Abstract
Plant residues derived from the agro-industrial sector and their disposal are still unsolved issues despite the various research and applications. The current study assessed the possible peat substitution in growing media with solid residues derived from the distillation of Origanum dubium Boiss (OD) and Sideritis cypria Post (SC) after essential oils production. Residues of OD and SC (0–5–10–20% v/v) and their mixture (OD + SC) were examined to partially substitute peat for the production of pansy (Viola × wittrockiana) plants. The presence of OD, SC, and OD + SC residues increased the pH, the electrical conductivity, the organic matter content, and the levels of minerals in the growing media mixtures compared to sole peat while decreasing the total porosity (up to 36.8%) and the available free air (up to 58.3%). The OD residues’ presence in the peat-based media revealed more detrimental effects than the SC, while the OD + SC mixture received intermediate effects. Low residue ratios (≤5% OD, ≤10% SC, ≤10% OD + SC) sustain plant growth and photosynthetic activity of plants, while higher ratios decrease plant growth, chlorophyll content and mineral accumulation due to inappropriate growing media properties, causing plant oxidative stress. This was verified using the increase in malondialdehyde (up to 4.5 times) and hydrogen peroxide (up to 2.1 times) content in plants and the activation of several non-enzymatic antioxidant processes, including total phenols, flavonoids, and antioxidant capacity of the plants. Therefore, OD, SC, and OD + SC at 5%, 10%, and 10%, respectively, can partially substitute peat, as they result in appropriate plant growth and development. However, the growing media’s properties must be improved to ensure adequate yields as well.
1. Introduction
Medicinal and aromatic plants (MAPs) have attracted a lot of research interest recently, especially those from the Lamiaceae family, since they contain bioactive compounds that may be used in the food industry in addition to the cosmetic and pharmaceutical sectors [1]. MAP material can be used in raw, dried, or frozen form to produce essential oils (EOs) and plant extracts with a variety of biocidal properties and applications [2]. Due to rising demands on the MAP industry, there were previously indications for a greater collection of wild species. This threatened or caused the extinction of some endemic species, alarming biodiversity in several locations [3]. Sustainable harvesting of wild species is a fundamental conservation strategy; however, unrestricted wild species collection occurs frequently because of the huge demand for raw biomass and lack of knowledge, legislation, and policy guidance [4]. Moreover, the composition and the biocidal effects of the EOs and extracts are influenced by the soil type, growing techniques, season, altitude, and other climatic variables; the pharmaceutical sector has recently demanded a steady biomass supply and steady composition of the MAP components [5,6,7]. In that sense, not only common MAP species but also wild MAP species are brought into cultivation systems in open fields or intensive greenhouse conditions, and lately under soilless cultivation/hydroponics cultivation systems [8]. Recent observations refer to 3227 MAP species from 235 distinct plant families that are commercially cultivated [3].
The herbal, fragrance, pharmaceutical, and cosmetics manufacturers generate an array of waste materials, both solid and liquid, such as the residues from the distillation of the EOs or other unutilized parts of the plants [9]. However, the term “waste” is getting less popular nowadays due to the notions of circular economy, recycling, and sustainability. The terms “leftovers”, “residues”, or “by-products” are more attaching, acceptable, and preferable. Notable, leftover feedstock is any biological substance that is inadvertently generated during the manufacturing process [10]. According to Olofsson and Börjesson [11], residual biomass is often referred to as a by-product, and, based on how it is managed, it could become a waste. The effective use and recycling of leftover biomass produced by the MAP sector are significant for revenue generation, sustainable development, and social benefits [12]. MAP residues should be managed and recycled in an environmentally and ecologically appropriate manner, as failing to do so may result in environmental hazards and issues affecting both the ecosystems and human health [13].
One of the main MAPs used to produce the EOs is mainly via distillation (including hydrodistillation, steam-distillation, hydro steam-distillation), extraction with organic solvents, or mechanical. Each one of these methods is time and cost-consuming [14]. On top of that, huge amounts of biomass residues produced from MAP distillation go underutilized, lowering agricultural added value [15]. MAP residues are predicted to account for more than 200,000 tons globally each year [16]. As a result, effective reuse of these leftovers might bring additional income for aromatic plant producers. During EO production, the obtained yield is usually quite low (up to 8%), and therefore, huge amounts of leftovers and hydro-distilled water, commonly known as hydrosol, are left behind to be appropriately handled as by-products, if possible [10]. The majority of these residues are discarded as wasteful, and they usually undergo processes such as burning or being tossed in landfills [1,17], causing environmental constraints and wasting underutilized resources. Indeed, several valuable bioactive components, such as phenolics and antioxidants, remain in the residues and could be successfully recovered [18,19]. However, materials such as the remaining hydrosol are getting attention and are being examined as natural preservatives in the food sector [20].
One of the common practices for handling plant by-products is to process them in composting in order to obtain a stable organic product rich in nutrients that can be used as organic fertilizer and/or soil improver [21,22]. Lately, several other uses of MAP leftovers have been reported. Herb residues derived after decoction can be used for gas, liquid or solid fuel production. However, the high wet biomass and the complex phytochemical compositions of different herbs render it difficult for these materials to be employed as feed stock for gasification or to be used in other techniques [12]. Residues after distillation of roses (Rosa damascena Mill. var. trigintipetala) are used successfully as an ingredient in animal feed and for a variety of medicinal reasons [23]. Another application for herb residue is as a food supplement for piglets after fermentation [18], while Aloe vera (L.) residues are introduced in the food supply of lactating cows [24]. Additional uses encompass distilled lavender stalks utilized as a bioaggregate for building material [25].
The utilization of crop residues in agriculture, either in composted or raw form, as organic fertilizers and soil improvers, has been explored on several occasions [21,26]. However, the use of these types of residues as a growing media component has received less attention [27,28,29]. This is contradictory to the urgent need for peat replacement, as peat is the principal growing media component used in agriculture due to its well-established physicochemical properties. Peat is used in almost 80% of growing media preparation, and only 20% of the growing media used in the European Union (EU) comprise materials other than peat [30]. The horticultural sector uses 14–20% of the total collected peat, while a large part of it is used for energy production processes [29]. Because peatlands are recognized as natural habitats for a variety of species and are protected areas, the extraction of peat, a nonrenewable resource, has prompted several environmental concerns. The use of peat urgently needs to be reduced, and alternative components for the partial peat substitute are under evaluation [31]. Various peat substitutes have been explored up to now, counting agro-industry residues, composted or raw, as growing media in soilless culture or pot-based culture for different crops [22,29,32]. When a growing medium is prepared, materials with unique physicochemical properties can be combined, and the produced mixtures can be enriched with minerals by adding fertilizers. Even though residue composting can create a stable material to be used in agriculture, which is a well-known way of residue management, farmers often use raw materials for their crop production and/or in nurseries/greenhouses. Some examples include the use of raw rice and kenaf for Pinus halepensis (Mill.) seedlings production [33], olive-mill waste (OMW), grape-mill waste (GMW) and MAP-residues in vegetable production [34,35], and even shredded paper waste for plant production [29,36]. Depending on the material employed, the growing/environmental conditions, and the plant species under consideration, these factors provide various restrictions for further implementation.
