Hemp (Cannabis sativa L.) Tolerates Chelator Stress Showing Varietal Differences and Concentration Dependence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Seed Germination Test
2.3. Pot Culture of Plants
2.4. Parameters and Test Methods
2.4.1. Seed Germination Indices
2.4.2. Plant Growth and Physiological Indices
2.5. Data Process and Analysis
3. Results
3.1. Effect of Chelators on Seed Germination of Seven Hemp Cultivars
3.1.1. Effect of Chelators on Hemp Seed Germination
3.1.2. Effect of EDTA on Seed Germination
3.1.3. Effect of CA on Seed Germination
3.2. Effects of Chelators on Plant Growth and Physiological Attributes of Two Hemp Cultivars
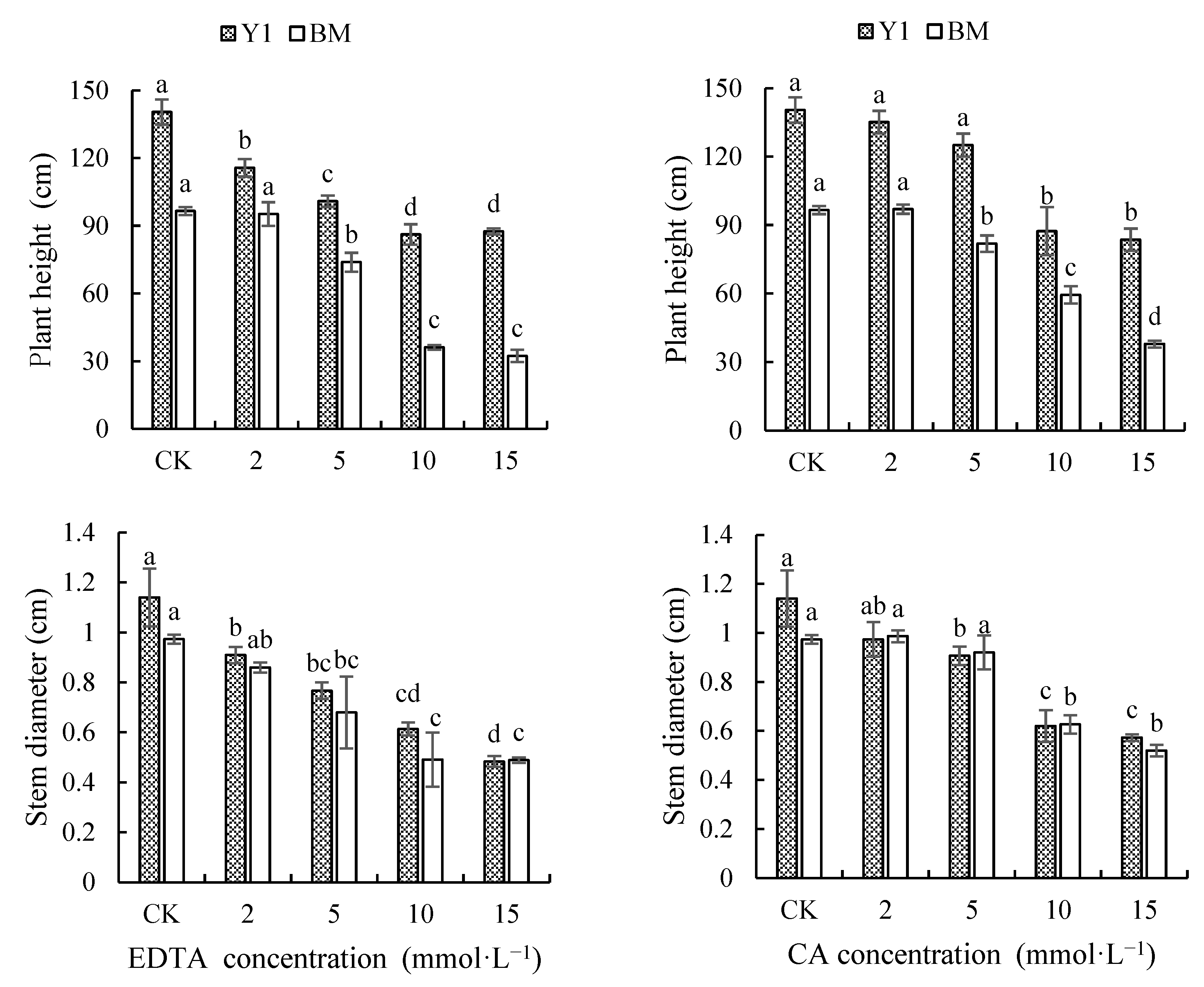
3.2.1. Effect of Chelators on Plant Growth of Hemp Cultivars
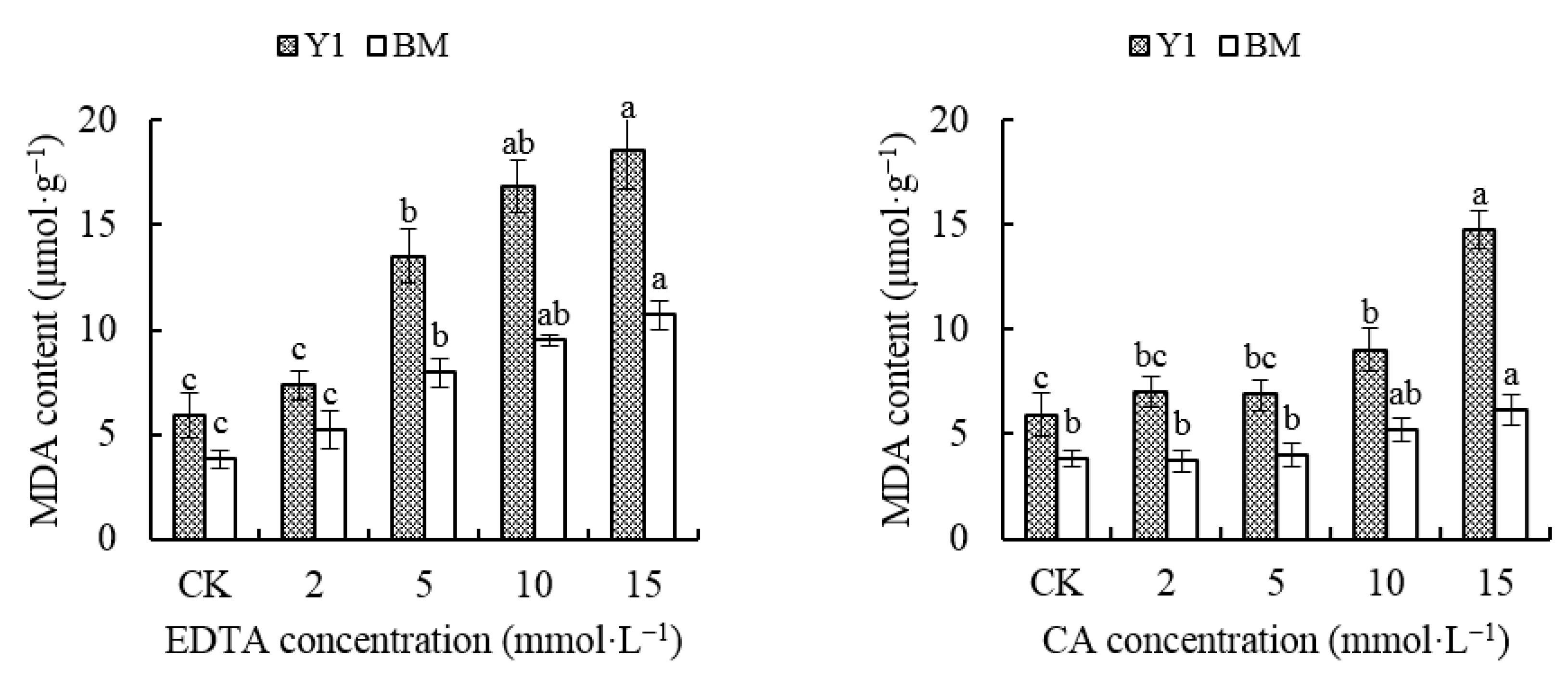
3.2.2. Effect of Chelators on MDA Content in Hemp Leaves
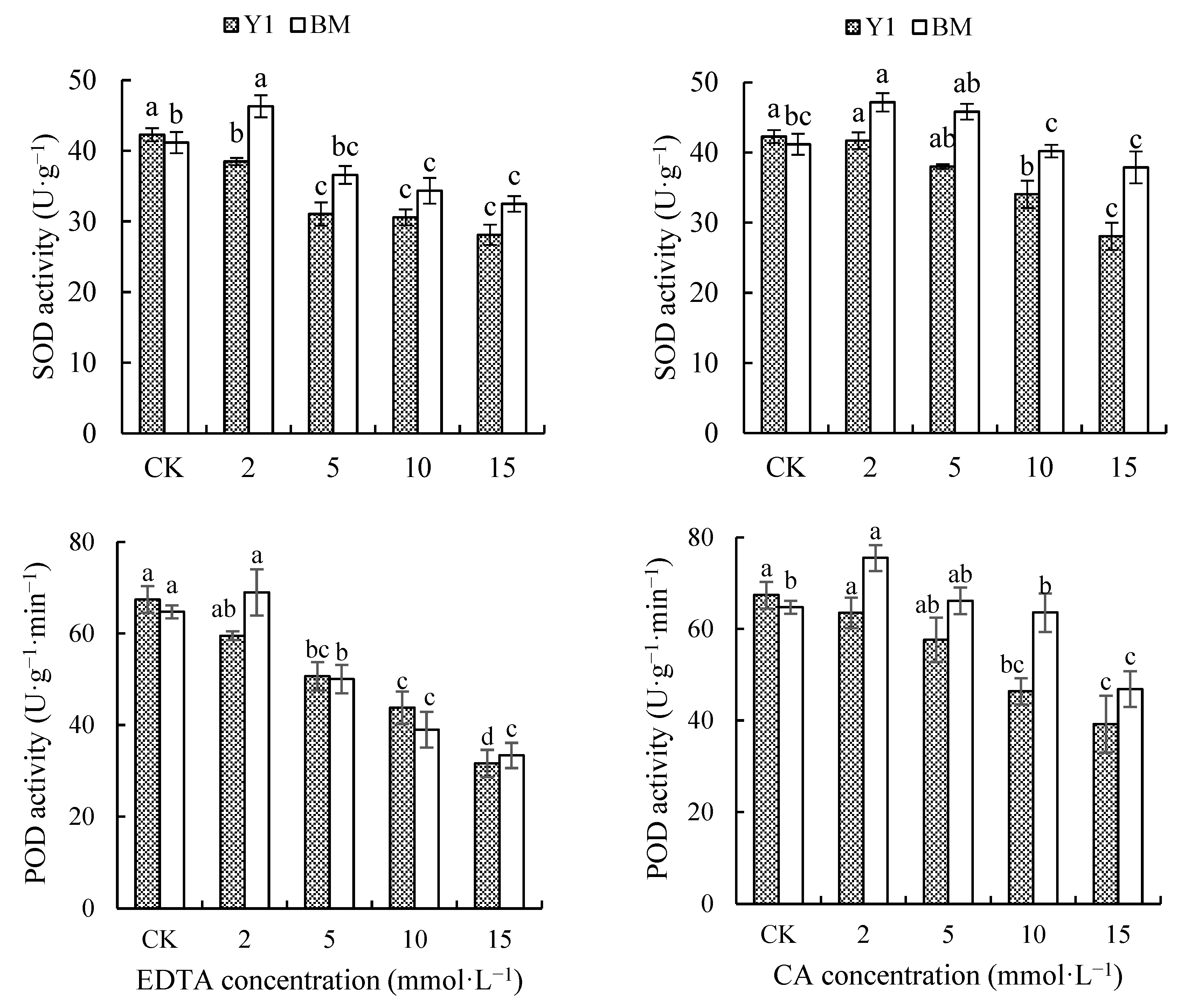
3.2.3. Effect of Chelators on Activities of SOD and POD in Hemp Leaves
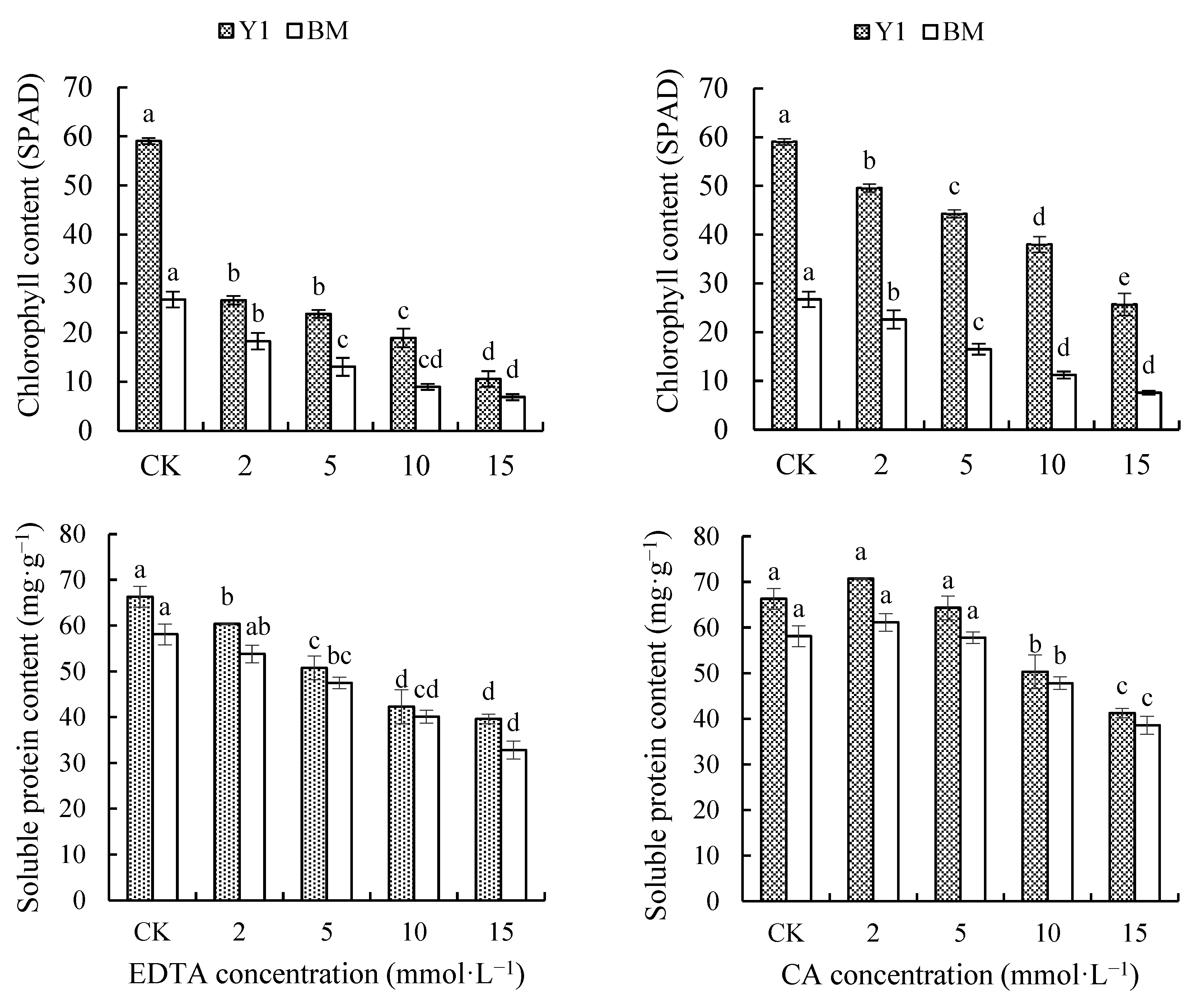
3.2.4. Effect of Chelators on Contents of Chlorophyll and SP in Hemp Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.C.; Zhang, T.; Stoffella, P.J. Heavy metal contamination of soils sources, indicators, and assessment. J. Environ. Indic. 2015, 9, 17–18. [Google Scholar]
- Ministry of Environmental Protection and Ministry of Land and Resources of China. National Survey Report of Soil Contamination Status of China; Department of Environment and Natural Resources: Beijing, China, 2014; pp. 26–27. [Google Scholar]
- Yang, S.C.; Nan, Z.R.; Zeng, J.J. Current situation of soil contaminated by heavy metals and research advances on the remediation techniques. J. Anhui Agric. Sci. 2006, 34, 549–552. [Google Scholar]
- Su, C.; Jiang, L.Q.; Zhang, W.J. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Crit. 2014, 3, 24–38. [Google Scholar]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.X.; Qin, Y.; Deng, S.H. Progress of phytoremediation on soil polluted by heavy metal. Hubei For. Sci. Technol. 2016, 45, 40–43+63. [Google Scholar]
- Yang, P.; Chen, X.Y.; Tian, L.L.; Zhang, K.K.; Xu, L.L.; Guan, P. Effects of EDTA mixed cadmium stress on the seed germination an physiological resistance characters of initial growth in Astragalus sinicus. J. Mt. Agric. Biol. 2016, 35, 26–30+80. [Google Scholar]
- Udawatta, R.P.; Motavalli, P.P.; Garrett, H.E.; Krstansky, J.J. Nitrogen losses in runoff from three adjacent agricultural watersheds with claypan soils. Agric. Ecosyst. Environ. 2006, 117, 39–48. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E.; Imborvungu, J.A. Removal of heavy metals from a contaminated soil using organic chelating acids. Int. J. Environ. Sci. Technol. 2010, 7, 485–496. [Google Scholar] [CrossRef]
- Jalali, M.; Khanboluki, G. Redistribution of fractions of zinc, cadmium and lead in calcareous soils treated with EDTA. Arch. Agron. Soil Sci. 2007, 53, 147–160. [Google Scholar] [CrossRef]
