Magnetic Seed Treatment Modulates Phenolic and Fatty Acid Metabolism of Sunflower under Water Scarcity
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Source and Selection of Levels
2.2. Experimental Plan
2.3. Estimation of Photosynthetic Pigments
2.4. Determination of Total Phenolics, Flavonoids, and Total Protein Contents
2.5. Estimation of Reducing Sugar Contents and Total Soluble Sugars
2.6. Determination of Total Anthocyanin Content
2.7. Determination of % Oil Content
2.8. Estimation of Nutritional Value and Metabolizable Energy
2.9. Determination of Growth and Yield Attributes
2.10. Quantification of Free Proline
2.11. Determination of Catalase (CAT) Activity
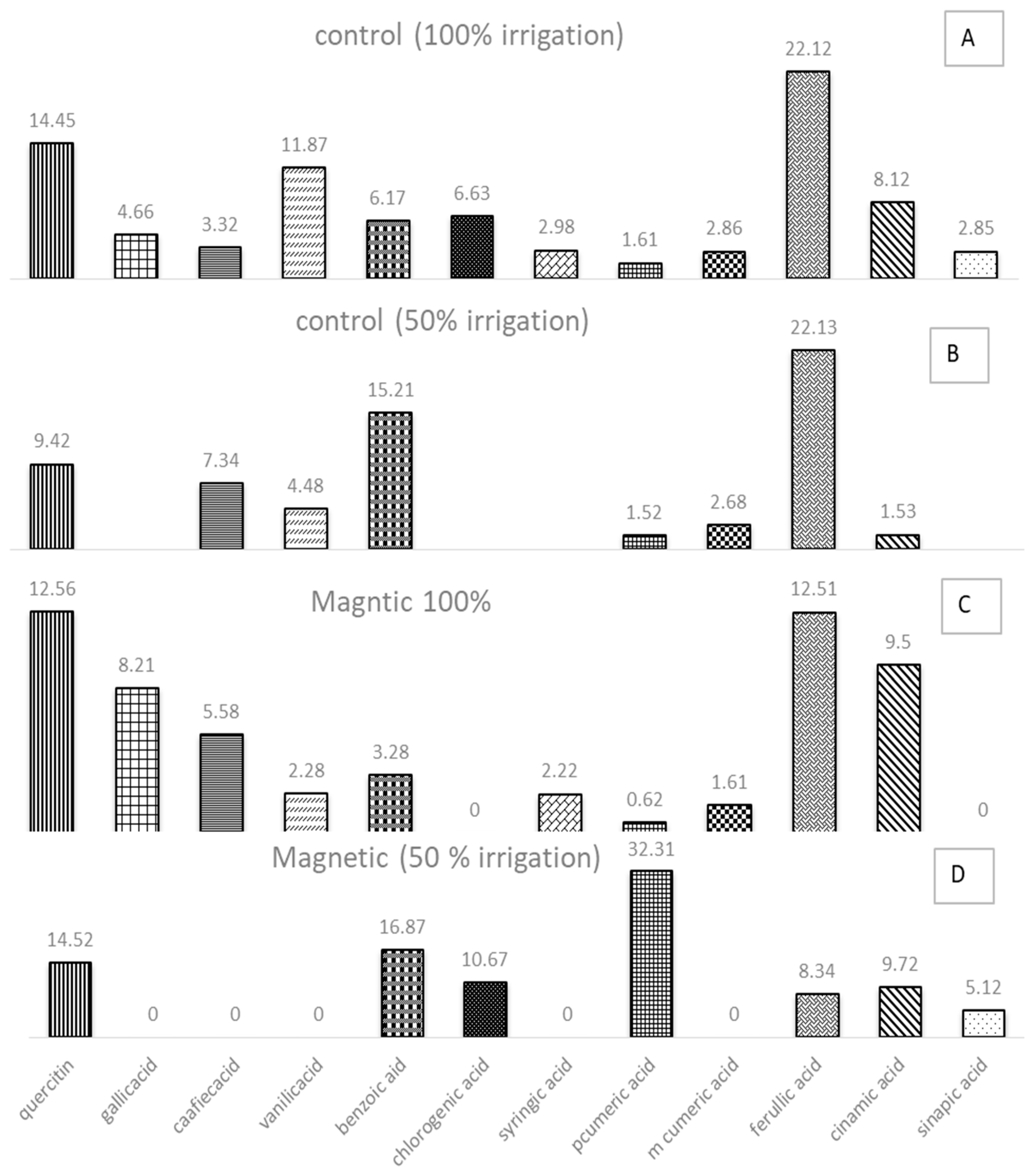
2.12. HPLC Based Phenolic Profiling
2.13. GC-Based Fatty Acid Profiling
2.14. Statistical Analysis
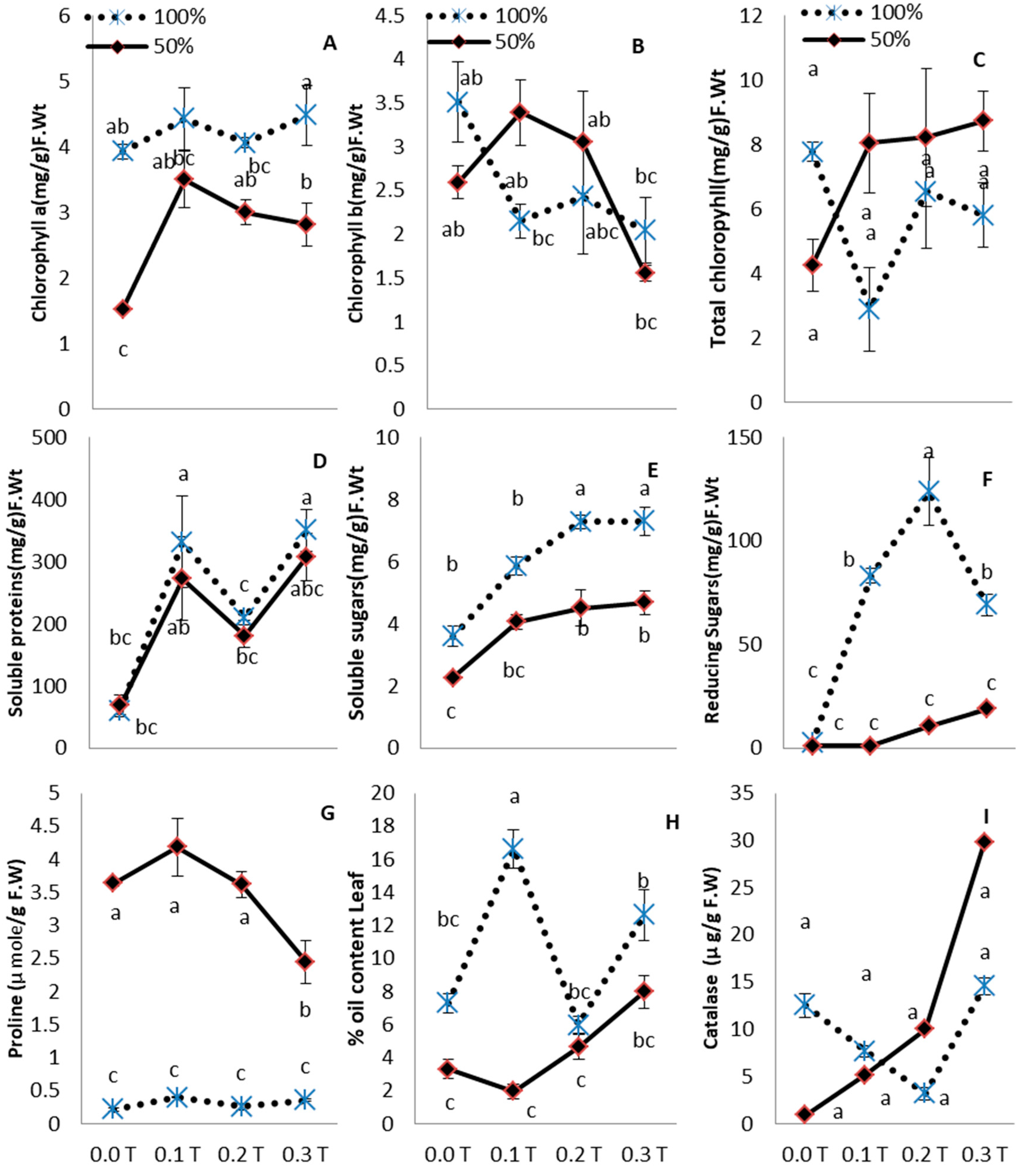
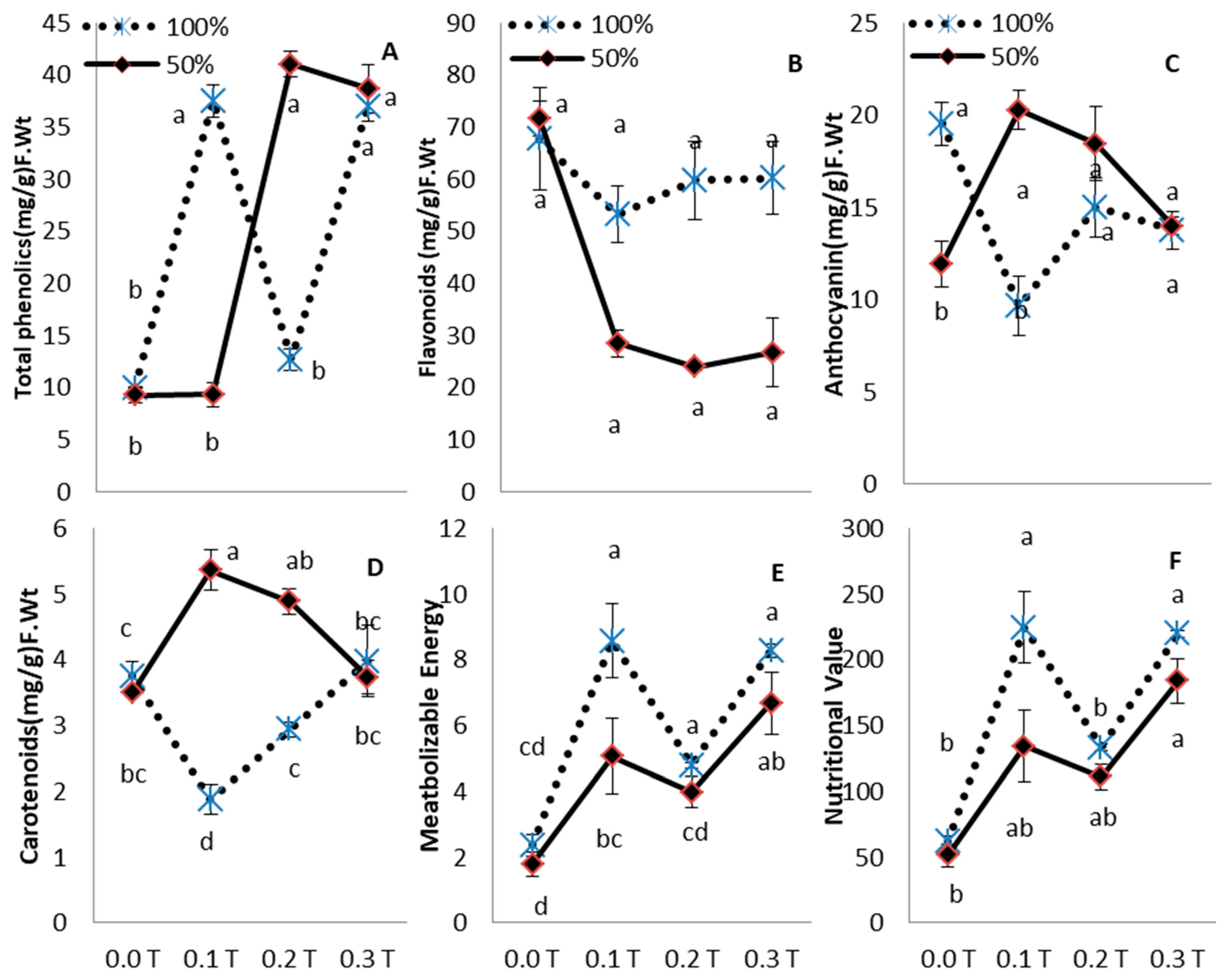
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havrysh, V.; Kalinichenko, A.; Mentel, G.; Mentel, U.; Vasbieva, D.G. Husk energy supply systems for sunflower oil mills. Energies 2020, 13, 361. [Google Scholar] [CrossRef]
- Khan, M.Y.; Ahmad-II, N.; Khan, M.N.; Padhi, S.K. Characterization of Used Sunflower Oil (USO) for utilization as CI engine fuel. In Proceedings of the All India Seminar on Catalysing Vision, New Delhi, India, 12 February 2020; pp. 6–7. [Google Scholar]
- Stahle, D.W. Anthropogenic mega drought. Science 2020, 368, 238–239. [Google Scholar] [CrossRef]
- Kaya, C.; Şenbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef] [PubMed]
- Kosar, F.; Akram, N.A.; Ashraf, M.; Ahmad, A.; Alyemeni, M.N.; Ahmad, P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant 2020, 172, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Ö.; Nalçaiyi, A.B.; Erdal, Ş.Ç.; Pekcan, V.; Kaya, Y.; Çiçek, N.U.N.; Ekmekçi, Y.İ.N. Analysis of drought response of sunflower inbred lines by chlorophyll a fluorescence induction kinetics. Photosynthetica 2020, 58, 163–172. [Google Scholar] [CrossRef]
- Escalante, M.; Vigliocco, A.; Moschen, S.; Fernández, P.; Heinz, R.; Garcia-Garcia, F.; Alemano, S. Transcriptomic analysis reveals a differential gene expression profile between two sunflower inbred lines with different ability to tolerate water stress. Plant Mol. Biol. Rep. 2020, 38, 222–237. [Google Scholar] [CrossRef]
