Potential Use of Wheat Straw, Grape Pomace, Olive Mill Wastewater and Cheese Whey in Mixed Formulations for Silage Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Silage Preparation
2.2. Chemical Analysis
Determination of Total Phenolic Content
2.3. Fermentation Characteristics
2.3.1. pH Determination
2.3.2. Flieg’s Point Calculation
2.3.3. Lactic and Volatile Fatty Acids
2.4. Statistical Analyses
3. Results and Discussion
3.1. Experiment 1—Chemical Composition
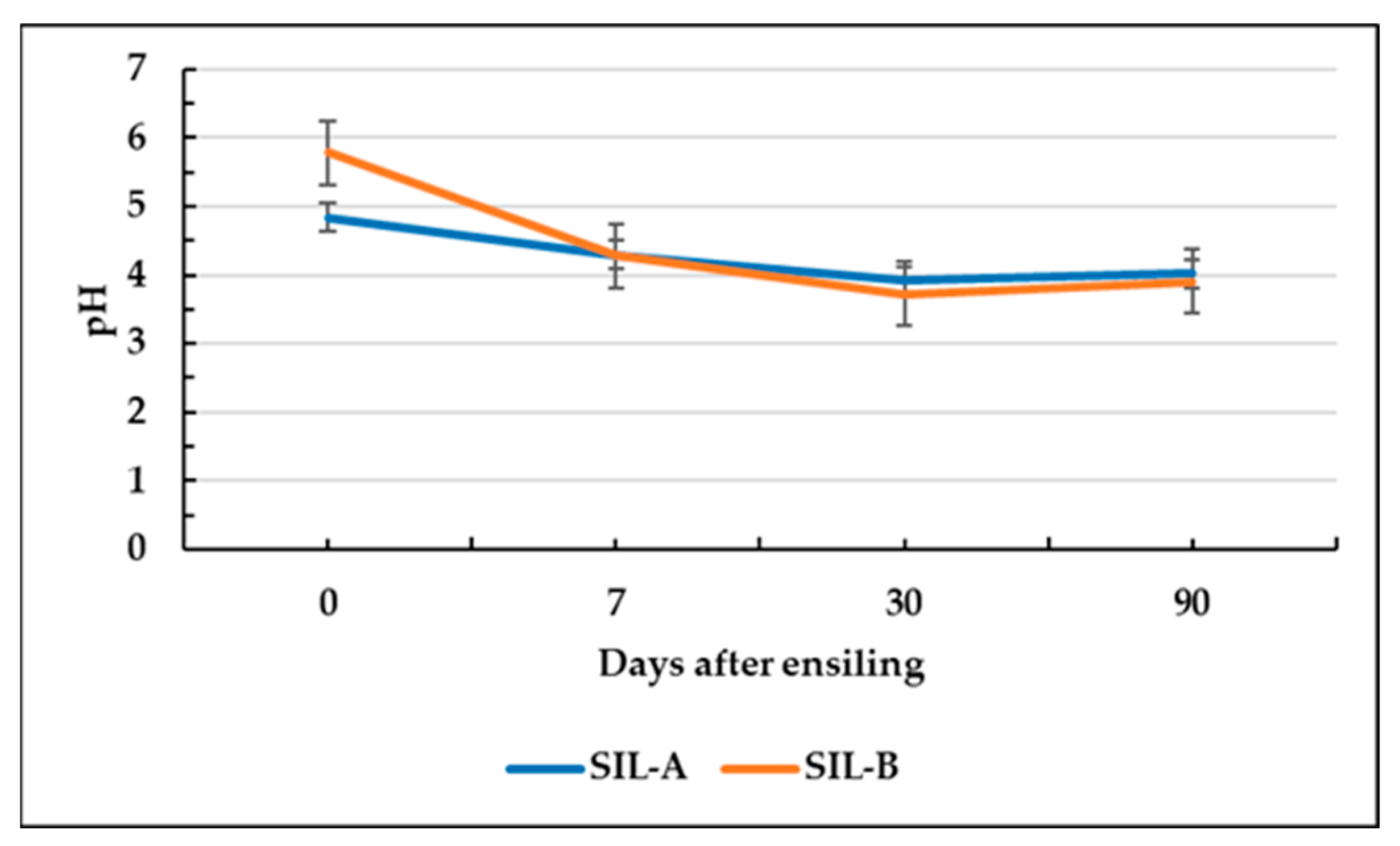
3.2. Fermentation Characteristics
3.3. Experiment 2—Chemical Composition
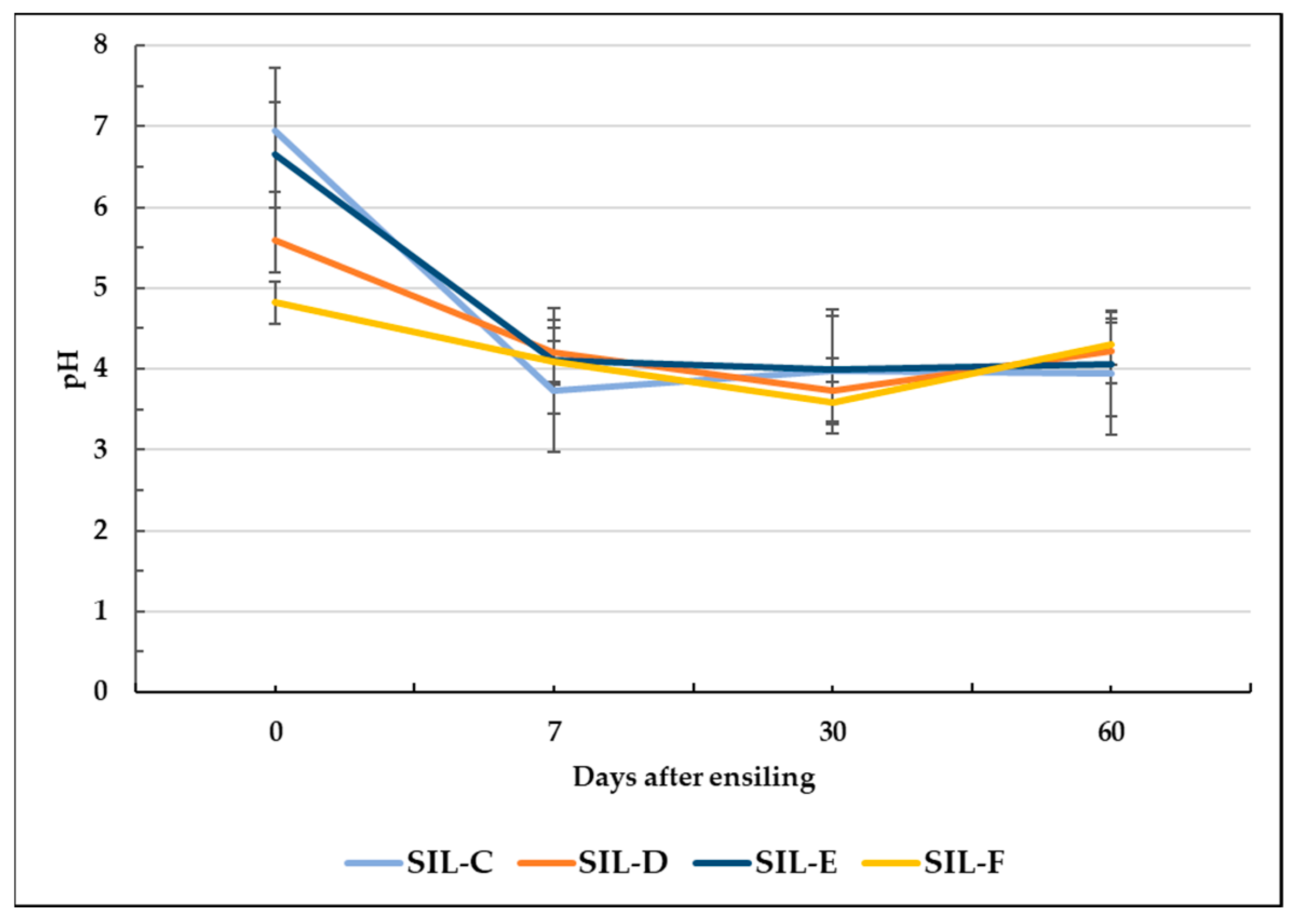
3.4. Fermentation Characteristics
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés- García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, s295–s309. [Google Scholar] [CrossRef]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of feeding plant by-products: A review of the implications for ruminant meat production. Anim. Feed Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Yang, K.; Qing, Y.; Yu, Q.; Tang, X.; Chen, G.; Fang, R.; Liu, H. By-Product feeds: Current understanding and future perspectives. Agriculture 2021, 11, 207. [Google Scholar] [CrossRef]
- Ominski, K.; McAllister, T.; Stanford, K.; Mengistu, G.; Kebebe, E.G.; Omonijo, F.; Cordeiro, M.; Legesse, G.; Wittenberg, K. Utilization of By-Products and Food Waste in Livestock Production Systems: A Canadian Perspective. Available online: https://academic.oup.com/af/article/11/2/55/6276828 (accessed on 25 August 2023).