- Meers, E.; Tack, F.M.G.; Verloo, M.G. Degradability of ethylenediaminedisuccinic acid (EDDS) in metal contaminated soils: Implications for its use soil remediation. Chemosphere 2008, 70, 358–363. [Google Scholar] [CrossRef]
- RöMkens, P.; Bouwman, L.; Japenga, J.; Draaisma, C. Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ. Pollut. 2002, 116, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Chen, L.S.; Zhu, Y. Effects of EDTA and EM composite agent on seed germination, seedling growth and lead uptake of ryegrass under lead stress. Guangdong Seric. 2020, 54, 22–25. [Google Scholar]
- Lee, J.; Sung, K. Effects of chelates on soil microbial properties, plant growth and heavy metal accumulation in plants. Ecol. Eng. 2014, 73, 386–394. [Google Scholar] [CrossRef]
- Wang, Y.K.; Chen, M.; Zhang, J.Q.; Yuan, B. Research progress of chelate- induced phytoremediation of soil contaminated by heavy metals. J. Hubei Polytech. Univ. 2014, 30, 30–32+40. [Google Scholar]
- Shi, Q.L. Leaching Characteristics od Pb and Cd in the Contaminated Soils by Organic Chelating Agents and Biosurfactant. Master’s Thesis, Southwest University, Chongqing, China, 2015. [Google Scholar]
- Wang, S.Y.; Duo, L.A.; Zhao, S.L. Effects of biodegradable chelator on growth and physiology of Festuca arundinacea seedlings. Acta Hortic. Sin. 2017, 44, 2186–2194. [Google Scholar]
- Diarra, I.; Kotra, K.K.; Prasad, S. Assessment of biodegradable chelating agents in the phytoextraction of heavy metals from multi-metal contaminated soil. Chemosphere 2021, 273, 128483. [Google Scholar] [CrossRef]
- Liu, F.H.; Hu, H.R.; Du, G.H.; Deng, G.; Yang, Y. Ethnobotanical research on origin, cultivation, distribution and utilization of hemp (Cannabis sativa L.) in China. Indian J. Tradit. Knowl. 2017, 16, 235–242. [Google Scholar]
- Galić, M.; Perčin, A.; Zgorelec, Ž.; Kisić, I. Evaluation of heavy metals accumulation potential of hemp (Cannabis sativa L.). J. Cent. Eur. Agric. 2019, 20, 700–711. [Google Scholar]
- Xu, Y.P.; Lü, P.; Zhang, Q.Y.; Guo, R.; Deng, G.; Guo, H.Y.; Yang, M. Comparison of the absorption and accumulation characteristics of five heavy metals among different industrial hemp varieties. J. Agric. Resour. Environ. 2020, 37, 106–114. [Google Scholar]
- Placido, D.F.; Lee, C.C. Potential of Industrial Hemp for Phytoremediation of Heavy Metals. Plants 2022, 11, 595. [Google Scholar] [CrossRef]
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.-G.; Komnou, A.A.; Vasilou, C. Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis Sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Wu, Y.; Trejo, H.X.; Chen, G.; Li, S. Phytoremediation of contaminants of emerging concern from soil with industrial hemp (Cannabis sativa L.): A review. Environ. Dev. Sustain. 2021, 23, 14405–14435. [Google Scholar] [CrossRef]
