Abstract
The domestication of vines started in Asia 11,000 years ago, although it was not until the 19th century that oenology was established as a scientific discipline thanks to the research of Louis Pasteur on the role of microorganisms in wine fermentation. At the present time, the progression in next-generation sequencing (NGS) technologies is helping to facilitate the identification of microbial dynamics during winemaking. These advancements have aided winemakers in gaining a more comprehensive understanding of the role of microbiota in the fermentation process, which, in turn, is ultimately responsible for the delivery of provisioning (wine features and its production), regulating (such as carbon storage by vineyards, regulation of soil quality, and biocontrol of pests and diseases) or cultural (such as aesthetic values of vineyard landscapes, scholarly enjoyment of wine, and a sense of belonging in wine-growing regions) ecosystem services. To our knowledge, this is the first review of the state of knowledge on the role of microbiota in the delivery of ecosystem services in the wine sector, as well as the possibility of valuing them in monetary terms by operating logic chains, such as those suggested by the SEEA-EA framework. This paper concludes with a review of management practices that may enhance the value of microbiota ecosystem services and the role of smart farming in this task.
1. Introduction
The human transformation of nature on a global scale, especially in recent decades, has led to a marked decline in indicators related to ecosystem health and biodiversity [1] and, with it, a decline in the benefits that all humans derive from ecosystem services. Ecosystem services can be defined as the conditions and processes through which natural ecosystems and their component species sustain and enable human life [2,3]. The main classifications group them into three broad categories: provisioning, regulating, and cultural [4,5]. The ecosystem services concept is the main tool used for calculating the value of natural capital [6].
Minimising the negative consequences of human transformations on the environment requires that each production sector adopts practices to reduce (or even neutralise) its net impact on ecosystems and biodiversity, while keeping (or even increasing) the flow of benefits that we obtain from nature. One of the production sectors with the greatest impact is agriculture, which is required to meet the food demands of the growing world population [7,8]. In fact, one of the main challenges for agriculture in the 21st century is the generation of multifunctional landscapes in which food is produced (provisioning service) at the same time as it promotes the supply of many other regulating and cultural services [9,10].
The wine sector is no stranger to this goal. Currently, vineyards occupy 7.3 million hectares worldwide, producing up to 260 million hectolitres to satisfy an estimated world consumption of 236 million hectolitres [11]. Efforts are being made in different wine regions in the form of pilot projects to integrate ecosystem services, biodiversity, and multifunctionality as relevant elements in vineyard management decision making [12,13,14,15,16,17]. Nevertheless, despite being a provider of relevant ecosystem services, the role of microbiota is commonly ignored in most approaches [18].
Agriculture that is not only environmentally friendly but also a producer of nature has the potential to provide nature-based solutions. Within such a paradigm, this review aims to compile and critically analyse the state of knowledge on the role of microbiota in the provision of ecosystem services in the wine sector, especially in vineyards but also in wineries. This review is structured into several sections, including a historical overview of the main scientific advances on microbiota and wine, the concept of terroir and the biogeography of the microbiota, the ecosystem services provided by the microbiota both in vineyards and in wineries, the impact that agricultural management systems have on this microbiota and therefore on ecosystem services, and methodologies for the quantification of this contribution in economic terms. The potential and limitations of this approach are also discussed, as well as the main lines of future research and possible applications.
2. Microbiota and Wine: The Evolution of Our Scientific Knowledge
Approximately 11,000 years ago, vines were domesticated in Western Asia to yield table and wine grapes [19], with evidence of oenological practices around 7000 years ago (5400–5000 BC) in the South Caucasus region [20]. These first wines were probably the serendipitous combination of chemistry and biology, specifically the microbiota present in grapes [21]. Domesticated varieties from Western Asia spread into other parts of the world through early agricultural communities. In Egypt, archaeological remains have provided evidence of grapevine cultivation on the Nile River since the Predynastic Period (4000–3100 BC) [22]. Jars with tartaric acid and tartrate residues are also proof from 4000 years ago of wine fermentation and storage processes in China (Jiahu region) [23,24]. In Europe, vine cultivation spread along trade routes, with vineyards planted on the Iberian Peninsula as early as the third millennium BC, even before the Phoenicians arrived [25]. In Ancient Greece (800 BC), wine was considered a basic element of everyday life, prompting the planting of vines in Greek colonies. However, the most advanced domestication of wine took place in the Roman Empire. The Romans already made the first grafts in the 1st century BC [26], when they began to use not only traditional amphorae but also wooden vats (51 BC) to preserve wine. However, considering microbiota, it has not yet been possible to find samples of microorganisms from before 1000 BC, with Saccharomyces cerevisiae being the first fermenting yeast to be identified [27,28].
Although oenology has evolved historically along with the production and elaboration of wine itself, it was not established as a scientific discipline until the 19th century with the research carried out by Pasteur on the role of microorganisms in wine fermentation. Pasteur obtained the first patent on alcoholic fermentation in 1857 and described the role of yeast strains in the production of different wines [29,30]. He also studied viticultural diseases and obtained a patent on the preservation of wine through pasteurisation [31]. Thanks to his contribution, in 1890, Hermann Müller carried out the first inoculation of grape must with a pure yeast culture, thus bringing forth the concept of controlled fermentation, which has survived to the present day with certain improvements in the control and reliability of the process [32]. Oenology has continued to evolve since then, studying the interactions of microbiota in both alcoholic fermentation and malolactic fermentation for wine production [33,34]. Another research effort has focused on the process of the domestication of oenological yeast strains, i.e., the artificial selection of strains with improved desirable characteristics that are adapted to environments with different stress factors, such as changes in temperature, acidity, and oxidative stress [35,36]. Now we know that S. cerevisiae stands out among the large number of yeast species involved in the different stages of fermentation due to its high fermentative capacity, ethanol tolerance, and strong resistance to the toxicity of different metabolites, thus being able to prevail in the final stages of fermentation [37,38,39,40]. Considering these properties, optimal hybrid starters formed by S. cerevisiae and Saccharomyces spp. or S. cerevisiae and non-Saccharomyces have been created for controlled fermentation, guaranteeing the predictability and reproducibility of wines over time [41,42]. At present, the use of starter cultures is widespread in wineries, modulating the aromatic profile of the wine because of their enzymes, such as esterase, β-glucosidase, proteolytic, and pectinolytic enzymes. In addition, starters may increase the glycerol content and thus decrease the alcohol content of wines [43,44]. At the same time, however, the use of starters represents a risk for the loss of genetic diversity in indigenous yeast populations [45].
In recent years, next-generation sequencing of DNA (NGS) techniques has enabled researchers to study the genomes of entire microbial communities, including those of unculturable organisms. Particularly, the use of techniques based on high-throughput sequencing (HTS) and omics, such as metabarcoding, meta-transcriptomics, meta-proteomics, and metabolomics, has opened up a new scenario in wine research. These technologies provide a powerful approach to a more complete understanding of the complexity of microbial communities in different environmental niches, helping greater monitoring and diagnosis, among other aspects [46,47,48]. Thus, HTS-based studies have allowed for the identification of microbial dynamics during winemaking, helping winemakers better understand and control the fermentation process and improve the final product’s quality [49,50,51,52,53]. In 1996, S. cerevisiae was the first eukaryotic organism for which a complete genome sequence was obtained [54]. It is now known that, as a result of the domestication process, more than 90% of the fermenting yeasts of S. cerevisiae in wineries around the world come from the same cluster as a consequence of their domestication [55]. The advances are even greater in the role played by microbiota in vineyards. NGS has enabled the identification and characterisation of diverse microbial populations in the vineyard ecosystem, facilitating the description of the virome [56], mycobiome [57], and bacteriome [58] of vineyards in different parts of the world. These developments have provided insights into the potential of the microbial communities that play essential roles in vineyards’ health and productivity [59] in processes such as nutrient cycling, disease suppression, or plant–microbe interactions [60,61]. Moreover, NGS has aided in deciphering the microbial components that contribute to the distinct characteristics of wines from different regions, the terroir [62,63,64]. New NGS advances allow for an ever-deeper understanding of the microbiome of vineyards. For example, the use of amplicon sequence variants (ASVs) instead of operational taxonomic units (OTUs) since 2013 has allowed for a finer distinction between sequences, enabling a more precise taxonomic identification, which is very useful for taxonomic groups that have close taxa with different functionalities in vineyards [65,66,67,68]. However, synthetic genomics—the construction of viruses, bacteria, and eukaryotic cells whose genome has been completely synthesised in the laboratory [69]—allows for the design of a generation of industrial microorganisms that will introduce notable improvements in aspects such as the organoleptic properties of wine or the reduction in ethanol concentration in light-bodied wines [70,71,72]. In this regard, strains that favour certain aromas have already been produced through the engineering of metabolic pathways and the fusion of synthetic enzymes [70,73,74].
Overall, the information obtained through these methods has the potential to transform vineyard management practices and contribute to more sustainable and efficient wine production [75,76,77].
3. Terroir and Microbiota of Wine
When tasting a wine, some of the first perceived differentiating aspects (such as aroma, acidity, and colour) are related to the area in which the physicochemical, biological, and cultural interactions of the vineyard take place: the terroir [69,78,79]. This term is frequently used nowadays, not without controversy and despite its poor widespread understanding [76,80]. In the traditional definition of the International Wine Organisation (OIV), the terroir initially referred to an “area in which collective knowledge of the interactions between the identifiable physical and biological environment and applied vitivinicultural practices is developed”, including specific soil, topography, climate, landscape characteristics, and biodiversity features (OIV/VITI Regulation 333/2010). Given advances in the understanding of the role of microbiota in wine and vineyard distinctiveness, recent studies within the literature already consider it an integral part of this terroir [81,82,83].
In biogeographical terms, since microorganisms operate in a particular ecological niche with specific environmental conditions, spatial differences can be found in the microbial communities present in each region [49,84,85]. It has been demonstrated that the distribution of the microbiota is influenced by geographical, environmental, and management factors, generating a region-specific microbial terroir that contributes distinctive qualities to the wines [61,86,87,88,89,90] (Figure 1). Research on this microbial terroir has shown that some OTUs of the microbiome can act as a geographical signature of a vineyard or wine region [91].
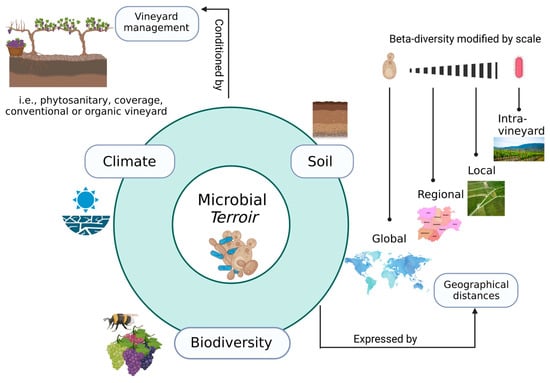
Figure 1.
Factors such as climate, soil, and surrounding biodiversity condition the microbiome of the specific microbial terroir in each vineyard (centre of the figure). These factors are shaped by crop management practices (upper left corner). The interaction between these elements defines a biogeography of microbiota in vineyards with differences expressed at different geographical distances and in which fungi are more site-specific and bacteria tend to have more cosmopolitan distributions (right side).
Differences in the spatial patterns of microbial communities in vineyards are expressed at different scales. Considerable differences have been observed between vineyards in different parts of the world [92] and between wine-growing regions [93,94,95,96]; however, landscape factors and local variations in the ecological niche are known to produce significant differences at the local scale [97,98,99,100]. Intra-vineyard variability is also a significant factor as differences in microbial composition can be even greater than between plots [101,102]. Hence, it is possible to identify a hierarchy within spatial distances, following a broad trend whereby the diversity of microbiota rises with the distance [63], while a common core of genera, or even species, has been described globally [92] (Figure 1).