Ornamental seedlings and pot production in nurseries and greenhouses are expanding [37]. One of the most popular bedding plants is the garden pansy (Viola × wittrockiana) [38], and apart from its decorative use, the pansy flowers are edible, rich in antioxidants, carotenoids, flavonoids and minerals [39]. Several studies implemented on pansies have examined various growing media components, such as biochar [40] and animal tissue compost [41], but the use of MAP residues has not yet been investigated on pansies. Therefore, the primary goal of this research was to investigate the use of residues (OD and SC) derived by the MAP sector to partially substitute peat during the growing of pansies. Additionally, the putative benefits observed when different residues are mixed (OD + SC) were also investigated in order to merge their properties in the growing media mixture.
2. Materials and Methods
2.1. Plant Material and Growing Media Preparation
The study took place at the soilless cultures’ greenhouse facilities of the Cyprus University of Technology in Limassol, Cyprus. The multi-spam greenhouse has a North–South orientation, is covered with transparent polyethylene material and is equipped with an automated climate control system. Commercial peat (professional-grade, Gebr. Brill Substrate GmbH and Co. KG, Georgsdorf, Germany) was used as the reference/control growing media material. Commercially produced fertilizers (Novatec, simple superphosphate, and potassium sulfate) were also used to enhance peat with minerals to obtain levels of 75 mg N L−1, 50 mg P2O5 L−1, and 125 mg K2O L−1 (w/v), respectively, achieving the acceptable mineral levels for a substrate mixture. Peat and fertilizers were then carefully blended.
The plant material of Origanum dubium Boiss. (OD) and Sideritis cypria Post. (SC) The wastes used for the production were provided by the Department of Agriculture’s Sector of Medicinal and Aromatic Plants (Nicosia, Cyprus). Plants were conventionally cultivated, and the applied practices included annual tillage, pruning, use of fertilizers or semi-composted manure, and crop protection with pesticides, all typical for the region of study. Before being subjected to steam-hydrodistillation, plants were air-dried in the shade. The solid residues generated by the steam-hydrodistillation process of the EOs’ extraction were used as the waste material under evaluation. For the EO extraction, a semi-commercial EO distillatory equipment of 60 L capacity was used. After the EOs distillation, the crop residues were air dried (to a moisture level ≤10%) and were then chopped to produce a powder (laboratory grinding mill, Polymix® System PX-MFC 90d, Kinematica GmbH, Eschbach, Germany). The physicochemical properties of the OD 100% and SC 100% material were analyzed, as described in Section 2.2.
Peat (P) was used as the main component to prepare the different growing media under evaluation. By proportionally substituting peat at different ratios (at 5–10–20%, based on volume) with residues from OD or SC or their combination, 10 different mixes (v/v) were produced: for the oregano residues, (1) 100% peat (P; control), (2) P:OD 95:5 (OD 5%), (3) P:OD 90:10 (OD 10%), (4) P:OD 80:20 (OD 20%); for the Sideritis residues, (1) 100% peat (P; control), (5) P:SC 95:5 (SC 5%), (6) P:SC 90:10 (SC 10%), (7) P:SC 80:20 (SC 20%); for the residues mixtures, (1) 100% peat (P; control), (8) P:OD:SC 95:2.5:2.5 (OD + SC 5%), (9) P:OD:SC 90:5:5 (OD + SC 10%), and (10) P:OD:SC 80:10:10 (OD + SC 20%). In each mixture, 10% of perlite was added to improve aeration and drainage [31]. Prior to the seedlings’ transplanting, all the mixtures were sampled and analyzed for their physicochemical features. The OD and SC levels chosen were based on pilot trials and/or earlier studies that have employed plant wastes in the examined growing media [29,34].
Viola × wittrockiana Gams (garden pansy) seedlings were purchased at the growing stage of 2-true leaves from a commercial nursery. The 10 different growing mixtures under examination were placed in 0.5 L plastic pots before the seedling’s transplanting. Each pot contained one single seedling, while each mixture was tested with 12 replicate pots. Seedlings were irrigated every second day, while no fertilizers, insecticides, or other plant protection products were used. The pots were placed on plastic trays to retain the drained solution after each watering. During the cultivation phase, temperature, humidity, and light conditions were recorded and averaged 19.3 °C, 59.4%, and 1426.2 Lux, respectively.
2.2. Growing Media Properties
The physicochemical properties of all the produced mixtures and the raw plant wastes were evaluated. The growing media total porosity (TP), air-filled porosity (AFP), available water-holding capacity (AWHC), and bulk density (BD) by volume were assessed according to the European Standard EN 13041 [42], as described previously [29]. One portion of growing media was mixed with five portions (1:5 v/v) of distilled water, and the extracts were used to measure the pH and electrical conductivity (EC). Organic matter content and carbon (both expressed in percentage) were calculated after the media had been burnt at 550 °C in an ash furnace [34]. After the ash samples had been acid digested (2 N HCl), the produced extracts were subjected to analysis of macronutrients, using a flame photometer (Lasany Model 1832, Lasany International, Panchkula, India), for potassium (K) and sodium (Na) determination, while phosphorus (P) was measured using spectrophotometry (Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA). The contents of magnesium (Mg) and calcium (Ca) were determined using an atomic absorption spectrophotometer (PG Instruments AA500FG, Leicestershire, UK). Nitrogen (N) was measured using the Kjeldahl method (BUCHI, Digest Automat K-439 and Distillation Kjelflex K-360, Flawil, Switzerland) as described previously [29]. Data were expressed in g kg−1 of dry weight. The C/N ratio was also determined as an indicator of the decomposition status of the organic material.
2.3. Plant Growth, Physiology, and Mineral Analysis
After 28 days of cultivation, several growth-related parameters were measured on six plants per treatment. The number of leaves and flowers on each plant and the plant’s height were recorded. Plants were then harvested, and the aerial fresh biomass was weighted (g) and dried, and the percentage (%) of dry matter content was then calculated.
As part of the study, relative chlorophyll content (optical chlorophyll meter SPAD-502, Minolta, Osaka, Japan) and chlorophyll fluorescence parameters were used to estimate the efficiency of photosystem II to determine if environmental factors were affecting photosynthesis. The fluorescence of leaf chlorophyll was observed on two fully developed leaves per plant (Opti-Sciences fluorometer OS-30p, Hertfordshire, UK). The leaf chlorophyll and carotenoid contents were also calculated (6 replications/treatment) using dimethyl sulfoxide (DMSO) as solvent. The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophylls (total Chl), and carotenoid were expressed as mg per g of fresh weight (mg Chl g−1 fw or mg Car g−1 fw) [34].