- De Vos, B.; Souza, M.F.; Michels, E.; Meers, E. Industrial hemp (Cannabis sativa L.) in a phytoattenuation strategy: Remediation potential of a Cd, Pb and Zn contaminated soil and valorization potential of the fibers for textile production. Ind. Crops Prod. 2022, 178, 114592. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, Y.T.; Zhou, Y.; Liu, H.; Li, X.; Du, G.H.; Liu, F.H. Chelators could enhance hemp efficacy in remediation of Pb contamination in culture substrate. J. Yunnan Univ. Nat. Sci. Ed. 2022, 44, 1297–1304. [Google Scholar]
- Chen, Z.Y. Research of Seed Germination, Plant Growth and Physiology of Hemp Variety under Heavy Metal Pb Stress. Master’s Thesis, Yunnan University, Kunming, China, 2016. [Google Scholar]
- Hu, H.R. Effect of Salinity on Seed Germination and Seeding Growth of Hemp. Master’s Thesis, Yunnan University, Kunming, China, 2015. [Google Scholar]
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2006; pp. 260–261, 167–169, 184–185. [Google Scholar]
- Zhang, Z.L.; Zhai, W.J. Plant Physiological Experiment Instruction; Higher Education Press: Beijing, China, 2004. [Google Scholar]
- Liu, H. Research on Pollution Effects of Chelating Agents in Chelate-Induced Phytoremediation Technology. Master’s Thesis, Northeast Forestry University, Harbin, China, 2010. [Google Scholar]
- Zhang, Y.; Deng, B.; Li, Z. Inhibition of NADPH oxidase increases defense enzyme activities and improves maize seed germination under Pb stress. Ecotoxicol. Envion. Saf. 2018, 158, 187–192. [Google Scholar] [CrossRef]
- Li, H.Y. The Effects of Exogenous Citric and Oxalic Acid on Cadmium Induced Physiological Response in Boehmeria nivea (L.) Gaud. Master’s Thesis, Hunan University, Changsha, China, 2014. [Google Scholar]
- Wang, X.Y.; Peng, W.B. Effect of organic acids and boron and zinc on metabolism of active oxygen during filling period and seed weight of wheat. Sci. Agric. Sin. 1995, 28, 69–74. [Google Scholar]
- Sillanp, M.; Oikari, A. Assessing the impact of complexation by EDTA and DTPA on heavy metal toxicity using microtox bioassay. Chemosphere 1996, 32, 1485–1497. [Google Scholar] [CrossRef]
- Xu, W.J.; Guo, J.; Zhao, M.; Wang, R.Y.; Hou, S.Z.; Yang, Y.; Zhong, B.; Guo, H.; Liu, C.; Shen, Y.; et al. Research progress of soil plant root exudates in heavy metal contaminated soil. J. Zhejiang A F Univ. 2017, 34, 1137–1148. [Google Scholar]
- Liu, L. The Regulation and Mechanism of Phytohormone on Rice Seed Germination and Seeding Root Growth under Salinity. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Ding, Z.H.; Hu, X.; Zhang, Y.F. Metal uptake of wheat seedlings and metal distribution after the addition of EDTA, [S, S]-EDDS and DTPA. Ecol. Environ. Sci. 2010, 19, 97–101. [Google Scholar]
- Ma, Y.; Zhao, G.L.; Wang, X.F.; Cheng, J.M. Remediation of lead and cadmium contaminated soil with chelate induced Beta vulgaris Var. Cicla L. Chin. J. Soil Sci. 2021, 52, 416–424. [Google Scholar]