Isolation by geographic distance is somewhat shaped by environmental heterogeneity, which defines microbiota spatial patterns in vineyards. Climate and soil differences between areas contribute to microbial community dissimilarities between vineyards [103,104]. At the wine-growing landscape scale, the factors behind these spatial patterns are also related to vineyard practices [97,105,106,107], grape variety [85,92,98], and microclimate conditions [98,99,108,109]. The surrounding ecosystems also seem to be involved in the composition of microbial communities in vineyards. Thus, nearby forests shape the fungal communities in the vineyard, not only because of the natural yeasts they host but also because of the social insects (e.g., wasps) that act as vectors for transporting microorganisms from the forest to the grapes [110,111,112,113].
The microbiomes of vineyards have also shown significant differences between the soil and the vines themselves. The soil acts as the main reservoir of microbiota and shows a certain level of concurrence with the different parts of the vine (roots, leaves, flowers, and grapes) [58,91]. While bacterial diversity decreases from the soil to the aerial parts [82,114], the main change in fungal-specific diversity occurs between the soil and the rhizosphere [115].
The variety of vines also conditions the microbial communities present in vineyards. For example, bacterial species, such as Enterococcus, Massilia, Kocuria, Pseudomonadales, and Pantoea, are more likely to appear in varieties, such as Merlot, Syrah, Cabernet Sauvignon, and Zinfandel, while species such as Bacillus, Turicibacter, and Romboutsia have a greater prevalence in Pinot Noir. As for fungal species, Cladosporium, Phoma, and Sporormiella appear in Zinfander, Lon, and Gem [108,116,117].
The spatial patterns of fungi and bacteria generally differ, which is partly explained by variations in responses to environmental effects or vineyard management practices [118,119,120,121,122,123]. On a global scale, although spatial distance explains beta diversity in both, climate is more related to alpha diversity in fungi than in bacteria [63], with some bacteria also responding to climate variables [104]. In addition, the dispersal of fungi by wind is probably more limited than that of bacteria due to the larger spore size compared to bacterial cells [112,124,125]. As a result, the diversity of fungal communities increases with distance, a pattern that is much less marked in bacterial genera. Thus, while fungi are more site-specific, bacteria tend to have more broad-based distributions but with notable local and intra-vineyard differences at the same time [87,93,126]. Overall, the fungi, which are more representative of each territory, define the microbial geographical signature of the different winegrowing regions and vineyards to a greater extent [94,127].
Microbiome differences can appear not only in space but also in time. Although inter-annual variations in the microbiota of a vineyard have barely been studied, some research has found that vintage can significantly affect the biodiversity of vineyard-associated microorganisms [85,128,129,130]. In contrast, other studies have shown that differences between vintages are not significant [102]. Moreover, during the vine growing period, alpha diversity experiences a decline, while beta diversity undergoes an increase. This trend implies that microbiomes are progressively reshaped by interactions between hosts and microbes [104].
What happens in the vineyard is transferred to the winery. All ecological interactions that take place between the microbiota, the plant, and the environment play an important role in the outcome of wine fermentation. In fact, it has been shown that up to 60% of microbial diversity must come from taxa derived from the soil, leaves, and grapes of the vine [96,108,117,131], while the communities present in that which is freshly harvested must be more similar to those found in berries; as fermentation progresses, they become more and more similar to the communities present in the vine bark [94,132,133]. Thus, it is possible to identify microbial biomarkers associated with each terroir among the different stages of fermentation and for different wine grape varieties [96]. Moreover, winery surfaces harbour seasonally fluctuating populations of bacteria and fungi with relevance to wine fermentation [134].
4. Ecosystem Services Provided by Microbiota in Vineyards and Wines
Microbiota provide ecosystem services both for vineyards and for the quality of the final product, wine. This review uses the classification proposed by the United Nations System of Environmental-Economic Accounting, which identifies three main groups: provisioning, regulating, and cultural ecosystem services [5].
4.1. Provisioning Ecosystem Services
Within the category of provisioning ecosystem services, microbiota are closely linked to wine production through fermentation. They also provide remarkable genetic diversity that can benefit the wine industry and wine cultures.
4.1.1. Biomass (Crop) Provisioning Services
Without microbiota, humans would simply not have wine. It plays a fundamental role in fermentation, transforming grape must into wine. Thanks to studies based on HTS, the existence of a large microbial pool that includes fungi and bacteria in spontaneous wine fermentation has been proven [47,51,53,135]. The different strains of oenological yeast have been “domesticated” over the years, adapting by evolutionary mechanisms to environments with many stress factors in the fermentation process [35,36,136]. Thus, regarding alcoholic fermentation, indigenous species of the genera Hanseniaspora, Candida, Pichia, and Metschnikowia are involved in the first steps of the process, producing secondary metabolites (such as acids, alcohols, and esters) and enzymes (such as esterases, lipases, and proteases) that can affect the final quality of the wine [137]. These species can grow at low ethanol concentrations, but when this exceeds 5–7% and the abundance of fermented sugars begins to decrease, they start to decline and die [138]. Other yeasts, such as species of Brettanomyces, Kluyveromyces, Schizosaccharomyces, Torulaspora, and Zygosaccharomyces, may also be present during fermentation and subsequently in wine, some of which are capable of adversely affecting sensory quality [35]. Due to its high fermentation capacity, ethanol tolerance, and strong resistance to the toxicity of different metabolites, S. cerevisiae prevails in the final stages of fermentation [38,41], being nearly unique in those fermentations with commercial starters, or else, accompanied by other species, such as Torulaspora delbrueckii, Zygosaccharomyces fermentati, Kluyveromyces thermotolerans, Hanseniaspora guilliermondii, and Dekkera anomala in spontaneous fermentation [49,96,137]. For malolactic fermentation, Oenococcus oeni is the dominant species [139,140,141]. Apart from the gradual growth of different yeast species during fermentation, the existence of the underlying successional development of different strains within each species has also been described [138].
Microbiota play a remarkable role in the organoleptic characteristics of wine. For example, beneficial yeast species of Debaryomyces may produce enzymes, such as β-glucosidases, which increase the concentration of desirable organoleptic compounds in wines [142]. Lachancea thermotolerans, Pichia kluyveri, Rhodotorula mucilaginosa, and Metschnikowia spp. may improve the flavour and aroma of wine [33,41,76,143]. Species of bacteria, such as those included in the genus Lactobacillus, contribute to the synthesis of methyl and isobutyl esters and the formation of red and black fruity wine fragrances. Fructobacillus is closely related to the synthesis of aromatic alcohols and the generation of fruity flavours [144].
In spontaneous fermentation, the soil microorganisms that are present in grape berry and those present only in berries (coming from insects, birds, etc.) produce wines with greater complexity than those fermented with pure starters, providing a bouquet of flavours perceived as more attractive to consumers [131,132,145,146]. These spontaneously fermented wines are practically impossible to reproduce in later vintages or in some regions, mainly due to terroir differences [21,42]. However, these wines are unpredictable due to fermentation arrests and can deteriorate due to the appearance of certain undesirable yeast species, such as Brettanomyces spp. [147].
4.1.2. Genetic Material Services
The oenological microbiota constitutes a reservoir of great genetic diversity, with a wide variety of Saccharomyces and non-Saccharomyces yeast species and strains, which stems from mechanisms such as heterozygosity, nucleotide and structural variations, horizontal gene transfer, and intraspecific hybridisation [39,148]. This genetic diversity is likely to provide resilience to climate change in wine production [47,50,135] and can be exploited to obtain higher quality wines [149,150,151] or to generate non-GMO hybrids to be used as commercial starters that do not suppress the native microbial flora [152]. The species most sought after are those that ferment well and produce less ethanol, more glycerol, and more attractive aroma compounds. Some of these yeasts are non-Saccharomyces, such as Hanseniaspora vineae and Metschnikowia fructicola [152], or other species of Saccharomyces belonging to the sensu stricto complex [153,154], such as S. kudriavzevii, which can produce more aroma compounds and even higher amounts of other alcohols, such as phenylethanol. Saccharomyces uvarum also produces more aromatic compounds, such as alcohols and esters. Saccharomyces bayanus is cryotolerant, allowing for fermentation at lower temperatures [32,155,156,157,158,159]. In addition, the sequential fermentation of S. cerevisiae with non-Saccharomyces yeasts, such as Meyerozyma guilliermondii and Hanseniaspora uvarum, enhances floral and fruity aromas in wines [150].
4.2. Regulating Ecosystem Services
The interactions between biological communities (including microbiota) and the physical and chemical properties of the soil environment are fundamental to the processes, functions, and ecosystem services provided by nature in vineyards, such as carbon storage [78], regulation of soil quality [160], formation of soil structure [161], or the biocontrol of pests and diseases [78,162,163].
4.2.1. Carbon Storage
As vineyards are a potential source of carbon storage [164], with differences among vine ages [165] and grape varieties [166], soil microorganisms are of great importance in regulating organic carbon dynamics [167], as taxa such as Patescibacteria, Synergistetes, Chloroflexi, Actinobacteria, Deinococcus-Thermus, and Atribacteria can degrade organic carbon to produce organic acids [168].
4.2.2. Soil Quality Regulation Services
Microbial communities play a pivotal role in shaping soil nutrient dynamics, and any shift in their activities and functions has the potential to jeopardise soil biogeochemical cycles, ultimately impacting the availability of nutrients to plants [78,169,170,171]. Thus, there are specific microbial consortia that lead to nitrogen fixation and nutrient mineralisation, metabolising, for example, recalcitrant forms of N, K, and P to release these essential elements for vine nutrition [172,173,174].
4.2.3. Soil and Sediment Retention Services
Given the characteristics of the crop, its management, and its location in topographically complex areas, vineyards present a particularly favourable context for soil loss compared to other agricultural land [175,176]. The microbiome can contribute to reducing this problem. In this regard, the arbuscular mycorrhizal fungi (AMF) of the subphylum Glomeromycotina promote the formation of soil aggregates and thus the prevention of soil erosion [177,178,179,180,181]. AMF develops a dense mycelial network in the soil [182,183], which, together with the secretion of sticky substances comprised of proteins [184], can have a binding action on soil particles and improve their structure, leading to increased structural stability and soil quality [185,186,187]. Thus, a reduction in AFM is expected to increase the risk of erosion [188].
4.2.4. Biological Control Services
Understanding the microbial ecology of vineyards has implications for disease management and the development of more sustainable and eco-friendly approaches to protecting grapevines. Bacteria present in the rhizosphere and endosphere of vine shoots and branches, such as Achromobacter xylosoxidans, Bacillus subtilis, and Pseudomonas fluorescens, can produce siderophores that limit the availability of iron, thus reducing the presence of pathogenic microorganisms. Some bacteria also degrade virulence factors (e.g., oxalic acid) produced by plant pathogens, thus reducing the severity of damage [189]. By making good use of the functionalities provided by the microbiome, chemical products are starting to be replaced by selected bacterial strains, endophytic fungi, and yeasts that show defensive responses to grapevine pathogens [190], such as powdery mildew (Uncinula necator), downy mildew (Plasmopara viticola), or Botrytis cinerea, which can negatively affect the quality of the final product [191,192]. This biocontrol is provided by species such as Lysobacter capsici (AZ78), Trichoderma spp. [193,194,195,196,197], and Aureobasidium pullulans [198,199,200]. The use of these microorganisms instead of chemical fungicides helps in the production of certified organic wines [201,202]. Biological control of microorganisms is an active field of research in which significant progress is being made. For instance, strains of Arthrobacter spp., Rhodococcus spp., and Bacillus mycoides with an excellent ability to reduce the growth of mycotoxins from the fungi Aspergillus carbonarius, A. niger, and A. flavus have recently been isolated from organic vineyard soils [203]. In addition, some yeasts derived from grape must are also effective against pathogens such as B. cinerea [204], making them potential candidates for industrial application as biological control agents.
4.2.5. Nursery Population and Habitat Maintenance Services
Microbiota can provide better soil conditions for vine development. For example, the fungus Aerobasidium pullulans is known to metabolise inorganic sulphur used as a fertiliser and pesticide and to absorb copper employed as a fungicide, which in high concentrations is toxic to the plant [198,199].
4.3. Cultural Ecosystem Services
Beyond the provisioning services, an ancestral culture has been created around wine, expressed in a rich tangible and intangible heritage that is ultimately based on the fermentation process carried out by the microbiota. These cultural services are expressed in the form of enotourism, the aesthetic values of vineyard landscapes, the scientific development of oenology, the scholarly enjoyment of wine, the identity and sense of belonging in wine-growing regions, symbolism, and even certain spiritual values [15,78,205].