- Sharma, R.; Pandey, S.T.; Verma, O.; Srivastava, R.C.; Guru, S.K. Physiological seedling vigour parameters of wheat as influenced by different seed invigoration techniques. Int. J. Chem. Stud. 2020, 8, 1549–1552. [Google Scholar] [CrossRef]
- Kataria, S.; Rastogi, A.; Bele, A.; Jain, M. Role of nitric oxide and reactive oxygen species in static magnetic field pre-treatment induced tolerance to ambient UV-B stress in soybean. Physiol. Mol. Biol. Plants 2020, 26, 931–945. [Google Scholar] [CrossRef]
- Hammad, M.; Albaqami, M.; Pooam, M.; Kernevez, E.; Witczak, J.; Ritz, T.; Ahmad, M. Cryptochrome mediated magnetic sensitivity in arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem. Photobiol. Sci. 2020, 19, 341–352. [Google Scholar] [CrossRef]
- Tissot, N.; Ulm, R. Cryptochrome-mediated blue-light signalling modulates UVR8 photoreceptor activity and contributes to UV-B tolerance in Arabidopsis. Nat. Commun. 2020, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Turker, M.; Temirci, C.; Battal, P.; Erez, M. The effects of an artificial and static magnetic field on plant growth, chlorophyll and phytohormone levels in maize and sunflower plants. Phyton Ann. Rei Bot. 2007, 46, 271–284. [Google Scholar]
- Mahajan, T.S.; Pandey, O.P. Effect of electric and magnetic treatments on germination of bitter gourd (Momordica charantia) seed. Int. J. Agric. Biol. 2015, 17, 351–356. [Google Scholar]
- Davies, B.H.; Goodwin, T.W. Chemistry and biochemistry of plant pigments. Carotenoids 1976, 2, 38–165. [Google Scholar]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Marinova, D.; Atanassova, M.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technnol. Metall. 2005, 40, 255–260. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Estimation of reducing sugar by dinitrosalicylic acid method. Anal. Chem. 1972, 31, 426–428. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Laboratory manual for physiological studies of rice. Int. Rice Res. Philipp. 1976, 23, 61–66. [Google Scholar]