- Guo, H.; Zhuang, J.J. Effect of citric acid amendment on cadmium uptake and translocation by three ornamental plants. J. Anhui Agric. Univ. 2021, 48, 121–127. [Google Scholar]
- Chen, Y.X.; Lin, Q.; Luo, Y.M.; He, Y.F.; Zhen, S.J.; Yu, Y.L.; Tian, G.M.; Wong, M.H. The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 2003, 50, 807–811. [Google Scholar] [CrossRef]
- Najeeb, U.; Xu, L.; Ali, S.; Jilani, G.; Gong, H.J.; Shen, W.Q.; Zhou, W.J. Citric acid enhances the phytoextraction of manganese and plant growth by alleviating the ultrastructural damages in Juncus effusus L. J. Hazard. Mater. 2009, 170, 1156–1163. [Google Scholar] [CrossRef]
- Aderholt, M.; Vogelien, D.L.; Koether, M.; Greipsson, S. Phytoextraction of contaminated urban soils by Panicum virgatum L. enhanced with application of a plant growth regulator (BAP) and citric acid. Chemosphere 2017, 175, 85–96. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Heavy metal accumulation and anti-oxidative feedback as a biomarker in Seagrass Cymodocea serrulata. Sustainability 2020, 12, 2841. [Google Scholar] [CrossRef]
- Abedi, T.; Paknyat, H. Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J. Genet. Plant Breed. 2010, 46, 27–34. [Google Scholar] [CrossRef]
- Zhu, S.J.; Gui, S.B.; Xu, W.; Xiang, P.; Meng, W.; Zhu, J.; Li, K.K. Experimental research on the tolerance of four emergent plants under high nitrogen and phosphorus pollution conditions. China Rural. Water Hydropower 2022, 2, 34–38. [Google Scholar]
- Tauqeer, H.M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Iftikhar, U.; Ahmad, R.; Farid, M.; Abbasi, G.H. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016, 126, 138–146. [Google Scholar] [CrossRef]
- Araújo, R.P.d.; Almeida, A.-A.F.d.; Pereira, L.S.; Mangabeira, P.A.O.; Souza, J.O.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Photosynthetic, antioxidative, molecular and ultrastructural responses of young cacao plants to Cd toxicity in the soil. Ecotoxicol. Environ. Saf. 2017, 144, 148–157. [Google Scholar] [CrossRef]
- Li, F.M.; Xiong, Z.T.; Hu, H.Y. Effects of chelating agents on toxicity of copper to Elsholtzia splendens. Environ. Sci. 2003, 24, 96–100. [Google Scholar]
- Meers, E.; Qadir, M.; De Caritat, P.; Tack, F.M.; Du Laing, G.; Zia, M.H. EDTA-assisted Pb phytoextraction. Chemosphere 2009, 74, 1279–1291. [Google Scholar]
| Parameter | Variance Source | SS | df | MS | F |
|---|---|---|---|---|---|
| Germination potential | Chelators | 0.430 | 1 | 0.430 | 86.594 ** |
| Concentrations (con.) | 2.137 | 4 | 0.534 | 107.667 ** | |
| Cultivars (var.) | 1.413 | 6 | 0.235 | 47.447 ** | |
| Chelator × con. | 0.110 | 4 | 0.027 | 5.525 ** | |
| Chelator × var. | 0.286 | 6 | 0.048 | 9.611 ** | |
| Con. × var. | 0.397 | 24 | 0.017 | 3.331 ** | |
| Chelator × con. × var. | 0.129 | 24 | 0.005 | 1.080 | |
| Error | 0.695 | 140 | 0.005 | ||
| Sum | 5.596 | 209 | |||
| Germination rate | Chelators | 0.659 | 1 | 0.659 | 109.019 ** |
