Abstract
Maize is part of the traditional diet of Mexico and other Latin American countries. The diverse varieties of maize produced by adaptation to different regions and climates are known as creole or native maize. The characteristics and nutritional contributions of each of these native maize are relatively diverse areas of study. This work specifically analyzes the nutritional aspects of creole maize grown in the Sierra Gorda in Querétaro. For this, a proximal chemical analysis was carried out, with a quantification of polyphenolic compounds and antioxidant capacities using the ABTS (2,20-azinobis (3-ethyl-benzothiazolin,6-sulfonic acid) and DPPH (2,2-difenil-1-picrilhidrazilo) methods to examine four landraces and one commercial hybrid control. The results indicate that, in general, the landraces present similar—and, in some cases, higher—nutritional indices than the commercial hybrid. With regard to polyphenolic compounds, the Elotes Occidentales and Tuxpeño varieties present greater amounts of total phenols and antiradical activity (ARA), as well as a higher concentration of flavonoids and percentage inhibition, respectively. Condensed tannins are identified both in the creole varieties and in the commercial hybrid. Creole maize presents important nutritional characteristics and can be a good source of antioxidant compounds, which can help the population as a source of food and in the prevention of chronic diseases.
1. Introduction
For various Central American countries, maize (Zea mays L.) is a core component of the diet and culture [1]. In Mexico, maize is a staple food product that is consumed in many forms, and natural and human selection processes have led to the development of specific varieties for each ecological niche or micro-region, generating what is known as creole maize [2,3,4]. The term “creole maize” is used to denote a maize variety native to a community, region, or state, and as such, it presents heterogeneous characteristics in its cultivation, color, texture, crop cycle, type of grain, hardness, and even type of use. Additionally, creole or native maize has ancestral roots in the life of Mexicans [5]. In Mexico, 65% of the maize cultivated in areas under rainfed conditions is grown from native seeds, and the rest is hybrid maize [6]. Most of the creole maize crops are destined for self-consumption in the region where they are grown. The nutraceutical properties of this type of maize benefit the populations that consume them.
Among the most important nutraceutical compounds found in creole maize are polyphenolic compounds. These include anthocyanins, which are the main natural pigments present in native maize, providing the grain with a high added value and making them more attractive for various industries. These bioactive compounds present in pigmented corn show a wide range of healthy properties such as anti-inflammatory, antibacterial, anticancer, neuroprotective, lipid-lowering, anti-obesogenic, and antioxidant properties [7,8,9]. An antioxidant compound is a substance that, even at low concentrations, helps reverse oxidative stress by significantly reducing or inhibiting substrate oxidation. Oxidative stress and inflammation have been associated with various diseases, including obesity, which is considered co-responsible for the increase in morbidity and mortality in recent decades [10]. Polyphenolic compounds have been located in the pericarp, aleurone, endosperm, and embryo of maize [11]. Within these compounds, the presence of phenolic acids and flavonoids, mainly ferulic acid and anthocyanins, respectively, has been reported. Both phenols and anthocyanins have been reported to have beneficial effects on human health, highlighting their antioxidant activity [8,9]. However, there is little scientific information on the amount of polyphenolic compounds that are present in native corn and how useful they could be as a natural source of antioxidants and thereby contribute to the reduction in diseases associated with oxidative stress, in addition to promoting the cultivation of native corn as a nutraceutical food. With this in mind, the objective of this study was to determine the content of polyphenolic compounds in terms of total phenols, total flavonoids, condensed tannins, and total carotenoids as well as the antioxidant capacities in grains of creole maize from the Sierra Gorda in Querétaro, specifically in the area of influence, which is the municipality of Arroyo Seco. The information generated from this study can be the basis for selecting creole maize that can be used to increase nutraceutical quality.
2. Materials and Methods
2.1. Seed Collection
The seeds were collected in ears with leaves and were obtained directly from the producers’ plots, which were located at different points of the Sierra Gorda in Querétaro, in order to obtain a representative sample of the area. Each variety was assigned a field label number for identification. No chemical preservatives were applied.
- Blanco Criollo Maize (1798) Collection date: August 2021. Collection site: Delegation of Concá, Arroyo Seco, Qro. Name of the producer who donated the seed: Rafael Elías Arvizu;
- Pinto Maize (1796) Collection date: April 2022 Collection site: Delegation of Concá. Arroyo Seco, Qro. Name of the producer who donated the seed: Francisco Yáñez Balderas;
- Amarillo Maize (1795) Collection date: April 2022. Collection site: Delegation of Concá. Arroyo Seco, Qro. Name of the producer who donated the seed: Adán Arredondo Arvizu;
- Morado Maize (1797) Collection date: April 2022 Collection site: La Presa, municipality of Lagunillas, SLP. Name of the producer who donated the seed: Hilario Baca Mancilla.
Figure 1 shows photographs of the representative ears of the creole maize obtained. The selection criteria were based on the producer’s observation: health and size of the ear, grain size, number of grains on the ear, and thickness of the cob. Each sample was assigned an identifying number, and the samples were then sent to the seed laboratory of the Faculty of Natural Sciences (UAQ) for the morphological and agronomic characterization of the maize varieties. The commercial hybrid maize sample (A 7573) was purchased from the Asgrow brand seed trading house.

Figure 1.