- Zhang, Y.; Ghaly, A.E.; Li, B. Physical properties of wheat straw varieties cultivated under different climatic and soil conditions in three continents. Am. J. Eng. Appl. Sci. 2012, 5, 98–106. [Google Scholar]
- Kapellakis, I.E.; Tsagarakis, K.P.; Avramaki, C.; Angelakis, A.N. Olive mill wastewater management in river basins: A case study in Greece. Agric. Water Manag. 2006, 82, 354–370. [Google Scholar] [CrossRef]
- Comegna, A.; Coppola, A.; Dragonetti, G. Time domain reflectometry for dielectric characterization of olive mill wastewater contaminated soils. J. Agric. Eng. 2020, 51, 248–254. [Google Scholar] [CrossRef]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K. Olive mill wastewater treatment. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, V., Eds.; Springer: Boston, MA, USA, 2007; pp. 133–157. [Google Scholar]
- El Hassani, F.Z.; Fadile, A.; Faouzi, M.; Zinedine, A.; Merzouki, M.; Benlemlih, M. The long term effect of Olive Mill Wastewater (OMW) on organic matter humification in a semi-arid soil. Heliyon 2020, 6, e03181. [Google Scholar] [CrossRef]
- Khdair, A.; Abu-Rumman, G. Sustainable environmental management and valorization options for olive mill byproducts in the middle east and North Africa (MENA) region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Botella, C.; de Ory, I.; Webb, C.; Cantero, D.; Blandino, A. Hydrolytic enzyme production by Aspergillus awamorion grape pomace. Biochem. Eng. J. 2005, 26, 100–106. [Google Scholar] [CrossRef]
- Domingos, J.M.B.; Martinez, G.A.; Scoma, A.; Fraraccio, S.; Kerckhof, F.-M.; Boon, N.; Reis, M.A.M.; Fava, F.; Bertin, L. Effect of operational parameters in the continuous anaerobic fermentation of cheese whey on titers, yields, productivities and microbial community structure. ACS Sustain. Chem. Eng. 2016, 5, 1400–1407. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Casamassima, D.; Palazzo, M.; D’Alessandro, A.G.; Colella, G.E.; Vizzarri, F.; Corino, C. The effects of lemon verbena (Lippia citriodora) verbascoside on the productive performance, plasma oxidative status, and some blood metabolites in suckling lambs. J. Anim. Feed Sci. 2013, 22, 204–212. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.M.; Sancho, A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Zervas, G. The effect of dietary inclusion of olive tree leaves and grape marc on the content of conjugated linoleic acid and vaccenic acid in the milk of dairy sheep and goats. J. Dairy Res. 2008, 75, 270–278. [Google Scholar] [CrossRef]
- D’Alessandro, A.G.; Martemucci, G. As verbascoside supplementation affects metabolic profile, oxidative status, and quality of milk and meat: The studies on donkeys. In Research Aspects in Agriculture and Veterinary Science; BP International: London, UK, 2022; pp. 65–80. [Google Scholar]
- Berbel, J.; Posadillo, A. Opportunities for the bioeconomy of olive oil byproducts. Biomed. J. Sci. Tech. Res. 2018, 2, 2094–2096. [Google Scholar]
- Flores, D.R.M.; da Fonseca, P.A.F.; Schmitt, J.; Tonetto, C.J.; Rosado, A.G.J.; Hammerschmitt, R.K.; Facco, D.B.; Brunetto, G.; Nornberg, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on productive performance, carcass characteristics, and blood parameters. Liv. Sci. 2020, 240, 104169. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Petrotos, K.; Kokka, S.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with polyphenolic byproduct from olive mill waste water processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Makri, S.; Kafantaris, I.; Stagos, D.; Chamokeridou, T.; Petrotos, K.; Gerasopoulos, K.; Mpesios, A.; Goutzourelas, N.; Kokkas, S.; Goulas, P.; et al. Novel feed including bioactive compounds from winery wastes improved broilers’ redox status in blood and tissues of vital organs. Food Chem. Toxicol. 2017, 102, 24–31. [Google Scholar] [CrossRef]
- Bonos, E.; Skoufos, I.; Petrotos, K.; Giavasis, I.; Mitsagga, C.; Fotou, K.; Vasilopoulou, K.; Giannenas, I.; Gouva, E.; Tsinas, A.; et al. Innovative Use of Olive, Winery and CheeseWaste By-Products as Functional Ingredients in Broiler. Nutrition. Vet. Sci. 2022, 9, 290. [Google Scholar]