| Concentrations (con.) | 2.336 | 4 | 0.584 | 96.564 ** | |
| Cultivars (var.) | 1.701 | 6 | 0.283 | 46.866 ** | |
| Chelator × con. | 0.142 | 4 | 0.036 | 5.872 ** | |
| Chelator × var. | 1.191 | 6 | 0.198 | 32.819 ** | |
| Con. × var. | 0.224 | 24 | 0.009 | 1.540 | |
| Chelator × con. × var. | 0.179 | 24 | 0.007 | 1.234 | |
| Error | 0.847 | 140 | 0.006 | ||
| Sum | 7.278 | 209 | |||
| Radicle length | Chelators | 774.445 | 1 | 774.445 | 3794.947 ** |
| Concentrations (con.) | 453.141 | 4 | 113.285 | 555.122 ** | |
| Cultivars (var.) | 71.177 | 6 | 11.863 | 58.131 ** | |
| Chelator × con. | 191.16 | 4 | 47.79 | 234.182 ** | |
| Chelator × var. | 51.681 | 6 | 8.614 | 42.208 ** | |
| Con. × var. | 56.968 | 24 | 2.374 | 11.631 ** | |
| Chelator × con. × var. | 55.871 | 24 | 2.328 | 11.407 ** | |
| Error | 57.14 | 280 | 0.204 | ||
| Sum | 1711.583 | 349 | |||
| Embryonic shoot length | Chelators | 106.426 | 1 | 106.426 | 833.218 ** |
| Concentrations (con.) | 143.868 | 4 | 35.967 | 281.59 ** | |
| Cultivars (var.) | 15.738 | 6 | 2.623 | 20.535 ** | |
| Chelator × con. | 12.043 | 4 | 3.011 | 23.572 ** | |
| Chelator × var. | 10.118 | 6 | 1.686 | 13.202 ** | |
| Con. × var. | 3.298 | 24 | 0.137 | 1.076 | |
| Chelator × con. × var. | 7.075 | 24 | 0.295 | 2.308 ** | |
| Error | 35.764 | 280 | 0.128 | ||
| Sum | 334.33 | 349 |
| Cultivar | EDTA Con. (mmol·L−1) | GP (%) | GR (%) | RL (cm) | SL (cm) |
|---|---|---|---|---|---|
| Y1 | 0 | 53.33 ± 3.33 a | 57.78 ± 2.22 a | 4.42 ± 0.62 a | 3.72 ± 0.41 a |
| 2 | 27.78 ± 1.11 b | 34.44 ± 2.94 b | 1.44 ± 0.36 b | 1.32 ± 0.22 b | |
| 5 | 26.67 ± 3.33 b | 30.00 ± 3.33 b | 0.66 ± 0.12 bc | 0.86 ± 0.17 bc | |
| 10 | 24.44 ± 2.94 bc | 32.22 ± 2.94 b | 0.24 ± 0.05 c | 0.66 ± 0.17 bc | |
| 15 | 27.78 ± 1.11 b | 28.89 ± 2.22 b | 0.15 ± 0.02 c | 0.44 ± 0.05 c | |
| 25 | 17.78 ± 1.11 c | 18.89 ± 2.22 c | 0.11 ± 0.02 c | 0.26 ± 0.02 c | |
| Y7 | 0 | 53.33 ± 5.09 a | 70.00 ± 5.09 a | 3.96 ± 0.64 a | 3.52 ± 0.40 a |
| 2 | 53.33 ± 6.67 a | 65.56 ± 4.01 ab | 1.08 ± 0.10 b | 1.58 ± 0.16 b | |
| 5 | 50.00 ± 3.33 a | 55.56 ± 4.01 b | 0.60 ± 0.13 bc | 0.88 ± 0.10 c | |
| 10 | 35.56 ± 2.94 b | 42.22 ± 2.94 c | 0.36 ± 0.07 bc | 0.64 ± 0.04 c | |
| 15 | 27.78 ± 2.94 b | 28.89 ± 2.94 d | 0.24 ± 0.05 bc | 0.42 ± 0.06 c | |
| 25 | 25.56 ± 4.01 b | 27.78 ± 2.94 d | 0.09 ± 0.01 c | 0.42 ± 0.05 c | |
| BM | 0 | 66.67 ± 3.85 a | 93.33 ± 1.92 a | 5.66 ± 1.28 a | 3.49 ± 0.34 a |
| 2 | 67.78 ± 2.94 a | 83.33 ± 1.92 ab | 1.29 ± 3.13 b | 2.51 ± 0.30 b | |
| 5 | 52.22 ± 4.01 a | 74.44 ± 4.01 bc | 0.49 ± 0.12 bc | 1.29 ± 0.22 c | |
| 10 | 51.11 ± 9.88 a | 74.44 ± 4.84 bc | 0.29 ± 0.61 bc | 0.84 ± 0.09 d | |
| 15 | 32.22 ± 4.01 b | 60.00 ± 5.09 c | 0.14 ± 0.03 bc | 0.75 ± 0.09 d | |
| 25 | 21.11 ± 4.01 b | 58.89 ± 9.69 c | 0.06 ± 0.01 c | 0.60 ± 0.12 d | |
| YW6 | 0 | 38.39 ± 4.44 a | 51.11 ± 4.84 a | 2.50 ± 0.11 a | 3.14 ± 0.39 a |