4.3.1. Recreation-Related Services
Enotourism includes all tourist activities related to the world of wine: wine tasting, visits to vineyards and wineries in different wine-growing regions, festivals, and other organised wine-related events [206]. The differences between wine production terroirs are what give “typicity” and “identity” to the wine produced in different territories and, ultimately, a “sense of place” to the communities linked to its production.
4.3.2. Visual Amenity Services
The beauty of the landscape increases the market value of wine-related products [15,79,205]. Given that humans aesthetically prefer healthy plants and that the fungal and bacterial diversity of leaves is closely related to the health status of grapevines [133], the microbiota is also related to the visual appreciation of vineyard landscapes.
4.3.3. Education, Scientific, and Research Services
The diversity provided by the different terroirs encourages scientific research in the field of oenological microbiology, aimed at unravelling the interactions between the microbiota, the vine, and the final product [78,207].
4.3.4. Spiritual, Artistic, and Symbolic Services
Wine has been linked to various myths, rites, and religious cults for thousands of years [208,209,210,211]. The Greek god Dionysus, reinterpreted as Bacchus in Roman mythology, was the revealer of wine culture. Among Egyptian deities, Hathor, the goddess of wine, was carved into the amphorae used to store it, while Osiris gave the people instructions on how to harvest the vine and store the wine. The Sumerians incorporated the goddess Geshtinanna, a name that means “mother vine”, as found in various inscriptions.
5. Economic Valuation of Microbiota Ecosystem Services in Vineyards and Wine
Ecosystem services provided by microbiota for vineyards and wine are valuable because they increase society’s well-being. The conceptual connections between microbiota natural capital and the enhancement of well-being can be thought of as a sequence that links the ecosystems, the intermediate ecosystem services, the final ecosystem services, and the value created for the beneficiaries, such as the one in Figure 2.
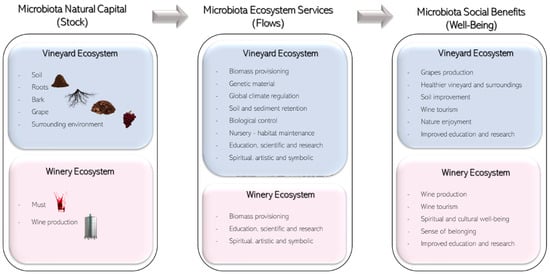
Figure 2.
Social benefits of vineyard and winery microbiota as natural capital.
Turning these value connections into monetary terms may be useful for decision-making (see below). This can be done by operating standard economic valuation techniques, which are frequently applied for the economic valuation of environmental assets and the ecosystem services they provide. These techniques and their results are not without debate; however, their general acceptance is higher if the value connections are clear, the beneficiaries are identified, and the benefits for them can be assessed to some extent.
In March 2021, the United Nations Statistical Commission posed the UN System of Environmental–Economic Accounting–Ecosystem Accounting (UN SEEA-EA) as the new standard for countries to report the state of their natural capital. This framework is set to define the common ground for the economic valuation of ecosystem services in the coming years; thus, it is worth drawing from it when dealing with specific elements in this field.
The UN SEEA-EA advocates the use of real or inferred exchange prices as the main source of information for the economic valuation of ecosystem services rather than estimated shadow prices. Hence, valuation techniques based on market prices or costs are preferred over beliefs and judgments, whether they come from experts or users. The identification of logic chains for value assessment, such as the one shown in Figure 3, also suggests that this new framework is a proper way to disclose and arrange the valuation steps from the ecosystem service to its economic value.
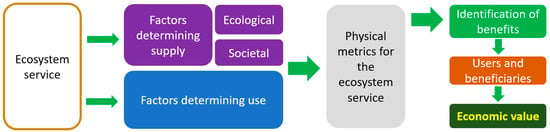
Figure 3.
Simplified logic chain from an ecosystem service to its economic value.
There are several reasons why estimating the economic value of ecosystem services from microbiota may be useful. First, the use of monetary terms allows for the aggregation of different physical flows. Second, it provides a framework in which the services from diverse ecosystems can be weighed and compared. Third, it may be useful to make informed preservation decisions. Fourth, it may help to identify new promising lines for research.
To reach these goals, the economic valuation of the ecosystem services provided by microbiota in vineyards and wines cannot cover the whole set of values from the entire microbiota at once for a very simple reason: without microbiota, we would not have wine; hence, we might obtain the wrong result that the value of microbiota equals the total value added by the wine industry, neglecting the rest of the contributions and, of course, missing the point [18]. Instead, we must necessarily focus on the value added by certain specific microorganisms, i.e., the marginal added value to vineyards and wine due to their presence or the marginal lost value due to their disappearance.
It is beyond the scope of this paper to provide economic valuations for the ecosystem services of microbiota, but a few examples of applications and methods will help explain the way in which they are calculated. In all cases, logic chains following the outline in Figure 3 can be identified for each ecosystem service to produce an economic value estimate once the benefits and beneficiaries have been bounded. The most visible case, of course, is provisioning services since microbiota play a central role in fermentation or in the production of enzymes that improve organoleptic characteristics. Provisioning services are usually valued by market data, and microbiota are no exception. The easy way to do this would be to start from the value of the final product (grapes or wine) and subtract all values not provided by microbiota, including winery installations, machinery, labour, and fertilisers. Since these latter values are usually hard to estimate, the most convenient way to estimate the economic value of microbiota provisioning services, when possible, might be from the comparison between the produced values with and without the presence of a certain type of microbiota or from the extra premium consumers are willing to pay for organic wines.
Regarding regulating ecosystem services, the main difficulty arises from the fact that, in many cases, their effects are not directly noticeable; hence, the use of market data is not so straightforward. The fraction of carbon storage that can be attributed to microbiota, for instance, can be calculated in tonnes and then valued from the standpoint of its replacement cost, i.e., the monetary cost of CO2 allowances needed to equalise such a level of carbon storage. A different example is biological control services, which can be valued by calculating the avoided cost, i.e., the cost of the chemical products that can be replaced by bacterial strains, endophytic fungi, or yeasts that show defensive responses to grapevine pathogens. The main obstacle is that substitutability rates have not yet been clearly determined in the literature; thus, estimating actual cost avoidance becomes a complex task.
The economic valuation of cultural ecosystem services Is also performed using sources of information related to market data, although its use is not straightforward. Recreation services can be valued by considering the cost of enjoying them, usually by applying travel cost methods. Hence, for instance, the value of cultural services from specific microbiota may be assessed by calculating the differential amount of money that people are willing to pay to visit organic vineyards or wineries using indigenous yeasts.
6. Vineyard Management and Microbiota Ecosystem Services
Differences in vineyard management can modify microbial communities [212] and, by this means, have an impact on the type and quantity of ecosystem services provided. Some studies suggest that, together with geographical location and climate, vineyard management is related to the diversity of yeast taxa present in grapes [74,103,213,214]. In terms of bacterial composition, vineyard management seems to have a direct impact on the composition of its communities [162,215]. These differences may also reach and materialise in the must microbiome [216], although other authors have found no evidence of change [107,212].
Management practices, such as cover crop use, tillage, and soil amendments, can influence vineyard microbial communities [78]. Green cover in vineyards can increase microbial biodiversity [217], enhance soil health and nutrient cycling, and promote a more resilient and balanced vineyard ecosystem. The presence of green cover increases the input of organic matter into the soil through plant residues and root exudates. As these materials decompose, they provide a source of nutrients for the microbial community, improving soil fertility and supporting the growth and health of grapevines. At the same time, green cover contributes by preventing erosion, regenerating soil biodiversity [13,218], and improving visual amenity services [219]. Nonetheless, empirical evidence has indicated that the outcomes of inter-row management may fluctuate due to underlying edaphoclimatic conditions [220]. Considering agricultural inputs, organic amendments, such as manure and compost, are a direct source of carbon for soil microbiota and other organisms, leading to increased plant growth and the return of plant residues to the environment [221,222]. Comparatively, organic fertilisation systems lead to a notable increase in microbial biomass, induce shifts in the structure and composition of the soil microbial community, and enhance microbial activity, as opposed to the inorganic fertilisation approach [223,224]. In addition, the use of nitrogen fertilisers can lead to soil acidification, with considerable negative effects on the microbiota present in the soil [225,226,227]. Fungicides alter the microbial communities on the surface of grapes, with the effect of those used in organic agriculture (sulphur, copper) being stronger than those used in conventional agriculture [221,228,229,230]. Chemical herbicides, such as glyphosate, have also been shown to induce alterations in soil microorganisms [231].
Since management practices affect the microbiota, it is no surprise that the sort of cultivation system, whether conventional, organic, or biodynamic, also plays a role, as some research has shown [107,232,233,234]. Higher fungal diversity is found in organic and biodynamic viticulture, which seems to be related to organic fertilisers [198,199]. In organic vineyards, there is a higher presence of Aureobasidium pullulans (which can metabolise inorganic sulphur and absorb copper), while in conventionally managed vineyards, Sporidiobolus pararoseus (carotenoid producer) is predominant [235]. However, higher levels of pathogenic Alternaria spp. And yeasts Rhodotorula and Sporidiobolus have been detected in conventional versus organic vineyards, forming a general pattern of reduced fungal diversity with a predominance of a few fungal taxa in these conventional vineyards, probably because of the use of systemic fungicides [201]. Other types of management can also affect vineyard microbiota. For instance, altered vineyard microclimates under rain shelters reduce diseases caused by Alternaria and Colletotrichum spp. [130].
7. Towards Smart Farming: Microbiota as a Nature-Based Solution in Vineyards and Wineries
In the coming decades, the agricultural sector will face major challenges in providing food for a growing world population, but intensive cropping based on the use of mineral fertilisers, agrochemicals, and water will continue producing a negative impact on biodiversity and ecosystem services [236]. Wine production is no exception to these environmental problems [237]. Indeed, in this type of crop, certain risks can prove to be even more severe. The placement of vineyards on steep slopes, coupled with irregular rainfall patterns (exacerbated by climate change), can lead to substantial soil erosion and degradation, significantly compromising soil structure and overall productivity [238,239,240].
In this context, “smart farming” can bring cutting-edge technology into play to enhance agricultural production in terms of quality, quantity, and sustainability [241]. Since the primary objectives of smart farming involve precise and location-specific interventions, microbiota are called upon to play a major role in these advances [242]. By nurturing a diverse and thriving microbial community, vineyard managers can promote environmentally friendly grape cultivation practices. However, although there are already many commercialised products based on the use of microbiota, such as biocontrol agents, biostimulants, and biofertilisers, intelligent management should go further and promote the sort of microbiota that provides relevant ecosystem services, while reducing the one that can provide negative functionalities as a nature-based solution in each vineyard. Therefore, there is a need to move quickly from promise to practice [243], generalising the ecosystem services approach to vineyard management practices, including microbiota.