Mineral content in pansy leaves was assessed on four replications for each growing media (2 pooled plants per replication). The plant tissue was air dried (~0.45 g) at 65 °C till constant weight, ashed at 550 °C in a furnace and acid-digested with hydrochloric acid (2 N HCl) [34]. The obtained extracts contained all the inorganic minerals. Phosphorus content was assessed via spectrophotometry (Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA), and K, Na, Mg, and Ca were measured using Ion Chromatography (ICS-3000, Dionex Aquion, Sunnyvale, CA, USA) and an IonPac CS19 analytical column (4 × 250 mm, Dionex Corporation, Sunnyvale, CA, USA). N was assessed with the Kjeldahl method, as described previously. Data were expressed in g kg−1 of dry weight.
2.4. Total Phenolics, Total Flavonoids, and Antioxidant Activity
For every treatment, six fresh leaf samples (pooled from two individual plants/samples) were processed with 10 mL of 50% (v/v) methanol, and the extraction was enabled using ultrasonication [43]. Extracts were then centrifuged, and the supernatant was stored at 4 °C until analysis (within 48 h) of the antioxidant activity employing three assays (ferric-reducing antioxidant power- FRAP; 2,2-diphenyl-1-picrylhydrazyl -DPPH; and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid -ABTS). The extracts were also used to determine the total amount of phenolic and flavonoid content.
Total phenols were quantified using the methanolic extracts with the Folin–Ciocalteu method (at 755 nm; using a microplate spectrophotometer Thermo Scientific, Multiskan GO), as described previously [43]. Results were expressed in gallic acid equivalents (mg GAE g−1 of fw). The content of total flavonoids was determined based on the aluminium chloride colourimetric method [44]. The absorbance was measured at 510 nm. Results were expressed in rutin equivalents (mg rutin g−1 of fw).
The free radical-scavenging activity was determined as described previously [45]. Briefly, the DPPH radical scavenging activity of the plant methanolic extracts was measured at 517 nm; FRAP activity was measured at 593 nm, as described in Chrysargyris et al. [45]. The ABTS assay was implemented according to the methodology described by Woidjylo et al. [46]. Results were expressed as Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) equivalents (mg trolox g−1 of fw).
2.5. Lipid Peroxidation, Hydrogen Peroxide Content and Antioxidant Enzymes Activity
The content of hydrogen peroxide (H2O2) was determined by employing the method described by Loreto and Velikova [47] from six samples (two individual plants were pooled/sampled) for each treatment. The absorbance of samples and standards (5 to 1000 μM of H2O2) was measured at 390 nm, and results were expressed as μmol H2O2 g−1 fresh weight.
Lipid peroxidation was assessed according to De Azevedo Neto et al. [48] and measured in terms of malondialdehyde content (MDA). The absorbance was determined at 532 nm and corrected for non-specific absorbance at 600 nm. MDA amount was determined using the extinction coefficient of 155 mM cm−1. Results were expressed as nmol of MDA g−1 fresh weight.
The activity of antioxidant enzymes was assayed, as it was described by Chrysargyris et al. [29]. Superoxide dismutase (SOD) (EC 1.15.1.1), catalase (CAT) (EC 1.11.1.6) and peroxidase (POD) (EC 1.11.1.6) activities were determined, and results were expressed as enzyme units per mg of protein. The protein content was determined using the Bradford method, using bovine serum albumin as a reference.
2.6. Statistical Analysis
Measurements were performed in four to six biological replications/treatments (each replication consisted of a poll of two individual measures/samples). Statistical analysis was carried out using the SPSS statistical software (SPSS v.22; IBM, Armonk, NY, USA). Data means were also compared with one-way analysis of variance (ANOVA), and Duncan’s multiple range test was used for the comparison of treatment means at p < 0.05.
3. Results and Discussion
Certain leftovers of plant origin provide suitable material that can be used in growing media mixtures for the production of seedlings or cuttings in nurseries or even to grow plants in pots. Parameters such as the pH, the salinity level, and the phytochemical profile (as the polyphenol levels) may limit the use and application of such residues since plants may experience phytotoxic stress as a result of their effect [49]. In this perspective, examining the impacts of growing media composition on plant growth and production is a highly complex task that necessitates the consideration of various physical, chemical, and biological factors. In the present study, the use of the MAP residues (OD and SC) in different ratios (5%, 10% and 20%) and their mixture indicated that they are a source of minerals and that they substantially contribute to the properties of the produced growing media mixtures, when they were employed in different ratios together with peat (Table 1, Table 2 and Table 3). In specific, the OD waste material was slightly acidic, with a pH value to be averaged at 6.03, with high organic matter content and bulk density, and increased levels of minerals such as N (10.57 g kg−1), K (13.59 g kg−1), P (2.85 g kg−1), Mg (2.73 g kg−1), Ca (19.79 g kg−1), and Na (3.92 g kg−1), which led to increased fertility by the high EC levels, when compared to peat (Table 1). Moreover, OD had lower total porosity (i.e., 71.91%) than the recommended thresholds (≥85% TP) for growing media [28,50], and that was reflected in the low air-filled porosity (i.e., 1.83%). In the case of SC, it was revealed that SC had almost neutral pH (averaged in 6.84), increased levels of organic material and available water-holding capacity, as well as high levels of N (12.95 g kg−1), K (14.59 g kg−1), P (1.61 g kg−1), Na (5.82 g kg−1), Ca (21.53 g kg−1), and Mg (1.71 g kg−1), which reflected the high levels of the EC (Table 2). The SC residues had a high C/N ratio of 42.3, which indicated that the material was not stable. The complete decomposition of the organic matter did not take place. This was most likely related to the inappropriate conditions for the decomposition process in terms of moisture, temperature, oxygen, and available N. The SC had also lower Ca levels and bulk density compared to peat.

Table 1.
The physicochemical properties of the growing media (peat and Origanum dubium Boiss. residue (OD)) prior to plant transplantation.

Table 2.
The physicochemical properties of the growing media (peat and Sideritis cypria Post. residue (SC)) prior to plant transplantation.

Table 3.
The physicochemical properties of the growing media (peat, Origanum dubium Boiss. residue (OD) and Sideritis cypria Post. residue (SC)) prior to plant transplantation.