- Wang, J.; Wang, J.Q.; Bu, D.P.; Guo, W.J.; Song, Z.T.; Zhang, J.Y. Effect of storing total mixed rations anaerobically in bales on feed quality. Anim. Feed Sci. Technol. 2010, 161, 94–102. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Chen, Y.; Miron, D.; Raviv, Y.; Nahim, E.; Bloch, A.; Yosef, E.; Nikbahat, M.; Miron, J. Preservation of total mixed rations for dairy cows in bales wrapped with polyethylene stretch film—A commercial scale experiment. Anim. Feed Sci. Technol. 2011, 164, 125–129. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, Y.; Hirakubo, T.; Fukui, H.; Matsuyama, H. Fermentation characteristics and microorganism composition of total mixed ration silage with local food by-products in different seasons. Anim. Sci. J. 2011, 82, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, G.; Yuan, X.; Shimojo, M.; Yu, C.; Shao, T. Effect of applying molasses and propionic acid on fermentation quality and aerobic stability of total mixed ration silage prepared with whole-plant corn in Tibet. Asian-Australas. J. Anim. Sci. 2014, 27, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, J.O.; Danés, M.A.C.; Casagrande, D.R.; Bernardes, T.F. Total mixed ration silage containing elephant grass for small-scale dairy farms. Grass Forage Sci. 2018, 73, 717–726. [Google Scholar] [CrossRef]
- Bueno, A.V.I.; Lazzari, G.; Jobim, C.C.; Daniel, J.L.P. Ensiling total mixed ration for ruminants: A review. Agronomy 2020, 10, 879. [Google Scholar] [CrossRef]
- Han, Z.; Xu, G.; Wang, S.; Dai, T.; Dong, D.; Zong, C.; Shao, T. Antimicrobial effects of four chemical additives on fermentation quality, aerobic stability, and in vitro ruminal digestibility of total mixed ration silage prepared with local food by-products. Anim. Sci. J. 2022, 93, e13755. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, G.; Zhao, J.; Zhihao Dong, Z.; Junfeng Li, J.; Shao, T. Fermentation quality, aerobic stability and in vitro gas production kinetics and digestibility in total mixed ration silage treated with lactic acid bacteria inoculants and antimicrobial additives. It. J. Anim. Sci. 2023, 22, 430–441. [Google Scholar] [CrossRef]
- Petrotos, K.; Papaioannou, C.; Kokkas, S.; Gkoutsidis, P.; Skoufos, I.; Tzora, A.; Bonos, E.; Tsinas, A.; Giavasis, I.; Mitsagga, C. Optimization of the composition of a novel bioactive silage produced by mixing of ground maize grains with olive mill waste waters, grape pomace and feta cheese whey. AgriEngineering 2021, 3, 868–894. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kostsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos, K.; Stagos, D.; Mitsagga, C.; Giavasisi, I.; et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2017, 101, 108–121. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Journal—Association of Official Analytical Chemists: Arlington, VA, USA, 2002. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Ortoger, R.M.; Lamuela-Ravendo, R.M. Analysis of total phenols and others oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Meth. Enzymol. 1999, 299, 152–173. [Google Scholar]
- Dong, Z.; Yuan, X.; Wen, A.; Desta, S.T.; Shao, T. Effects of calcium propionateon the fermentation quality and aerobic stability of alfalfa silage. Asian-Australas. J. Anim. Sci. 2017, 30, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Madrid, J.; Martınez-Teruel, A.; Hernandez, F.; Megıas, M.D. A comparative study on the determination of lactic acid in silage juice by colorimetric, high-performance liquid chromatography and enzymatic methods. J. Sci. Food Agric. 1999, 79, 1722–1726. [Google Scholar] [CrossRef]
- Lashkari, S.; Taghizadeh, A.; Seifdavati, J.; Salem, A.Z.M. Qualitative characteristics, microbial populations and nutritive values of orange pulp ensiled with nitrogen supplementation. Slovak J. Anim. Sci. 2014, 47, 90–99. [Google Scholar]
- Monllor, P.; Romero, G.; Muelas, R.; Sandoval-Castro, C.A.; Sendra, E.; Ramón Díaz, J. Ensiling process in commercial bales of horticultural by-products from artichoke and broccoli. Animals 2020, 10, 831. [Google Scholar] [CrossRef]
- McDonald, P.; Hunderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Cambrian Printers Ltd.: Aberystwyth, Welsh, 1991. [Google Scholar]
- Santoso, B.; Hariadi, B.T.; Alimuddin Seseray, D.Y. Fermentation characteristics of rice crop residue-based silage created by epiphytic and commercial LAB. Media Peternak. 2012, 35, 60–66. [Google Scholar] [CrossRef][Green Version]
- Ward, R.T.; de Ondarza, M.B. Fermentation Analysis of Silage: Use and Interpretation; Cumberland Valley Analytical Services, Inc.: Hagerstown, MD, USA, 2008; Available online: https://www.foragelab.com/Media/Fermentation-Silage-NFMP-Oct-2008.pdf (accessed on 12 March 2022).