- Nozzolillo, C. Anthocyanin pigments in pea seedlings: Genetically controlled and environmentally influenced. Can. J. Bot. 1978, 56, 2890–2897. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Indrayan, A.K.; Sharma, S.; Durgapal, D.; Kumar, N.; Kumar, M. Determination of nutritive value and analysis of mineral elements for some medicinally valued plants from Uttaranchal. Curr. Sci. 2005, 89, 1252–1255. [Google Scholar]
- Macrae, J.; Smith, J.; Dewey, P.; Brewer, A.; Brown, D.S.; Walker, A. The efficiency of utilization of metabolizable energy and apparent absorption of amino acids in sheep given spring-and autumn-harvested dried grass. Br. J. Nutr. 1985, 54, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Paquot, C. Standard Methods for the Analysis of Oils, Fats and Derivatives; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hussain, M.S.; Dastgeer, G.; Afzal, A.M.; Hussain, S.; Kanwar, R.R. Eco-friendly magnetic field treatment to enhance wheat yield and seed germination growth. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100299. [Google Scholar] [CrossRef]
- Dziwulska-Hunek, A.; Kornarzyńska-Gregorowicz, A.; Niemczynowicz, A.; Matwijczuk, A. Influence of electromagnetic stimulation of seeds on the photosynthetic indicators in Medicago sativa L. leaves at various stages of development. Agronomy 2020, 10, 594. [Google Scholar] [CrossRef]
- Abdollahi, F.; Amiri, H.; Niknam, V.; Ghanati, F.; Mahdigholi, K. Effects of static magnetic fields on the antioxidant system of almond seeds. Russ. J. Plant Physiol. 2019, 66, 299–307. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, W.; Hu, X.; Liu, M.; Xu, X.; Hu, F.; Huang, J. Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci. Rep. 2019, 9, 14384. [Google Scholar] [CrossRef]
- Krishnan, V.; Gothwal, S.; Dahuja, A.; Vinutha, T.; Singh, B.; Jolly, M.; Sachdev, A. Enhanced nutraceutical potential of gamma irradiated black soybean extracts. Food Chem. 2018, 245, 246–253. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Shomali, A.; Seifikalhor, M.; Lastochkina, O. Calcium Signaling in Plants under Drought. Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Piacentini, M.P.; Fraternale, D.; Piatti, E.; Ricci, D.; Vetrano, F.; Dachà, M.; Accorsi, A. Senescence delay and change of antioxidant enzyme levels in Cucumis sativus L. etiolated seedlings by ELF magnetic fields. Plant Sci. 2001, 161, 45–53. [Google Scholar] [CrossRef]