There is still important work to be done in the identification of the taxa present in the microbial communities of vineyards, with some poorly described groups in which it is not yet possible to identify genera or species [63]. Furthermore, another major challenge is still to assign species to ecosystem functions. This is not straightforward, as redundancies are common and different microbial communities may provide the same function [244]. While NGS techniques allow for the identification of taxa, cultivable or not, present in an environmental sample, they are not intended to define the roles that taxa play within the microbial community in which they are part. Laboratory experiments with a given taxon are only partially useful because, in the vineyard, this taxon is part of a complex microbial community that can modify its functionality. In this sense, to study the role of ecological, structural, and functional characteristics in a controlled way in the laboratory without losing the complexity of the environmental samples, artificial synthetic communities are generated. These synthetic communities maintain the essential characteristics of the communities found naturally in the vineyard while reducing the number of components. Thus, it is possible to study the roles and functionality of the different taxa given the interactions within the microbial community. The generation of these synthetic communities requires the isolation of microorganisms, either individually or by the encapsulation of microspheres of a small number of microorganisms or by the sequential layering of microbes in a synthetic biofilm [245,246], as a preliminary step to the complete study of the metabolic interactions between different isolates [247,248,249]. The study of these synthetic communities generates invaluable information on the contribution of the community to the overall ecosystem function. However, there are still several limitations, including how to manage the knowledge acquired in synthetic communities when dealing with naturally established communities, intercellular communication between single and multiple species, and how cell consortia can be optimised or controlled in the long term [250,251]. Once the functionalities provided by specific taxa are identified, it is necessary to investigate how the population dynamics of these taxa and of different microbial strains respond to different agricultural practices. It is also necessary to further investigate the factors affecting the microbiome—a set that is formed by microorganisms, their genes, and their metabolites in each ecological niche—and the species composition of microbial communities, especially those related to vineyard management practices (such as green soils, tillage, surrounding vegetation, and soil amendments). This knowledge can enable the implementation of advantageous strategies, for instance, considering the constraints imposed by the limited dispersal of fungi and the significant influence of environmental conditions on bacteria [61]. The room for biotechnological applications and developments is also wide and very relevant, exploiting microbial properties, such as the ability to sustain and replicate themselves without requiring repeated inoculation [252]. More research efforts should focus on how this microbiota is finally transferred to the properties of the wine and contributes to its quality and to the valorisation of the terroir, which involves improving our knowledge on the value of ecosystem services.
In terms of the valuation of ecosystem services, apart from the use values related to the provisioning, regulating, and cultural services, the maintenance of microbiota also has an option value by keeping open the possibility of a future benefit from microbial communities [253,254], be it in the form of provisioning, regulating, or cultural services. Furthermore, microbiota in vineyards also offer an insurance value in terms of an increase in ecosystem resilience, which serves as a safety net against global change, protecting humans and ecosystems from potential welfare losses [255,256,257,258]. This insurance value reduces the likelihood of future declines in the supply of ecosystem services resulting from changes in microbial communities [255,259].
Overall, the analysis of the ecosystem services provided by microorganisms in vineyards and wine production reveals the essential contribution of these invisible communities to the functioning and sustainability of viticultural systems. Microorganisms are key players in achieving optimal outcomes with their diverse influences, from soil health and vine vitality to fermentation and the sensorial profile of wine. Their complex and symbiotic interaction with the viticultural environment triggers a range of benefits, including the enhancement of final product quality and vineyard resilience against adverse factors. This analysis not only highlights the importance of a holistic approach to winemaking but also the potential for future research to understand the mechanisms behind the delivery of ecosystem services, thereby promoting more sustainable and resilient agricultural and winemaking practices [260].
Author Contributions
Conceptualization, V.J.C.-R., F.R.-L. and I.G.-I.; methodology, I.G.-I., V.J.C.-R., F.R.-L. and M.T.; validation, I.G.-I., V.J.C.-R., F.R.-L. and M.T.; formal analysis, I.G.-I., V.J.C.-R. and F.R.-L.; investigation, I.G.-I., V.J.C.-R. and F.R.-L.; resources, I.G.-I. and V.J.C.-R.; data curation, I.G.-I. and V.J.C.-R.; writing—original draft preparation, I.G.-I., V.J.C.-R. and F.R.-L.; writing—review and editing, F.R.-L., V.J.C.-R. and M.T.; visualization, I.G.-I., V.J.C.-R. and. F.R.-L.; supervision, V.J.C.-R., F.R.-L. and M.T.; project administration, F.R.-L.; funding acquisition, F.R.-L. and V.J.C.-R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from Junta de Castilla y León research grant SA126P20, Análisis y valoración del capital natural asociado al sector vitivinícola de Castilla y León, for the period 2021–2023.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Díaz, S., Settele, J., Brondízio, E.S., Ngo, H.T., Guèze, M., Agard, J., Arneth, A., Balvanera, P., Brauman, K.A., Butchart, S.H.M., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2019; 56p. [Google Scholar]
- Fu, B.; Wang, S.; Su, C.; Forsius, M. Linking ecosystem processes and ecosystem services. Curr. Opin. Environ. Sustain. 2013, 5, 4–10. [Google Scholar] [CrossRef]
- Daily, G.C. Nature’s Services. Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 1997; 392p, ISBN 1-55963-475-8. [Google Scholar]
- Haines-Young, R.; Potschin, M.B. Common International Classification of Ecosystem Services (CICES) v5.1 and Guidance on the Application of the Revised Structure. 2018. Available online: www.cices.eu (accessed on 14 January 2023).
- United Nations. System of Environmental-Economic Accounting—Ecosystem Accounting (SEEA EA). 2021. Available online: https://seea.un.org/ecosystem-accounting (accessed on 15 January 2023).
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, E.; Grasso, M.; Hannon, B.; Limburgo, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Ali, G.; Dahlhaus, P. Roles of Selective Agriculture Practices in sustainable agriculture performance: A systematic review. Sustainability 2022, 14, 3185. [Google Scholar] [CrossRef]
- Robertson, G.P.; Swinton, S.M. Reconciling agricultural productivity and environmental integrity: A grand challenge for agriculture. Front. Ecol. Environ. 2005, 3, 38–46. [Google Scholar] [CrossRef]
- Huang, J.; Tichit, M.; Poulot, M.; Darly, S.; Li, S.; Petit, C.; Aubry, C. Comparative review of multifunctionality and ecosystem services in sustainable agriculture. J. Environ. Manag. 2015, 149, 138–147. [Google Scholar] [CrossRef]
- TEEB. TEEB for Agriculture & Food: Scientific and Economic Foundations; UN Environment: Geneva, Switzerland, 2018. [Google Scholar]
- OIV-International Organisation of Vine and Wine. State of the World Vine and Wine Sector 2021; OIV-International Organisation of Vine and Wine: Dijon, France, 2022; 20p. [Google Scholar]
- Bindi, M.; Nunes, P.A.L.D. Vineyards and vineyard management related to ecosystem services: Experiences from a wide range of enological regions in the context of global climate change. J. Wine Econ. 2016, 11, 66–68. [Google Scholar] [CrossRef]
- Paiola, A.; Assandri, G.; Brambilla, M.; Zottini, M.; Pedrini, P.; Nascimbene, J. Exploring the potential of vineyards for biodiversity conservation and delivery of biodiversity-mediated ecosystem services: A global-scale systematic review. Sci. Total Environ. 2020, 706, 135839. [Google Scholar] [CrossRef]
- Winkler, K.J.; Viers, J.H.; Nicholas, K.A. Assessing ecosystem services and multifunctionality for vineyard systems. Front. Environ. Sci. 2017, 5, 15. [Google Scholar] [CrossRef]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: A meta-analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef]
- Garcia, L.; Celette, F.; Gary, C.; Ripoche, A.; Valdes-Gomez, H.; Metay, A. Management of service crops for the provision of ecosystem services in vineyards: A review. Agric. Ecosyst. Environ. 2018, 251, 158–170. [Google Scholar] [CrossRef]
- Candiago, S.; Winkler, K.J.; Giombini, V.; Giupponi, C.; Vigl, L.E. An ecosystem service approach to the study of vineyard landscapes in the context of climate change: A review. Sustain. Sci. 2023, 18, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Han, K.Y.; Kröger, L.; Buchholz, F.; Dewan, I.; Quaas, M.; Schulenbur, H.; Reusch, T.B.H. The economics of microbiodiversity. Ecol. Econ. 2023, 204, 107664. [Google Scholar] [CrossRef]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.F.; et al. Dual domestications and origin of traits in grapevine evolution. Science 2023, 379, 892–901. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.E.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From vineyard soil to wine fermentation: Microbiome approximations to explain the “terroir” concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Guasch Jané, M.R.; Fonseca, S.; Ibrahim, M. “IREP EN KEMET” Wine of ancient Egypt: Documenting the viticulture and winemaking scenes in the Egyptian tombs. ISPRS J. Photogramm. Remote Sens. 2013, 2, 157–161. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, H. Chapter 1: The history of Chinese wine. In Overview of Wine in China; EDP Sciences: Les Ulis, France, 2022; pp. 1–8. [Google Scholar] [CrossRef]
- Stevenson, A.C. Studies in the vegetational history of S. W. Spain II: Palynological investigations at Laguna de Las Madres, S.W. Spain. J. Biogeogr. 1985, 12, 293–314. [Google Scholar] [CrossRef]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- Cavalieri, D.; McGovern, P.E.; Hartl, D.L.; Mortimer, R.; Polsinelli, M. Evidence for S. cerevisiae fermentation in ancient wine. J. Mol. Evol. 2003, 57, 226–232. [Google Scholar] [CrossRef]
- da Silva Fernandes, F.; de Souza, E.S.; Carneiro, L.M.; Alves Silva, J.P.; de Souza, J.V.B.; da Silva Batista, J. Current ethanol production requirements for the yeast Saccharomyces cerevisiae. Int. J. Microbiol. 2022, 2022, 7878830. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L. Etudes sur le vin. Imprimeurs Imperials; BNF: Paris, France, 1866. [Google Scholar]