- Kung, L.J.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Wang, B.; Yu, Z.; Wu, Z. The effects of stage of maturity and lactic acid bacteria inoculants on the ensiling characteristics, aerobic stability and in vitro digestibility of whole-crop oat silages. Grassl. Sci. 2021, 67, 55–62. [Google Scholar] [CrossRef]
- Shinners, K.J.; Wepner, A.D.; Muck, R.E.; Weimer, P.J. Aerobic and anaerobic storage of single-pass, chopped corn stover. Bioenerg. Res. 2011, 4, 61–75. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Bovera, F.; Piccolo, G.; Infascelli, F. In vitro fermentation kinetics of carbohydrate fractions of fresh forage, silage and hay of Avena sativa. J. Sci. Food Agric. 2005, 85, 1838–1844. [Google Scholar] [CrossRef]
- Amer, S.; Seguin, P.; Mustafa, A.F. Effects of feeding sweet sorghum silage on milk production of lactating dairy cows. J. Dairy Sci. 2012, 95, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.J. Understanding the biology of silage preservation to maximize quality and protect the environment. In Proceedings of the California Alfalfa & Forage Symposium and Corn/Cereal Silage Conference, Visalia, CA, USA, 1–2 December 2010. [Google Scholar]
- Belém, C.D.S.; de Souza, A.M.; de Lima, P.R.; de Carvalho, F.A.L.; Ávila Queiroz, M.A.; Matiuzzi da Costa, M. Digestibility, fermentation and microbiological characteristics of Calotropis procera silage with different quantities of grape pomace. Ciênc. Agrotecnologia 2016, 40, 698–705. [Google Scholar] [CrossRef]
- Castle, M.E.; Watson, J.N. Silage and milk production: Studies with molasses and formic acid additives for grass silage. Grass Forage Sci. 1985, 40, 85–92. [Google Scholar] [CrossRef]
- Wróbel, B.; Nowak, J.; Fabiszewska, A.; Paszkiewicz-Jasinska, A.; Przystupa, W. Dry matter losses in silages resulting from epiphytic microbiota activity—A comprehensive study. Agronomy 2023, 13, 450. [Google Scholar] [CrossRef]
- Day, C.A.; Liscansky, S.G. Agricultural alternatives. In Environmental Biotechnology; Forster, C.F., Wase, D.A.J., Eds.; Ellis Horwood Ltd.: Chichester, UK, 1987; pp. 234–294. [Google Scholar]
- Stadhouders, J.; Spoelstra, S.F. Prevention of the contamination of raw milk by making a good silage. Bull. Int. Dairy Fed. 1990, 251, 40–46. [Google Scholar]
- Muck, R.E. Factors influencing silage quality and their implications for management. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- Xu, D.; Ding, Z.; Wang, M.; Bai, J.; Ke, W.; Zhang, Y.; Guo, X. Characterization of the microbial community, metabolome and biotransformation of phenolic compounds of sainfoin (Onobrychis viciifolia) silage ensiled with or without inoculation of Lactobacillus plantarum. Bioresour. Technol. 2020, 316, 123910. [Google Scholar] [CrossRef] [PubMed]
- Peyrat, J.; Nozièrea, P.; Le Morvana, A.; Férardc, A.; Protinc, P.V.; Baumont, R. Effects of ensiling maize and sample conditioning on in situ rumen degradation of dry matter, starch and fibre. Anim. Feed Sci. Technol. 2014, 196, 12–21. [Google Scholar] [CrossRef]
- Gerlach, K.; Weiss, K.; Ross, F.; Busher, W.; Sudekum, K.H. Aerobic exposure of grass silages and its impact on dry matter intake and preference by goats. Small Rum. Res. 2014, 117, 131–141. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.F.; Scott, M.B.; Tweed, J.K.S.; Minchin, F.R.; Davies, D.R. Effects of polyphenol oxidase on lipolysis and proteolysis of red clover silage with and without a silage inoculant (Lactobacillus plantarum L54). Anim. Feed Sci. Technol. 2008, 144, 125–136. [Google Scholar] [CrossRef]