Distillation residues often contain micronutrients, including iron, copper, manganese and zinc, in moderately adequate quantities together with a series of macronutrients as 1.35–1.80% N, 0.45–0.60% P, and 2.00–2.25% K [51]. This indicates that the examined MAP residues could be considered as quite fertile material in terms of macronutrient content. The mineral status of the residues is of great interest, as it has been noted that recycling distillation residue can meet up to half of the nutritional needs of Japanese mint (Mentha arvensis L.) and mustard (Brassica juncea L.) [52]. Moreover, the addition of EO distillation residues from mint promotes the productivity of mustard and upgrades the physico-chemical characteristics of soil [52]. Nevertheless, the micronutrients were not assessed in the current analysis; this will be necessary to consider in upcoming studies. Due to the wide variety of raw materials and the stage of decomposition, there are no reference values for raw material qualities; there are more reports on composted materials, which are a more stable entity. Nevertheless, the properties of the OD and SC fell within acceptable bounds, with the exception of the OD air-fill porosity, OD total porosity, OD container capacity, and OD- and SC-electrical conductivity [50]. The EC of OD and SC were 1.98 and 1.16 mS cm−1, respectively, which were below the values of EC of 4 mS cm−1, a level considered tolerable by plants of medium sensitivity [53], suggesting that the residues can be used safely without causing phytotoxicity in young plants.
The incorporation of the MAP residues in the growing media affected the physicochemical features of the tested mixtures (Table 1, Table 2 and Table 3). The presence of SC increased the pH of the mixture compared to peat, at SC 20%, which is in accordance with previous reports [31]. Moreover, the pH was increased at the 20% OD + SC mixtures, too, whereas the pH values were measured above the recommended levels (5.3–6.5) [50]. Organic matter content and organic carbon were at similar or slightly increased levels with the presence of OD or SC in the mixtures compared to peat (control), and this was probably due to the relevantly low ratios (up to 20%) of the residues incorporated in the peat mixtures. Considering that the soil organic matter is in low levels (i.e., up to 4–5%), indicating poor soil fertility, the soil enrichment with organic material is of great interest in the Mediterranean basin as well as in other parts of the world. Therefore, MAP industries can provide their residues as organic biomass for soil organic matter enrichment or growing media amendments [10].
It is referenced that MAP residues, via the decomposition process of the organic matter, can enrich soils with N, P, and K [54]. This was observed for the tested MAP-enriched growing media in the present study, where the N, K, Mg, P and Na levels were considerably (p < 0.001) higher than in peat. The MAP-based media (OD, SC, and OD + SC) exhibited N of 8.81–15.82 g kg−1, K of 3.14–7.35 g kg−1, P of 1.42–2.57 g kg−1, Mg of 1.06–2.32 g kg−1, Ca of 17.04–22.52 g kg−1, and Na of 1.05–1.67 g kg−1. In most cases, the mineral levels observed in the mixture of OD + SC were at the intermediate levels of the examined OD and SC. According to Abad et al. [50], the recommended EC values for growing media are ≤0.5 mS cm−1; however, the EC of the examined growing media were higher (varying from 0.84 to 1.76 mS cm−1). Similarly, composted residues derived from the winery-distillery process, when used as a partial substitute for peat-based growing media, revealed higher than the recommended EC values, ranging from 0.61–2.41 mS cm−1 [28]. Detrimental effects of the high EC (2.3–2.7 mS cm−1) were evidenced when spent mushroom residue was substituted peat in Gossypium herbaceum and Talinum paniculatum seedlings, while T. paniculatum seedlings grown in 75–100% spent mushroom residue died [55]. Indeed, the high EC values, which reflect the high mineral availability to the plant’s roots, can be handled by adequate irrigation and fertilization management or by employing inert materials (i.e., perlite, pumice, sand, etc.) in the case of elevated mineral levels [56].
The chemical features of a growing media, including the pH, EC, cation and anion exchange capacity, can be adjusted/fine-tuned after the media preparation and/or during plant growth; however, the same cannot be achieved for the physical properties of the media. Features such as total porosity, available water-holding capacity, air-filled porosity and bulk density cannot be adjusted after the transplanting of the plants. That indicates that the physical properties of a growing media are fundamental for successful crop cultivation in soilless cultures. Both OD and SC residues decreased the total porosity, available water-holding capacity and air capacity of the growing media, while between the two, OD caused stronger decreases than the SC, and their mixture revealed intermediate values. Air capacity ≥ 10% is mandatory for growing media, although the recommended values are from 20 to 30% [50]. In this case, ≥10% OD in the growing media resulted in unaccepted levels of AFP (≤10%); however, this was alleviated when OD was mixed with SC, and the final AFP of the OD + SC mixtures was ≥10%. The OD low porosity and AFP were related to the very low particle sizes (≤0.4 mm) and dust size material that derived after the shredding process, while SC had average particle sizes of 3.6 mm [57]. However, mixing residue materials of various particle sizes, as in the case of OD + SC, is an effective way to overcome shortfalls in the appropriate physical properties of the growing media. Another way to improve the features of the mixtures is to introduce higher ratios of inert materials frequently used on growing media, i.e., perlite, sand, etc., to increase the particle sizes and, therefore, to increase total porosity and AFP. OD could probably be shredded in larger particles than the ones in the present study, and this can be considered in the future. The above restriction to AFP mirrors the unfavourable outcomes found in the current study’s use of OD at 10–20%.
The chemical properties of the growing media were also assessed at the end of the crop cultivation (Table 4). Regarding OD-based media, the pH, EC, organic matter and organic C were increased at ≥ OD 10%, while the high levels of P, Mg, Ca and Na have remained at low OD levels and/or control. At the end of the cultivation period, K levels in OD-based media were higher compared to peat (control), while N content remained unchanged (Table 4). In SC-based media, the high ratio of 20% of SC in the growing media revealed high pH, organic C, organic matter and K, P, and Mg levels compared to the control treatment. The EC, N, Ca, and Na levels were similar in all SC-based media compared to peat (Table 4). The properties of the mixtures of the residues (OD + SC) appeared to be between the corresponding values of OD and SC, in most cases, while at OD + SC 20%, increased the levels of pH, organic C, organic matter and N, K, and P content, and decreased Ca content compared to the peat (Table 4).

Table 4.
The physicochemical properties of the growing media (peat, Origanum dubium Boiss. residue (OD) and Sideritis cypria Post. residue (SC)) after plant growth (end of experiment).
The relevant changes in the properties from the start to the end of the crop duration are presented in Figure S1. At the end of the cultivation period, the high ratios of the OD-based media (i.e., 20% OD) had lower mineral levels (N, K, P, Mg, and Ca), while the SC 20% had lower N and K levels compared to the start of the cropping period. Indeed, the addition of the residue’s mixture in all ratios (i.e., 5%, 10% and 20% OD + SC) exhibited less minerals in most cases compared to peat at the end of the pansy cultivation (Figure S1). Sodium was accumulated in all treatments with residues, as well as in peat, at the end of the cultivation, probably due to the partial release of Na from the media, the accumulations of Na from the irrigation water and/or the Na availability/release from the media due to the antagonism with other cations, primarily K and secondarily with Ca and Mg. No significant changes took place from the initial to the final pH values of each growing media, which is well explained due to the short cultivation period.
