–100. [Google Scholar] [CrossRef]
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and Phytotoxic Risk Assessment of Fresh and Treated Hydrochar from Hydrothermal Carbonization Compared to Biochar from Pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile Organic Compounds in Biochar—A Potential Source of Contamination—Phytotoxic Effects on Cress Seed (Lepidium sativum) Germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef]
- Ruzickova, J.; Koval, S.; Raclavska, H.; Kucbel, M.; Svedova, B.; Raclavsky, K.; Juchelkova, D.; Scala, F. A Comprehensive Assessment of Potential Hazard Caused by Organic Compounds in Biochar for Agricultural Use. J. Hazard. Mater. 2021, 403, 123644. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O.; Graham, M.; Wüst, D. Inherent Organic Compounds in Biochar–Their Content, Composition and Potential Toxic Effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef]
- Cordella, M.; Torri, C.; Adamiano, A.; Fabbri, D.; Barontini, F.; Cozzani, V. Bio-Oils from Biomass Slow Pyrolysis: A Chemical and Toxicological Screening. J. Hazard. Mater. 2012, 231–232, 26–35. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the Total and Bioavailable Polycyclic Aromatic Hydrocarbons and Dioxins in Biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Mackowiak, C.L.; Comerford, N.B.; Moline, E.F.D.V.; Shirley, J.P.; Guimaraes, D.V. Pyrolysis Methods Impact Biosolids-Derived Biochar Composition, Maize Growth and Nutrition. Soil Tillage Res. 2017, 165, 59–65. [Google Scholar] [CrossRef]
- Hilber, I.; Bastos, A.C.; Loureiro, S.; Soja, G.; Marsz, A.; Cornelissen, G.; Bucheli, T.D. The different faces of biochar: Contamination risk versus remediation tool. J. Environ. Eng. Landsc. Manag. 2017, 25, 86–104. [Google Scholar] [CrossRef]
- Fabbri, D.; Rombolà, A.G.; Torri, C.; Spokas, K.A. Determination of Polycyclic Aromatic Hydrocarbons in Biochar and Biochar Amended Soil. J. Anal. Appl. Pyrolysis 2013, 103, 60–67. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Park, Y.-K. Overview of Biochar Production from Preservative-Treated Wood with Detailed Analysis of Biochar Characteristics, Heavy Metals Behaviors, and Their Ecotoxicity. J. Hazard. Mater. 2020, 384, 121356. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M. Biochar Properties Regarding to Contaminants Content and Ecotoxicological Assessment. J. Hazard. Mater. 2013, 260, 375–382. [Google Scholar] [CrossRef]
- Chandra, S.; Bhattacharya, J. Influence of Temperature and Duration of Pyrolysis on the Property Heterogeneity of Rice Straw Biochar and Optimization of Pyrolysis Conditions for Its Application in Soils. J. Clean. Prod. 2019, 215, 1123–1139. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Modification of Biochar Surface by Air Oxidation: Role of Pyrolysis Temperature. Biomass Bioenergy 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Baltrėnaitė-Gedienė, E. Dependence of Pyrolysis Temperature and Lignocellulosic Physical-Chemical Properties of Biochar on Its Wettability. Biomass Convers. Biorefin. 2021, 11, 2775–2793. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Q.; Yang, R.; Lin, W.; Wang, H.; Zhou, W.; Qi, Z.; Ouyang, L. Slight Carbonization as a New Approach to Obtain Peat Alternative. Ind. Crops Prod. 2023, 202, 117041. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- De Jager, M.; Giani, L. An Investigation of the Effects of Hydrochar Application Rate on Soil Amelioration and Plant Growth in Three Diverse Soils. Biochar 2021, 3, 349–365. [Google Scholar] [CrossRef]
- Ordonez-Loza, J.; Chejne, F.; Jameel, A.G.A.; Telalovic, S.; Arrieta, A.A.; Sarathy, S.M. An Investigation into the Pyrolysis and Oxidation of Bio-Oil from Sugarcane Bagasse: Kinetics and Evolved Gases Using TGA-FTIR. J. Environ. Chem. Eng. 2021, 9, 106144. [Google Scholar] [CrossRef]