- Carrau, F.; Gaggero, C.; Aguilar, P.S. Yeast diversity and native vigor for flavor phenotypes. Trends Biotechnol. 2015, 33, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Legout, S. Louis Pasteur: Between myth and reality. Biomolecules 2022, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Marsit, S.; Dequin, S. Diversity and adaptative evolution of Saccharomyces wine yeast: A review. FEMS Yeast Res. 2015, 15, fov067. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderón, F.; Benito, S. Outlining the influence of non-conventional yeasts in wine ageing over lees. Yeast 2016, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Jovial, N.P.; Gianvito, P.D.; Rantsiou, K.; Cocolín, L. Microbial interactions in winemaking: Ecological aspects and effect on wine quality. Trends Food Sci. Technol. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Bauer, F.F.; Pretorius, I.S. Yeast stress response and fermentation efficiency: How to survive the making of wine—A review. S. Afr. J. Enol. Vitic. 2000, 21, 27–51. [Google Scholar] [CrossRef]
- Querol, A.; Pérez-Torrado, R.; Alonso-Del-Real, J.; Minebois, R.; Stribny, J.; Oliveira, B.M.; Barrio, E. New trends in the uses of yeasts in oenology. Adv. Food Nutr. Res. 2018, 85, 177–210. [Google Scholar] [CrossRef]
- Ortiz-Álvarez, R.; Ortega-Arranz, H.; Ontiveros, V.J.; Celis, M.; Ravarani, C.; Acedo, A.; Belda, I. Network properties of local fungal communities reveal the anthropogenic disturbance consequences of farming practices in vineyard soils. Msystems 2021, 6, e00344-21. [Google Scholar] [CrossRef]
- Gonzalez, R.; Morales, P. Truth in wine yeast. Microb. Biotechnol. 2022, 15, 1339–1356. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.Y.; Han, D.Y.; Duan, S.F.; Wang, Q.M. The ecology and evolution of the baker’s yeast Saccharomyces cerevisiae. Genes 2022, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torrado, R.; Barrio, E.; Querol, A. Alternative yeasts for winemaking: Saccharomyces non-cerevisiae and its hybrids. Crit. Rev. Food Sci. Nutr. 2017, 58, 1780–1790. [Google Scholar] [CrossRef]
- Sidari, R.; Ženišová, K.; Tobolková, B.; Belajová, E.; Cabicarová, T.; Bučková, M.; Puškárová, A.; Planý, M.; Kuchta, T.; Pangallo, D. Wine yeasts selection: Laboratory characterization and protocol review. Microorganisms 2021, 9, 2223. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Maicas, S. Application of non-Saccharomyces yeasts to wine-making process. Fermentation 2016, 2, 14. [Google Scholar] [CrossRef]
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Patterns of genetic diversity and the invasion of commercial starters in Saccharomyces cerevisiae vineyard populations of Santorini Island. Foods 2020, 9, 561. [Google Scholar] [CrossRef]
- Engel, S.R.; Dietrich, F.S.; Fisk, D.G.; Binkley, G.; Balakrishnan, R.; Costanzo, M.C.; Dwight, S.S.; Hitz, B.C.; Karra, K.; Nash, R.S.; et al. The reference genome sequence of Saccharomyces cerevisiae: Then and now. G3 Genes Genomes Genet. 2014, 4, 389–398. [Google Scholar] [CrossRef]
- Belda, I.; Gobbi, A.; Ruiz, J.; de Celis, M.; Ortiz-Álvarez, R.; Acedo, A.; Santos, A. Microbiomics to define wine terroir. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Belda, I.; Palacios, A.; Fresno, J.; Ortega, H.; Acedo, A. WineSeq: A new tool for the study of the functional biodiversity of soils, and its use as a biomarker and guide for vitiviniculture practices. BIO Web Conf. 2017, 9, 01012. [Google Scholar] [CrossRef]
- Li, R.; Yang, S.; Lin, M.; Guo, S.; Han, X.; Ren, M.; Du, L.; Song, Y.; You, Y.; Zhan, J.; et al. The biogeography of fungal communities across different Chinese wine-producing regions associated with environmental factors and spontaneous fermentation performance. Front. Microbiol. 2022, 12, 636639. [Google Scholar] [CrossRef]
- Portillo, M.C.; Mas, A. Analysis of microbial diversity and dynamics during wine fermentation of Grenache grape variety by high-throughput barcoding sequencing. LWT Food Sci. Technol. 2016, 72, 317–321. [Google Scholar] [CrossRef]
- Stefanini, I.; Albanese, D.; Cavazza, A.; Franciosi, E.; De Filippo, C.; Donati, C.; Cavalieri, D. Dynamic changes in microbiota and mycobiota during spontaneous ‘Vino Santo Trentino’ fermentation. Microb. Biotechnol. 2016, 9, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Sirén, K.; Mak, S.S.T.; Fischer, U.; Hansen, L.H.; Gilbert, M.T.P. Multi-omics and potential applications in wine production. Curr. Opin. Biotechnol. 2019, 56, 172–178. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Joseph, C.L.; Allen, G.; Benson, A.K.; Mills, D.A. Next-generation sequencing reveals significant bacterial diversity of botrytized wine. PLoS ONE 2012, 7, e36357. [Google Scholar] [CrossRef]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M.; et al. Life with 6000 genes. Science 1996, 274, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Legras, J.L.; Merdinoglu, D.; Cornuet, J.M.; Karst, F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 2007, 16, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, B.; Freeborough, M.H.; Maree, H.J.; Celton, J.M.; Rees, D.J.G.; Burger, J.T. Deep sequencing analysis of viruses infecting grapevines: Virome of a vineyard. Virology 2010, 400, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Setati, M.E.; Jacobson, D.; Bauer, F.F. Sequence-based analysis of the Vitis vinifera L. cv Cabernet sauvignon grape must mycobiome in three South African Vineyards employing distinct agronomic systems. Front. Microbiol. 2015, 6, 1358. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The soil microbiome influences grapevine-associated microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef]
- Cobos, R.; Ibáñez, A.; Diez-Galán, A.; Calvo-Peña, C.; Ghoreshizadeh, S.; Coque, J.J.R. The grapevine microbiome to the rescue: Implications for the biocontrol of trunk diseases. Plants 2022, 11, 840. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Nityagovsky, N.N.; Suprun, A.R.; Ananev, A.A.; Dubrovina, A.S.; Kiselev, K.V. The diversity of fungal endophytes from wild grape Vitis amurensis Rupr. Plants 2022, 11, 2897. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Albanese, D.; Stegen, J.; Franceschi, P.; Coller, E.; Zanzotti, R.; Ioriatti, C.; Stefani, E.; Pindo, M.; Cestaro, A.; et al. Distinct and temporally stable assembly mechanisms shape bacterial and fungal communities in vineyard soils. Microb. Ecol. 2023, 86, 337–349. [Google Scholar] [CrossRef]
- Fabres, P.J.; Collins, C.; Cavagnaro, T.R.; Rodríguez-López, C.M. A concise review on multi-omics data integration for terroir analysis in Vitis vinifera. Front. Plant Sci. 2017, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Acedo, A.; Imam, N.; Santini, R.G.; Ortiz-Álvarez, R.; Ellegaard-Jensen, L.; Belda, I.; Hansen, L.H. A global microbiome survey of vineyard soils highlights the microbial dimension of viticultural terroirs. Commun. Biol. 2022, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Afonso, I.M.; Pereira, J.; Rocha, R.; Rodrigues, A.S. Epiphitic microbiome of Alvarinho wine grapes from different geographic regions in Portugal. Biology 2023, 12, 146. [Google Scholar] [CrossRef]
- Chiarello, M.; McCauley, M.; Villéger, S.; Jackson, C.R. Ranking the biases: The choice of OTUs vs. ASVs in 16S rRNA amplicon data analysis has stronger effects on diversity measures than rarefaction and OUT identity threshold. PLoS ONE 2022, 17, e0264443. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- García-López, R.; Cornejo-Granados, F.; Lopez-Zavala, A.A.; Cota-Huízar, A.; Sotelo-Mundo, R.; Gómez-Gil, B.; Ochoa-Leyva, A. OTUs and ASVs produce comparable taxonomic and diversity from shrimp microbiota 16S profiles using tailored abundance filters. Genes 2021, 12, 564. [Google Scholar] [CrossRef]
- Glassman, S.I.; Martiny, J.B.H. Broadscale ecological patterns are robust to use of exact sequence variants versus Operational Taxonomic Units. mSphere 2018, 3, e00148-18. [Google Scholar] [CrossRef]
- Venter, J.C.; Glass, J.I.; Hutchison, C.A.; Vashee, S. Synthetic chromosomes, genomes, viruses and cells. Cell 2022, 185, 2708–2724. [Google Scholar] [CrossRef]
- Pretorius, I.S. Synthetic genome engineering forging new frontiers for wine yeast. Crit. Rev. Biotechol. 2017, 37, 112–136. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S.; Boeke, J.D. Yeast 2.0-connecting the dots in the construction of the world’s first functional synthetic eukaryotic genome. FEMS Yeast Res. 2018, 18, foy032. [Google Scholar] [CrossRef] [PubMed]
- Kutyna, D.R.; Onetto, C.A.; Williams, T.C.; Goold, H.D.; Paulsen, I.T.; Pretorius, I.S.; Johnson, D.L.; Borneman, A.R. Construction of a synthetic Saccharomyces cerevisiae pan-genome neo-chromosome. Nat. Commun. 2022, 13, 3628. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tasting the terroir of wine yeast innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Visualizing the new frontiers in wine yeast research. FEMS Yeast Res. 2022, 22, foac010. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the vineyard to the winery: How microbial ecology drives regional distinctiveness of wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Pio Rossetti, A.; Battistelli, N.; Zulli, C.; Cichelli, A.; Arfelli, A.; Arfelli, G.; Tofalo, R. Impact of vineyard management on grape fungal community and Montepulciano d’Abruzzo wine quality. Food Res. Int. 2022, 158, 111577. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.G.; Steenwerth, K.L.; Mills, D.A.; Cantu, D.; Bokulich, N.A. Sources and assembly of microbial communities in vineyards as a functional component of winegrowing. Front. Microbiol. 2021, 12, 673810. [Google Scholar] [CrossRef]
- Giffard, B.; Winter, S.; Guidoni, S.; Nicolai, A.; Castaldini, M.; Cluzeau, D.; Coll, P.; Cortet, J.; Le Cadre, E.; d’Errico, G.; et al. Vineyard management and its impacts on soil biodiversity, functions, and ecosystem services. Front. Ecol. Evol. 2022, 10, 850272. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Seguin, G. The concept of Terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Lewin, B. Wine Myths and Reality; Wine Appreciation Guild: San Francisco, CA, USA, 2010; 636p, ISBN 1934259519. [Google Scholar]
- Gilbert, J.A.; van der Lelie, D.; Zarraonaindia, I. Microbial terroir for wine grapes. Proc. Natl. Acad. Sci. USA 2014, 111, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Lauga, B.; Miot-Sertier, C.; Mercier, A.; Lonvaud, A.; Soulas, M.L.; Soulas, G.; Masneuf-Pomarède, I. Characterization of epiphytic bacterial communities from grapes, leaves, bark and soil of grapevine plants grown, and their relations. PLoS ONE 2013, 8, e73013. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Silva, A.; Laureano, G.; Pereira, M.; Días, R.; Moreira da Silva, J.; Oliveira, N.; Gouveia, C.; Cruz, C.; Gama-Carvalho, M.; Alagna, F.; et al. A new perspective for vineyard terroir identity: Looking for microbial indicator species by long read nanopore sequencing. Microorganisms 2023, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Martiny, J.B.H.; Bohannan, B.J.M.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage and climate. Proc. Nail. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- Rivas, G.A.; Guillade, A.C.; Semorile, L.C.; Delfederico, L. Influence of climate on soil and wine bacterial diversity on a vineyard in a non-traditional wine region in Argentina. Front. Microbiol. 2021, 12, 726384. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Q.; Zhang, P.; Chen, D.; Howell, K.S. The fungal microbiome is an important component of vineyard ecosystems and correlates with regional distinctiveness of wine. mSphere 2020, 5, e00534-20. [Google Scholar] [CrossRef]
- Burns, K.N.; Kluepfel, D.A.; Strauss, S.L.; Bokulich, N.A.; Cantu, D.; Steenwerth, K.L. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: Differentiation by geographic features. Soil Biol. Biochem. 2015, 91, 232–247. [Google Scholar] [CrossRef]
- Vadour, E.; Costantini, E.; Jones, G.V.; Mocali, S. An overview of the recent approaches for terroir functional modelling, footprinting and zoning. Soil Discuss. 2015, 1, 827–906. [Google Scholar] [CrossRef]