The impacts of the selected MAP residues as a partial substitute component to peat in the growing media on pansy’s growth features are shown in Table 5. The presence of OD in the growing media reduced plant growth with significant effects at OD 20% for the plant height (up to 42.8%) and the number of flowers (up to 39.8%) produced, and this was additionally reflected in the decrease in the fresh biomass at ≥10% OD (up to 52.4%), compared to the plants grown in peat (control). The addition of SC in the media revealed plant height, leaf number and fresh weight decrease at 20% SC (up to 41.6%, 23.9% and 36.9%, respectively), compared to the lower ratios of SC and/or control treatment. Similar findings were observed with the combination of OD + SC, as leaf and flower number and plant fresh weight decreased at OD + SC by 20% (up to 39.1%, 100%, and 55.4%, respectively), and plant height decreased (up to 24.6%) at ≥10% OD + SC, compared to the control (Table 5). In previous studies, the effect of OD and SC was varied: decreased plant growth for sowthistle (Sonchus oleraceus L.) at 20–40% OD and at 40% SC [35] while in purslane (Portulaca oleracea L.), the detrimental effects on plant growth were more profound even in lower MAP ratios [57]. When ornamental plants, including marigold (Calendula officinalis L.), petunia (Petunia × hybrita L.) and matthiola (Matthiola incana L.), were grown in peat with olive-mill residues, plant growth was decreased at >10% of OMW [29]. Polyphenols in OMW are highly responsible for such a decrease [29]. Reduced plant height without compromising plant biomass is not always a bad thing for the production of ornamentals since, in a nursery setting, it could make it simpler to store and move smaller seedlings [58]. The residue materials used in the present study already had a high C/N ratio (i.e., >26) (Table 1, Table 2 and Table 3), which indicates an unstable material that continues to decompose, using for that process a part of the available N present in the mixtures, instead for the needs of the plant. This was evidenced even at the end of the experiment (Table 4) when the N levels were lower at high MAP ratios growing media (Figure S1) without observing higher biomass (Table 1, Table 2 and Table 3). Therefore, a part of the N was used by microorganisms to decompose the organic material. Even though the mineral status of the examined materials was enriched, the decreased plant growth was associated with the inadequate properties of the mixtures, including the total porosity and air-filled porosity, as reported in previous studies [35]. The OD air-filled porosity can be improved by chopping larger particle sizes or by adding inert material with higher particles, such as the perlite, leading to higher plant growth and development.

Table 5.
The effect of growing media (peat, O. dubium residue—OD, and S. cypria residue—SC) on the pansy seedlings’ height (cm), leaf and flower number, above ground fresh weight (fw; g plant−1), dry weight (dw; g plant−1) and dry matter content (%) on plants grown in the greenhouse/nursery.
The photosynthetic capacity of the plants is closely associated with the leaf pigment content and the leaf stomatal closure. The contents of chlorophyll a, chlorophyll b, total chlorophylls, carotenoids and SPAD values were significantly decreased (from 3.8% up to 40.5%) in pansy grown in mixtures with SC 20% and OD + SC 20%, compared to plants grown in 100% peat (Table 6). Indeed, the high levels of residues in the case of OD (i.e., OD 20%), decreased leaf chlorophyll fluorescence (up to 7.6%) and SPAD values (up to 55.6%) but not the chlorophylls and carotenoids content. The increase in chlorophyll degradation or a decline in chlorophyll synthesis could be the cause of the reduction in chlorophyll content. Chl b is changed into Chl a via the degradation of chlorophyll [59]; this could account for the decrease in chlorophyll content and a rise in the ratio of Chl a to Chl b when leaves are subjected to stress conditions at SC 20% and OD + SC 20%. The carotenoid:chlorophyll ratio was raised in plants grown with OD ≥10%, which actually mirrored the decrease in leaf chlorophyll fluorescence and, therefore, led to the decrease in the leaf photosynthetic capacity and plant growth, as observed in Table 5 [60]. However, plants may partially overcome the chlorophylls decrease and the relevant stress conditions when they are subjected to complete fertigation with hydroponic nutrient solution, as was evidenced in ornamental plants grown in 30% olive mill residues [29]. As mentioned earlier, leaf stomatal closure also affects the photosynthetic capacity of plants. Even though leaf stomatal conductance was not determined in the present study, there is evidence from previous studies where sowthistle plants grown in MAP residues-based media revealed decreases in leaf stomatal conductance even at low rates of the residues in the mixtures when compared to the control (100% peat) treatment [35]. Plants react to various stress conditions by closing their stomata, as has been previously reported in tomato (Solanum lycopersicum Mill.) plants that grew in sand and were irrigated with olive mill wastewater; in this case, irrigation water provoked a stress condition via by increasing the EC of the material [61].

Table 6.
The effect of growing media (peat, O. dubium residue—OD, and S. cypria residue—SC) on the pansy chlorophyll fluorescence (Fv/Fm), SPAD values, chlorophylls (Chl a, Chl b, total Chls; mg g−1 fw), and carotenoids (mg g−1 fw) levels as well as their ratios of Chl a:Chl b and carotenoids:total Chls on plants grown in the greenhouse/nursery.
Table 7 presents the mineral content as accumulated in pansies grown in OD- or SC- or OD + SC-based growing media after a 28-day cultivation period. The addition of OD into the peat-based media revealed pansy N content decrease, but K content increased at ≥10% OD while Ca, Mg, and Na contents decreased at OD 20%. Interestingly, phosphorus was accumulated in plants grown in the control treatment (peat). In the case of SC addition in the growing media, N content decreased, and K content increased at SC by 20%. Calcium content was decreased at ≥SC 10%, and Mg and Na contents were decreased at ≥SC 5% compared to the control treatment. Phosphorus remained unaffected among the SC-based treatments and averaged in 6.89 g P kg−1 dry weight. Combining the OD and SC residues in peat-based media, N levels were increased at OD + SC 5% compared to peat or mixtures with higher ratios (≥10% OD + CS). Potassium, Ca, Mg, and Na levels of the plants of the OD + SC mixtures were in intermediate values of the single OD or SC values. Phosphorus did not differ among the tested treatments.

Table 7.
Effects of growing media (Peat, O. dubium residue—OD, S. cypria residue—SC, and their mixtures) on mineral element contents (g kg−1 dry weight) in pansy plants grown in the greenhouse/nursery.
Considering the immature stage of the utilized residues, when OD, SC and OD + SC were used in high ratios, that practically means that a higher amount of immature residues would undergo the decomposition process, and available N would be consumed partially during this process by microorganisms rather than it would be accumulated inside the plant tissue (Table 7 and Figure S1). This was mirrored by the lower N accumulation in plants grown at high residue ratios (Table 7). It is worth indicating that not all N was available to the plants since a large part of N was mainly organic and needed to be first subjected to mineralization before it could be used by the plants. Additionally, mineral shortage was evidenced at the end of the experiment, especially at the high OD and OD + SC residue levels, which was reflected in the decreased plant growth in conjunction with the inappropriate growing media properties. Indeed, the SC residues were more fertile than the OD (Table 1 and Table 2), and mineral shortage at the end of the study was less evident in the cases of N and K (Figure S1). One way to adjust any mineral shortages during plant growth is to provide fertilizers based on the growing media mineral levels [29,62].