- Tabacco, E.; Borreani, G.; Crovetto, G.M.; Galassi, G.; Colombo, D.; Cavallarin, L. Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage. J. Dairy Sci. 2006, 89, 4736–4746. [Google Scholar] [CrossRef]
- Guo, X.S.; Ding, W.R.; Zhou, H. Characterization of protein fractions and amino acids in ensiled alfalfa treated with different chemical additives. Anim. Feed Sci. Technol. 2008, 142, 89–98. [Google Scholar] [CrossRef]
- Ke, W.C.; Yang, F.Y.; Undersander, D.J.; Guo, X.S. Fermentation characteristics, aerobic stability, proteolysis and lipid composition of alfalfa silage ensiled with apple or grape pomace. Anim. Feed Sci. Technol. 2015, 202, 12–19. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Dong, Z.; Chen, L.; Shao, T. Inclusion of alfalfa improves nutritive value and in vitro digestibility of various straw–grass mixed silages in Tibet. Grass Forage Sci. 2018, 73, 694–704. [Google Scholar] [CrossRef]
- Niderkorn, V.; Baumont, R. Associative effects between forages on feed intake and digestion in ruminants. Animal 2009, 3, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.T.; Van-Den, T.A.; Roger, A.; McFeeters, F.; Roger, L.; Thompson, K.V.; Pecota, G.; Yencho, C. Antioxidant activities, phenolic and βcarotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar]
- Martemucci, G.; Portincasa, P.; Centonze, V.; Mariano, M.; Khalil, M.; D’Alessandro, A.G. Prevention of oxidative stress and diseases by antioxidant supplementation. Med. Chem. 2023, 19, 509–537. [Google Scholar] [CrossRef] [PubMed]
- Abarghoei, M.; Rouzbehan, Y.; Alipour, D. Nutritive value and silage characteristics of whole and partly stoned olive cakes treated with molasses. J. Agr. Sci. Technol. 2011, 13, 709–716. [Google Scholar]
- Gefrom, A.; Ott, E.M.; Hoedtke, S.; Zeyner, A. Effect of ensiling moist field bean (Vicia faba), pea (Pisum sativum) and lupine (Lupinus spp.) grains on the contents of alkaloids, oligosaccharides and tannins. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling grape pomace with and without addition of a Lactiplantibacillus plantarum strain: Effect on polyphenols and microbiological characteristics, in vitro nutrient apparent digestibility, and gas emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of phenolic compounds in grape stem extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef]
- Kim, Y.I.; Oh, Y.K.; Park, K.K.; Kwak, W.S. Ensiling characteristics and the in situ nutrient degradability of a by-product feed-based silage. Asian Australas. J. Anim. Sci. 2014, 27, 201–208. [Google Scholar] [CrossRef]
- Chamberlain, D.G. The silage fermentation in relation to the utilization of the nutrients in the rumen. Process. Biochem. 1987, 22, 60–63. [Google Scholar]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Kung, A.P., Jr. The effects of lactobacillus buchneri with or without a homolactic bacterium on the fermentation and aerobic stability of corn silages made at different locations. J. Dairy Sci. 2010, 93, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Zhao, C.; Feng, P.; Wang, Y.; Zhao, X.; Luo, D.; Cheng, L.; Liu, D.; Fang, I. Effects of feeding ground versus pelleted total mixed ration on digestion, rumen function and milk production performance of dairy cows. Int. J. Dairy Technol. 2019, 73, 22–30. [Google Scholar] [CrossRef]
- Moselhy, M.A.; Borba, J.P.; Borba, A.E. Improving the nutritive value, in vitro digestibility and aerobic stability of Hedychium gardnerianum silage through application of additives at ensiling time. Anim. Feed Sci. Technol. 2015, 206, 8–18. [Google Scholar] [CrossRef]



| Silage | WS | GP | CW | OMWW | W |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| SIL-A | 40 | 20 | 0 | 5 | 35 |