- Intani, K.; Latif, S.; Islam, M.; Müller, J. Phytotoxicity of Corncob Biochar before and after Heat Treatment and Washing. Sustainability 2018, 11, 30. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, P.; Guo, Z.; Sun, H.; Li, D.; Xue, L.; Feng, Y.; Yu, S.; Yang, L.; Xing, B. Raw Material of Water-Washed Hydrochar Was Critical for the Mitigation of GHGI in Infertile Paddy Soil: A Column Experiment. Biochar 2021, 3, 381–390. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Rafique, M.I.; Ahmad, M.; Ahmad, M.; Hussain, A.; Usman, A.R.A. Pyrolytic and Hydrothermal Carbonization of Date Palm Leaflets: Characteristics and Ecotoxicological Effects on Seed Germination of Lettuce. Saudi J. Biol. Sci. 2019, 26, 665–672. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dicke, C.; Kalderis, D.; Kern, J. Rice Husks and Their Hydrochars Cause Unexpected Stress Response in the Nematode Caenorhabditis Elegans: Reduced Transcription of Stress-Related Genes. Environ. Sci. Pollut. Res. 2015, 22, 12092–12103. [Google Scholar] [CrossRef]
- Bahcivanji, L.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. The Effect of Post-Pyrolysis Treatment on Waste Biomass Derived Hydrochar. Waste Manag. 2020, 106, 55–61. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, S.; Zhong, Q.; Wang, G.; Pan, X.; Xu, X.; Zhou, W.; Li, T.; Luo, L.; Zhang, Y. Soil Washing Remediation of Heavy Metal from Contaminated Soil with EDTMP and PAA: Properties, Optimization, and Risk Assessment. J. Hazard. Mater. 2020, 381, 120997. [Google Scholar] [CrossRef]
- Abumaizar, R.J.; Smith, E.H. Heavy Metal Contaminants Removal by Soil Washing. J. Hazard. Mater. 1999, 70, 71–86. [Google Scholar] [CrossRef]
- Carril, P.; Ghorbani, M.; Loppi, S.; Celletti, S. Effect of Biochar Type, Concentration and Washing Conditions on the Germination Parameters of Three Model Crops. Plants 2023, 12, 2235. [Google Scholar] [CrossRef]
- Dikinya, O.; Areola, O. Comparative Analysis of Heavy Metal Concentration in Secondary Treated Wastewater Irrigated Soils Cultivated by Different Crops. Int. J. Environ. Sci. Technol. 2010, 7, 337–346. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of Soil Washing on Heavy Metal Removal and Soil Quality: A Two-Sided Coin. Ecotoxicol. Environ. Saf. 2020, 203, 110981. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Liu, X.; Yuan, L.; Liu, X.; Deng, H. Comparative Study on Washing Effects of Different Washing Agents and Conditions on Heavy Metal Contaminated Soil. Surf. Interfaces 2021, 27, 101563. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Kanyerere, T.; Wang, Y.; Sun, M. Washing Reagents for Remediating Heavy-Metal-Contaminated Soil: A Review. Front. Earth Sci. 2022, 10, 901570. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Wang, G.; Zhang, S.; Cheng, Z.; Li, T.; Yang, Z.; Xian, J.; Yang, Y.; Zhou, W. Removal of Heavy Metals from Industrial Sludge with New Plant–Based Washing Agents. Chemosphere 2020, 246, 125816. [Google Scholar] [CrossRef]
- Branzini, A.; Zubillaga, M.S. Assessing Phytotoxicity of Heavy Metals in Remediated Soil. Int. J. Phytoremediation 2010, 12, 335–342. [Google Scholar] [CrossRef]
- Gitipour, S.; Mohebban, A.; Ghasemi, S.; Abdollahinejad, M.; Abdollahinejad, B. Evaluation of Effective Parameters in Washing of PAH-Contaminated Soils Using Response Surface Methodology Approach. Int. J. Environ. Sci. Technol. 2020, 17, 683–694. [Google Scholar] [CrossRef]
- Bilgin, M.; Tulun, Ş. Biodrying for Municipal Solid Waste: Volume and Weight Reduction. Environ. Technol. 2015, 36, 1691–1697. [Google Scholar] [CrossRef]
- Liu, P.Y.; Liu, H.; Li, Y.J.; Dong, C.X. Remediation of Arsenic Contaminated Soils and Treatment of Washing Effluent Using Calcined Mn-Fe Layered Double Hydroxide. Adv. Mater. Res. 2014, 955–959, 2014–2021. [Google Scholar] [CrossRef]
- Maity, J.P.; Huang, Y.M.; Hsu, C.-M.; Wu, C.-I.; Chen, C.-C.; Li, C.-Y.; Jean, J.-S.; Chang, Y.-F.; Chen, C.-Y. Removal of Cu, Pb and Zn by Foam Fractionation and a Soil Washing Process from Contaminated Industrial Soils Using Soapberry-Derived Saponin: A Comparative Effectiveness Assessment. Chemosphere 2013, 92, 1286–1293. [Google Scholar] [CrossRef]