Various abiotic and biotic stress factors can provoke the active formation of free radicals, as the increased stress conditions reflect the more active oxidation processes that occur in the plant, as the plants lose their antioxidant capacity [63]. Plants respond to stress by activating a number of enzymatic and non-enzymatic processes [64]. Induced stress conditions were found by MAP supplementation. The total phenols production is one of the non-enzymatic antioxidant processes. Total phenols content in pansies was increased at ≥OD 10% (up to 62.1%; Figure 1(A1)), at SC 5% and SC 20% (up to 21.3%; Figure 1(B1)) compared to the control treatment. Moreover, the content of total phenols increased at OD + SC 20% (1.4 times; Figure 1(C1)). The antioxidant capacity of pansy grown in SC-based media was not affected (Figure 1(B2–B4)). However, pansies grown in OD-based media revealed increased levels of DPPH and FRAP at ≥OD 10% and ABTS at OD 10% compared to the peat treatment. The mixtures of the residues (OD + SC 20%) also produced pansy plants with increased antioxidant levels in terms of DPPH, FRAP and ABTS (Figure 1(C2–C4)). The content of total flavonoids was increased at the OD 20% (up to 3.1 times; Figure 1(A5)) and at OD + SC 20% (up to 3.2 times; Figure 1(C5)) since SC addition did not affect the flavonoids content (Figure 1(B5)). In general, the OD supplementation increased the antioxidant capacity of the pansy plants, whereas SC did not affect the capacity. In the case of pansies, the observations of increased antioxidant capacity could be valuable in case the increase in antioxidants could be carried over to the plant’s flowers, which are edible flowers, as successfully reported when pansies were grown in hydroponics [65].
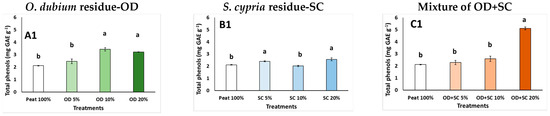
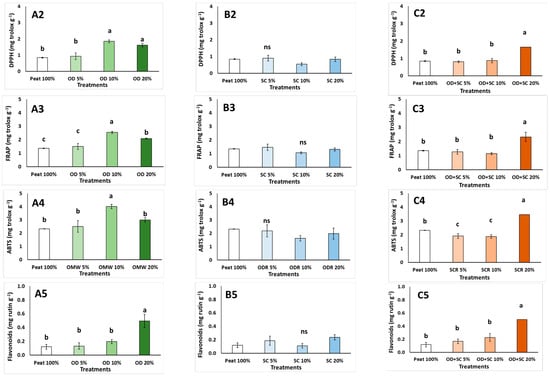
Figure 1.
Effects of growing media (Peat, O. dubium residue—OD, S. cypria residue—SC, and their mixtures) on total phenols (mg GAE g−1 fw), antioxidant activity (DPPH, FRAP, ABTS: mg trolox g−1 fw), and flavonoids (mg rutin g−1 fw) in pansy plants (OD: (A1–A5); SC: (B1–B5); OD + SC: (C1–C5)). Values are mean ± SD (n = 6). Mean values followed by the same letter do not differ significantly at p ≥ 0.05, according to Duncan’s Multiple Range Test. ns: no significance.
MDA generation, which is associated with increasing lipid peroxidation under stressful situations, is one of the most frequently utilized stress markers, where excessive levels of H2O2 generation signify a stressful situation for cellular damage. MDA levels increase as the first stage in detoxifying reactive oxygen species (ROS). MDA levels increased as the OD levels were increased in the growing media, revealing the highest values at the ≥OD 10% (up to 2-fold) compared to the control treatment (Table 8). However, SC presence reflected lighter/no stress when used in a low ratio of 5–10% SC, as MDA levels were statistically increased (1.5 times) only in the case of SC 20% compared to peat treatment. Considering the residues mixture, MDA was increased up to 4.5 times at OD + SC 20% compared to peat or ≤10% OD + SC mixtures. A similar trend to the MDA production was found in the levels of H2O2 content (Table 8). Plants are able to detoxify the ROS accumulation by activation of the enzymatic and non-enzymatic antioxidant process [64], whereas the increased MDA was in conjunction with the increase in total phenols, antioxidants and flavonoids at the higher residue’s levels in the growing media (Table 7). As for the enzymatic activity, increased SOD activity was found for plants grown at the higher ratios of 20% of OD and SC, which is in alignment with the increased H2O2 levels measured at these ratios. SOD comes as the first defence mechanism to collect reactive oxygen species and transform them into H2O2 molecules. The lower CAT activity at the same treatments indicates that the enzyme has not yet been activated, while the increased values in the rest of the OD and SC treatments reveal that the enzymes were active, and they removed the additional H2O2 from the cells. POD values also indicated that OD and SC-based mixtures increased along with the rise of the percentage inside the medium to remove hydrogen peroxide overflow. It seems that the induced stress caused by the OD and SC residues in the ratio of ≤10% can be soothed by the antioxidant mechanism of the plant itself by inducing both enzymatic and non-enzymatic pathways.

Table 8.
Effects of growing media (Peat, O. dubium residue—OD, S. cypria residue—SC, and their mixtures) on hydrogen peroxide—H2O2 (μmol g−1) and lipid peroxidation—MDA (nmol g−1) in pansy plants grown in the greenhouse/nursery.
To produce seedlings, cuttings and potted ornamental plants under nursery conditions, it is important to make sure that plants are grown promptly, are in good condition for the market needs, and are cost-effective. Selecting peat substitutes that are environmentally friendlier with desired physicochemical properties is also crucial when introducing them to growing media mixtures [31]. In that sense, future research should focus on adjusting growing media components quantities while always considering the amount and regularity of fertilization and watering.