Photographs of the creole maize from the Sierra Gorda in Querétaro. (A) Blanco Criolle Maize (1798) = Raton Tuxpeño Norteño, (B) Pinto Criolle Maize (1796) = Tuxpeño, (C) Amarillo Maize (1795) = Hybrid Northern Tuxpeño, (D) Morado Maize (1797) = Elotes Occidentales, and (E) Comercial Hybrid Maize (A 7573).
2.2. Morphological and Agronomic Characterization of Seeds
Each variety was identified according to the descriptions of the ear, plant, ear morphology, and days to flowering in comparison with a representative sampling of all the varieties [6,12,13,14], in collaboration with Dr. Rafael Ortega Paczka, a specialist in the subject and researcher at the Chapingo Autonomous University. As a result, the following identification was obtained:
Blanco Criollo Maize (1798): found in the literature as “Raton-Tuxpeño Norteño” with the characteristics of “Ratón” predominating and “Tuxpeño Norteño”, a native variety of the region, as a secondary variety. Similarly, the Morado Maize (1797) was identified as “Elotes Occidentales”, a native variety. Two of the maize types—Amarillo Maize (1795) (Hybrid Tuxpeño Norteño) and Pinto criolle Maize (1796) (Improved Tuxpeño)—showed a combination with improved maize, meaning they were possibly crossed with commercial varieties, although the producer categorizes them as creole maize. The commercial hybrid maize (variety A 7573) was a creole hybrid of northern Tuxpean maize from the Caribbean, which possessed the characteristics of a commercial variety. Based on the morphological characterization, the samples were assigned the name of the identified variety.
2.3. Sowing
The seeds obtained from the producers of the Sierra Gorda de Querétaro were grown in an area of 1 ha; a total of 5 plots were established with 5 types of maize (Ratón Tuxpeño Norteño, Tuxpeño improved, Tuxpeño Norteño Hybrid, Elotes Occidental, and Commercial White Hybrid). First, the soil was prepared with the cultures taken, followed by analysis of the subsoil, tracking, furrowing, and sowing; once the soil was prepared, the furrows were traced 75 cm apart, and seeds were planted along each furrow 20 cm apart, with a planting density of 70 thousand per ha. Each planting plot contained ten rows for each type of maize. The plots were spaced 15 m apart, as a barrier to avoid cross-contamination by pollination. Each plot was sown with a lapse of three weeks from the previous one; the white maize was sown first, then, after 21 days, the yellow maize was sown, and so on. Thus, the lag between the sowing of each variety prevented a coincidence of blooms and the contamination of the crop by cross-pollination. This process was carried out three times during the same year to obtain three independent repetitions for each of the crops.
2.4. Irrigation
A slow-flow 0.5” diameter drip irrigation tape was placed, with drippers spaced each 15 cm in a single row; each dripper yields 0.7 gallons per hour. The irrigation was applied every eight days according to the need of the crop, supplying an average irrigation sheet of 20 cm in depth, supported by the readings of an irrometer brand tensiometer, which was maintained in a range between 12 and 15 kPa.
2.5. Nutrition
In the development stage, 30 days after sowing (daw), 4 kg of 46% urea and 10 kg of FOLI-GREEN 19%–19%–19% fertilizer balanced in NPK and enriched with boron and molybdenum were applied via drip. Iron, manganese, zinc, and copper chelated with the disodium salt of 2,2′,2″,2″-(Ethane-1,2-diyldinitrilo)tetra acetic acid (EDTA) were also added in mineral form.
2.6. Plagues, Diseases, and Weed Control
In general, there were no significant pests to control; a preventive application of Bacillus HD® (Bacillus turingensis variety Kurastaki strain HD-1 and HD-3, with a power of 42,000 UI/mg of the product) was enacted in order to control fall armyworms, and this application was sprayed onto the crop using a backpack-type sprinkler. The dose used was 3 g/L of water at a frequency of once a week via foliar application. Regarding weed control, this was done mechanically; no chemical products were used for this purpose.
2.7. Harvest and Sampling
Once the cultivation cycle was completed and before harvest, plants (3 plants) from different points of the plot were selected for the collection of the ears (3 ears per collection point), approximately 90 days after observing the state of the dry grain, leaving the ear to dry naturally on the plant and then being hand-picked so as to not mistreat the grains. The choice of the plants was made based on location, discarding those that were on the edges of the plot. Healthy, pest-free, vigorous plants with a homogeneous color in their leaves and stems were selected. Once the plant was dry, the cob was harvested. The criteria for the selection of the cob were as follows: it needed to be free of pests, a criterion related to the formation of the grain lines that formed the cob, a criterion related to the filling and number of grains that formed the lines of the cob, homogeneity in the color of the grain depending on the type of variety, and the size of the cob (the largest were selected in terms of the characteristics of each crop). The ears were collected and labeled individually for further processing. From the selected ears, the grains at the ends were discarded, and only the grains from the central area were used for subsequent analyses.
2.8. Proximal Chemical Analysis
Ash and moisture content was determined by the AOAC method (1975); protein content was determined by the MacroKjeldahl method, using a nitrogen-to-protein conversion factor of 6.25 following the NMX-F-068-S-198; the ethereal extract was determined based on the Soxhlet extraction technique (NMX-Y-103-SCFI-2004); and carbohydrates and crude fiber were determined according to NMX-Y-097-1974 and NMX-Y-94-SCFI-200, respectively.