| 2 | 33.33 ± 7.70 a | 34.44 ± 5.88 b | 1.38 ± 0.21 b | 2.06 ± 0.28 b | |
| 5 | 33.33 ± 1.92 a | 33.33 ± 7.70 b | 0.80 ± 0.20 c | 1.30 ± 0.36 c | |
| 10 | 27.78 ± 2.94 a | 34.44 ± 2.22 b | 0.38 ± 0.10 d | 0.90 ± 0.18 cd | |
| 15 | 27.78 ± 6.76 a | 27.78 ± 2.94 b | 0.26 ± 0.05 d | 0.52 ± 0.06 cd | |
| 25 | 26.67 ± 5.09 a | 27.78 ± 4.84 b | 0.16 ± 0.02 d | 0.48 ± 0.04 d | |
| Q1 | 0 | 45.56 ± 1.11 a | 64.44 ± 4.01 a | 8.30 ± 0.59 a | 4.06 ± 0.19 a |
| 2 | 42.22 ± 2.94 a | 53.33 ± 3.85 ab | 0.92 ± 0.21 b | 1.52 ± 0.25 b | |
| 5 | 37.78 ± 4.01 a | 52.22 ± 1.11 bc | 0.70 ± 0.17 b | 1.14 ± 0.23 bc | |
| 10 | 37.78 ± 4.01 a | 42.22 ± 2.94 bc | 0.30 ± 0.03 b | 0.76 ± 0.07 cd | |
| 15 | 37.78 ± 2.22 a | 41.11 ± 4.01 c | 0.12 ± 0.02 b | 0.58 ± 0.04 d | |
| 25 | 20.00 ± 1.92 b | 25.56 ± 4.84 d | 0.11 ± 0.02 b | 0.54 ± 0.07 d | |
| W1 | 0 | 53.33 ± 1.92 a | 70.00 ± 3.85 a | 6.70 ± 0.28 a | 3.28 ± 0.26 a |
| 2 | 52.22 ± 8.01 a | 58.89 ± 7.29 ab | 1.66 ± 0.15 b | 2.40 ± 0.43 b | |
| 5 | 48.89 ± 2.94 ab | 54.44 ± 5.88 ab | 0.94 ± 0.09 c | 1.34 ± 0.24 c | |
| 10 | 44.44 ± 7.29 ab | 46.67 ± 6.94 bc | 0.68 ± 0.08 cd | 1.10 ± 0.11 cd | |
| 15 | 35.56 ± 1.11 bc | 44.44 ± 2.22 bc | 0.28 ± 0.07 de | 0.88 ± 0.14 cd | |
| 25 | 27.78 ± 4.01 c | 34.44 ± 4.84 c | 0.16 ± 0.02 e | 0.58 ± 0.04 d | |
| J1 | 0 | 35.56 ± 2.94 a | 58.89 ± 2.22 a | 3.18 ± 0.35 a | 3.32 ± 0.14 a |
| 2 | 32.22 ± 2.94 a | 47.78 ± 2.94 ab | 1.06 ± 0.29 b | 1.78 ± 0.10 b | |
| 5 | 17.78 ± 2.94 b | 43.33 ± 6.94 bc | 0.70 ± 0.15 bc | 1.20 ± 0.07 c | |
| 10 | 13.33 ± 3.85 bc | 35.56 ± 5.56 bc | 0.50 ± 0.13 bc | 0.94 ± 0.09 cd | |
| 15 | 7.78 ± 2.94 c | 30.00 ± 3.85 c | 0.36 ± 0.10 c | 0.78 ± 0.11 d | |
| 25 | 5.56 ± 1.11 c | 28.89 ± 2.94 c | 0.12 ± 0.02 c | 0.56 ± 0.06 d |
| Cultivar | CA Con. (mmol·L−1) | GP (%) | GR (%) | RL (cm) | SL (cm) |
|---|---|---|---|---|---|
| Y1 | 0 | 54.67 ± 3.93 a | 65.67 ± 2.94 ab | 5.10 ± 0.48 a | 3.88 ± 0.17 a |
| 2 | 56.67 ± 3.76 a | 75.33 ± 4.01 a | 5.08 ± 0.42 a | 3.88 ± 0.08 a | |
| 5 | 52.33 ± 2.23 ab | 60.33 ± 3.33 b | 5.18 ± 0.29 a | 3.54 ± 0.24 a | |
| 10 | 48.00 ± 1.00 ab | 60.00 ± 5.09 b | 4.02 ± 0.24 b | 2.54 ± 0.13 b | |
| 15 | 42.33 ± 3.93 bc | 56.67 ± 5.09 b | 2.76 ± 0.27 c | 1.36 ± 0.10 c | |
| 25 | 35.67 ± 2.96 c | 36.67 ± 3.85 c | 1.74 ± 0.23 d | 1.14 ± 0.07 c | |
| Y7 | 0 | 51.33 ± 2.94 a | 65.33 ± 3.93 ab | 4.78 ± 0.17 ab | 2.08 ± 0.17 b |
| 2 | 59.00 ± 2.22 a | 77.00 ± 5.77 a | 5.26 ± 0.31 a | 2.84 ± 0.20 a | |
| 5 | 59.67 ± 3.33 a | 73.33 ± 5.24 a | 4.34 ± 0.18 b | 2.00 ± 0.12 b | |
| 10 | 38.76 ± 2.94 b | 53.33 ± 3.33 bc | 3.14 ± 0.14 c | 1.72 ± 0.15 b | |
| 15 | 30.00 ± 3.85 bc | 48.00 ± 4.93 cd | 1.36 ± 0.22 d | 1.16 ± 0.09 c | |
| 25 | 23.33 ± 3.85 c | 35.67 ± 2.96 d | 0.82 ± 0.14 d | 0.84 ± 0.13 c | |
| BM | 0 | 54.33 ± 4.84 b | 74.67 ± 4.01 a | 5.40 ± 0.24 a | 3.08 ± 0.10 a |
| 2 | 72.00 ± 4.84 a | 76.67 ± 5.09 a | 5.82 ± 0.40 a | 2.80 ± 0.17 ab | |