- Gayevski, V.; Goddard, M.R. Geographic delineations of yeast communities and populations associated with vines and wines in New Zealand. ISME J. 2012, 6, 1281–1290. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Sandionigi, A.; Guzzetti, L.; Galimberti, A.; Grando, M.S.; Tardaguila, J.; Labra, M. Geographical and cultivar features differentiate grape microbiota in Northern Italy and Spain vineyards. Front. Microbiol. 2018, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Tronchoni, J.; Setati, M.E.; Fracassetti, D.; Valdetara, F.; Maghradze, D.; Foschino, R.; Curiel, J.A.; Morales, P.; Gonzalez, R.; Vigentini, I.; et al. Identifying the main drivers in microbial diversity for cabernet sauvignon cultivars from Europe to South Africa: Evidence for a cultivar-specific microbial fingerprint. J. Fungi 2022, 8, 1034. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Tsai, P.; Anfang, N.; Ross, H.A.; Goddard, M.R. Pyrosequencing reveals regional differences in fruit-associated fungal communities. Environ. Microbiol. 2014, 16, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Morrison-Whittle, P.; Goddard, M.R. From vineyard to winery: A source map of microbial diversity driving wine fermentation. Environ. Microbiol. 2018, 20, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kioroglou, D.; Kraeva-Deloire, E.; Leigh, M.; Schmidtke, L.M.; Mas, A.; Portillo, M.C. Geographical origin has a greater impact on grape berry fungal community than grape variety and maturation state. Microorganisms 2019, 7, 669. [Google Scholar] [CrossRef] [PubMed]
- Kamilari, E.; Mina, M.; Karallis, C.; Tsaltas, D. Metataxonomic analysis of grape microbiota during wine fermentation reveals the distinction of Cyprus regional terroirs. Front. Microbiol. 2021, 12, 726483. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.F.; Migicovsky, Z.; Trello, G.E.; Miller, A.J. Grapevine bacterial communities display compartment-specific dynamics over space and time within the Central Valley of California. Environ. Microbiome 2023, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.C.; Franquès, J.; Araque, I.; Reguant, C.; Bordons, A. Bacterial diversity of grenache and carignan grape surface from different vineyards at Priorat wine region (Catalonia, Spain). Int. J. Food Microbiol. 2016, 219, 56–63. [Google Scholar] [CrossRef]
- Knight, S.J.; Karon, O.; Goddard, M.R. Small scale fungal community differentiation in a vineyard system. Food Microbiol. 2020, 87, 103358. [Google Scholar] [CrossRef]
- Yan, H.; Ge, C.; Zhou, J.; Li, J. Diversity of soil fungi in the vineyards of Changli región in China. Can. J. Microbiol. 2022, 68, 341–352. [Google Scholar] [CrossRef]
- Setati, M.E.; Jacobson, D.; Andong, U.; Bauer, F. The vineyard yeast microbiome, a mixed model microbial map. PLoS ONE 2012, 7, e52609. [Google Scholar] [CrossRef]
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Biogeographical regionalization of wine yeast communities in Greece and environmental drivers of species distribution at a local scale. Front. Microbiol. 2021, 12, 705001. [Google Scholar] [CrossRef]
- Zhou, J.; Cavagnaro, T.R.; De Bei, R.; Nelson, T.M.; Stephen, J.R.; Metcalfe, A.; Gilliham, M.; Breen, J.; Collins, C.; Rodríguez López, C.M. Wine terroir and the soil bacteria: An amplicon sequencing-based assessment of the Barossa Valley and its sub-Regions. Front. Microbiol. 2021, 11, 597944. [Google Scholar] [CrossRef]
- Wei, R.T.; Chen, N.; Ding, Y.T.; Wang, L.; Gao, F.F.; Zhang, L.; Liu, Y.H.; Li, H.; Wang, H. Diversity and dynamics of epidermal microbes during grape development of cabernet sauvignon (Vitis vinifera L.) in the ecological viticulture model in Wuhai, China. Front. Microbiol. 2022, 13, 935647. [Google Scholar] [CrossRef]
- Kecskeméti, E.; Berkelmann-Löhnertz, B.; Reineke, A. Are epiphytic microbial communities in the carposphere of ripening grape clusters (Vitis vinifera L.) different between conventional, organic, and biodynamic grapes? PLoS ONE 2016, 11, e0160852. [Google Scholar] [CrossRef]
- Grangeteau, C.; Roullier-Gall, C.; Rousseaux, S.; Gougeon, R.D.; Schmitt-Kopplin, P.; Alexandre, H.; Guilloux-Benatier, M. Wine microbiology is driven by vineyard and winery anthropogenic factors. Microb. Biotechnol. 2017, 10, 354–370. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Lee, S.A.; Goddard, M.R. Fungal communities are differentially affected by conventional and biodynamic agricultural management approaches in vineyard ecosystems. Agric. Ecosyst. Environ. 2017, 246, 306–313. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Chen, X.; Cui, B.; Bai, Z.; Zhuang, G. Variety features differentiate microbiota in the grape leaves. Can. J. Microbiol. 2020, 66, 653–663. [Google Scholar] [CrossRef]
- Martins, G.; Casini, C.; Da Costa, J.P.; Geny, L.; Lonvaud, A.; Masneuf-Pomarède, I. Correlation between water activity (aw) and microbial epiphytic communities associated with grape berries. OENO One 2020, 54, 49–61. [Google Scholar] [CrossRef]
- Mozzachiodi, S.; Bai, F.Y.; Baldrian, P.; Bell, G.; Boundy-Mills, K.; Buzzini, P.; Čadež, N.; Cubillos, F.A.; Dashko, S.; Dimitrov, R.; et al. Yeasts from temperate forests. Yeast 2022, 39, 4–24. [Google Scholar] [CrossRef]
- Valentini, B.; Barbero, F.; Casacci, L.P.; Luganini, A.; Stefanini, I. Forest influence yeast populations vectored by insects into vineyards. Front. Microbiol. 2022, 13, 1039939. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Sánchez, R.; Castañeda, L.E.; Godoy, K.; Barbosa, O. Is microbial terroir related to geographic distance between vineyards? Environ. Microbiol. Rep. 2017, 9, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, J.; Franco, L.M.; Cools, T.L.; De Meester, L.; Michiels, J.; Wenseleers, T.; Hassan, B.A.; Yaksi, E.; Verstrepen, K.J. The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep. 2014, 9, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Lampis, S.; Vallini, G. Diversity, distribution and functional role of bacterial endophytes in Vitis vinifera. In Endophytes: Biology and Biotechnology; Maheshwari, D.K., Ed.; Springer: Cham, Switzerland, 2017; Volume 5, pp. 233–266. [Google Scholar] [CrossRef]
- Martínez-Diz, M.P.; Andrés-Sodupe, M.; Bujanda, R.; Díaz-Losada, E.; Eichmeier, A.; Gramaje, D. Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol. 2019, 41, 234–244. [Google Scholar] [CrossRef]
- Bao, L.; Sun, B.; Wei, Y.; Xu, N.; Zhang, S.; Gu, L.; Bai, Z. Grape cultivar features differentiate the grape rhizosphere microbiota. Plants 2022, 11, 1111. [Google Scholar] [CrossRef]
- Cureau, N.; Threlfall, R.; Marasini, D.; Lavefve, L.; Carbonero, F. Year, location, and variety impact on grape-associated mycobiota of Arkansas-grown wine grapes for wine production. Microb. Ecol. 2021, 82, 845–858. [Google Scholar] [CrossRef]
- Paolinelli, M.; Martinez, L.E.; García-Lampasona, S.; Diaz-Quirós, C.; Belmonte, M.; Ahumada, G.; Pirrone, M.A.; Farber, M.D.; Escoriaza, G.; Longone, V.; et al. Microbiome in soils of Mendoza: Microbial resources for the development of agroecological management in viticulture. OENO One 2023, 57, 191–205. [Google Scholar] [CrossRef]
- Ghiță, S.; Hnatiuc, M.; Ranca, A.; Artem, V.; Mădălina-Andreea, C. Studies on the short-term effects of the cease of pesticides use on vineyard microbiome. In Vegetation Dynamics, Changing Ecosystems and Human Responsibility; Hufnagel, L., El-Esawi, M.A., Eds.; Intechopen: London, UK, 2023; pp. 1–10. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. mBio 2016, 7, e00631-16. [Google Scholar] [CrossRef]
- Knight, S.J.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Pölme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Quang Thu, P.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 6213. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Smets, W.; Moretti, S.; Denys, S.; Lebeer, S. Airborne bacteria in the atmosphere: Presence, purpose, and potential. Atmos. Environ. 2016, 139, 214–221. [Google Scholar] [CrossRef]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Coller, E.; Cestaro, A.; Zanzotti, R.; Bertoldi, D.; Pindo, M.; Larger, S.; Albanese, D.; Mescalchin, E.; Donati, C. Microbiome of vineyard soils is shaped by geography and management. Microbiome 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Steenwerth, K.L.; Morelan, I.; Stahel, R.; Figueroa-Balderas, R.; Cantu, D.; Lee, J.; Runnebaum, R.C.; Poret-Peterson, A.T. Fungal and bacterial communities of “Pinot noir” must: Effects of vintage, growing region, climate, and basic must chemistry. PeerJ 2021, 9, e10836. [Google Scholar] [CrossRef]
- Sabate, J.; Cano, J.; Esteve-Zarzoso, B.; Guillamon, J.M. Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol. Res. 2002, 157, 267–274. [Google Scholar] [CrossRef]
- Vigentini, I.; De Lorenzis, G.; Fabrizio, V.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Imazio, S.; Failla, O.; Foschino, R. The vintage effect overcomes the terroir effect: A three year survey on the wine yeast biodiversity in Franciacorta and Oltrepò Pavese, two northern Italian vine-growing areas. Microbiology 2015, 161, 362–373. [Google Scholar] [CrossRef]
- Huang, R.; Shen, L.; Yu, H.; Jiang, J.; Qin, Y.; Liu, Y.; Zhang, J.; Song, Y. Evaluation of rain-shelter cultivation mode effects on microbial diversity during Cabernet Sauvignon (Vitis vinifera L.) maturation in Jingyang, Shaanxi, China. Food Res. Int. 2022, 156, 111165. [Google Scholar] [CrossRef]
- Conacher, C.G.; Luyt, N.A.; Naidoo-Blassoples, R.K.; Rossouw, D.; Setati, M.E.; Bauer, F.F. The ecology of wine fermentation: A model for the study of complex microbial ecosystems. Appl. Microbiol. Biotechnol. 2021, 105, 3027–3043. [Google Scholar] [CrossRef]
- Alonso, A.; de Celis, M.; Ruiz, J.; Vicente, J.; Navascués, E.; Acedo, A.; Ortiz-Álvarez, R.; Belda, I.; Santos, A.; Gómez-Flechoso, M.A.; et al. Looking at the origin: Some insights into the general and fermentative microbiota of vineyard soils. Fermentation 2019, 5, 78. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Cardoso, R.; Custódio, V.; Fernandes, J.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Wine fermentation microbiome: A landscape from different Portuguese wine appellations. Front. Microbiol. 2015, 6, 905. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Ohta, M.; Richardson, P.M.; Mills, D.A. Monitoring seasonal changes in winery-resident microbiota. PLoS ONE 2013, 8, e66437. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Hawley, E.; Kopf, S.; DeScenzo, R.; Sealock, S.; Henick-Kling, T.; Hess, M. Insights into the bacterial community and its temporal succession during the fermentation of wine grapes. Front. Microbiol. 2015, 6, 809. [Google Scholar] [CrossRef] [PubMed]
- Querol, A.; Fernández-Espinar, M.T.; del Olmo, M.; Barrio, E. Adaptative evolution of wine yeast. Int. J. Food Microbiol. 2003, 86, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tello, J.; Cordero-Bueso, G.; Aporta, I.; Cabellos, J.M.; Arroyo, T. Genetic diversity in commercial wineries: Effects of the farming system and vinification management on wine yeasts. J. Appl. Microbiol. 2011, 112, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Fleet, G.H.; Heard, G.M. Lactic acid bacteria associated with wine grapes from several Australian vineyards. J. Appl. Microbiol. 2006, 100, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Henick-Kling, T. Modification of wine flavour by malolactic fermentation. In Proceedings of the 10th International Oenological Symposium, Breisach, Germany, 3–5 May 1993; International Association for Winery Technology and Management: London, UK, 1993; pp. 290–306. [Google Scholar]