2.9. Polyphenolic Compounds
2.9.1. Extraction of Polyphenolic Compounds
The polyphenolic compounds were extracted according to the methodology described by Cardador-Martínez et al. [15], with some modifications. Two hundred milligrams of ground criollo maize was mixed with 10 mL of methanol (HPLC Grade, 99.98%, Sigma-Aldrich, St. Louis, MO, USA), agitated continuously for 30 min in an ultrasonic bath (ELMASONIC P, Multifrequency, Singen (Hohentwiel), Germany) at a temperature of 25 °C, then centrifuged (Themo Scientific, Waltham, MA, USA) at 5000× g rpm for 10 min at 4 °C. The resulting supernatant was used for subsequent analysis.
2.9.2. Total Flavonoids
For the quantification of total flavonoids, an aliquot of the methanolic extract (50 μL) was added to 180 μL of methanol and 20 μL of a 2-aminoethyldiphenylborate solution (1%) in a 96-well microplate. Absorbance was recorded using the Multiskan Go (model 51119300, Thermos Scientific, Vantaa, Finland) microplate reader with 404 nm filters. A rutin standard was prepared in methanol. Extract absorption was compared with that of a rutin standard curve (up to 2 μg/mL). The sample was tested in triplicate. The results obtained were expressed as a mg equivalent of rutin (RE)/100 g of the sample [16].
2.9.3. Total Phenols
The phenol content was determined using the Folin–Ciocalteu method [17]. For the quantification of these compounds, a calibration curve was made with a known concentration of gallic acid (0, 1, 2, 4, 5, 8, 10, 16, and 20 μg). A total of 460 μL of distilled water, 250 μL of the Folin–Ciocalteu reagent (1 N), and 1250 μL of the Na2CO3 solution (20%) were added to 40 µL of the methanolic extract, and this was allowed to stand for 2 h in the dark for the identification of compounds via color change. The blank was prepared using a Folin–Ciocalteu reagent and Na2CO3. After a period of time, absorbance at 760 nm was measured in a spectrophotometer (Genesys 10S UV-Vis, Thermo Fisher Scientific, Waltham, MA, USA). Gallic acid was used for the calibration curve. The sample was tested in triplicate, and the results were expressed as a mg gallic acid equivalent (GAE)/100 g of the sample.
2.9.4. Condensed Tannins
The quantification of condensed tannins was carried out according to the methodology described by Feregrino-Pérez et al. [18], adapted to a 96-well microplate. A total of 50 μL of the sample supernatant was taken and mixed with 200 μL of 0.5% of a vanillin reagent (1% vanillin and 8% HCl in a 1:1 ratio). The blank was prepared using 50 μL of methanol and 200 μL of 4% HCl. For the total content of condensed tannins, quantification was performed via spectrophotometry (Multiskan Go, model 51119300, thermos Scientific, Vantaa, Finland) at an absorbance of 492 nm, using (+)-catequin (up to 0.1 mg/mL) as a reference standard. The sample was tested in triplicate. The concentration was expressed as mg equivalents of (+) catechin (CE)/100 g of the sample.
2.9.5. Anthocyanins
The anthocyanin content was determined using the method described by Abdel-Aal and Hucl [19]. A total of 500 milligrams of the sample were weighed and mixed with 25 mL of acidified ethanol (ethanol: HCl 1N). This solution was stirred at 8000 rpm for 30 min, and the pH was adjusted to 1.0 with 4 N HCl. The solution was then centrifuged at 5000 rpm for 15 min. Finally, 240 µL of the resulting sample was taken and read at an absorbance of 535 nm (Genesys 10S UV-Vis, Thermo Fisher Scientific, Waltham, MA, USA). The results were expressed in mg equivalents of cyanidin 3-glucoside (CGE)/per gram of the sample.
2.10. Antioxidant Capacity
2.10.1. DPPH
Antioxidant activity was determined using the DPPH free radical scavenging assay (DPPH), following the method developed by Brand and Williams [20] and modified by Fukumoto and Mazza [21] for microplate use. The DDPH (diphenyl-picryl-hydrazyl) reagent was prepared in a 25 mL flask, adjusted with methanol, and added to the supernatant resulting from the extraction. Absorbance was measured in a spectrophotometer (Multiskan Go, model 51119300, thermos Scientific, Vantaa, Finland) at 532 nm every 10 min for 90 min.
The plate was covered and kept in the dark at room temperature between readings. A standard curve with Trolox at 0.05–0.8 mM concentrations was used. The antiradical activity (ARA) was calculated by the percentage of DPPH decolorization using Equation (1), where Sabs is the absorbance of the sample at 532 nm, and Cabs is the absorbance of the control (the absence of an antioxidant).
ARA = (1 − Sabs/Cabs) × 100.