| 5 | 59.00 ± 1.11 b | 79.67 ± 3.33 a | 4.36 ± 0.14 b | 2.42 ± 0.16 bc | |
| 10 | 47.67 ± 2.94 bc | 61.00 ± 1.11 b | 3.12 ± 0.12 c | 2.04 ± 0.12 cd | |
| 15 | 35.67 ± 4.84 cd | 52.33 ± 4.44 bc | 2.50 ± 0.13 c | 1.80 ± 0.11 d | |
| 25 | 27.67 ± 4.01 d | 45.67 ± 2.94 c | 1.08 ± 0.24 d | 1.04 ± 0.12 e | |
| YW6 | 0 | 63.33 ± 5.09 a | 82.00 ± 4.93 ab | 4.02 ± 0.34 c | 3.52 ± 0.14 a |
| 2 | 59.00 ± 4.01 ab | 88.00 ± 4.93 a | 5.66 ± 0.32 a | 2.96 ± 0.16 b | |
| 5 | 63.33 ± 3.85 a | 75.33 ± 3.93 abc | 4.98 ± 0.09 b | 2.54 ± 0.12 c | |
| 10 | 48.00 ± 5.88 bc | 69.00 ± 9.87 bc | 2.92 ± 0.17 d | 1.62 ± 0.18 d | |
| 15 | 36.67 ± 3.85 c | 61.00 ± 4.16 c | 1.98 ± 0.09 e | 1.16 ± 0.12 e | |
| 25 | 35.67 ± 2.94 c | 43.33 ± 3.33 d | 0.92 ± 0.09 f | 0.86 ± 0.12 e | |
| Q1 | 0 | 45.67 ± 4.84 b | 63.33 ± 3.33 b | 7.68 ± 0.31 b | 2.42 ± 0.09 b |
| 2 | 60.00 ± 5.09 a | 78.67 ± 4.44 a | 8.48 ± 0.43 a | 2.96 ± 0.20 a | |
| 5 | 53.33 ± 5.09 ab | 58.00 ± 4.84 b | 4.08 ± 0.31 c | 2.34 ± 0.13 b | |
| 10 | 43.67 ± 3.33 bc | 52.00 ± 5.88 b | 2.84 ± 0.11 d | 1.56 ± 0.12 c | |
| 15 | 31.33 ± 2.94 cd | 35.67 ± 2.94 c | 1.28 ± 0.18 e | 1.18 ± 0.17 cd | |
| 25 | 20.00 ± 3.85 d | 32.33 ± 2.94 c | 0.70 ± 0.10 e | 0.90 ± 0.07 d | |
| W1 | 0 | 62.00 ± 1.11 a | 66.67 ± 1.92 ab | 7.44 ± 0.23 b | 3.38 ± 0.17 a |
| 2 | 63.33 ± 3.85 a | 71.00 ± 4.84 a | 9.64 ± 0.57 a | 3.58 ± 0.18 a | |
| 5 | 60.00 ± 1.92 a | 64.33 ± 1.11 ab | 7.98 ± 0.33 b | 3.38 ± 0.21 a | |
| 10 | 46.67 ± 6.94 b | 55.33 ± 6.19 b | 4.90 ± 0.34 c | 2.60 ± 0.17 b | |
| 15 | 30.00 ± 3.85 c | 39.00 ± 2.22 c | 2.72 ± 0.16 d | 2.10 ± 0.26 bc | |
| 25 | 17.67 ± 2.94 d | 32.33 ± 2.22 c | 1.28 ± 0.17 e | 1.54 ± 0.20 c | |
| J1 | 0 | 25.33 ± 4.01 a | 32.00 ± 4.84 ab | 4.14 ± 0.21 a | 3.40 ± 0.15 ab |
| 2 | 28.67 ± 2.94 a | 40.00 ± 5.77 a | 4.12 ± 0.20 a | 3.60 ± 0.21 a | |
| 5 | 28.00 ± 5.88 a | 39.00 ± 4.84 a | 2.92 ± 0.22 b | 2.94 ± 0.19 b | |
| 10 | 21.00 ± 4.84 a | 31.33 ± 2.94 ab | 2.32 ± 0.17 b | 2.12 ± 0.11 c | |
| 15 | 17.67 ± 2.22 a | 25.67 ± 2.94 ab | 1.40 ± 0.27 c | 1.36 ± 0.10 d | |
| 25 | 16.67 ± 1.92 a | 22.33 ± 4.01 b | 1.26 ± 0.19 c | 1.00 ± 0.18 d |
| Index | Chelator | Concentrations of Chelator (mmol·L−1) | ||||
|---|---|---|---|---|---|---|
| 2 | 5 | 10 | 15 | 25 | ||
| GP | EDTA | 0.90 ± 0.06 b | 0.77 ± 0.05 b | 0.67 ± 0.05 b | 0.57 ± 0.05 a | 0.42 ± 0.04 a |
| CA | 1.15 ± 0.06 a | 1.08 ± 0.06 a | 0.85 ± 0.05 a | 0.65 ± 0.04 a | 0.51 ± 0.03 a | |
| GR | EDTA | 0.80 ± 0.03 b | 0.73 ± 0.04 b | 0.66 ± 0.03 b | 0.56 ± 0.03 b | 0.47 ± 0.03 b |
| CA | 1.16 ± 0.06 a | 1.02 ± 0.03 a | 0.85 ± 0.03 a | 0.73 ± 0.04 a | 0.56 ± 0.02 a | |
| RL | EDTA | 0.31 ± 0.03 b | 0.17 ± 0.02 b | 0.10 ± 0.01 b | 0.06 ± 0.01 b | 0.03 ± 0.01 b |
| CA | 1.15 ± 0.04 a | 0.92 ± 0.05 a | 0.63 ± 0.03 a | 0.38 ± 0.03 a | 0.22 ± 0.02 a | |
| SL | EDTA | 0.56 ± 0.04 b | 0.34 ± 0.02 b | 0.25 ± 0.02 b | 0.19 ± 0.01 b | 0.15 ± 0.01 b |
| CA | 1.07 ± 0.04 a | 0.90 ± 0.03 a | 0.67 ± 0.03 a | 0.48 ± 0.03 a | 0.35 ± 0.02 a | |