4. Conclusions
The present study assessed the partial peat substitution with residues derived from the MAP industry for the pansies’ seedling production. Both the MAP residues (OD and SC) and their combination (OD + SC) had a significant effect on the physicochemical characteristics of the produced media when they were added to the peat-based mixtures. The primary barriers were the declining total and air-fill porosities of the growing media, while the addition of residues enhanced the medium’s organic matter and mineral content, making them more nutrient-dense for the plants. However, the N levels were in shortage due to the partial decomposition of the residues, and more N is suggested to be added in future studies. High levels of OD, SC, and OD + SC inhibited plant growth and photosynthetic-related parameters, as well as mineral accumulation in the plant tissue. The decrease was mainly due to the inappropriate growing media properties and, to a lesser extent, to the mineral shortage in the growing mixtures. Plants grown at low ratios of 5% OD, 5–10% SC and 5–10% OD + SC revealed some positive effects with slight increase or maintenance in decorative-related qualities of plants such as leaf and flower numbers produced as well fresh biomass. However, greater ratios of residues (≥5% OD, ≥20% SC, ≥20% OD + SC) decreased the number of flowers, plant height and plant fresh weight up to 39.8%, 42.8%, 52.4% for OD, up to 0%, 41.6% 36.9% for SC, and up to 100%, 32.4%, 55.4% for OD + SC, respectively. Those decreases are related to the plant’s oxidative stress, with lipid peroxidation to be evidenced and the activation of various non-enzymatic antioxidant mechanisms, including increased levels of polyphenols, flavonoids, and antioxidants. Finally, the current study indicates that OD, SC and OD + SC can be used in the growing media at low ratios of 5%, 10%, and 10%, respectively, maintaining the growth and photosynthetic capacity of plants, with partial peat substitution. However, it is necessary to investigate potential additional fertigation as well as improve the growing media properties with materials of larger particle sizes to improve media porosity and, as a result, features such as air and water capacity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14010187/s1, Figure S1: Changes of growing media (Peat, O. dubium residue -OD, S. cypria residue -SC and their mixtures) chemical properties (% of changes from initial to final material) after plant harvesting (at the end of the growing period).
Author Contributions
Conceptualization, A.C. and N.T.; methodology, A.C. and N.T.; software, A.C.; validation, A.C. and N.T.; formal analysis, A.C. and N.T.; investigation, A.C. and N.T.; resources, N.T.; data curation, A.C.; writing—original draft preparation, A.C. and N.T.; writing—review and editing, A.C. and N.T.; visualization, A.C. and N.T.; supervision, N.T.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors declare data availability only upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, J.; Chen, Y.; Wang, K.; Huang, Y.; Wang, H. Re-utilization of Chinese medicinal herbal residues improved soil fertility and maintained maize yield under chemical fertilizer reduction. Chemosphere 2021, 283, 131262. [Google Scholar] [CrossRef] [PubMed]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crop. Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef]
- Brinckmann, J.A.; Kathe, W.; Berkhoudt, K.; Harter, D.E.V.; Schippmann, U. A New Global Estimation of Medicinal and Aromatic Plant Species in Commercial Cultivation and Their Conservation Status. Econ. Bot. 2022, 76, 319–333. [Google Scholar] [CrossRef]
- Schippmann, U.; Leaman, D.; Cunningham, A.B. A Comparison of Cultivation and Wild Collection of Medicinal and Aromatic Plants Under Sustainability Aspects. In Medicinal and Aromatic Plants; Bogers, R.J., Cracker, L.E., Lange, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 75–95. ISBN 1402054491. [Google Scholar]
- Usano-Alemany, J.; Herraiz-Peñalver, D.; Cuadrado, J.; Díaz, S.; Santa-Cruz, M.; Palá-Paúl, J. Seasonal variation of the essential oils of Salvia lavandulifolia: Antibacterial activity. J. Essent. Oil-Bear. Plants 2012, 15, 195–203. [Google Scholar] [CrossRef]
- De Oliveira, B.M.S.; Blank, A.F.; Nizio, D.A.D.C.; Nogueira, P.C.D.L.; Arrigoni-Blank, M.D.F.; Bacci, L.; Melo, C.R.; Nascimento, L.F.D.A.; Sampaio, T.S. Chemical analyses of the essential oils from Varronia curassavica accessions in two seasons. J. Essent. Oil Res. 2020, 32, 494–511. [Google Scholar] [CrossRef]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental effects on yield and composition of essential oil in wild populations of spike lavender (Lavandula latifolia medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Petropoulos, S.A.; Fernandes, Â.; Barros, L.; Tzortzakis, N.; Ferreira, I.C.F.R. Effect of phosphorus application rate on Mentha spicata L. grown in deep flow technique (DFT). Food Chem. 2019, 276, 84–92. [Google Scholar] [CrossRef]
- Navarrete, A.; Herrero, M.; Martín, A.; Cocero, M.J.; Ibáñez, E. Valorization of solid wastes from essential oil industry. J. Food Eng. 2011, 104, 196–201. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crop. Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Olofsson, J.; Börjesson, P. Residual biomass as resource—Life-cycle environmental impact of wastes in circular resource systems. J. Clean. Prod. 2018, 196, 997–1006. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Dong, L.; Jing, Y. An innovative example of herb residues recycling by gasification in a fluidized bed. Waste Manag. 2013, 33, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Jin, J.; Zheng, Y.; Li, S. Current Advances of Resource Utilization of Herbal Extraction Residues in China. Waste Biomass Valorization 2021, 12, 5853–5868. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from Citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’ distillation Mohamed. Flavour Fragr. J. 2008, 22, 494–504. [Google Scholar] [CrossRef]
- Saha, A.; Tripathy, V.; Basak, B.B.; Kumar, J. Entrapment of distilled palmarosa (Cymbopogon martinii) wastes in alginate beads for adsorptive removal of methylene blue from aqueous solution. Environ. Prog. Sustain. Energy 2018, 37, 1942–1953. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Lu, Q.; Li, C. Comprehensive utilization of Chinese medicine residues for industry and environment protection: Turning waste into treasure. J. Clean. Prod. 2021, 279, 123856. [Google Scholar] [CrossRef]
- Xiao, D.; Shao, H.; Huo, Y.; Agung Nugroho, W.; Ifeoluwa Ogunniran, B.; Fan, W.; Huo, M. Reclamation of ginseng residues using two-stage fermentation and evaluation of their beneficial effects as dietary feed supplements for piglets. Waste Manag. 2022, 154, 293–302. [Google Scholar] [CrossRef]
- Gavarić, N.; Kladar, N.; Mišan, A.; Nikolić, A.; Samojlik, I.; Mimica-Dukić, N.; Božin, B. Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind. Crop. Prod. 2015, 74, 457–464. [Google Scholar] [CrossRef]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J. Infect. Public Health 2020, 13, 58–67. [Google Scholar] [CrossRef]
- Zhou, Y.; Manu, M.K.; Li, D.; Johnravindar, D.; Selvam, A.; Varjani, S.; Wong, J. Effect of Chinese medicinal herbal residues compost on tomato and Chinese cabbage plants: Assessment on phytopathogenic effect and nutrients uptake. Environ. Res. 2023, 216, 114747. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Ali, E.F.; Al-Yasi, H.M.; Majrashi, A.; Farahat, E.A.; Eid, E.M.; Galal, T.M. Chemical and Nutritional Characterization of the Different Organs of Taif’s Rose (Rosa damascena Mill. var. trigintipetala) and Possible Recycling of the Solid Distillation Wastes in Taif City, Saudi Arabia. Agriculture 2022, 12, 1925. [Google Scholar]