2.10.2. ABTS
The antioxidant activity of the maize extracts was determined according to the method developed by Nenadis et al. [22], modified for a microplate using 2,2′-azinobis (3-ethyl-benzothiazolin,6-sulfonic acid) (ABTS). The radical cation was prepared with 7 nM ABTS stock solution and 140 nM potassium persulfate (1/1, v/v), and the mixture was left for 12 h until the reaction was completed and the absorbance was stable. The ABTS+ solution was diluted with ethanol to an absorbance of 0.7 ± 0.05 nm at 730 nm for measurement. The samples were prepared with the extract (0.1 mL) and ABTS+ (0.9 mL) for 45 s, and measurements were taken at 730 nm with a spectrophotometer (Multiskan Go, model 51119300, thermos Scientific, Vantaa, Finland). Methanol and ABTS+ were used as a control, and a mixture of the sample with methanol was used as a blank. The ABTS antioxidant capacity was calculated using Equation (2) and expressed as percent of inhibition.
% Inhibition = ((Inicialabs − finalabs)/(Inicialabs)) × 100
2.10.3. FRAP
A ferric-reducing antioxidant power (FRAP) assay was performed according to the method described by Benzie and Strain [23] at a temperature of 37 °C. A total of 60 µL of the prepared sample and 240 µL of a solution composed of the acetate buffer [10:1:1 (300 mM, pH 3.6), TPTZ (10 mM in 40 mM HCl), and ferric chloride (20 mM)] were added. The plate was allowed to settle for 1 h. Subsequently, it was read at an absorbance of 593 nm. The absorbance values were compared with the curve made with Trolox. The results were expressed in micromoles of the Trolox equivalent (TE) per gram of dry matter (DM).
2.10.4. Statistical Analysis
The results were expressed as the mean of three repetitions with their standard deviations (SD). Statistical analysis was performed using the IBM SPSS Statistics 25.0 program (Document number 589145; IBM Corporation 2021, Armonk, NY, USA). A significant difference of p < 0.05 was used. A Pearson correlation analysis was performed with the values of the antioxidant capacity and phenolic compounds variables.
3. Results and Discussion
3.1. Proximal Chemical Analysis
Table 1 shows the results obtained from the proximal analysis (moisture, ashes, lipids, protein, carbohydrates, and crude fiber). The protein content ranged from 8.12% to 11.16%, with the Hybrid White Maize (commercial A7573) presenting the lowest percentage and the Hybrid Northern Tuxpeño (commercial) presenting the highest. The lipid content ranged from 1.95% to 4.21%, with the highest proportion of oil occurring in Hybrid White Maize (commercial A7573) and the lowest occurring in Elotes Occidentales (Native Race). Martínez et al. [24] propose that, for a maize to be considered to have nutritional attributes, it must include at least 8% protein, a criterion met by all the varieties evaluated in this work. This means they can be considered a good nutritional source of protein.

Table 1.
Proximal chemical analysis of creole maize from the Sierra Gorda in Querétaro expressed in g/100 g of the sample.
The values obtained in this work coincide with those reported by Vázquez et al. [25], in which a range of 6.9 to 12.5% protein is indicated for creole maize, and Broa-Rojas et al. [26], who report an interval of 7.6 to 11.5% for native pigmented maize from the state of Morelos. They do not, however, coincide with the values reported by Gaytán-Martínez et al. [5], who found an interval of 9.7 to 11.9% for native Mexican maize. Additionally, it has been reported that the protein concentration directly influences the hardness of the grain, presumably because there are more protein bodies (prolamins) surrounding the starch granules in the endosperm [27]. Accordingly, the more protein there is, the harder the grain and vice versa.
With regard to lipid content, the values reported in this work are lower than those presented for other Mexican landraces [25,26,28], where values from 4.5 to 5.2% are reported for native Mexican maize. These differences can be attributed to the varying climatic conditions of the places where the native maize analyzed were cultivated, as well as the quality and nutrients available in the soil and the amount of rainfall during cultivation, since these variables influence the synthesis of macro-compounds, as indicated by Vázquez-Carrillo et al. [29].
In general, the carbohydrates present in maize grains provide indirect information about the starch content, which forms 87% of the endosperm, while the crude fiber is found in a greater proportion in the seminal cover or pericarp, forming 85 to 87% of its weight [30]. The data shown in Table 1 show carbohydrates ranging from 70 to 76% and crude fiber from 1.47 to 2.90%, where Hybrid White Maize (A7573) has the highest amount of carbohydrates and the lowest amount of crude fiber, and Elotes Occidentales (Native Race) contains the lowest proportion of carbohydrates and the highest proportion of crude fiber. As previously mentioned, carbohydrates provide information regarding the starch content present in the maize kernel. Starch from native maize is of interest to the food industry, mainly due to its very specific rheological properties such as viscosity, granularity, and digestibility; therefore, these varieties are a potentially important source of this type of compound.
3.2. Polyphenolic Compounds
The concentration of polyphenolic compounds found in the analysis of native maize samples from the Sierra Gorda of Querétaro are shown in Table 2.

Table 2.
Content of polyphenolic compounds in landrace maize samples from the Sierra Gorda in Querétaro.
Creole maize contains compounds that provide added value for the consumer, benefiting the health of the population or region where they are grown and consumed. These include polyphenolic compounds, which are secondary metabolites present in plants. There is a wide diversity of secondary metabolites, which differ in structure and activity. However, polyphenolic compounds are the most abundant in plants and play a significant role in their survival, increasing the immune system and inducing adaptation, reproductivity, growth, and development processes, among others [31]. Additionally, the concentration of these compounds has been found to depend on cultivation conditions, substrates used, climate and geographical conditions, as well as biotic and abiotic factors, such as the increase in solar radiation, as well as water scarcity, which induces the production of flavonoids [32].