- Navazio, L.; Formentin, E.; Cendron, L.; Szabò, I. Chloroplast calcium signaling in the spotlight. Front. Plant Sci. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Momayyezi, M.; McKown, A.D.; Bell, S.C.; Guy, R.D. Emerging roles for carbonic anhydrase in mesophyll conductance and photosynthesis. Plant J. 2020, 101, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Ogée, J.; Wingate, L.; Genty, B. Estimating mesophyll conductance from measurements of C18OO photosynthetic discrimination and carbonic anhydrase activity. Plant Physiol. 2018, 178, 728–752. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Zhang, Z.; Liu, X.; Hsu, C.C.; Du, Y.; Wang, P. A RAF-SnRK2 kinase cascade mediates early osmotic stress signaling in higher plants. Nat. Commun. 2020, 11, 613. [Google Scholar] [CrossRef]
- Azri, W.; Cosette, P.; Guillou, C.; Rabhi, M.; Nasr, Z.; Mliki, A. Physiological and proteomic responses to drought stress in leaves of two wild grapevines (Vitis sylvestris): A comparative study. Plant Growth Regul. 2020, 91, 37–52. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zuo, Z.C.; Zhao, X.Y.; Li, X.; Klejnot, J.; Li, Y.; Lin, C.T. Blue-light-independent activity of Arabidopsis cryptochromes in the regulation of steady-state levels of protein and mRNA expression. Mol. Plant 2008, 1, 167–177. [Google Scholar] [CrossRef]
- Qu, X.; Wang, H.; Chen, M.; Liao, J.; Yuan, J.; Niu, G. Drought stress–induced physiological and metabolic changes in leaves of two oil tea cultivars. J. Am. Soc. Hortic. Sci. 2019, 144, 439–447. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Y.; Wu, H.; Bai, J. Drought stress-induced autophagy gene expression is correlated with carbohydrate concentrations in Caragana korshinskii. Protoplasma 2020, 257, 1211–1220. [Google Scholar] [CrossRef]
- Zareei, E.; Zaare-Nahandi, F.; Oustan, S.; Hajilou, J. Effects of magnetic solutions on some biochemical properties and production of some phenolic compounds in grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 253, 217–226. [Google Scholar] [CrossRef]