- Singh, P.; Hundal, J.S.; Patra, A.K.; Wadhwa, M.; Sharma, A. Sustainable utilization of Aloe vera waste in the diet of lactating cows for improvement of milk production performance and reduction of carbon footprint. J. Clean. Prod. 2021, 288, 125118. [Google Scholar] [CrossRef]
- Ratiarisoa, R.V.; Magniont, C.; Ginestet, S.; Oms, C.; Escadeillas, G. Assessment of distilled lavender stalks as bioaggregate for building materials: Hygrothermal properties, mechanical performance and chemical interactions with mineral pozzolanic binder. Constr. Build. Mater. 2016, 124, 801–815. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Saez-Tovar, J.; Martinez-Sabater, E.; Gruda, N.S.; Egea-Gilabert, C. Promising composts as growing media for the production of baby leaf lettuce in a floating system. Agronomy 2020, 10, 1540. [Google Scholar] [CrossRef]
- Bolechowski, A.; Moral, R.; Bustamante, M.A.; Bartual, J.; Paredes, C.; Pérez-Murcia, M.D.; Carbonell-Barrachina, A.A. Winery-distillery composts as partial substitutes of traditional growing media: Effect on the volatile composition of thyme essential oils. Sci. Hortic. 2015, 193, 69–76. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Tzionis, A.; Prasad, M.; Tzortzakis, N. Alternative soilless media using olive-mill and paper waste for growing ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 35915–35927. [Google Scholar] [CrossRef]
- Gruda, N. Current and future perspective of growing media in Europe. Acta Hortic. 2012, 960, 37–43. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Bustamante, M.A.; Amara, M.B.; Tittarelli, F. The challenge of peat substitution in organic seedling production: Optimization of growing media formulation through mixture design and response surface analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Tsakaldimi, M. Kenaf (Hibiscus cannabinus L.) core and rice hulls as components of container media for growing Pinus halepensis M. seedlings. Bioresour. Technol. 2006, 97, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Olive-mill and grape-mill waste as a substitute growing media component for unexploded vegetables production. Sustain. Chem. Pharm. 2023, 31, 100940. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Goumenos, C.; Tzortzakis, N. Use of Medicinal and Aromatic Plant Residues for Partial Peat Substitution in Growing Media for Sonchus oleraceus Production. Agronomy 2023, 13, 1074. [Google Scholar] [CrossRef]
- Tebenkova, D.N.; Lukina, N.V.; Vorobyev, R.A.; Orlova, M.A.; Gagarin, Y.N. Germination and biometric parameters of seedlings grown on solid pulp and paper waste medium. Contemp. Probl. Ecol. 2015, 8, 892–900. [Google Scholar] [CrossRef]
- Salachna, P. Trends in Ornamental Plant Production. Horticulturae 2022, 8, 413. [Google Scholar] [CrossRef]
- Gandolfo, E.; Hakim, G.; Geraci, J.; Feuring, V.; Giardina, E.; Benedetto, A. Responses of Pansy (Viola wittrockiana Gams.) to the Quality of the Growing Media. Am. J. Exp. Agric. 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Nobile, C.; Denier, J.; Houben, D. Linking biochar properties to biomass of basil, lettuce and pansy cultivated in growing media. Sci. Hortic. 2020, 261, 109001. [Google Scholar] [CrossRef]
- Getter, K.L.; Rozeboom, D.W. Animal tissue compost as a potential substrate amendment for production of four annual floriculture crops. Horttechnology 2014, 24, 686–695. [Google Scholar] [CrossRef]
- European Standard EN 13041; Soil Improvers and Growing Media—Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space. European Committee for Standardization: Brussels, Belgium, 1999.
- Tzortzakis, N.; Pitsikoulaki, G.; Stamatakis, A.; Chrysargyris, A. Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System. Agronomy 2022, 12, 386. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Kloukina, C.; Vassiliou, R.; Tomou, E.-M.E.-M.; Skaltsa, H.; Tzortzakis, N. Cultivation strategy to improve chemical profile and anti-oxidant activity of Sideritis perfoliata L. subsp. perfoliata. Ind. Crop. Prod. 2019, 140, 111694. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Rinaldi, S.; De Lucia, B.; Salvati, L.; Rea, E. Understanding complexity in the response of ornamental rosemary to different substrates: A multivariate analysis. Sci. Hortic. 2014, 176, 218–224. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Gupta, N. Integrated nutrient management in menthol mint cultivation utilizing mint residue fertilizer. J. Med. Aromat. Plant Sci. 1999, 21, 1058–1063. [Google Scholar]
- Patra, D.D.; Anwar, M.; Chand, S. Integrated nutrient management and waste recycling for restoring soil fertility and productivity in Japanese mint and mustard sequence in Uttar Pradesh, India. Agric. Ecosyst. Environ. 2000, 80, 267–275. [Google Scholar] [CrossRef]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of composts in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhan, S.; Yu, H.; Xue, X.; Hong, N. The effects of temperature and catalysts on the pyrolysis of industrial wastes (herb residue). Bioresour. Technol. 2010, 101, 3236–3241. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, S.; Jin, A.; Tang, J.; Luo, Y. The use of un-composted spent mushroom residue as a replacement of peat in substrates for Gossypium herbaceum and Talinum paniculatum. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12193. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Romero, A.M.; Pereira, H.; Borges, P.; Cabral, F.; Vasconcelos, E. Evaluation of a compost obtained from forestry wastes and solid phase of pig slurry as a substrate for seedlings production. Bioresour. Technol. 2007, 98, 3294–3297. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Louka, S.; Petropoulos, S.A.; Tzortzakis, N. Soilless Cultivation of Portulaca oleracea Using Medicinal and Aromatic Plant Residues for Partial Peat Replacement. Horticulturae 2023, 9, 474. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kargas, G.; Lytra, I. Olive-mill waste compost as a growth medium component for foliage potted plants. HortScience 2005, 40, 1746–1750. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Kiarostami, K.; Mohseni, R.; Saboora, A. Biochemical changes of Rosmarinus officinalis under salt stress. J. Stress Physiol. Biochem. 2010, 6, 114–122. [Google Scholar]
- Ouzounidou, G.; Asfi, M.; Sotirakis, N.; Papadopoulou, P.; Gaitis, F. Olive mill wastewater triggered changes in physiology and nutritional quality of tomato (Lycopersicon esculentum Mill.) depending on growth substrate. J. Hazard. Mater. 2008, 158, 523–530. [Google Scholar] [CrossRef]
- Carmona, E.; Moreno, M.T.; Avilés, M.; Ordovás, J. Use of grape marc compost as substrate for vegetable seedlings. Sci. Hortic. 2012, 137, 69–74. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of abiotic stress factors on the antioxidant properties and polyphenols profile composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, I.; Chrysargyris, A.; Finimundy, T.C.; Carocho, M.; Santos-Buelga, C.; Calhelha, R.C.; Tzortzakis, N.; Barros, L.; Heleno, S.A. Magnesium and manganese induced changes on chemical, nutritional, antioxidant and antimicrobial properties of the pansy and Viola edible flowers. Food Chem. 2024, 438, 137976. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).