The results obtained in this work indicate a higher proportion of total phenols in all the samples over other polyphenolic compounds analyzed (total flavonoids and condensed tannins). Likewise, a wide range of total phenol concentration was observed, the highest being 5482 mg GAE/100 g sample for Elotes Occidentales (Native Race) and the lowest being 202 mg GAE/100 g for the hybrid Northern Tuxpeño (commercial). This breadth of range, which has been evaluated by quantifying total polyphenols using the Folin–Ciocalteu reagent method, has been reported by other authors as well: quantities of 1756 mg of gallic acid equivalent/100 g of the sample was reported for a purple maize variety with the Andean genotype [33], and 266 mg of equivalent gallic acid/100 g of the sample was reported for purple maize varieties with the Mexican genotype [34]; in Mexican white maize, quantities of 260 mg of equivalent gallic acid/100 g of the sample have been reported. It is also likely that varieties of maize with a high anthocyanin profile contain a higher concentration of phenolic compounds, as one study found 320 mg of equivalent gallic acid/100 g of the sample [34]. Additionally, the most widely reported phenolic compounds in maize are protocatechuic acids such as ferulic acid and p-coumaric acid, which are abundant in pigmented maize varieties [35,36], where concentrations of 129,985 and 130,297 mg of ferulic acid/100 g of the sample were found in blue and red maize, respectively [32]. Ruiz-Torres et al. [37] also report variability in the total phenol content among the 38 maize samples analyzed, 33 of which corresponded to simple crosses and 5 to controls.
A wide range of concentrations was also found in total flavonoids and condensed tannins, where results range from 65.78 to 121.33 mg RAE/100 g of the sample for total flavonoids and from 1.16 to 4.96 mg CEA/100 g of the sample for condensed tannins. Existing studies primarily report on the presence of anthocyanins, which are the flavonoids that contribute significantly to antioxidant activity. This study is one of the first to quantify the presence of total flavonoids, and the first to report on the presence of condensed tannins, in maize.
The maize samples presented significant differences (p > 0.05) in anthocyanin content, ranging from 69.585 ± 2.74 to 293.24 ± 11.62 mg CGE/g of the sample (Table 2). Among the various samples, purple maize presented the highest anthocyanin content. The lack of coloration in the other varieties of maize would indicate low levels of this compound. These results concur with those of López-Martínez et al. [38], where purple maize was shown to have similar amounts of anthocyanins to those of this study and higher amounts compared to other varieties. The synthesis of these compounds is related to the genetic variety of maize, as well as the agroclimatic conditions. Environmental factors, such as light and temperature, can have an impact on the biosynthesis of anthocyanins [39]. In other studies, it has been observed that purple maize contains large amounts of bioactive compounds, such as anthocyanins, which are vital for its coloring and for its antibacterial and antioxidant properties, which help to prevent chronic diseases [40].
Regarding their nutraceutical contribution, these compounds are of interest since they have biological properties, including antioxidant activities, that are beneficial to human health, such as anti-cancer, anti-mutagenic, and anti-inflammatory properties, among others [7,8,9].
3.3. Antioxidant Activity
The inhibition percentage and the antiradical (antioxidant) activity of landrace maize from Sierra Gorda obtained in this work are shown in Table 3.

Table 3.
Antioxidant capacities (ABTS, DPPH, and FRAP) for landrace maize samples from the Sierra Gorda in Querétaro.
Using the ABTS technique, the samples presented a percentage of inhibition greater than 85% in all cases, while the antiradical activity (by DPPH assay) ranged from 33 to 41%. The results indicate that the Elotes Occidentales maize (Native Race) presents the highest percentage of antioxidant activity in the DPPH technique, and it also presents the highest concentration of total phenols, suggesting a linear relationship between the content of phenolic compounds and antioxidant activity, as reported by Wang and Lin [41] for raspberry and blackberry samples. However, it differs from what was reported by Ruíz-Torres et al. [37], where no direct association was found between phenolic compounds with antioxidant capacity in the 38 maize samples analyzed. In this study, the Tuxpeño (enhanced) sample presented the highest inhibition percentage (97%), coinciding with it also presenting the highest concentration of flavonoid-type compounds and condensed tannins, which may contribute to this activity. It should be noted that antioxidant activity is important not just for the plant but for the consumer as well, because it allows for the reduction of reactive oxygen species (ROS) generated by endogenous, extracellular, and intracellular mechanisms. ROS are oxygen-derived molecules that are characterized by a short lifetime and high reactivity resulting from the mispairing of electrons in the outermost shell of the atom. ROS can damage the organism because of their oxidizing property, and because excess production of these molecules can alter cell integrity and cause DNA damage, leading, in turn, to chronic diseases. Fruits, vegetables, and seeds in general contain compounds with antioxidant activity. Studies indicate that cereals can contribute more abundantly to this activity than fruits and vegetables. In the case of maize, however, there is a discrepancy regarding the antioxidant capacity, since this varies depending on the variety evaluated, the cultivation conditions, the biotic and abiotic conditions, etc. Furthermore, the antioxidant activity of maize is not limited to inhibiting the formation of ROS; it has also been found to contribute to the regulation of cellular enzymatic elements for defense against oxidative stress. López-Martínez et al. [42] demonstrated that some components of maize can enhance QR enzyme activity. In addition, Del Pozo-Insfran et al. [8] found that Mexican maize varieties have a greater capacity to inhibit ROS formation than other maize varieties.