- Babeanu, C.; Dodocioiu, A.M.; Buzatu, D.G. Variation of chlorophylls and osmolytes content in three cultivars of wheat under drought stress. Ann. Univ. Craiova-Agric. Mont. Cadastre Ser. 2019, 49, 293–298. [Google Scholar]
- Prazeres, C.S.; Coelho, C.M.M. Osmolyte accumulation and antioxidant metabolism during germination of vigorous maize seeds subjected to water deficit. Acta Sci. Agron. 2020, 42, e42476. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Borisova-Mubarakshina, M.M.; Ignatova, L.K.; Fedorchuk, T.P.; Nadeeva-Zhurikova, E.M.; Ivanov, B.N. Role of plant carbonic anhydrases under stress conditions. Plant Stress Physiol. 2021, 4, 7–38. [Google Scholar]
- Ghonaim, M.M.; Mohamed, H.I.; Omran, A.A. Evaluation of wheat (Triticum aestivum L.) salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet. Resour. Crop Evol. 2021, 68, 227–242. [Google Scholar] [CrossRef]
- He, Y.; Xie, Y.; Li, X.; Yang, J. Drought tolerance of transgenic rice overexpressing maize C4-PEPC gene related to increased anthocyanin synthesis regulated by sucrose and calcium. Biol. Plant 2020, 64, 136–149. [Google Scholar] [CrossRef]
- Naderi, S.; Fakheri, B.A.; Maali-Amiri, R.; Mahdinezhad, N. Tolerance responses in wheat landrace Bolani are related to enhanced metabolic adjustments under drought stress. Plant Physiol. Biochem. 2020, 150, 244–253. [Google Scholar] [CrossRef]
- Christie, J.M.; Briggs, W.R. Blue light sensing in higher plants. J. Biol. Chem. 2001, 276, 11457–11460. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Ang, L.H.; Puente, P.; Deng, X.W.; Wei, N. Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 1998, 10, 673–683. [Google Scholar] [CrossRef]
- Harris, S.R.; Henbest, K.B.; Maeda, K.; Pannell, J.R.; Timmel, C.R.; Hore, P.J.; Okamoto, H. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 2009, 6, 1193–1205. [Google Scholar] [CrossRef]
- Hamidi, H.; Masoudian, N.; Ebadi, M.; Roudi, B.; Khajehzadeh, M.H. How nitric oxide down-regulates cryptochrome 1 from canola in blue but not red light, and reduces hypocotyl dwarfism in lead and drought stress. Polish J. Environ. Stud. 2020, 29, 1161–1167. [Google Scholar] [CrossRef]
