The Physiological Mechanism of Melatonin Enhancing the Tolerance of Oat Seedlings under Saline–Alkali Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Solution Preparation
2.3. Cultivating Experimental Materials
2.4. MT Treatment
2.4.1. Agronomic Characteristics
2.4.2. Chlorophyll Content
2.4.3. Photosynthetic Parameters
2.4.4. Leaf ABA, GA3, IAA, and TAM Contents
2.4.5. Mineral Elements
2.5. Data Visualization and Statistical Analysis
3. Results
3.1. Effect of MT Treatment on Agronomic Traits of Oat Seedlings under Saline–Alkali Stress
3.2. Effect of MT Treatment on the Photosynthetic Physiology of Oat Seedlings under Saline–Alkali Stress
3.3. Effect of MT Treatment on the Endogenous Hormone Content of Oat Seedling Leaves under Saline–Alkali Stress
3.4. Effect of MT Treatment on the Mineral Element Content of Oat Seedlings under Saline–Alkali Stress
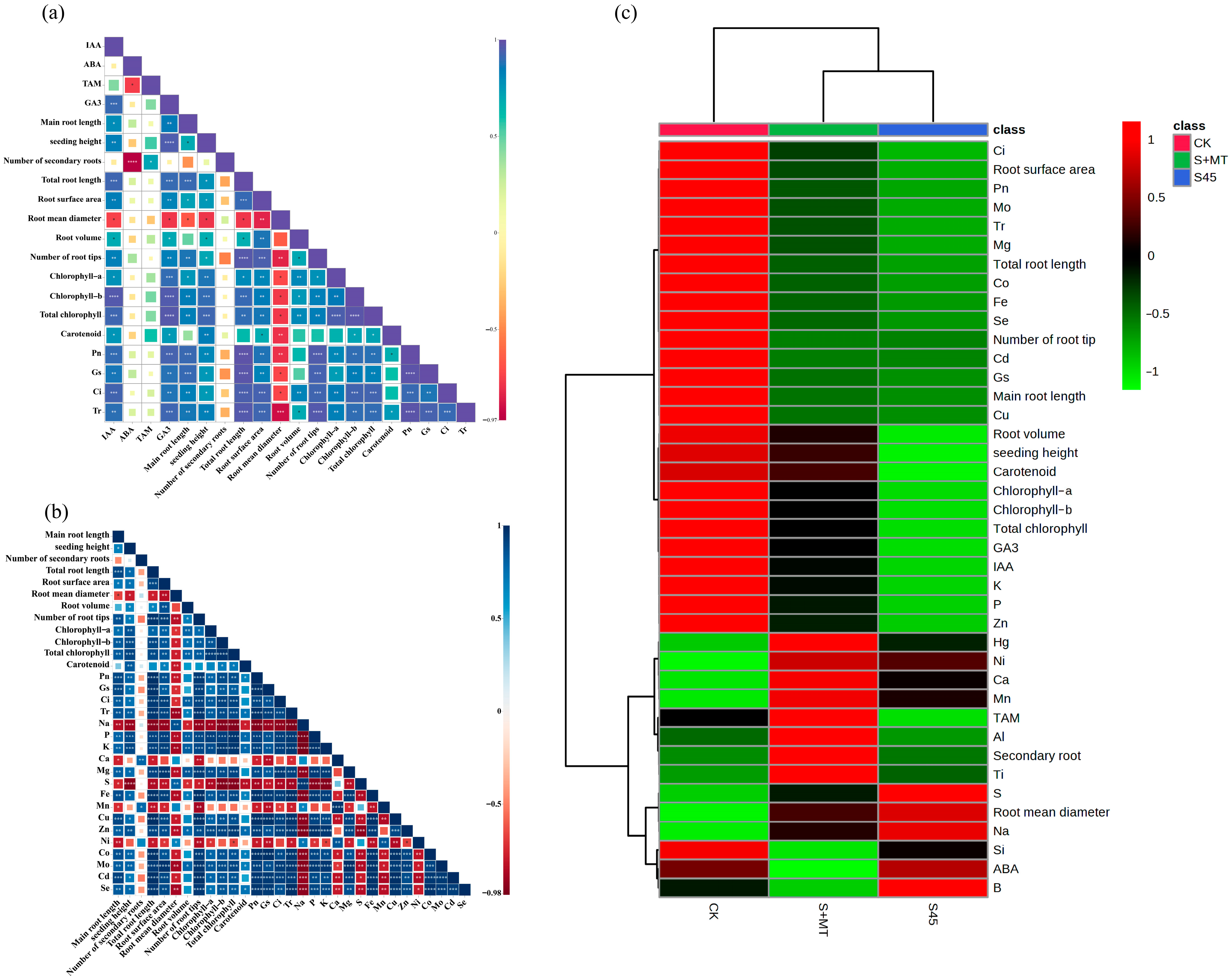
3.5. Correlation Analysis and Hierarchical Clustering Analysis of MT Treatment on Agronomic and Physiological Indicators of Oat Seedlings under Saline–Alkali Stress
4. Discussion
4.1. MT Treatment Effect on Phenotypic Characteristics
4.2. MT Treatment Effects on Photosynthetic Physiology
4.3. Effect on Endogenous Hormones
4.4. Effect on Mineral Element Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Takano, T.; Liu, S. Screening and evaluation of saline–alkaline tolerant germplasm of rice (Oryza sativa L.) in soda saline–alkali soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.; Ling, N.; Zhu, C.; Chi, F.; Li, W.; Hao, X.; Zhang, W.; Bian, J.; Chen, L.; et al. Exploring Soil Factors Determining Composition and Structure of the Bacterial Communities in Saline-Alkali Soils of Songnen Plain. Front. Microbiol. 2020, 10, 2902. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Mishra, B.; Gupta, S. Effects of soil salinity and alkalinity on grain quality of tolerant, semi-tolerant and sensitive rice genotypes. Rice Sci. 2013, 20, 284–291. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2020, 15, e0244365. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Yang, K. Klebsiella variicola improves the antioxidant ability of maize seedlings under saline-alkali stress. PeerJ 2021, 9, e11963. [Google Scholar] [CrossRef]
- Yang, L.; You, J.; Li, J.; Wang, Y.; Chan, Z. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611. [Google Scholar] [CrossRef]
- Sun, J.; He, L.; Li, T. Response of seedling growth and physiology of Sorghum bicolor (L.) Moench to saline-alkali stress. PLoS ONE 2019, 14, e0220340. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, G.; Chang, Y.; Lin, D.; Reiter, R.J.; He, C.; Shi, H. Melatonin biosynthesis enzymes recruit WRKY transcription factors to regulate melatonin accumulation and transcriptional activity on W-box in cassava. J. Pineal. Res. 2018, 65, e12487. [Google Scholar] [CrossRef]
- Khan, M.S.S.; Ahmed, S.; Ikram, A.U.; Hannan, F.; Yasin, M.U.; Wang, J.; Zhao, B.; Islam, F.; Chen, J. Phytomelatonin: A key regulator of redox and phytohormones signaling against biotic/abiotic stresses. Redox Biol. 2023, 64, 102805. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 1, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Seybold, H.; Trempel, F.; Ranf, S.; Scheel, D.; Romeis, T.; Lee, J. Ca2+ signalling in plant immune response: From pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol. 2014, 204, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Silalert, P.; Pattanagul, W. Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12417. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Ren, C.; Zhan, C.; Wang, C.; Li, J.; Ren, Q.; Liang, X.; Wei, L.; Xiang, D.; et al. Physiological and Biochemical Mechanisms of Exogenous Melatonin Regulation of Saline–Alkali Tolerance in Oats. Agronomy 2023, 13, 1327. [Google Scholar] [CrossRef]