Using the Ferric reducing Antioxidant Power (FRAP) assay, the values found in the different maize samples ranged from 2.77 to 3.97 µmol TE/g DM. The samples presented a significant difference (p > 0.05), with the exception of the hybrid white maize. The purple maize presented a higher antioxidant capacity than the other samples. This could be because there are more anthocyanins in purple maize, giving it a greater capacity to reduce ferric ions [43]. Other studies have found that phenolic acids and anthocyanins are the main antioxidant compounds in maize. These latter components also account for the coloring of some varieties. Therefore, colored maize generally has a higher antioxidant capacity even than some cereals such as oats, wheat, and rice [44].
A positive correlation was observed between the content of total phenolic compounds and ARA (DPPH) and, to a lesser extent, between FRAP and anthocyanin content. The largest positive correlation found was between ARA (DPPH) and anthocyanin content. This indicates that the anthocyanins found in the different maize samples analyzed contribute to the donation of hydrogen to the 2,2-diphenyl-1-picrylhydrazyl radical, which indicates that they are excellent antioxidants. A positive association was also observed between condensed tannins and % ABTS inhibition. Negative correlations were also observed between % ABTS inhibition and anthocyanin content and between ARA (DPPH) and FRAP. This indicates that the different phenols analyzed (total phenolic compounds, anthocyanins, total flavonoids, and condensed tannins) influence the antioxidant capacity found by the various techniques used (FRAP, DPPH, and ABTS) (Figure 2).
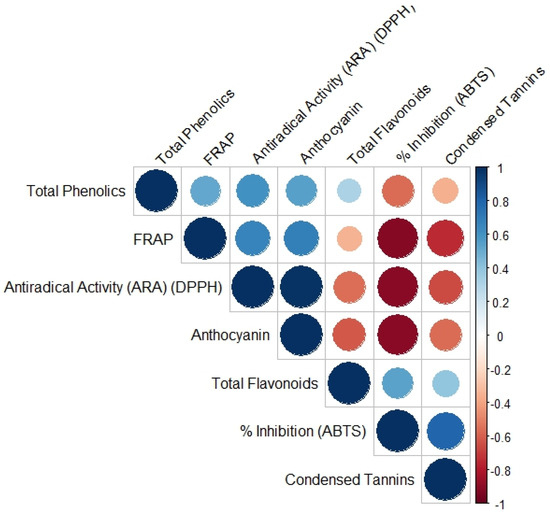
Figure 2.
Pearson correlation analysis between the content of the analyzed phenolic compounds and antioxidant capacity. The values analyzed in the correlation are as follows: Antiradical Activity (ARA) from 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP), the percentage of inhibition from 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), total phenols, anthocyanins, total flavonoids, and condensed tannins. The blue color indicates a correlation close to 1; red indicates a correlation close to −1. The size of the circle and the intensity of the color indicate the correlation between the two variables; the color gradient from blue to red indicates the extent to which they are proportional or inversely proportional to each other, respectively. All the tests were performed in triplicate.
There is still little information available on Mexican native maize; therefore, further study is needed on the contributions of these varieties and their role in the health of consumers.
4. Conclusions
Creole maize grown in the Sierra Gorda in Querétaro presents important nutritional characteristics. One of these is the percentage of protein it contains, as this is a relevant criterion for considering a food to be nutritious and is therefore beneficial to human nutrition. Likewise, the native maize evaluated here can be considered a good source of fiber, since it presented higher values than the control group. Fiber contributes in various ways to the health of consumers, whether by its prebiotic properties in regulating gastrointestinal transit, as a substrate for colonic bacteria, or its use in regulating glycemic and cholesterol inducers. The content of phenolic compounds varies in landrace maize from this region, but the Elotes Occidentales (Native Race) and Tuxpeño (enhanced), red, and pinto maize varieties contribute notably higher amounts of phenols and flavonoids than those reported for other landrace varieties. This means they have a greater antioxidant capacity and thus contribute to the health of the population that consumes them by inhibiting the formation of ROS. Additionally, this study quantified the presence of condensed tannins, compounds that had not been previously documented in creole or native maize, which add to the benefits of consuming these varieties. All of these findings highlight the importance of preserving the cultivation and diversification of these native varieties, highlighting the need for further research into the various compounds that these maize can offer to the population that consumes them as well, and for the population in general, as a staple of the traditional Mexican diet.
Author Contributions
Conceptualization, C.A.M.-C., R.G.-S. and J.L.C.-S.; methodology, A.A.F.-P.; formal analysis, A.A.F.-P., J.L.C.-S. and A.F.V.-M.; investigation, A.A.F.-P. and E.L.-S.; resources, A.M.-L.; data curation, R.G.-S., J.L.C.-S. and A.F.V.-M.; writing—original draft preparation, A.A.F.-P.; writing—review and editing, E.L.-S. and A.A.F.-P.; visualization, A.M.-L.; supervision, A.M.-L. and C.A.M.-C.; project administration, A.M.-L.; funding acquisition, E.L.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Programa para el Desarrollo Sustentable y Cuidado del Medio Ambiente del Estado de Querétaro, fiscal year 2021, through the project “Corredor Regional de Formación Integral para la Sustentabilidad en el Estado de Querétaro”.