| SIL-B | 60 | 0 | 35 | 5 | 0 |
| Experiment 2 | |||||
| SIL-C | 40 | 20 | 20 | 0 | 20 |
| SIL-D | 40 | 20 | 20 | 5 | 15 |
| SIL-E | 40 | 20 | 35 | 0 | 5 |
| SIL-F | 40 | 20 | 35 | 5 | 0 |
| WS | GP | CW | OMWW | |
|---|---|---|---|---|
| Dry matter (%) | 92.94 ± 0.84 | 51.63 ± 4.64 | 6.65 ± 0.35 | 10.18 ± 0.59 |
| Crude protein (% DM) | 3.18 ± 0.08 | 9.75 ± 2.19 | 11.30 ± 3.01 | - |
| Ether extract (% DM) | 1.20 ± 0.01 | 4.55 ± 0.21 | 0.79 ± 0.02 | 9.58 ± 0.16 |
| Ash (% DM) | 6.11 ± 0.06 | 6.76 ±1.60 | 7.53 ± 1.40 | 6.13 ± 0.17 |
| Crude fat (% DM) | 44.44 ± 0.62 | 20.72 ± 0.57 | - | - |
| NDF (% DM) | 73.00 ± 0.74 | 50.48 ± 0.95 | - | - |
| ADF (% DM) | 48.13 ±0.15 | 42.15 ± 1.49 | - | - |
| ADL (% DM) | 7.08 ± 0.07 | 15.39 ± 0.37 | - | - |
| Total phenols (mg GAE/100 g−1 DM) | 11.40 ± 0.10 | 37.50 ± 2.10 |
| Items | Treatment | Effect | ||||
|---|---|---|---|---|---|---|
| SIL-A | SIL-B | SEM | Treatment | Time | Interaction | |
| Dry matter (%) | * | ns | ns | |||
| 0 d | 47.62 a | 59.10 b | 0.185 | |||
| 7 d | 46.15 a | 58.87 b | 0.069 | |||
| 30 d | 47.55 a | 58.75 b | 0.225 | |||
| 90 d | 45.89 a | 58.58 b | 0.053 | |||
| Crude protein (%) | * | ns | ns | |||
| 0 d | 4.23 a | 3.44 b | 0.024 | |||
| 7 d | 3.93 | 3.28 | 0.029 | |||
| 30 d | 3.98 | 3.37 | 0.021 | |||
| 90 d | 4.04 | 3.77 | 0.029 | |||
| Ether extract (%) | * | ns | ns | |||
| 0 d | 1.87 a | 1.51 b | 0.017 | |||
| 7 d | 1.90 a | 1.45 b | 0.018 | |||
| 30 d | 1.88 a | 1.45 b | 0.013 | |||
| 90 d | 1.87 a | 1.46 b | 0.009 | |||
| Crude fiber (%) | ns | ns | ns | |||
| 0 d | 37.19 | 37.91 | 0.019 | |||
| 7 d | 37.27 | 38.32 | 0.056 | |||
| 30 d | 38.25 | 38.70 | 0.020 | |||
| 90 d | 37.28 | 37.78 | 0.020 | |||
| Ash (%) | ns | ns | ns | |||
| 0 d | 6.35 | 6.17 | 0.023 | |||
| 7 d | 6.46 | 6.21 | 0.020 | |||
| 30 d | 6.39 | 6.28 | 0.011 | |||
| 90 d | 6.28 | 6.30 | 0.014 | |||
| NDF (%) | ns | ns | ns | |||
| 0 d | 66.04 | 67.29 | 0.029 | |||
| 7 d | 67.98 | 69.30 | 0.043 | |||
| 30 d | 67.80 | 68.71 | 0.018 | |||
| 90 d | 66.21 | 68.84 | 0.011 | |||
| ADF (%) | ns | ns | ns | |||
| 0 d | 44.61 | 45.21 | 0.058 | |||
| 7 d | 46.78 | 47.14 | 0.010 | |||
| 30 d | 46.81 | 47.86 | 0.027 | |||
| 90 d | 45.58 | 47.49 | 0.027 | |||
| ADL (%) | * | ns | ns | |||
| 0 d | 8.87 a | 6.42 b | 0.027 | |||
| 7 d | 8.41 a | 6.46 b | 0.027 | |||
| 30 d | 8.27 a | 7.20 b | 0.037 | |||
| 90 d | 8.80 a | 7.12 b | 0.058 | |||
| Total phenols, mg GAE g−1 DM | ns | ns | ns | |||
| 0 d | 0.63 | 0.57 | 0.030 | |||
| 7 d | 0.61 | 0.59 | 0.020 | |||
| 30 d | 0.63 | 0.60 | 0.030 | |||
| 90 d | 0.60 | 0.58 | 0.010 | |||
| Treatment | Effect | |||||
|---|---|---|---|---|---|---|
| SIL-A | SIL-B | SEM | Treatment | Time | Interaction | |
| Lactic acid | ns | ** | ns | |||
| 0 d | nd | nd | ||||
| 7 d | 11.03 a | 12.29 a | 0.021 | |||
| 30 d | 19.84 b | 20.06 b | 0.018 | |||
| 90 d | 20.21 b | 19.93 b | 0.027 | |||
| Acetic acid | ** | ** | * | |||
| 0 d | nd | nd | ||||
| 7 d | 0.91 1a | 0.18 2a | 0.003 | |||
| 30 d | 0.78 1b | 0.23 2 | 0.001 | |||
| 90 d | 0.75 1b | 0.26 2b | 0.002 | |||
| Propionic acid | ns | ns | ns | |||
| 0 d | nd | nd | ||||
| 7 d | nd | nd | ||||
| 30 d | nd | nd | ||||
| 90 d | nd | nd | ||||
| Isobutyric acid | ** | ns | * | |||
| 0 d | nd | nd | ||||
| 7 d | 0.20 | nd | 0.002 | |||
| 30 d | 0.22 1 | 0.02 2 | 0.001 | |||
| 90 d | 0.271 | 0.05 2 | 0.001 | |||
| Butyric acid | * | ** | ** | |||
| 0 d | nd | nd | ||||
| 7 d | 0.18 1a | 0.08 2a | 0.002 | |||
| 30 d | 0.47 b | 0.51 b | 0.009 | |||
| 90 d | 0.57 b | 0.53 b | 0.008 | |||