- Sathe, S.K.; Zaffran, V.D.; Gupta, S.; Li, T. Protein Solubilization. J. Am. Oil Chem. Soc. 2018, 95, 883–901. [Google Scholar] [CrossRef]
- Pérez, S.G.-; Vereijken, J.M.; Koningsveld, G.A.; Gruppen, H.; Voragen, A.G.J. Physicochemical Properties of 2S Albumins and the Corresponding Protein Isolate from Sunflower (Helianthus annuus). J. Food Sci. 2005, 70, C98–C103. [Google Scholar] [CrossRef]
- Sripad, G.; Prakash, V.; Rao, M.S.N. Extractability of Polyphenols of Sunflower Seed in Various Solvents. J. Biosci. 1982, 4, 145–152. [Google Scholar] [CrossRef]
- Altland, J.E.; Owen, J.S.; Jackson, B.E.; Fields, J.S. Physical and Hydraulic Properties of Commercial Pine-Bark Substrate Products Used in Production of Containerized Crops. Am. Soc. Hortic. Sci. 2018, 53, 1883–1890. [Google Scholar] [CrossRef]
- Buamscha, M.G.; Altland, J.E.; Sullivan, D.M.; Horneck, D.A.; McQueen, J.P.G. Nitrogen Availability in Fresh and Aged Douglas Fir Bark. HortTechnology 2008, 18, 619–623. [Google Scholar] [CrossRef]
- Okada, S.; Iwasaki, A.; Kataoka, I.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity of Kiwifruit Leaves and Isolation of a Phytotoxic Substance. Sci. Hortic. 2019, 250, 243–248. [Google Scholar] [CrossRef]
- Chemetova, C.; Quilhó, T.; Braga, S.; Fabião, A.; Gominho, J.; Ribeiro, H. Aged Acacia melanoxylon Bark as an Organic Peat Replacement in Container Media. J. Clean. Prod. 2019, 232, 1103–1111. [Google Scholar] [CrossRef]
- Lescano, I.; Devegili, A.M.; Martini, C.; Tessi, T.M.; González, C.A.; Desimone, M. Ureide Metabolism in Arabidopsis thaliana Is Modulated by C:N Balance. J. Plant Res. 2020, 133, 739–749. [Google Scholar] [CrossRef]
- Liu, K.; He, Y.; Xu, S.; Hu, L.; Luo, K.; Liu, X.; Liu, M.; Zhou, X.; Bai, L. Mechanism of the Effect of pH and Biochar on the Phytotoxicity of the Weak Acid Herbicides Imazethapyr and 2,4-D in Soil to Rice (Oryza sativa) and Estimation by Chemical Methods. Ecotoxicol. Environ. Saf. 2018, 161, 602–609. [Google Scholar] [CrossRef]
- Akkermans, S.; Van Impe, J.F. Mechanistic Modelling of the Inhibitory Effect of pH on Microbial Growth. Food Microbiol. 2018, 72, 214–219. [Google Scholar] [CrossRef]
- Baker, J.R. Temperature and Enzyme Activity. J. Mar. Biol. Ass. 1927, 14, 723–727. [Google Scholar] [CrossRef]
- Jośko, I.; Dobrzyńska, J.; Dobrowolski, R.; Kusiak, M.; Terpiłowski, K. The Effect of pH and Ageing on the Fate of CuO and ZnO Nanoparticles in Soils. Sci. Total Environ. 2020, 721, 137771. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in Plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Ma, Y.B.; Nichols, D.G. Phytotoxicity and Detoxification of Fresh Coir Dust and Coconut Shell. Commun. Soil Sci. Plant Anal. 2004, 35, 205–218. [Google Scholar] [CrossRef]
- Witcher, A.L.; Blythe, E.K.; Fain, G.B.; Curry, K.J.; Pounders, C.T. Assessing Phytotoxicity in Fresh and Aged Whole Pine Tree Substrates©; IPPS—International Plant Propagator’s Society: Carlisle, PA, USA, 2011; Volume 61, pp. 477–482. [Google Scholar]
- Tuckeldoe, R.B.; Maluleke, M.K.; Adriaanse, P. The Effect of Coconut Coir Substrate on the Yield and Nutritional Quality of Sweet Peppers (Capsicum annuum) Varieties. Sci. Rep. 2023, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Miao, A.; Cao, J.; Wang, W.; Chen, W.; Pang, S.; He, X.; Ma, C. Study on the Effects of Rapid Aging Technology on the Aroma Quality of White Tea Using GC–MS Combined with Chemometrics: In Comparison with Natural Aged and Fresh White Tea. Food Chem. 2018, 265, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liao, J.; Zhou, B.; Yang, R.; Lin, W.; Zhang, D.; Wang, H.; Qi, Z. Rapidly Reducing Phytotoxicity of Green Waste for Growing Media by Incubation with Ammonium. Environ. Technol. Innov. 2023, 31, 103136. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; Van Winkel, A. Growing Media for Food and Quality of Life in the Period 2020-2050. Acta Hortic. 2021, 1305, 341–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).