Data Availability Statement
Data will be available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carvajal-Larenas, F.E.; Caviedes Cepeda, G.M. Análisis Comparativo de La Eficiencia Productiva Del Maíz En Sudamérica y El Mundo En Las Dos Últimas Décadas y Análisis Prospectivo En El Corto Plazo. Av. Cienc. Ing. 2019, 11, 1079. [Google Scholar] [CrossRef]
- FAO. El maíz En La Nutrición Humana. Available online: https://www.fao.org/3/t0395s/T0395S00.htm (accessed on 15 June 2023).
- Turrent-Fernández, A.; Wise, T.A.; Garvey, E. Factibilidad de alcanzar el potencial productivo de maíz de México. Mex. Rural Develop. Res. Rep. 2012, 24, 1–36. [Google Scholar]
- Cowan, C. Tras Los Pasos Del Maíz Criollo, 50 Año Después. Available online: https://www.cimmyt.org/es/noticias/tras-los-pasos-del-maiz-criollo-50-anos-despues/ (accessed on 22 May 2019).
- Gaytán-Martínez, M.; Figueroa-Cárdenas, J.D.D.; Reyes-Vega, M.D.L.L.; Morales-Sánchez, E.; Rincón-Sánchez, F. Selección De Maíces Criollos Para Su Aplicación En La Industria Con Base En Su Valor Agregado. RevFitotecMex 2013, 36, 339. [Google Scholar] [CrossRef]
- Comisión Nacional Para El Conocimiento y Uso de la Biodiversidad. CONABIO. Razas de maíz de México. Available online: https://www.biodiversidad.gob.mx/diversidad/alimentos/maices/razas-de-maiz (accessed on 27 April 2022).
- Luna-Vital, D.A.; Chatham, L.; Juvik, J.; Singh, V.; Somavat, P.; de Mejia, E.G. Activating Effects of Phenolics from Apache Red Zea mays L. on Free Fatty Acid Receptor 1 and Glucokinase Evaluated with a Dual Culture System with Epithelial, Pancreatic, and Liver Cells. J. Agric. Food Chem. 2019, 67, 9148–9159. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Ferron, L.; Papetti, A. Colored Corn: An Up-Date on Metabolites Extraction, Health Implication, and Potential Use. Molecules 2021, 26, 199. [Google Scholar]
- Frosi, I.; Balduzzi, A.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of Polyphenols from Corn Cob (Zea mays L.): Optimization of Different Green Extraction Methods and Efficiency Comparison. Food Bioprod. Process. 2024, 143, 212–220. [Google Scholar] [CrossRef]
- De Nisi, P.; Borlini, G.; Abbasi Parizad, P.; Scarafoni, A.; Sandroni, P.; Cassani, E.; Adani, F.; Pilu, R. Biorefinery approach applied to the valorization of purple corn cobs. ACS Sustain. Chem. Eng. 2021, 9, 3781–3791. [Google Scholar] [CrossRef]
- Chatham, L.A.; Paulsmeyer, M.; Juvik, J.A. Prospects for economical natural colorants: Insights from maize. Theor. Appl. Genet 2019, 132, 2927–2946. [Google Scholar] [CrossRef]
- Wellhausen, E.J.; Roberts, L.M.; Hernández, E. Razas de Maíz en México, Su Origen, Características y Distribución; Folleto Técnico No. 5; Oficina de Estudios Especiales, Secretaría de Agricultura y Ganadería: Michoacan, Mexico, 1951; 273p. [Google Scholar]
- Sánchez, J.J.; Goodman, M.M.; Stuber, C.W. Isozymatic and Morphological Diversity in the Races of Maize of Mexico. Econ. Botany 2000, 54, 43–59. [Google Scholar] [CrossRef]
- Ramírez-Galindo, J.; González-Santos, R. Conservación y Aprovechamiento Sostenible de la Diversidad de Maíces Nativos de México; Servicio Nacional de Inspección y Certificación de Semillas: Ciudad de México, Mexico, 2018; 135 p. [Google Scholar]
- Cardador-Martínez, A.; Loarca-Piña, G.; Oomah, B.D. Antioxidant Activity in Common Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and Antioxidative Activities in Common Beans (Phaseolus vulgaris L.): Phenolics and Antioxidative Activities in Common Beans. J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 9780121822002. [Google Scholar]
- Feregrino-Pérez, A.A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Loarca-Piña, G. Composition and Chemopreventive Effect of Polysaccharides from Common Beans (Phaseolus vulgaris L.) on Azoxymethane-Induced Colon Cancer. J. Agric. Food Chem. 2008, 56, 8737–8744. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chem. J. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of Scavenging Activity of Phenolic Compounds Using the ABTS•+ Assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Martínez, M.; Palacios, N.; Ortíz, R. Caracterización Nutricional Del Grano De 50 Accesiones De Maíz Cubano. Cultiv. Trop. 2009, 30, 80–88. [Google Scholar]
- Vázquez-Carrillo, M.G.; Pérez-Camarillo, J.P.; Hernández-Casillas, J.M.; Marrufo-Díaz, M.D.L.L.; Martínez-Ruiz, E. Calidad De Grano Y De Tortillas De Maíces Criollos Del Altiplano Y Valle Del Mezquital, México. RevFitotecMex 2010, 33, 49. [Google Scholar] [CrossRef]