- Qi, X.Y.; Wang, W.L.; Hu, S.Q.; Liu, M.Y.; Zheng, C.S.; Sun, X.Z. Effects of exogenous melatonin on photosynthesis and physiological characteristics of chrysanthemum seedlings under high temperature stress. Ying Yong Sheng Tai Xue Bao 2021, 32, 2496–2504. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal. Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Xu, B.; Li, J.; Huang, B. Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Front. Plant Sci. 2016, 7, 1500. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal. Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behavior in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 2018, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Nat. Res. Centre. 2020, 44, 18. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Chen, L.; Ding, C.; Tang, S.; Jiang, Y.; Liu, Z.; Li, G. Melatonin enhances Na+/K+ homeostasis in rice seedlings under salt stress through increasing the root H+-pump activity and Na+/K+ transporters sensitivity to ROS/RNS. Environ. Exp. Bot. 2021, 182, 104328. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Łabanowska, M.; Kurdziel, M.; Filek, M.; Wesełucha-Birczyńska, A. The impact of biochemical composition and nature of paramagnetic species in grains on stress tolerance of oat cultivars. J. Plant. Physiol. 2016, 199, 52–66. [Google Scholar] [CrossRef]
- Han, L.; Ma, F.; Liu, J.; Yu, S.; Liu, H.; Tan, L. Analysis of oat-straw salt ion accumulation and the potential for improving saline-alkali soils in coastal Hebei Province. Chin. J. Eco-Agric. 2012, 20, 1706–1712. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Chen, K.; Li, G. Spectrophotometric determination of chlorophylls in different solvents related to the leaf traits of the main tree species in Northeast China. IOP Conf. Ser. Earth Environ. Sci. 2021, 836, 012008. [Google Scholar] [CrossRef]
- Kraska, J.E.; Breitenbeck, G.A. Simple, robust method for quantifying silicon in plant tissue. Commun. Soil Sci. Plant Anal. 2010, 41, 2075–2085. [Google Scholar] [CrossRef]
- Lu, X.; Min, W.; Shi, Y.; Tian, L.; Li, P.; Ma, T.; Zhang, Y.; Luo, C. Exogenous melatonin alleviates alkaline stress by removing reactive oxygen species and promoting antioxidant defence in rice seedlings. Front. Plant Sci. 2022, 13, 849553. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Yu, J.; Lyv, J.; Zhang, J.; Ding, D.; Li, N.; Zhang, J.; Bakpa, E.P.; Yang, Y.; et al. Melatonin enhanced low-temperature combined with low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating root growth, antioxidant defense system, and osmotic adjustment. Front. Plant Sci. 2022, 13, 998293. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Min, W.; Akhtar, M.; Lu, X.; Bai, X.; Zhang, Y.; Tian, L.; Li, P. Melatonin enhances drought tolerance in rice seedlings by modulating antioxidant systems, osmoregulation, and corresponding gene expression. Int. J. Mol. Sci. 2022, 23, 12075. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin enhances seed germination and seedling growth of Medicago sativa under salinity via a putative melatonin receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, A.; Yu, H.; Li, W.; Liang, C.; Guo, S.; Zhang, R.; Chu, C. Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial effects of exogenous melatonin on overcoming salt stress in sugar beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, F.; Liu, Z.; Li, Y.; Di, X.; Wang, J.; Lin, J. Uniform water potential induced by salt, alkali, and drought stresses has different impacts on the seedling of Hordeum jubatum: From growth, photosynthesis, and chlorophyll fluorescence. Front. Plant Sci. 2021, 12, 733236. [Google Scholar] [CrossRef]
- Wang, K.; He, J.; Gao, Y.; Han, K.; Liu, J.; Wang, Y. Exogenous melatonin improved the growth and development of naked oat seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2022, 29, 88109–88118. [Google Scholar] [CrossRef]
- Wu, P.; Lyu, J.; Yu, J.H.; Liu, N.; Li, J.W.; Jin, L.; Jin, N.; Wang, S.Y. Effects of melatonin on photosynthetic properties and osmoregulatory substance contents of cucumber seedlings under salt-alkali stress. Ying Yong Sheng Tai Xue Bao 2022, 33, 1901–1910. [Google Scholar] [CrossRef]
- Wu, P.; Ma, Y.; Ahammed, G.J.; Hao, B.; Chen, J.; Wan, W.; Zhao, Y.; Cui, H.; Xu, W.; Cui, J.; et al. Insights into melatonin-induced photosynthetic electron transport under low-temperature stress in cucumber. Front. Plant Sci. 2022, 13, 1029854. [Google Scholar] [CrossRef]
- Shumskaya, M.; Wurtzel, E.T. The carotenoid biosynthetic pathway: Thinking in all dimensions. Plant Sci. 2013, 208, 58–63. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, J.; Wang, Y.; Kang, H.; Zeng, J. The tolerance to saline–alkaline stress was dependent on the roots in wheat. Physiol. Mol. Biol. Plants 2020, 26, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Hu, Q.; Zhao, Y.; Lin, X.; Zhang, J.; Zhang, J. Enhancing quinoa growth under severe saline-alkali stress by phosphate solubilizing microorganism Penicillium funicuiosum P1. PLoS ONE 2022, 17, e0273459. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Song, R.; Shao, H.; Song, F.; Xu, H.; Lu, Y. Silicon improves maize photosynthesis in saline-alkaline soils. Sci. World J. 2015, 2015, 245072. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Wang, J.; Qin, H.; Zhou, S.; Wei, P.; Zhang, H.; Zhou, Y.; Miao, Y.; Huang, R. The Ubiquitin-Binding Protein OsDSK2a Mediates Seedling Growth and Salt Responses by Regulating Gibberellin Metabolism in Rice. Plant Cell 2020, 32, 414–428. [Google Scholar] [CrossRef]
- Kumar, M.; Kesawat, M.S.; Ali, A.; Lee, S.C.; Gill, S.S.; Kim, A.H.U. Integration of Abscisic Acid Signaling with Other Signaling Pathways in Plant Stress Responses and Development. Plants 2019, 8, 592. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, C.; Xu, L.; Niu, H.; Liu, Q.; Huang, Y.; Lv, G.; Yang, H.; Li, M. Melatonin and indole-3-acetic acid synergistically regulate plant growth and stress resistance. Cells 2022, 11, 3250. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, S.; Lin, D.; Wei, Y.; Yan, Y.; Liu, G.; Reiter, R.J.; Chan, Z. Zinc finger of Arabidopsis thaliana 6 is involved in melatonin-mediated auxin signaling through interacting INDETERMINATE DOMAIN15 and INDOLE-3-ACETIC ACID 17. J. Pineal Res. 2018, 65, e12494. [Google Scholar] [CrossRef]