- Lonvaud-Funel, A. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Varela, C.; Borneman, A.R. Yeasts found in vineyards and wineries. Yeast 2017, 43, 111–128. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Yang, H.; Sun, L.; Xia, H.; Sun, W.; Wang, Z.; Zhang, J. Bacterial communities related to aroma formation during spontaneous fermentation of “cabernet sauvignon” wine in Ningxia, China. Foods 2022, 11, 2775. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Du, H.; Zhang, H.; Fang, C.; Jin, G.; Chen, S.; Wu, Q.; Zhang, Y.; Zhang, M.; Xu, Y. Geographically associated fungus-bacterium interactions contribute to the formation of geography-dependent flavor during high-complexity spontaneous fermentation. Microbiol. Spectr. 2022, 10, e0184422. [Google Scholar] [CrossRef] [PubMed]
- Tempère, S.; Marchal, A.; Barbe, J.C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The complexity of wine: Clarifying the role of microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Nature and origin of wine quality. In Wine Tasting: A Professional Handbook, 2nd ed.; Jackson, R.S., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 387–426. [Google Scholar]
- Guillamón, J.M.; Barrio, E. Genetic polymorphism in wine yeasts: Mechanisms and methods for its detection. Front. Microbiol. 2017, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Yang, Q.; Han, X.; Zhou, Z.; Mao, J. Saccharomyces cerevisiae strains with low-yield higher alcohols and high-yield acetate esters improve the quality, drinking comfort and safety of huangjiu. Food Res. Int. 2022, 161, 111763. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Peng, S.; Sam, F.E.; Zhu, Y.; Liang, L.; Li, M.; Wang, J. Indigenous non-Saccharomyces yeast with β-Glucosidase activity in sequential fermentation with Saccharomyces cerevisiae: A strategy to improve the volatile composition and sensory characteristics of wines. Front. Microbiol. 2022, 13, 845837. [Google Scholar] [CrossRef]
- Amato, M.; Ballco, P.; López-Galán, B.; De Magistris, T.; Verneau, F. Exploring consumers’ perception and willingness to pay for “Non-Added Sulphite” wines through experimental auctions: A case study in Italy and Spain. Wine Econ. Policy 2017, 6, 146–154. [Google Scholar] [CrossRef]
- Carrau, F.; Henschke, P. Hanseniaspora vineae and the concept of friendly yeasts to increase autochthonous wine flavor diversity. Front. Microbiol. 2021, 12, 702093. [Google Scholar] [CrossRef]
- Scannell, D.R.; Zill, O.A.; Rokas, A.; Payen, C.; Dunham, M.J.; Eisen, M.B.; Rine, J.; Johnston, M.; Hittinger, C.T. The awesome power of yeast evolutionary genetics: New genome sequences and strain resources for the Saccharomyces sensu stricto genus. G3 Genes Genomes Genet. 2011, 1, 11–25. [Google Scholar] [CrossRef]
- Borneman, A.R.; Pretorius, I.S. Genomic insights into the Saccharomyces sensu stricto complex. Genetics 2015, 199, 281–291. [Google Scholar] [CrossRef]
- Tapia, S.M.; Pérez-Torrado, R.; Adam, A.C.; Macías, L.G.; Barrio, E.; Querol, A. Adaptative evolution in the Saccharomyces kudriavzevii Aro4p promoted a reduced production of higher alcohols. Microb. Biotechnol. 2022, 15, 2958–2969. [Google Scholar] [CrossRef]
- Karabegović, I.; Malićanin, M.; Popović, N.; Stamenković Stojanović, S.; Lazić, M.; Stanojević, J.; Danilović, B. Native non-Saccharomyces yeasts as a tool to produce distinctive and diverse Tamjanika grape wines. Foods 2022, 11, 1935. [Google Scholar] [CrossRef]
- Alsammar, H.; Delneri, D. An update on the diversity, ecology and biogeography of the Saccharomyces genus. FEMS Yeast Res. 2020, 20, foaa013. [Google Scholar] [CrossRef]
- Pontes, A.; Hutzler, M.; Brito, P.H.; Sampaio, J.P. Revisiting the taxonomic synonyms and populations of Saccharomyces cerevisiae—Phylogeny, phenotypes, ecology and domestication. Microorganisms 2020, 8, 903. [Google Scholar] [CrossRef]
- Boynton, P.J.; Greig, D. The ecology and evolution of non-domesticated Saccharomyces species. Yeast 2014, 31, 449–462. [Google Scholar] [CrossRef]
- Zahid, M.S.; Hussain, M.; Song, Y.; Li, J.; Guo, D.; Li, X.; Song, S.; Wang, L.; Xu, W.; Wang, S. Root-zone restriction regulates soil factors and bacterial community assembly of grapevine. Int. J. Mol. Sci. 2022, 23, 15628. [Google Scholar] [CrossRef]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Pérès, G.; Rutgers, M. Soil biodiversity, biological indicators and soil ecosystem services—An overview of European approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- Burns, K.N.; Bokulich, N.A.; Cantu, D.; Greenhut, R.F.; Kluepfel, D.A.; O’Geen, A.T.; Strauss, S.L.; Steenwerth, K.L. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: Differentiation by vineyard management. Soil Biol. Biochem. 2016, 103, 337–348. [Google Scholar] [CrossRef]
- Darriaut, R.; Tran, J.; Martins, G.; Ollat, N.; Masneuf-Pomarède, I.M.; Lauvergeat, V. In grapevine decline, microbiomes are affected differently in symptomatic and asymptomatic soils. Appl. Soil Ecol. 2023, 183, 104767. [Google Scholar] [CrossRef]
- Williams, J.N.; Morandé, J.A.; Vaghti, M.G.; Medellín-Azuara, J.; Viers, J.H. Ecosystem services in vineyard landscapes: A focus on aboveground carbon storage and accumulation. Carbon Balance Manag. 2020, 15, 23. [Google Scholar] [CrossRef]
- Song, R.; Zhu, Z.; Zhang, L.; Li, H.; Wang, H. A simple method using an allometric model to quantify the carbon sequestration capacity in vineyards. Plants 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, T.; Gao, F.; Wei, R.; Wang, Z.; Li, H.; Wang, H. Carbon storage distribution characteristics of vineyard ecosystems in Hongsibu, Ningxia. Plants 2021, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Sun, q.; Guoa, S.; Wang, R.; Song, J. Responses of bacterial communities and their carbon dynamics to subsoil exposure on the Loess Plateau. Sci. Total Environ. 2021, 756, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yuan, J.; Yue, F.-J.; Li, S.; Wang, B.; Mohinuzzaman, M.; Liu, Y.; Senesi, N.; Lao, X.; Li, L.; et al. New insights into mechanisms of sunlight- and dark-mediated high-temperature can accelerate diurnal production-degradation transformation of lake fluorescent DOM. Sci. Total Environ. 2021, 760, 143377. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Nail. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Scandellari, F. Arbuscular mycorrhizal contribution to nitrogen uptake of grapevines. Vitis 2017, 56, 147–154. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.; Montier, C.; Jamague, M.; Daroussin, J.; King, D. Mapping erosion risk for cultivated soil in France. Catena 2001, 46, 207–220. [Google Scholar] [CrossRef]
- Brenot, J.; Quiquerez, A.; Petit, C.; Garcia, J.P. Erosion rates and sediment budgets in vineyards at 1-m resolution based on stock unearthing (Burgundy, France). Geomorphology 2008, 100, 345–355. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Kurtural, S.K. Inoculation with mycorrhizal fungi and irrigation management shape the bacterial and fungal communities and networks in vineyard soils. Microorganisms 2021, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Koch, B. Diversity of arbuscular mycorrhizal fungi in no-till and conventionally tilled vineyards. J. Appl. Bot. Food Qual. 2018, 91, 56–60. [Google Scholar] [CrossRef]
- Schreiner, R.P. Effects of native and nonnative arbuscular mycorrhizal fungi on growth and nutrient uptake of “Pinot noir” (Vitis vinifera L.) in two soils with contrasting levels of phosphorous. Appl. Soil Ecol. 2007, 36, 205–215. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mumey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Petgen, M.; Schropp, A.; Marschner, H.; Roemheld, V. Investigations on the Occurrence of Arbuscular Mycorrhizae in Some Grape Vine Nurseries and the Practical Management of Field Inoculation with Arbuscular Mycorrhizae; Mitteilungen-Biologische Bundesanstalt für Land-und Forstwirtschaft: Berlin, Germany, 1997; pp. 32–46. [Google Scholar]
- Cavagnaro, T.R.; Smith, F.A.; Smith, S.E.; Jakobsen, I. Functional diversity in arbuscular mycorrhizas: Exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant. Cell Environ. 2005, 28, 642–650. [Google Scholar] [CrossRef]
- Wilson, G.W.T.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 238, 325–333. [Google Scholar] [CrossRef]
- Caravaca, F.; Alguacil, M.M.; Azcón, R.; Roldán, A. Formation of stable aggregates in rhizosphere soil of Juniperus oxycedrus: Effect of AM fungi and organic amendments. Appl. Soil Ecol. 2006, 33, 30–38. [Google Scholar] [CrossRef]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Bergmann, J.; Verbruggen, E.; Veresoglou, S.D.; Lehmann, A. (2014) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol. 2014, 205, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef]
- Bettenfeld, P.; Cadena I Canals, J.; Jacquens, L.; Fernandez, O.; Fontaine, F.; van Schaik, E.; Courty, P.E.; Trouvelot, S. The microbiota of the grapevine holobiont: A key component of plant health. J. Adv. Res. 2022, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hanada, R.E.; Pomelia, A.W.V.; Soberanis, W.; Loguercio, L.L.; Pereira, J.O. Biocontrol potential of Trichoderma martiale against the black-pod disease (Phytophthora palmivora) of cacao. Biol. Control 2009, 50, 143–149. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Seem, R.C.; Pearson, R.C.; Wilcox, W.F.; Dunst, R.M. Effects of powdery mildew on vine growth, yield, and quality of concord grapes. Plant Dis. 2001, 85, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef] [PubMed]
- Darriaut, R.; Lailheugue, V.; Masneuf-Pomarède, I.; Marguerit, E.; Martins, G.; Company, S.; Ballestra, P.; Upton, S.; Ollat, N.; Lauvergeat, V. Grapevine rootstock and soil microbiome interactions: Keys for a resilient viticulture. Hortic. Res. 2022, 9, uhac019. [Google Scholar] [CrossRef]
- Mutawila, C.; Halleen, F.; Mostert, L. Optimisation of time of application of Trichoderma biocontrol agents for protection of grapevine pruning wounds. Aust. J. Grape Wine Res. 2016, 22, 279–287. [Google Scholar] [CrossRef]
- Perazzolli, M.; Antonielli, L.; Storari, M.; Puopolo, G.; Pancher, M.; Giovannini, O.; Pindo, M.; Pertot, I. Resilience of the natural phyllosphere microbiota of the grapevine to chemical and biological pesticides. Appl. Environ. Microbiol. 2014, 80, 3585–3596. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionst. Nat. Rev. Microbiol. 2004, 2, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; Cardoza, R.E.; Gutiérrez, S.; Casquero, P.A. Vineyard management and physicochemical parameters of soil affect native Trichoderma populations, sources of biocontrol agents against Phaeoacremonium minimum. Plants 2023, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Döring, J.; Collins, C.; Frisch, M.; Kauer, R. Organic and biodynamic viticulture affect biodiversity and properties of vine and wine: A systematic quantitative review. Am. J. Enol. Vitic. 2019, 70, 3. [Google Scholar] [CrossRef]
- Pancher, M.; Coel, M.; Corneo, P.E.; Longa, C.M.O.; Yousaf, S.; Pertot, I.; Campisano, A. Fungal endophytic communities in grapevines (Vitis vinifera L.) respond to crop management. Appl. Environ. Microbiol. 2012, 78, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Custódio, V.; Nunes, M.; Songy, A.; Rabenoelina, F.; Courteaux, B.; Clément, C.; Gomes, A.C.; Fontaine, F. Understand the potential role of Aureobasidium pullulans, a resident microorganism from grapevine, to prevent the infection caused by Diplodia seriata. Front. Microbiol. 2018, 9, 3047. [Google Scholar] [CrossRef] [PubMed]
- Kernaghan, G.; Mayerhofer, M.; Griffin, A. Fungal endophytes of wild and hybrid Vitis leaves and their potential for vineyard biocontrol. Can. J. Microbiol. 2017, 63, 583–8595. [Google Scholar] [CrossRef] [PubMed]
- Point, E.; Tyedmers, P.; Naugler, C. Life cycle environmental impacts of wine production and consumption in Nova Scotia, Canada. J. Clean. Prod. 2012, 27, 11–20. [Google Scholar] [CrossRef]
- De la Huerta-Bengoechea, P.; Gil-Serna, J.; Melguizo, C.; Ramos, A.J.; Prim, M.; Vázquez, C.; Patiño, B. Biocontrol of mycotoxigenic fungi using bacteria isolated from ecological vineyard soils. J. Fungi 2022, 8, 1136. [Google Scholar] [CrossRef]