- Abe, M.; Itoyama, R.; Komiyama, Y.; Ito, T.; Mashimo, T.; Tojo, S. Quantitative investigation of the Zeeman and Paschen-Back effects of the hyperfine structure during the rubidium 5 2 S 1/2→5 2 D 5/2 two-photon transition. Phys. Rev A 2019, 99, 053420. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharby, H.F.; Uddin, M.N.; Ali, M.A.; Anwar, Y.; Fang, X.W.; Hajar, A.S. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol. J. Environ. Stud. 2020, 29, 1625–1636. [Google Scholar] [CrossRef]
- de Souza-Torres, A.; Sueiro-Pelegrín, L.; Zambrano-Reyes, M.; Macías-Socarras, I.; González-Posada, M.; García-Fernández, D. Extremely low frequency non-uniform magnetic fields induce changes in water relations, photosynthesis and tomato plant growth. Int. J. Radiat. Biol. 2020, 96, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef]
- Vanhercke, T.; Petrie, J.R.; El Tahchy, A.; Singh, S.P.; Liu, Q. Industrial Research Organization CSIRO, 2014. Processes for Producing Hydrocarbon Products. U.S. Patent 8,735,111, 27 May 2014. [Google Scholar]
- Mazrou, R.M.; El-Shafie, S.E.; Afify, M. Integrated effect of magnetized water and biofertilizers for enhancing the productivity of Majorana hortensis L. MJPPF 2022, 7, 31–41. [Google Scholar] [CrossRef]
- Zamani, S.; Naderi, M.R.; Soleymani, A.; Nasiri, B.M. Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol. Environ. 2020, 190, 110017. [Google Scholar] [CrossRef]
| Stress Level | Manetic Treatments | Leaf Area | Leaf Number | Leaf Fresh Weight | Leaf Dry Weight | Plant Height | Capitulum Weight | % Oil Yield | No. of Achenes/Capitulum | 50 Achenes Weight | Achene Weight/Plant |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100% Irrigation | Control | 242.3 ab | 19.0 ab | 2.00 c | 0.38 a | 99.66 ab | 129.3 a | 14.8 b | 533 b | 2.55 abc | 16.66 cd |
| 0.1 T | 272.6 a | 24.3 a | 3.18 bc | 0.47 a | 118.6 a | 85.3 b | 15.6 b | 296 b | 2.78 a | 43 a | |
| 0.2 T | 216.5 ab | 20.3 ab | 4.11 ab | 0.54 a | 105.3 ab | 110.3 ab | 17.8 ab | 142 e | 2.91 a | 28.33 b | |
| 0.3 T | 125.3 b | 18.3 ab | 2.89 bc | 0.39 a | 81.66 b | 104.3 ab | 16.5.ab | 118 e | 1.97 c | 20 c | |