| At D30: | ||||||
| Total VFA | 1.47 | 0.76 | 0.164 | ** | ||
| Total acids | 21.31 | 20.84 | 0.292 | ns | ||
| % total VFA/total acids | 7.03 | 3.63 | 0.764 | ** | ||
| Treatment | Effect | |||||
|---|---|---|---|---|---|---|
| SIL-C | SIL-D | SIL-E | SIL-F | SEM | Treatment | |
| Dry matter | 50.32 | 50.75 | 50.61 | 50.27 | 0.085 | ns |
| Crude protein | 5.53 | 5.62 | 5.58 | 5.20 | 0.049 | ns |
| Ether extract | 1.87 | 2.04 | 2.01 | 1.93 | 0.013 | ns |
| Crude fiber | 36.51 | 35.95 | 36.34 | 36.91 | 0.016 | ns |
| Ash | 5.93 | 5.66 | 5.64 | 5.69 | 0.012 | ns |
| NDF | 65.74 | 64.09 | 64.11 | 64.35 | 0.021 | ns |
| ADF | 45.48 | 44.52 | 45.13 | 45.26 | 0.025 | ns |
| ADL | 8.75 | 8.21 | 8.67 | 8.85 | 0.025 | ns |
| Total phenols, mg GAE g−1 DM | 0.62 | 0.66 | 0.66 | 0.71 | 0.025 | ns |
| Treatment | Effect | |||||||
|---|---|---|---|---|---|---|---|---|
| SIL-C | SIL-D | SIL-E | SIL-F | SEM | Treatment | Time | Interaction | |
| Lactic acid | * | ** | * | |||||
| 0 d | nd | nd | nd | nd | ||||
| 7 d | 15.09 a | 16.88 a | 15.11 a | 17.33 a | 0.013 | |||
| 30 d | 28.21 1b | 29.88 1,2b | 30.27 2b | 30.91 2b | 0.017 | |||
| 90 d | 27.77 1b | 29.14 b | 29.93 b | 30.86 2b | 0.011 | |||
| Acetic acid | * | ** | ** | |||||
| 0 d | 0.74 1a | 0.53 a | 0.37 2a | 0.33 2a | 0.001 | |||
| 7 d | 2.68 1b | 2.97 2b | 2.62 1b | 2.88 2b | 0.002 | |||
| 30 d | 5.34 1c | 4.49 1c | 6.82 2c | 7.89 2c | 0.002 | |||
| 90 d | 5.43 1c | 4.50 1c | 7.03 2c | 7.92 2c | 0.003 | |||
| Propionic acid | ns | ns | ns | |||||
| 0 d | nd | nd | nd | nd | ||||
| 7 d | nd | 0.02 | 0.08 | 0.01 | 0.001 | |||
| 30 d | nd | 0.02 | 0.08 | 0.04 | 0.001 | |||
| 90 d | 0.02 | 0.02 | 0.06 | 0.01 | 0.001 | |||
| Isobutyric acid | * | ** | * | |||||
| 0 d | 0.08 a | 0.02 a | nd | 0.03 a | 0.001 | |||
| 7 d | 0.18 1b | 0.20 1b | 0.12 1,2 | 0.10 2a | 0.001 | |||
| 30 d | 0.12 1b | 0.20 1b | 0.16 1 | 0.60 2b | 0.002 | |||
| 90 d | 0.15 1b | 0.22 1b | 0.17 1 | 0.63 2b | 0.001 | |||
| Butyric acid | * | * | ns | |||||
| 0 d | nd | nd | nd | nd | ||||
| 7 d | 1.53 1,2a | 1.35 1 | 1.85 2a | 2.01 2a | 0.002 | |||
| 30 d | 1.86 1b | 1.35 2 | 2.09 1a | 2.22 1b | 0.001 | |||
| 90 d | 1.91 1b | 1.37 2 | 2.22 1b | 2.29 1b | 0.001 | |||
| At D30: | ||||||||
| Total VFA | 7.31 A | 6.11 B | 9.15 C | 10.77 D | 0.535 | ** | ||
| Total acids | 35.54 A | 35.94 AB | 39.07 Ca | 41.64 Cb | 0.780 | ** | ||
| % total VFA/total acids | 24.09 A | 16.58 B | 23.43 AC | 25.63 A | 0.508 | ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, A.G.; Dibenedetto, R.S.; Skoufos, I.; Martemucci, G. Potential Use of Wheat Straw, Grape Pomace, Olive Mill Wastewater and Cheese Whey in Mixed Formulations for Silage Production. Agronomy 2023, 13, 2323. https://doi.org/10.3390/agronomy13092323
D’Alessandro AG, Dibenedetto RS, Skoufos I, Martemucci G. Potential Use of Wheat Straw, Grape Pomace, Olive Mill Wastewater and Cheese Whey in Mixed Formulations for Silage Production. Agronomy. 2023; 13(9):2323. https://doi.org/10.3390/agronomy13092323
Chicago/Turabian StyleD’Alessandro, Angela Gabriella, Roberta Savina Dibenedetto, Ioannis Skoufos, and Giovanni Martemucci. 2023. "Potential Use of Wheat Straw, Grape Pomace, Olive Mill Wastewater and Cheese Whey in Mixed Formulations for Silage Production" Agronomy 13, no. 9: 2323. https://doi.org/10.3390/agronomy13092323
APA StyleD’Alessandro, A. G., Dibenedetto, R. S., Skoufos, I., & Martemucci, G. (2023). Potential Use of Wheat Straw, Grape Pomace, Olive Mill Wastewater and Cheese Whey in Mixed Formulations for Silage Production. Agronomy, 13(9), 2323. https://doi.org/10.3390/agronomy13092323