| Chelator and Con. (mmol·L−1) | Cultivar Y1 | Cultivar BM | |||||
|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Root | Stem | Leaf | ||
| CA | CK | 7.37 ± 0.41 a | 22.87 ± 0.55 a | 21.85 ± 0.88 a | 6.83 ± 0.32 a | 17.48 ± 0.36 b | 13.96 ± 0.30 a |
| 2 | 6.15 ± 0.12 b | 20.60 ± 0.58 b | 20.33 ± 0.48 a | 7.48 ± 0.17 a | 19.53 ± 0.35 a | 14.27 ± 0.23 a | |
| 5 | 4.43 ± 0.26 c | 18.33 ± 0.29 c | 15.67 ± 0.52 b | 5.03 ± 0.33 b | 15.80 ± 0.53 c | 11.82 ± 0.30 b | |
| 10 | 3.97 ± 0.25 c | 13.47 ± 0.66 d | 10.40 ± 1.27 c | 3.65 ± 0.22 c | 13.00 ± 0.53 d | 5.95 ± 0.31 c | |
| 15 | 2.46 ± 0.19 d | 8.47 ± 0.48 e | 7.80 ± 0.42 d | 1.89 ± 0.13 d | 6.07 ± 0.48 e | 3.99 ± 0.35 d | |
| EDTA | CK | 7.37 ± 0.41 a | 22.87 ± 0.55 a | 21.85 ± 0.88 a | 6.83 ± 0.32 a | 17.48 ± 0.36 a | 13.96 ± 0.30 a |
| 2 | 5.90 ± 0.17 b | 18.93 ± 0.53 b | 18.53 ± 0.47 b | 6.15 ± 0.20 a | 16.53 ± 0.47 a | 11.81 ± 0.29 b | |
| 5 | 3.93 ± 0.35 c | 17.20 ± 0.42 c | 15.27 ± 0.64 c | 5.15 ± 0.18 b | 15.06 ± 0.48 b | 9.52 ± 0.64 c | |
| 10 | 2.88 ± 0.24 d | 11.53 ± 0.47 d | 9.73 ± 0.24 d | 3.59 ± 0.39 c | 11.60 ± 0.40 c | 5.77 ± 0.50 d | |
| 15 | 1.81 ± 0.27 e | 6.94 ± 0.44 e | 5.83 ± 0.26 e | 1.77 ± 0.26 d | 6.26 ± 0.29 d | 3.93 ± 0.38 e | |
| Index | Chelator | Concentrations of Chelator (mmol·L−1) | |||
|---|---|---|---|---|---|
| 2 | 5 | 10 | 15 | ||
| Plant height | EDTA | 0.91 ± 0.04 b | 0.74 ± 0.02 b | 0.49 ± 0.05 b | 0.48 ± 0.07 a |
| CA | 0.98 ± 0.02 a | 0.87 ± 0.02 a | 0.62 ± 0.01 a | 0.50 ± 0.05 a | |
| Stem diameter | EDTA | 0.85 ± 0.04 b | 0.69 ± 0.02 b | 0.53 ± 0.03 a # | 0.47 ± 0.02 b |
| CA | 0.94 ± 0.05 a | 0.88 ± 0.05 a | 0.60 ± 0.04 a # | 0.53 ± 0.03 a | |
| Total biomass | EDTA | 0.87 ± 0.02 b | 0.74 ± 0.02 b | 0.51 ± 0.02 b | 0.30 ± 0.01 a * |
| CA | 0.99 ± 0.04 a | 0.80 ± 0.03 a | 0.56 ± 0.02 a | 0.34 ± 0.01 a * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Hu, J.; Zhang, Y.; Li, X.; Yang, Y.; Du, G.; Tang, K. Hemp (Cannabis sativa L.) Tolerates Chelator Stress Showing Varietal Differences and Concentration Dependence. Agronomy 2023, 13, 2325. https://doi.org/10.3390/agronomy13092325
Liu F, Hu J, Zhang Y, Li X, Yang Y, Du G, Tang K. Hemp (Cannabis sativa L.) Tolerates Chelator Stress Showing Varietal Differences and Concentration Dependence. Agronomy. 2023; 13(9):2325. https://doi.org/10.3390/agronomy13092325
Chicago/Turabian StyleLiu, Feihu, Jianming Hu, Yating Zhang, Xuan Li, Yang Yang, Guanghui Du, and Kailei Tang. 2023. "Hemp (Cannabis sativa L.) Tolerates Chelator Stress Showing Varietal Differences and Concentration Dependence" Agronomy 13, no. 9: 2325. https://doi.org/10.3390/agronomy13092325
APA StyleLiu, F., Hu, J., Zhang, Y., Li, X., Yang, Y., Du, G., & Tang, K. (2023). Hemp (Cannabis sativa L.) Tolerates Chelator Stress Showing Varietal Differences and Concentration Dependence. Agronomy, 13(9), 2325. https://doi.org/10.3390/agronomy13092325