| 50% Irrigation | Control | 139.7 b | 14.3 b | 3.32 bc | 0.54 a | 72.66 b | 036.0 c | 15.2 b | 194 de | 2.04 c | 11.00 de |
| 0.1 T | 156.3 b | 26 a | 2.13 c | 0.32 a | 88.00 b | 103.6 ab | 5.7 a | 583 b | 2.44 abc | 12.00 de | |
| 0.2 T | 167.8 a | 25.5 a | 4.76 a | 0.61 a | 97.66 b | 38 c | 9.6 c | 733 a | 1.91 c | 6.33 f | |
| 0.3 T | 243.0 ab | 25.6 a | 3.13 bc | 0.43 a | 84.33 b | 41 c | 20.4 d | 350 c | 2.19 abc | 6.66 ef |
| Irrigations | Seed Treatment | Palmitic Acid (%) | Stearic Acid (%) | Oleic Acid (%) | Linoleic Acid (%) | Sum | U/SRatio |
|---|---|---|---|---|---|---|---|
| 100% | Control | 8.01 | 4.11 | 36.51 | 50.76 | 99.39 | 46.957 |
| Magnetic | 8.9 | 4.11 | 36.69 | 50.86 | 100.56 | 46.51 | |
| 50% | Control | 8.41 | 6.58 | 31.82 | 44.02 | 90.83 | 43.634 |
| Magnetic | 6.13 | 2.98 | 75.28 | 12.57 | 96.96 | 80.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, B.; Nisa, Z.u.; Bashir, R.; Ali, N.; Mahmood, S.; Azeem, M.; Batool, F.; Wahid, A.; Iqbal, M.; Alsahli, A.A.; et al. Magnetic Seed Treatment Modulates Phenolic and Fatty Acid Metabolism of Sunflower under Water Scarcity. Agronomy 2023, 13, 2094. https://doi.org/10.3390/agronomy13082094
Afzal B, Nisa Zu, Bashir R, Ali N, Mahmood S, Azeem M, Batool F, Wahid A, Iqbal M, Alsahli AA, et al. Magnetic Seed Treatment Modulates Phenolic and Fatty Acid Metabolism of Sunflower under Water Scarcity. Agronomy. 2023; 13(8):2094. https://doi.org/10.3390/agronomy13082094
Chicago/Turabian StyleAfzal, Beenish, Zaib un Nisa, Rohina Bashir, Naila Ali, Saqib Mahmood, Muhammad Azeem, Fatima Batool, Abdul Wahid, Muhammad Iqbal, Abdulaziz Abdullah Alsahli, and et al. 2023. "Magnetic Seed Treatment Modulates Phenolic and Fatty Acid Metabolism of Sunflower under Water Scarcity" Agronomy 13, no. 8: 2094. https://doi.org/10.3390/agronomy13082094
APA StyleAfzal, B., Nisa, Z. u., Bashir, R., Ali, N., Mahmood, S., Azeem, M., Batool, F., Wahid, A., Iqbal, M., Alsahli, A. A., & Kaushik, P. (2023). Magnetic Seed Treatment Modulates Phenolic and Fatty Acid Metabolism of Sunflower under Water Scarcity. Agronomy, 13(8), 2094. https://doi.org/10.3390/agronomy13082094