- Broa Rojas, E.; Vázquez Carrillo, M.G.; Estrella Chulím, N.G.; Hernández Salgado, J.H.; Ramírez Valverde, B.; Bahena Delgado, G. Características Fisicoquímicas y Calidad de La Proteína de Maíces Nativos Pigmentados de Morelos En Dos Años de Cultivo. Remexca 2019, 10, 683–697. [Google Scholar] [CrossRef]
- Fox, G.; Manley, M. Hardness Methods for Testing Maize Kernels. J. Agric. Food Chem. 2009, 57, 5647–5657. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Moreno, Y.; García-Salinas, C.; Coutiño-Estrada, B.; Vidal-Martínez, V.A. Variabilidad En Contenido Y Tipos De Antocianinas En Granos De Color Azul/morado De Poblaciones Mexicanas De Maíz. RevFitotecMex 2013, 36, 285. [Google Scholar] [CrossRef]
- Vázquez-Carrillo, M.G.; Rojas-Martínez, I.; Santiago-Ramos, D.; Arellano-Vázquez, J.L.; Espinosa-Calderón, A.; García-Pérez, M.; Crossa, J. Stability Analysis of Yield and Grain Quality Traits for the Nixtamalization Process of Maize Genotypes Cultivated in the Central High Valleys of Mexico. Crop Sci. 2016, 56, 3090–3099. [Google Scholar] [CrossRef]
- Burge, R.M.; Duensing, W.J. Processing and Dietary Fiber Ingredient Applications Of Corn Bran. Cereal. Foods World 1989, 34, 535–538. [Google Scholar]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of stress and defense in plant secondary metabolites production. In Bioactive Natural Products for Pharmaceutical Applications, 1st ed.; Pal, D., Nayak, A.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 151–195. ISBN 978-3-030-54029-6. [Google Scholar]
- Rico-Chávez, A.K.; Franco, J.A.; Fernandez-Jaramillo, A.A.; Contreras-Medina, L.M.; Guevara-González, R.G.; Hernandez-Escobedo, Q. Machine Learning for Plant Stress Modeling: A Perspective towards Hormesis Management. Plants 2022, 11, 970. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stoichiometric and Kinetic Studies of Phenolic Antioxidants from Andean Purple Corn and Red-Fleshed Sweetpotato. J. Agric. Food Chem. 2003, 51, 3313–3319. [Google Scholar] [CrossRef]
- De La Parra, C.; Serna Saldivar, S.O.; Liu, R.H. Effect of Processing on the Phytochemical Profiles and Antioxidant Activity of Corn for Production of Masa, Tortillas, and Tortilla Chips. J. Agric. Food Chem. 2007, 55, 4177–4183. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic Compounds, Carotenoids, Anthocyanins, and Antioxidant Capacity of Colored Maize (Zea mays L.) Kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef]
- Ruiz-Torres, N.A.; Rincón-Sánchez, F.; Hernández-López, V.M.; Figueroa-Cárdenas, J.D.D.; Loarca-Piña, M.G.F. Determinación De Compuestos Fenólicos Y Su Actividad Antioxidante En Granos De Maíz. RevFitotecMex 2008, 31, 29. [Google Scholar] [CrossRef]
- López-Martínez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.-H.; Parkin, K.L.; Garcia, H.S. Antioxidant Activity, Phenolic Compounds and Anthocyanins Content of Eighteen Strains of Mexican Maize. LWT—Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Aguilar-Hernández, Á.D.; Salinas Moreno, Y.; Ramírez-Díaz, J.L.; Alemán-de La Torre, I.; Bautista-Ramírez, E.; Flores-López, H.E. Antocianinas y Color En Grano y Olote de Maíz Morado Peruano Cultivado En Jalisco, México. Remexca 2019, 10, 1071–1082. [Google Scholar] [CrossRef]
- Stănilă, A.; Daria Pop, T.; Maria Diaconeasa, Z. Purple Corn Cob: Rich Source of Anthocyanins with Potential Application in the Food Industry. In Flavonoid Metabolism—Recent Advances and Applications in Crop Breeding; Muhammad Khalid Abbas, H., Ahmad, A., Eds.; IntechOpen: Rijeka, Croatia, 2023; ISBN 9781803567044. [Google Scholar]
- Wang, S.Y.; Lin, H.-S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, L.X.; Parkin, K.L.; Garcia, H.S. Phase II-Inducing, Polyphenols Content and Antioxidant Capacity of Corn (Zea mays L.) from Phenotypes of White, Blue, Red and Purple Colors Processed into Masa and Tortillas. Plant Foods Hum. Nutr. 2011, 66, 41–47. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, W. Identification and Antioxidant Activity of Anthocyanins Extracted from the Seed and Cob of Purple Corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Kim, J.-T.; Chung, I.-M.; Kim, M.-J.; Lee, J.-S.; Son, B.-Y.; Bae, H.-H.; Go, Y.S.; Kim, S.-L.; Baek, S.-B.; Kim, S.-H.; et al. Comparison of Antioxidant Activity Assays in Fresh Purple Waxy Corn (Zea mays L.) during Grain Filling. Appl. Biol. Chem. 2022, 65, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).