- Munns, R. Approaches to identifying genes for salinity tolerance and the importance of timescale. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 25–38. [Google Scholar]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl– ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef]
- Wu, D.; Shen, Q.; Cai, S.; Chen, Z.H.; Dai, F.; Zhang, G. Ionomic responses and correlations between elements and metabolites under salt stress in wild and cultivated barley. Plant Cell Physiol. 2013, 54, 1976–1988. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil 2012, 352, 353–362. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Syeed, S.; Khan, N.A. Understanding the significance of sulfur in improving salinity tolerance in plants. Environ. Exp. Bot. 2011, 70, 80–87. [Google Scholar] [CrossRef]
- Pandya, D.H.; Mer, R.K.; Prajith, P.K.; Pandey, A.N. Effect of salt stress and manganese supply on growth of barley seedlings. J. Plant Nutr. 2004, 27, 1361–1379. [Google Scholar] [CrossRef]
- Yousfi, S.; Wissal, M.S.; Mahmoudi, H.; Abdelly, C.; Gharsalli, M. Effect of salt on physiological responses of barley to iron deficiency. Plant Physiol. Biochem. 2007, 45, 309–314. [Google Scholar] [CrossRef]
- Li, C.; Liang, B.; Chang, C.; Wei, Z.; Zhou, S.; Ma, F. Exogenous melatonin improved potassium content in Malus under different stress conditions. J. Pineal Res. 2016, 61, 218–229. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Ji, J.; He, X.; Zhang, J.; Shang, Y.; Liu, H.; Xu, J.; Liang, B. Ionomic combined with transcriptomic and metabolomic analyses to explore the mechanism underlying the effect of melatonin in relieving nutrient stress in apple. Int. J. Mol. Sci. 2022, 23, 9855. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant. 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Jiang, C.; Cui, Q.; Feng, K.; Xu, D.; Li, C.; Zheng, Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 82. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Guo, H.; Huang, Z.; Li, M.; Hou, Z. Growth, ionic homeostasis, and physiological responses of cotton under different salt and alkali stresses. Sci. Rep. 2020, 10, 21844. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liang, X.; Xiang, D.; Xu, W.; Wang, C.; Zhan, C.; Ren, C.; Wei, L.; Zhang, S.; Zhang, L.; et al. The Physiological Mechanism of Melatonin Enhancing the Tolerance of Oat Seedlings under Saline–Alkali Stress. Agronomy 2023, 13, 2343. https://doi.org/10.3390/agronomy13092343
Wang Q, Liang X, Xiang D, Xu W, Wang C, Zhan C, Ren C, Wei L, Zhang S, Zhang L, et al. The Physiological Mechanism of Melatonin Enhancing the Tolerance of Oat Seedlings under Saline–Alkali Stress. Agronomy. 2023; 13(9):2343. https://doi.org/10.3390/agronomy13092343
Chicago/Turabian StyleWang, Qiang, Xiaotian Liang, Dabing Xiang, Weiwei Xu, Chunlong Wang, Chao Zhan, Changzhong Ren, Liming Wei, Shuqiao Zhang, Li Zhang, and et al. 2023. "The Physiological Mechanism of Melatonin Enhancing the Tolerance of Oat Seedlings under Saline–Alkali Stress" Agronomy 13, no. 9: 2343. https://doi.org/10.3390/agronomy13092343
APA StyleWang, Q., Liang, X., Xiang, D., Xu, W., Wang, C., Zhan, C., Ren, C., Wei, L., Zhang, S., Zhang, L., Wang, J., & Guo, L. (2023). The Physiological Mechanism of Melatonin Enhancing the Tolerance of Oat Seedlings under Saline–Alkali Stress. Agronomy, 13(9), 2343. https://doi.org/10.3390/agronomy13092343