- Maluleke, E.; Jolly, N.P.; Patterton, H.G.; Setati, M.E. Antifungal activity of non-conventional yeasts against Botrytis cinerea and non-Botrytis grape bunch rot fungi. Front. Microbiol. 2022, 13, 986229. [Google Scholar] [CrossRef]
- Tempesta, T.; Giancristofaro, R.A.; Corain, L.; Salmaso, L.; Tomasi, D.; Boatto, V. The importance of landscape in wine quality perception: An integrated approach using choice-based conjoint analysis and combination-based permutation tests. Food Qual. Prefer. 2010, 21, 827–836. [Google Scholar] [CrossRef]
- Montella, M.M. Wine tourism and sustainability: A review. Sustainability 2017, 9, 113. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar] [CrossRef]
- Rosso, A.M. Beer and wine in antiquity: Beneficial remedy or punishment imposed by the Gods? Acta Med.-Hist. Adriat. 2012, 10, 237–262. [Google Scholar] [PubMed]
- Stanislawski, D. Dionysus westward: Early religion and the economic geography of wine. Geogr. Rev. 1975, 65, 4. [Google Scholar] [CrossRef]
- Kirkpatrick, J. The jews and their god of wine. Arch. Für Relig. 2014, 15, 167–186. [Google Scholar] [CrossRef]
- Onofri, L.; Boatto, V. On the economic valuation of cultural ecosystem services: A tale of myths, vine and wine. Ecosyst. Serv. 2020, 46, 101215. [Google Scholar] [CrossRef]
- Chou, M.Y.; Vanden Heuvel, J.; Bell, T.H.; Panke-Buisse, K.; Kao-Kniffin, J. Vineyard under-vine floor management alters soil microbial composition, while the fruit microbiome shows no corresponding shifts. Sci. Rep. 2018, 8, 11039. [Google Scholar] [CrossRef]
- Quiquerez, A.; García, J.P.; Dequiedt, S.; Djemiel, C.; Terrat, S.; Mathieu, O.; Sassi, A.; Ranjard, L. Legacy of land-cover changes on soil microbiology in Burgundy vineyards (Pernand-Vergelesses, France). OENO One 2022, 56, 2. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Arroyo, T.; Serrano, A.; Valero, E. Influence of different floor management strategies of the vineyard on the natural yeast population associated with grape berries. Int. J. Food Microbiol. 2011, 148, 23–29. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Bagheri, B.; Bauer, F.; Setati, M. The diversity and dynamics of indigenous yeast communities in grape must from vineyards employing different agronomic practices and their influence on wine fermentation. S. Afr. J. Enol. Vitic. 2015, 36, 243–251. [Google Scholar] [CrossRef]
- Capó-Bauçà, S.; Marqués, A.; Llopis-Vidal, N.; Bota, J.; Baraza, E. Long-term establishment of natural green cover provides agroecosystem services by improving soil quality in a Mediterranean vineyard. Ecol. Eng. 2019, 127, 285–291. [Google Scholar] [CrossRef]
- Daane, K.M.; Hogg, B.N.; Wilson, H.; Yokota, G.Y. Native grass ground covers provide multiple ecosystem services in Californian vineyards. J. Appl. Ecol. 2018, 55, 2473–2483. [Google Scholar] [CrossRef]
- Rodríguez-Entrena, M.; Colombo, S.; Arriaza, M. The landscape of olive groves as a driver of the rural economy. Land Use Policy 2017, 65, 164–175. [Google Scholar] [CrossRef]
- Chapela-Oliva, C.; Winter, S.; Ochoa-Hueso, R. Edaphoclimatic drivers of the effect of extensive vegetation management on ecosystem services and biodiversity in vineyards. Agric. Ecosyst. Environ. 2022, 339, 108115. [Google Scholar] [CrossRef]
- Bünemann, E.; Schwenke, G.; Zwieten, L. Impact of agricultural inputs on soil organisms—A review. Aust. J. Soil Res. 2006, 44, 379–406. [Google Scholar] [CrossRef]
- Okur, N.; Altindİşlİ, A.; Çengel, M.; Göçmez, S.; Kayikçioğlu, H.H. Microbial biomass and enzyme activity in vineyard soils under organic and conventional farming systems. Turk. J. Agric. For. 2009, 33, 413–423. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- García-Orenes, F.; Roldán, A.; Morugán-Coronado, A.; Linares, C.; Cerdà, A.; Caravaca, F. Organic fertilization in traditional Mediterranean grapevine orchards mediates changes in soil microbial community structure and enhances soil fertility. Land Degrad. Dev. 2016, 27, 1622–1628. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Yeoh, Y.K.; Kasinadhuni, N.R.; Lonhienne, T.G.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 2015, 5, 8678. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L.; Ivan, D.; Zottini, M. Management intensity and topography determined plant diversity in vineyards. PLoS ONE 2013, 8, e76167. [Google Scholar] [CrossRef] [PubMed]
- Amaral, H.F.; Schwan-Estrada, K.R.F.; de Sena, J.O.A.; Colozzi-Filho, A.; Andrade, D.S. Seasonal variations in soil chemical and microbial indicators under conventional and organic vineyards. Acta Sci.-Agron. 2023, 45, e56158. [Google Scholar] [CrossRef]
- Karimi, B.; Masson, V.; Guilland, C.; Leroy, E.; Pellegrinelli, S.; Giboulot, E.; Maron, P.A.; Ranjard, L. Ecotoxicity of copper input and accumulation for soil biodiversity in vineyards. Environ. Chem. Lett. 2021, 19, 2013–2030. [Google Scholar] [CrossRef]
- Ostandie, N.; Giffard, B.; Bonnard, O.; Joubard, B.; Richart-Cervera, S.; Thiéry, D.; Rusch, A. Multi-community effects of organic and conventional farming practices in vineyards. Sci. Rep. 2021, 11, 11979. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, V.; Comitini, F.; Ciani, M. Grape berry yeast communities: Influence of fungicide treatments. Int. J. Food. Microbiol. 2013, 161, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Mandl, K.; Cantelmo, C.; Gruber, E.; Faber, F.; Friedrich, B.; Zaller, J.G. Effects of glyphosate-, glufosinate- and flazasulfuron-based herbicides on soil microorganisms in a vineyard. Bull. Env. Contam. Toxic. 2018, 101, 562–569. [Google Scholar] [CrossRef]
- Probst, B.; Schüler, C.; Joergensen, R.G. Vineyard soils under organic and conventional management—Microbial biomass and activity indices and their relation to soil chemical properties. Biol. Fert. Soils 2008, 44, 443–450. [Google Scholar] [CrossRef]
- Vega-Avila, A.D.; Gumiere, T.; Andrade, P.A.M.; Lima-Perim, J.E.; Durrer, A.; Baigori, M.; Vazquez, F.; Andreote, F.D. Bacterial communities in the rhizosphere of Vitis vinifera L. cultivated under distinct agricultural practices in Argentina. Antonie Leeuwenhoek J. Microb. 2015, 107, 575–588. [Google Scholar] [CrossRef]
- Patrignani, F.; Montanari, C.; Serrazanetti, D.I.; Braschi, G.; Vernocchi, P.; Tabanelli, G.; Parpinello, G.P.; Versari, A.; Gardini, F.; Lanciotti, R. Characterisation of yeast microbiota, chemical and sensory properties of organic and biodynamic Sangiovese red wines. Ann. Microbiol. 2016, 67, 99–109. [Google Scholar] [CrossRef]
- Schmid, F.; Moser, G.; Müller, H.; Berg, G. Functional and structural microbial diversity in organic and conventional viticulture: Organic farming benefits natural biocontrol agents. Appl. Environ. Microb. 2011, 77, 2188–2191. [Google Scholar] [CrossRef]
- Power, A.G. Ecosystem services and agriculture: Trade-offs and synergies. Phil. Trans. R. Soc. B 2010, 365, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Viers, J.H.; Williams, J.N.; Nicholas, K.A.; Barbosa, O.; Kotzé, I.; Spence, L.; Webb, L.B.; Merenlender, A.; Reynolds, M. Vinecology: Pairing wine with nature. Conserv. Lett. 2013, 6, 287–299. [Google Scholar] [CrossRef]
- Cerdan, O.; Govers, G.; Le Bissonnais, Y.; Van Oost, K.; Poesen, J.; Saby, N.; Gobin, A.; Vacca, A.; Quinton, J.; Auerswald, K.; et al. Rates and spatial variations of soil erosion in Europe: A study based on erosion plot data. Geomorphology 2010, 122, 167–177. [Google Scholar] [CrossRef]
- Biddoccu, M.; Ferraris, S.; Opsi, F.; Cavallo, E. Long-term monitoring of soil management effects on runoff and soil erosion in sloping vineyards in Alto Monferrato (North–West Italy). Soil Till. Res. 2016, 155, 176–189. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Litskas, V.; Mandoulaki, A.; Antoniou, D.; Boyias, T.; Stavrinides, M.; Tzortzakis, N. Drought stress and soil management practices in grapevines in Cyprus under the threat of climate change. J. Water Clim. Chang. 2018, 9, 703–714. [Google Scholar] [CrossRef]
- Wolfert, S.; Ge, L.; Verdouw, C.; Bogaardt, M.J. Big data in smart farming—A review. Agric. Syst. 2017, 153, 69–80. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Guerry, A.D.; Polasky, S.; Lubchenco, J.; Chaplin-Kramer, R.; Daily, G.C.; Griffin, R.; Ruckelshaus, M.; Bateman, I.J.; Duraiappah, A.; Elmqvist, T.; et al. Natural capital and ecosystem services informing decisions: From promise to practice. Proc. Natl. Acad. Sci. USA 2015, 112, 7348–7355. [Google Scholar] [CrossRef]
- Chen, H.; Ma, K.; Lu, C.; Fu, Q.; Qiu, Y.; Zhao, J.; Huang, Y.; Yang, Y.; Schadt, C.W.; Chen, H. Functional redundancy in soil microbial community based on metagenomics across the globe. Front. Microbiol. 2022, 13, 878978. [Google Scholar] [CrossRef]
- Grosskopf, T.; Soyer, O.S. Synthetic microbial communities. Curr. Opin. Microbiol. 2014, 18, 72–77. [Google Scholar] [CrossRef]
- Gonçalves, O.S.; Creevey, C.J.; Santana, M.F. Designing a synthetic microbial community through genome metabolic modeling to enhance plant-microbe interaction. Environ. Microbiome 2023, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Suman, A.; Govindasamy, V.; Ramakrishnan, B.; Aswini, K.; SaiPrasad, J.; Sharma, P.; Pathak, D.; Annapurna, K. Microbial Community and Function-Based Synthetic Bioinoculants: A Perspective for Sustainable Agriculture. Front. Microbiol. 2022, 12, 805498. [Google Scholar] [CrossRef] [PubMed]
- Carlström, C.I.; Field, C.M.; Bortfeld-Miller, M.; Müller, B.; Sunagawa, S.; Vorholt, J.A. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nat. Ecol. Evol. 2019, 3, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, T.; Devers-Lamrani, M.; Spor, A.; Blouin, M. Community diversity determines the evolution of synthetic bacterial communities under artificial selection. Evolution 2022, 76, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.S.K.; Pretorius, I.S. Synthetic biology for the engineering of complex wine yeast communities. Nat. Food 2022, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Dixon, T.A.; Walker, R.S.K.; Pretorius, I.S. Visioning synthetic futures for yeast research within the context of current global technolopolitical trends. Yeast 2023, 40, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a key player in sustainable agriculture and human health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Traeger, C.P. On option values in environmental and resource economics. Resour. Energy Econ. 2014, 37, 242–252. [Google Scholar] [CrossRef]
- Pascual, U.; Termansen, M.; Hedlund, K.; Brussaard, L.; Faber, J.H.; Foudi, S.; Lemanceau, P.; Jørgensen, S.L. On the value of soil biodiversity and ecosystem services. Ecosyst. Serv. 2015, 15, 11–18. [Google Scholar] [CrossRef]
- Baumgärtner, S.; Strunz, S. The economic insurance value of ecosystem resilience. Ecol. Econ. 2014, 101, 21–32. [Google Scholar] [CrossRef]
- Dallimer, M.; Martin-Ortega, J.; Rendon, O.; Afionis, S.; Bark, R.; Gordon, I.J.; Paavola, J. Taking stock of the empirical evidence on the insurance value of ecosystems. Ecol. Econ. 2020, 167, 106451. [Google Scholar] [CrossRef]
- Paul, C.; Hanley, N.; Meyer, S.T.; Fürst, C.; Weisser, W.W.; Knoke, T. On the functional relationship between biodiversity and economic value. Sci. Adv. 2020, 6, 7712. [Google Scholar] [CrossRef] [PubMed]
- Primmer, E.; Paavola, J. Insurance value of ecosystems: An introduction. Ecol. Econ. 2021, 184, 107001. [Google Scholar] [CrossRef]
- Baumgärtner, S. The insurance value of biodiversity in the provision of ecosystem services. Nat. Resour. Model. 2007, 20, 87–127. [Google Scholar] [CrossRef]
- Colwell, R.R. Microbial diversity: The importance of exploration and conservation. J. Ind. Microbiol. Biotechnol. 1997, 18, 302–307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).